The Essential Role of H2S-ABA Crosstalk in Maize Thermotolerance through the ROS-Scavenging System
Abstract
:1. Introduction
2. Results
2.1. H2S and ABA Alone or in Combination Induces Thermotolerance
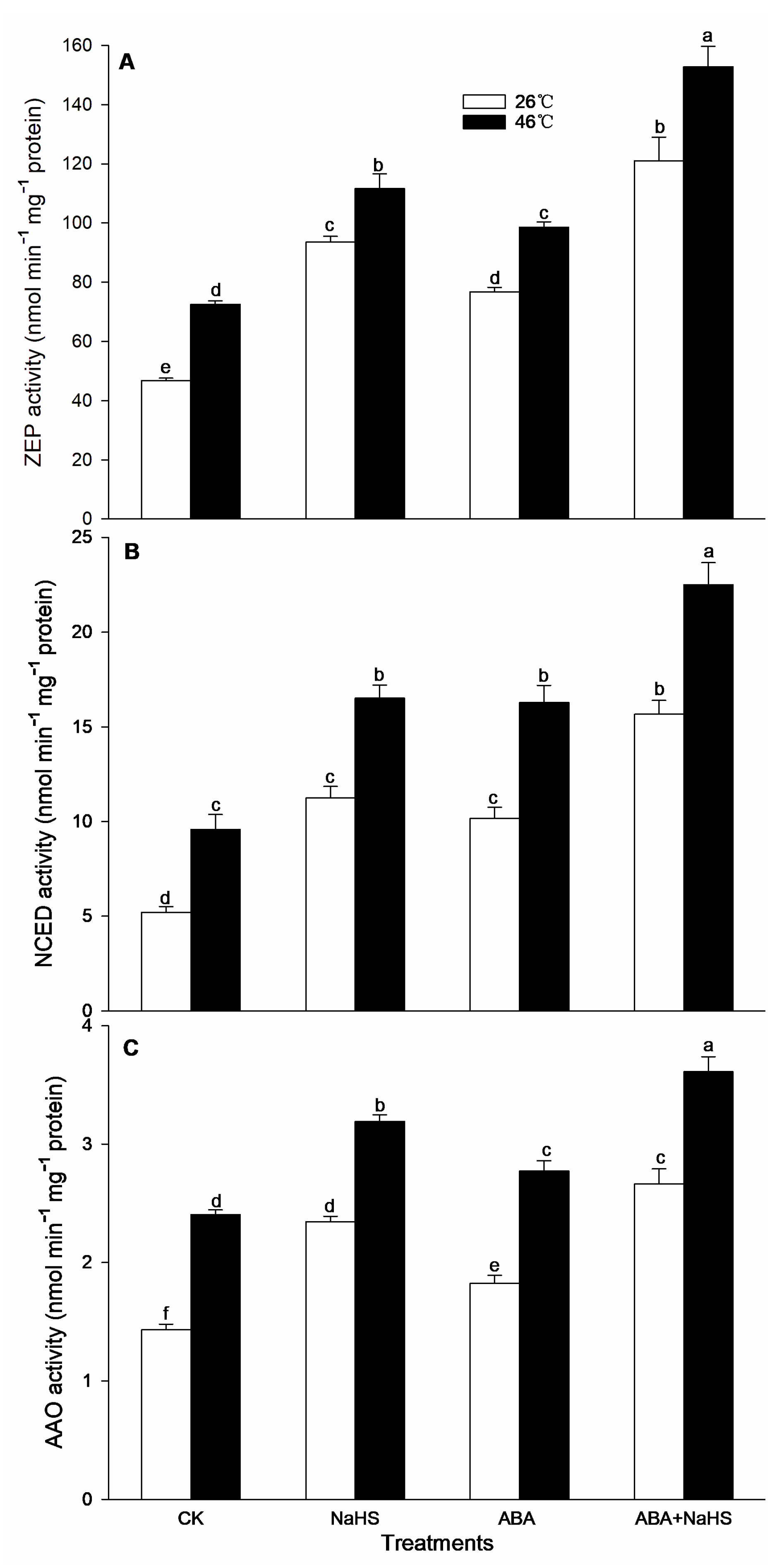
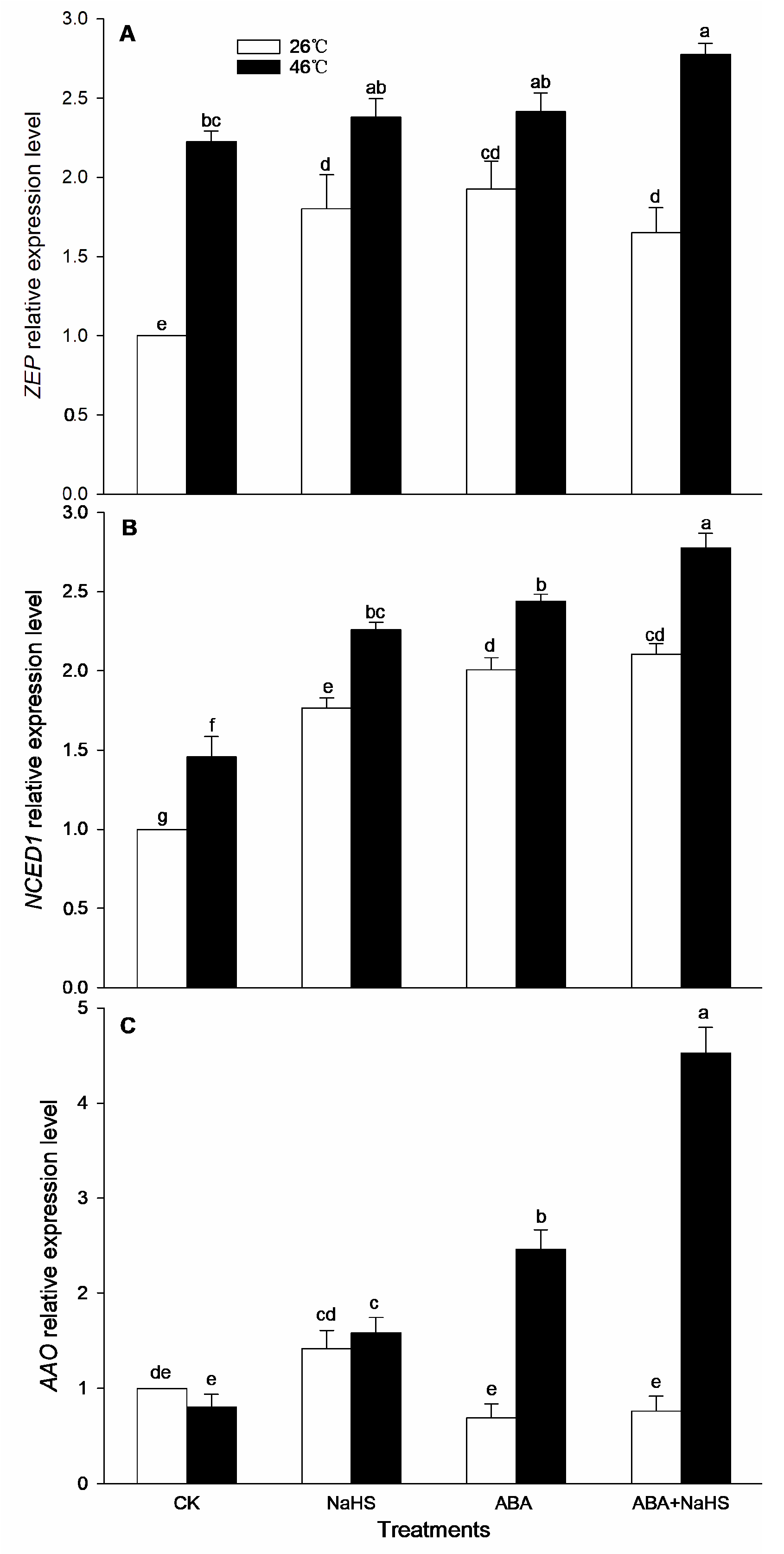
2.2. ABA Increases Endogenous H2S Level
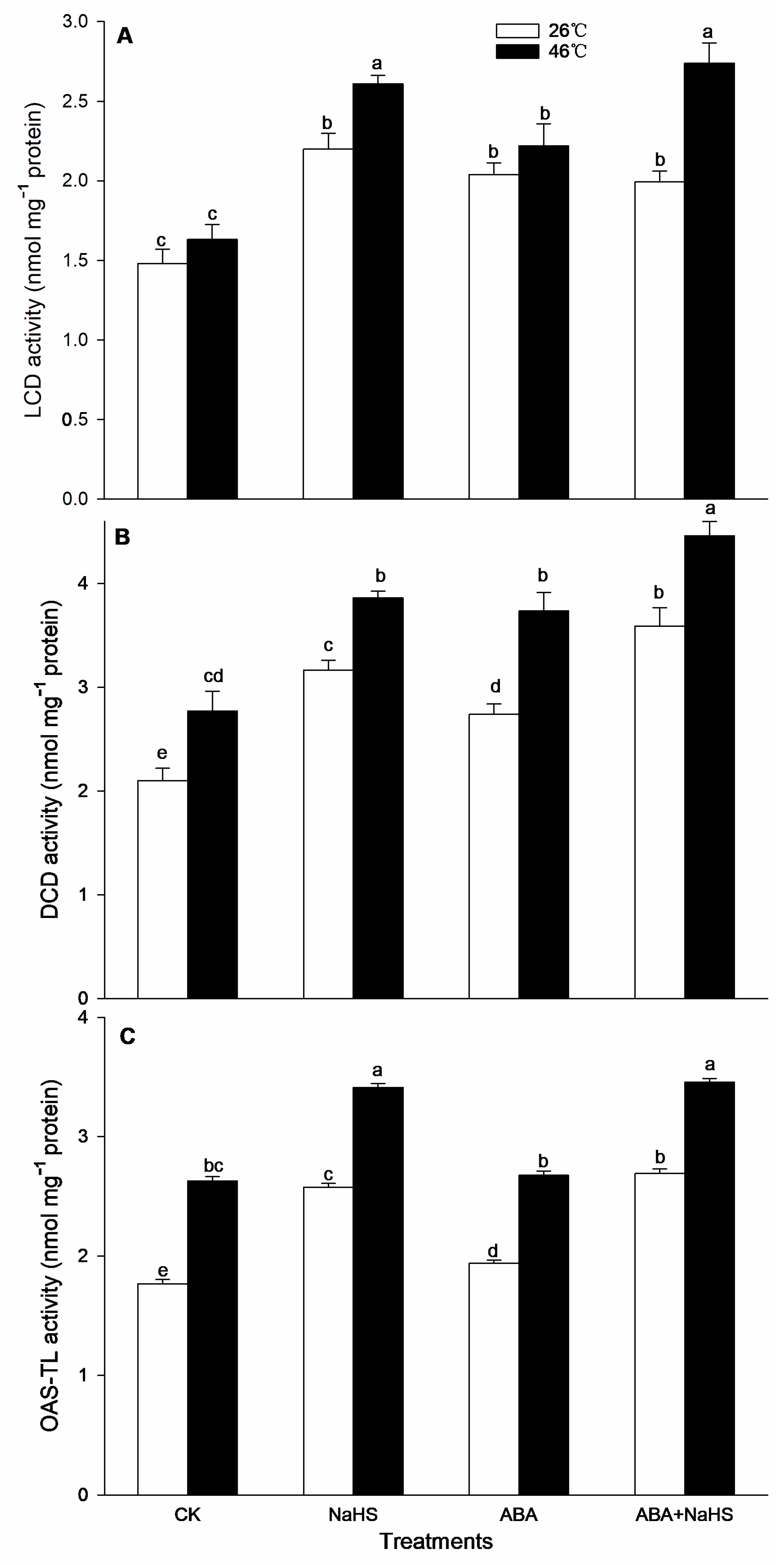
2.3. H2S Increases Endogenous ABA Level
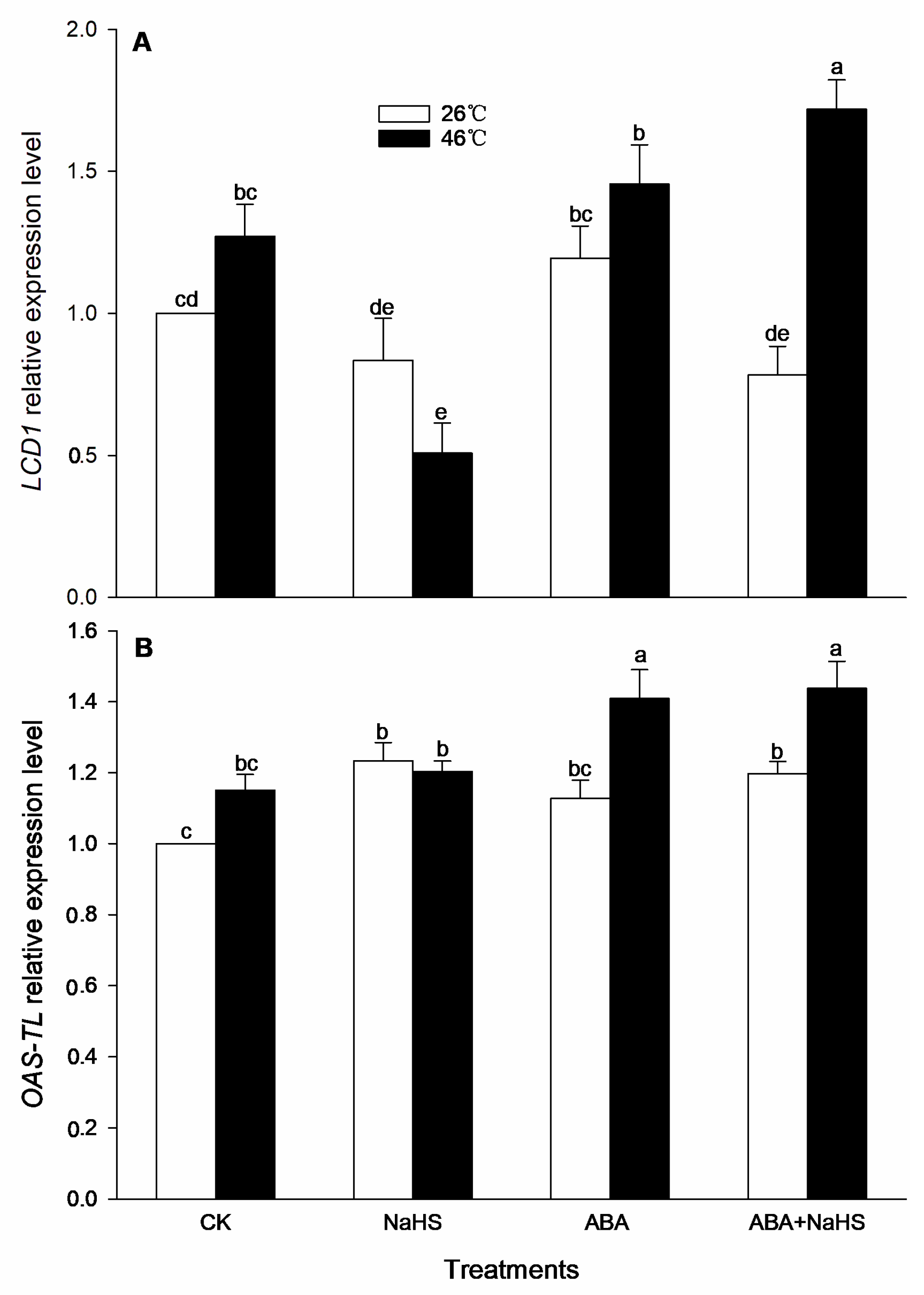
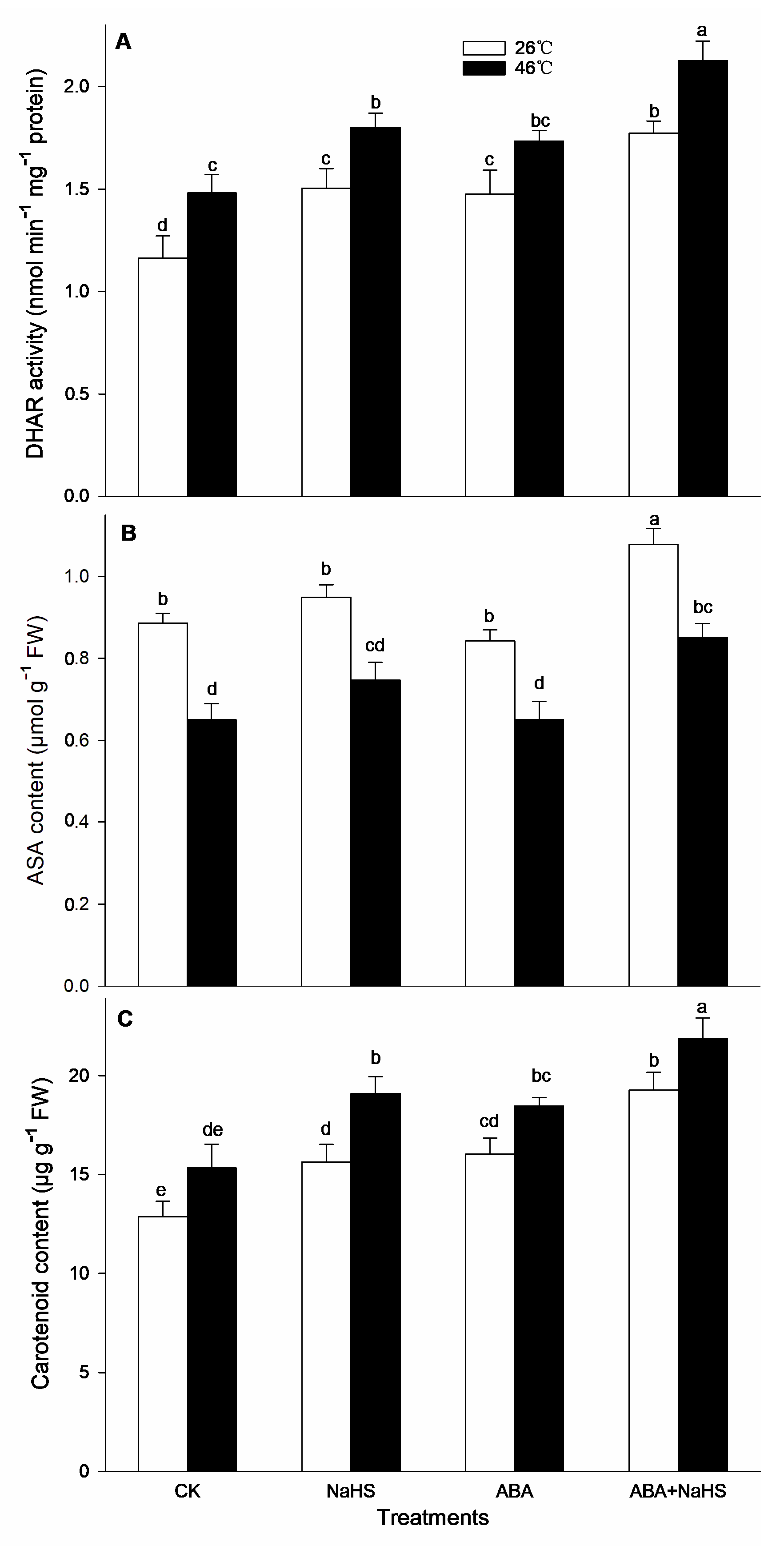
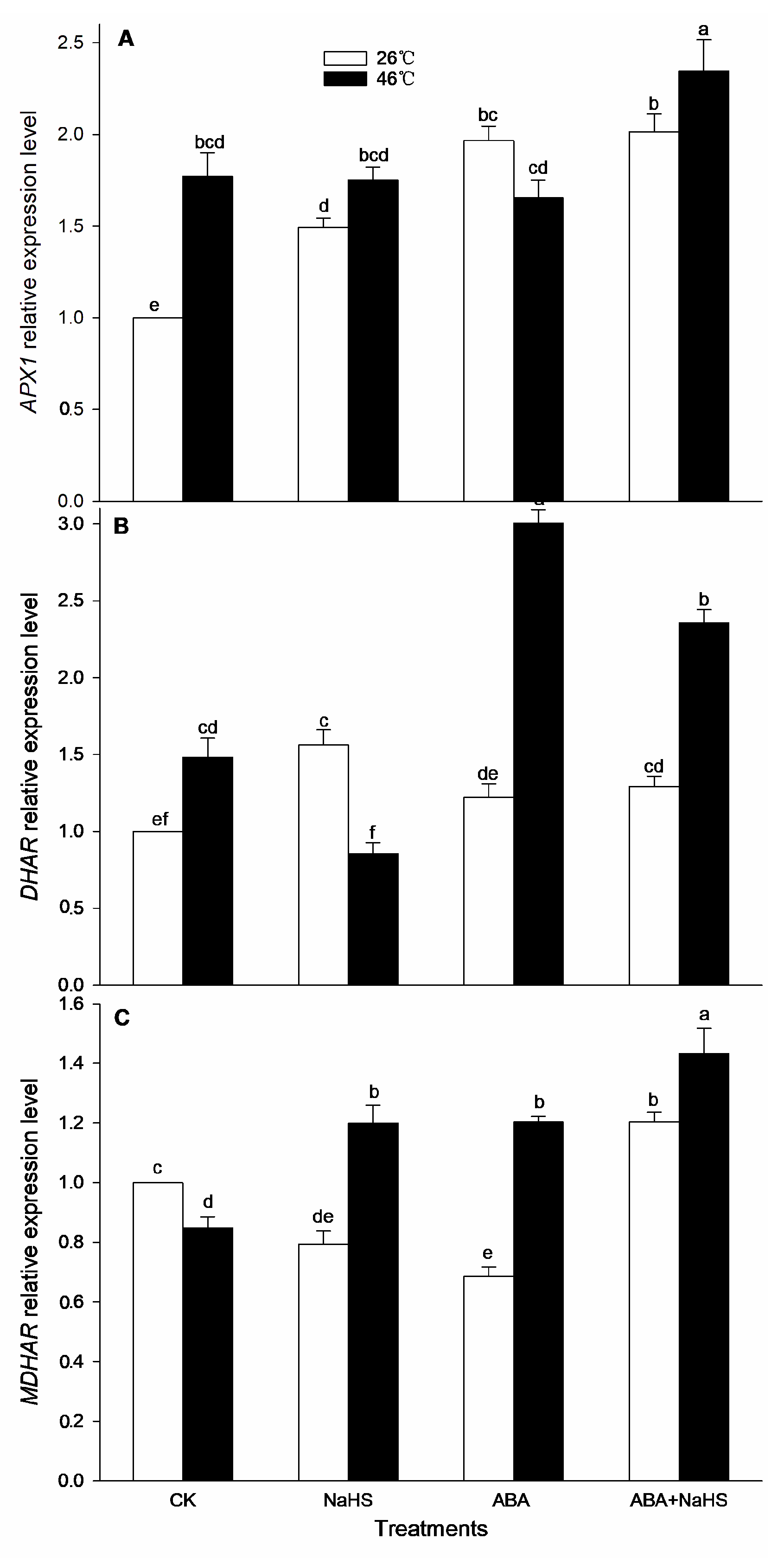
2.4. H2S-ABA Crosstalk Activates ROS-Scavenging System
2.5. H2S-ABA Crosstalk Modulates the ROS Level
2.6. Correlation among Indices
| r | H2S | LCD | DCD | OAS-TL |
|---|---|---|---|---|
| ABA | 0.672 * | 0.873 ** | 0.825 ** | 0.821 ** |
| ZEP | 0.589 | 0.724 ** | 0.703 ** | 0.862 ** |
| NCED | 0.727 ** | 0.604 * | 0.610 * | 0.661 * |
| AAO | 0.671 * | 0.787 ** | 0.614 * | 0.861 ** |
| r | SR | EL | TV | MDA |
|---|---|---|---|---|
| SR | 1 | |||
| EL | −0.561 ** | 1 | ||
| TV | 0.331 ** | −0.138 | 1 | |
| MDA | −0.622 ** | 0.175 | −0.515 ** | 1 |
| r | SR | POD | CAT | SOD | GR | APX | DHAR | MDHAR |
|---|---|---|---|---|---|---|---|---|
| SR | 1 | |||||||
| POD | 0.459 ** | 1 | ||||||
| CAT | 0.636 ** | 0.405 ** | 1 | |||||
| SOD | 0.085 | 0.114 | 0.288 ** | 1 | ||||
| GR | 0.456 ** | 0.339 ** | 0.374 ** | 0.134 | 1 | |||
| APX | 0.424 ** | 0.325 ** | 0.467 ** | 0.307 ** | 0.345 ** | 1 | ||
| DHAR | 0.471 ** | 0.387 ** | 0.324 ** | 0.167 | 0.385 ** | 0.267 * | 1 | |
| MDHAR | 0.245 * | 0.318 ** | 0.168 | −0.049 | 0. 348 ** | 0.016 | 0.103 | 1 |
| r | SR | AsA | DHA | FLA | TP | CAR | H2O2 | O2·− |
|---|---|---|---|---|---|---|---|---|
| SR | 1 | |||||||
| AsA | 0.303 ** | 1 | ||||||
| DHA | −0.75 | −0.73 | 1 | |||||
| FLA | 0.499 ** | 0.241 * | −0.138 | 1 | ||||
| TP | 0.515 ** | 0.306 ** | 0.017 | 0.351 ** | 1 | |||
| CAR | 0.513 ** | 0.362 ** | −0.248 * | 0.361 ** | 0.399 ** | 1 | ||
| H2O2 | 0.582 ** | 0.397 ** | 0.011 | 0.407 ** | −0.576 ** | 0.467 ** | 1 | |
| O2·− | −0.610 ** | −0.186 | −0.060 | −0.417 ** | −0.370 ** | −0.446 ** | −0.383 ** | 1 |
3. Discussion
4. Materials and Methods
4.1. Seedling Culture and Treatment
4.2. Measurement of Thermotolerance Parameters
4.3. Analysis of H2S Content and Its Metabolic Enzymes
4.4. Determination of ABA Content and Its Metabolic Enzymes
4.5. Estimation of ROS-Scavenging System
4.6. Determination of ROS
4.7. Quantification of Gene Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Xie, Y. Hydrogen sulfide signaling in plants. Antioxid. Redox Signal 2023, 39, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Zhang, J.; Xie, Y.; Romero, L.C.; Gotor, C. Hydrogen sulfide signaling in plant adaptations to adverse conditions: Molecular mechanisms. J. Exp. Bot. 2021, 72, 5893–5904. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Mubarik, M.S.; Anwar, S.; Zahra, N.; Sharif, Y.; Hafeez, M.B.; Zhang, C.; Corpas, F.J.; Chen, H. Hydrogen sulfide: An emerging component against abiotic stress in plants. Plant Biol. 2022, 24, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Min, X.; Zhou, Z.H. Hydrogen sulfide: A signal molecule in plant cross-adaptation. Front. Plant Sci. 2016, 7, 1621. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Fahad, S.; Ali, M.A.; Hussain, S.; Tariq, M.; Ilyas, F.; Ahmad, S.; Saud, S.; Hammad, H.M.; Nasim, W.; et al. Hydrogen sulfide: A novel gaseous molecule for plant adaptation to stress. J. Plant Growth Regul. 2021, 40, 2485–2501. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopouls, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef]
- Christou, A.; Filippou, P.; Manganaris, G.A.; Fotopoulos, V. Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 2014, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Rehman, A.; Azhar, M.T.; Hinze, L.; Qayyum, A.; Li, H.; Peng, Z.; Qin, G.; Jia, Y.; Pan, Z.; He, S.; et al. Insight into abscisic acid perception and signaling to increase plant tolerance to abiotic stress. J. Plant Inter. 2021, 16, 222–237. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Crosstalk between abscisic acid and nitric oxide under heat stress: Exploring new vantage points. Plant Cell Rep. 2021, 40, 1429–1450. [Google Scholar] [CrossRef]
- Quesada, V. Advances in the molecular mechanisms of abscisic acid and gibberellins functions in plants 2.0. Int. J. Mol. Sci. 2022, 23, 8524. [Google Scholar] [CrossRef]
- Habibpourmehraban, F.; Wu, Y.; Masoomi-Aladizgeh, F.; Amirkhani, A.; Atwell, B.J.; Haynes, P.A. Pre-treatment of rice plants with ABA makes them more tolerant to multiple abiotic stress. Int. J. Mol. Sci. 2023, 24, 9628. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef] [Green Version]
- Malini, M.K.; Karwa, S.; Priyadarsini, P.; Kumar, P.; Nagar, S.; Kumar, M.; Kumar, S.; Chinnusamy, V.; Pandey, R.; Pal, M. Abscisic-acid-modulated stomatal conductance governs high-temperature stress tolerance in rice accessions. Agriculture 2023, 13, 545. [Google Scholar] [CrossRef]
- Arif, Y.; Hayat, S.; Yusuf, M.; Bajguz, A. Hydrogen sulfide: A versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021, 158, 372–384. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.W. Nitric oxide acts as a key signaling molecule in plant development under stressful conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef]
- Boulc’h, P.N.; Caullireau, E.; Faucher, E.; Gouerou, M.; Guérin, A.; Miray, R.; Couée, I. Abiotic stress signalling in extremophile land plants. J. Exp. Bot. 2020, 71, 5771–5785. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Zhang, L.; Liu, X.; Yang, G.; Pei, Y. H2S is involved in ABA-mediated stomatal movement through MPK4 to alleviate drought stress in Arabidopsis thaliana. Plant Soil 2019, 435, 295–307. [Google Scholar] [CrossRef]
- Li, Z.G.; Jin, J.Z. Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tissue Organ Cult. 2016, 125, 207–214. [Google Scholar] [CrossRef]
- Perrella, G.; Bäurle, I.; van Zanten, M. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New Phytol. 2022, 234, 1144–1160. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Badalamenti, F.; Bernardeau-Esteller, J.; Ruiz, J.M.; Giacalone, V.M.; Procaccini, G.; Marín-Guirao, L. Thermo-priming increases heat-stress tolerance in seedlings of the Mediterranean seagrass P. oceanica. Mar. Pollut. Bull. 2022, 174, 113164. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Nikalje, G.C.; Zargar, S.M.; Suprasanna, P. Molecular insights into sensing, regulation and improving of heat tolerance in plants. Plant Cell Rep. 2022, 41, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Sehar, Z.; Gautam, H.; Iqbal, N.; Alvi, A.F.; Jahan, B.; Fatma, M.; Albaqami, M.; Khan, N.A. The functional inte between ethylene, hydrogen sulfide, and sulfur in plant heat stress tolerance. Biomolecules 2022, 12, 678. [Google Scholar] [CrossRef]
- Wang, R. Gasotransmitters; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Kolupaev, Y.E.; Karpets, Y.V.; Shkliarevskyi, M.A.; Yastreb, T.O.; Plohovska, S.H.; Yemets, A.I.; Blume, Y.B. Gasotransmitters in plants: Mechanisms of participation in adaptive responses. Open Agric. J. 2022, 16 (Suppl. M5), e187433152207050. [Google Scholar] [CrossRef]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric oxide and abscisic acid mediate heat stress tolerance through regulation of osmolytes and antioxidants to protect photosynthesis and growth in wheat plants. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Mir, I.R.; Khan, N.A. Hydrogen sulfide, ethylene, and nitric oxide regulate redox homeostasis and protect photosynthetic metabolism under high temperature stress in rice plants. Antioxidants 2022, 11, 1478. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’ippolito, I.; Khan, N.A. The Crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Islam, M.R.; Fen, B.; Chen, T.; Tao, L.; Fu, G. Role of abscisic acid in thermal acclimation of plants. J. Plant Biol. 2018, 61, 255–264. [Google Scholar] [CrossRef]
- Havva, E.N.; Yu, E.; Shkliarevskyi, M.; Kokorev, A.I.; Dmitriev, A.P. Hydrogen sulfide participation in the formation of wheat seedlings’ heat resistance under the action of hardening temperature. Cytol. Genet. 2022, 56, 218–225. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.P. Redox-mediated responses to high temperature in plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Zuther, E.; Jagadish, S.V.K.; KHincha, D. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 2018, 45, 212–217. [Google Scholar] [CrossRef]
- Dvorak, P.; Krasylenko, Y.; Zeiner, A.; Samaj, J.; Takac, T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Suzuki, N. Fine tuning of ROS, redox and energy regulatory systems associated with the functions of chloroplasts and mitochondria in plants under heat stress. Int. J. Mol. Sci. 2023, 24, 1356. [Google Scholar] [CrossRef]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling role of glutamate in plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [Green Version]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Yemets, A.I.; Yastreb, T.O.; Blume, Y.B. The role of nitric oxide and hydrogen sulfide in regulation of redox homeostasis at extreme temperatures in plants. Front. Plant Sci. 2023, 14, 1128439. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Van Breusegem, F. Improving oxidative stress resilience in plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Rosyida, R.; Kristanto, B.A. The role of antioxidant compounds in plant heat tolerance. Open Access Res. J. Life Sci. 2022, 4, 11–15. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2022, 427, 127891. [Google Scholar] [CrossRef]
- Joshi, R.; Chinnusamy, V. Antioxidant enzymes: Defense against high temperature stress. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: London, UK, 2014; pp. 369–396. [Google Scholar]
- Li, Z.G.; Yang, S.Z.; Long, W.B.; Yang, G.X.; Shen, Z.Z. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013, 36, 1564–1572. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, Y.; Ye, X.Y.; Li, Z.G. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front. Plant Sci. 2018, 9, 1288. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.G.; Xie, L.R.; Li, X.J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Wang, J.Q.; Xiang, R.H.; Li, Z.G. Key role of reactive oxygen species-scavenging system in nitric oxide and hydrogen sulfide crosstalk-evoked thermotolerance in maize seedlings. Front. Plant Sci. 2022, 13, 967968. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.Y.; Qiu, X.M.; Li, Z.G. Methylglyoxal triggers the heat tolerance in maize seedlings by driving AsA-GSH cycle and reactive oxygen species-/methylglyoxal-scavenging system. Plant Physiol. Biochem. 2019, 138, 91–99. [Google Scholar] [CrossRef]
- Ye, X.Y.; Qiu, X.M.; Sun, Y.Y.; Li, Z.G. Interplay between hydrogen sulfide and methylglyoxal initiates thermotolerance inmaize seedlings by modulating reactive oxidative species and osmolyte metabolism. Protoplasma 2020, 257, 1415–1432. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–2781. [Google Scholar]
- Qiu, X.M.; Sun, Y.Y.; Li, Z.G. Signaling molecule glutamic acid initiates the expression of genes related to methylglyoxal scavenging and osmoregulation systems in maize seedlings. Plant Signal. Behav. 2022, 17, 1994257. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-Q.; Xiang, R.-H.; Li, Z.-G. The Essential Role of H2S-ABA Crosstalk in Maize Thermotolerance through the ROS-Scavenging System. Int. J. Mol. Sci. 2023, 24, 12264. https://doi.org/10.3390/ijms241512264
Wang J-Q, Xiang R-H, Li Z-G. The Essential Role of H2S-ABA Crosstalk in Maize Thermotolerance through the ROS-Scavenging System. International Journal of Molecular Sciences. 2023; 24(15):12264. https://doi.org/10.3390/ijms241512264
Chicago/Turabian StyleWang, Jia-Qi, Ru-Hua Xiang, and Zhong-Guang Li. 2023. "The Essential Role of H2S-ABA Crosstalk in Maize Thermotolerance through the ROS-Scavenging System" International Journal of Molecular Sciences 24, no. 15: 12264. https://doi.org/10.3390/ijms241512264





