Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions
Abstract
:1. Introduction
2. Results
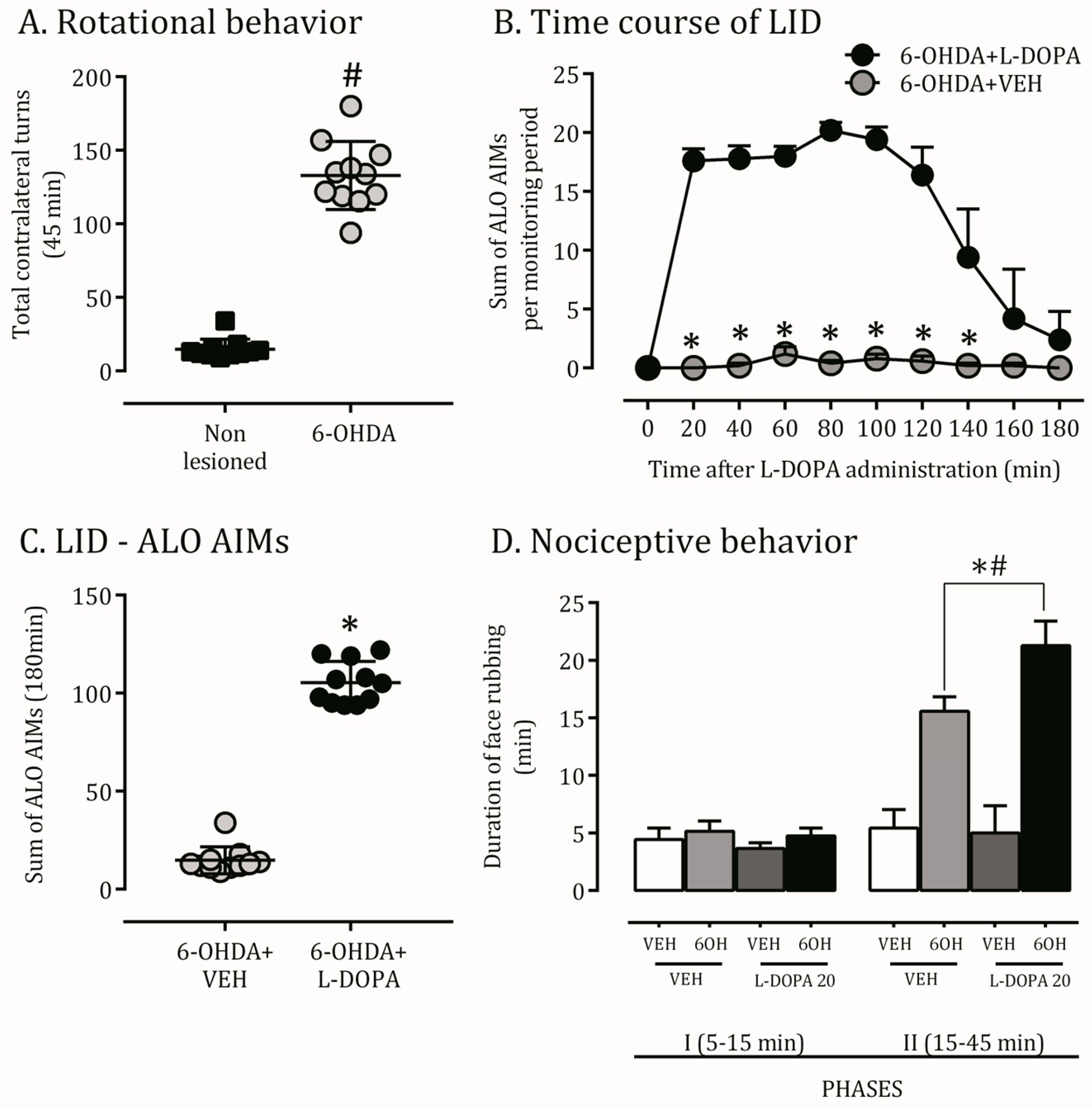
2.1. 6-OHDA Nigrostriatal Lesion Characterization and AIMs Scores
2.2. Orofacial Hyperalgesia Induced by 6-OHDA Lesion and LID
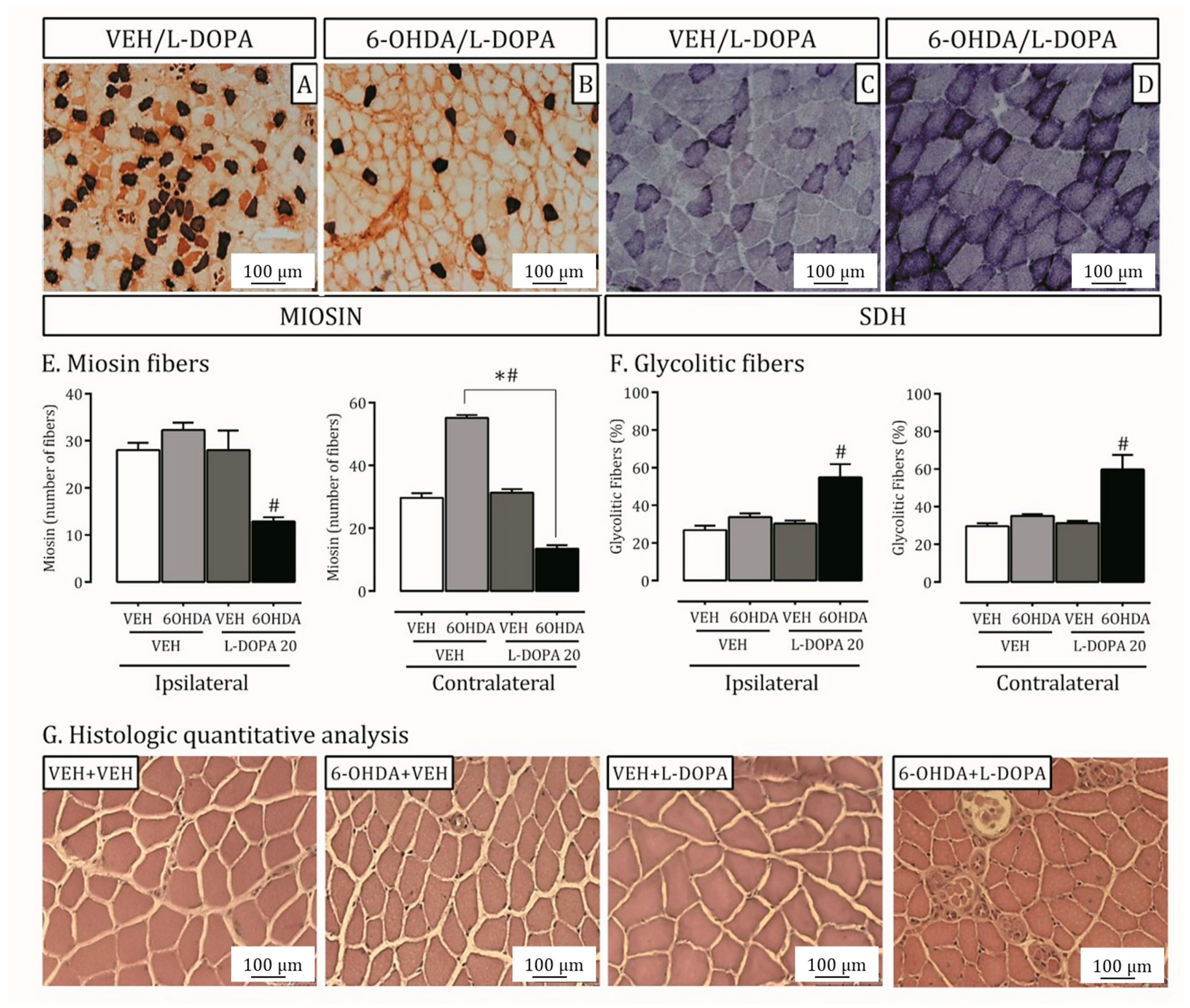
2.3. Parkinson’s Disease and LID Impact on Lateral Pterygoid Muscle
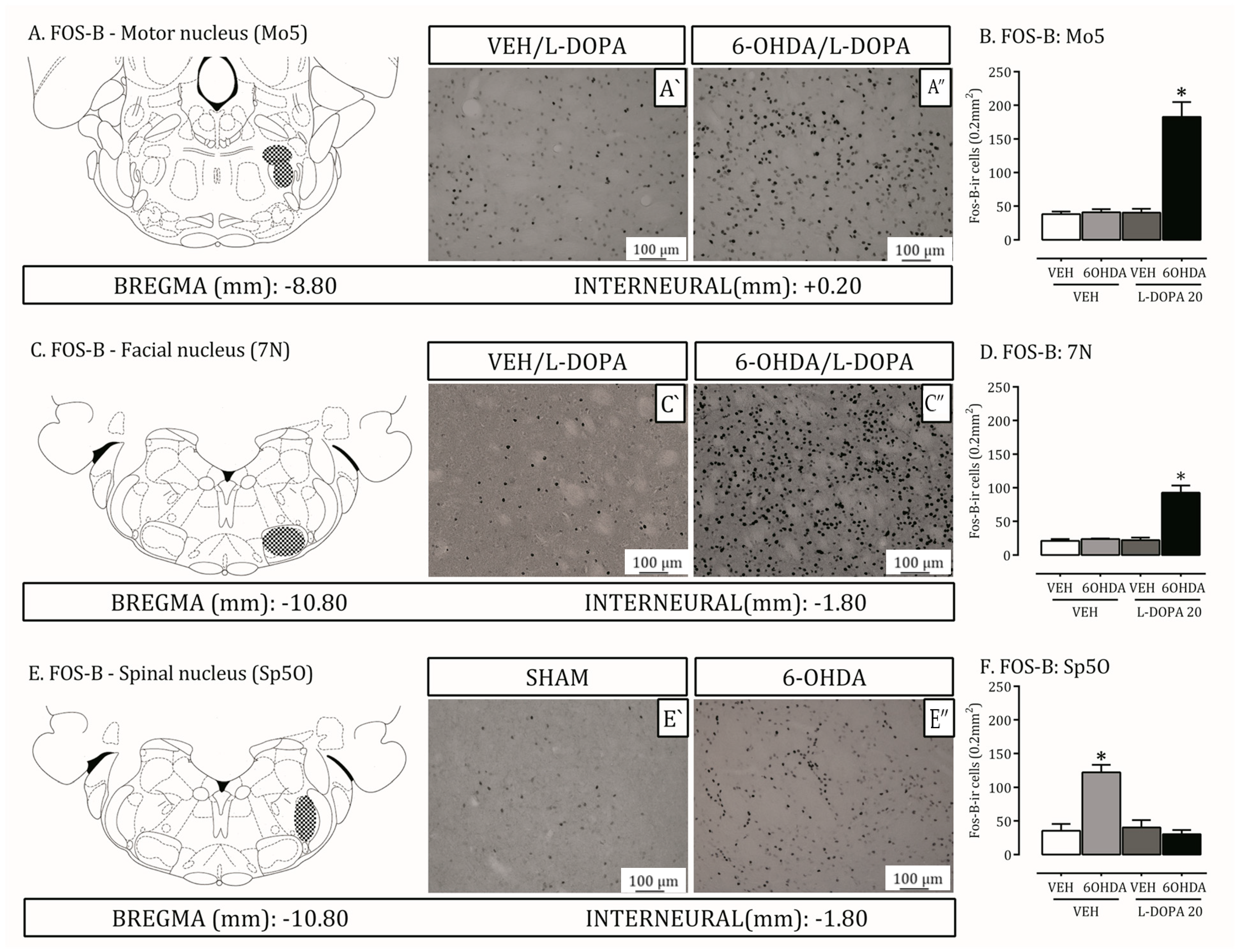
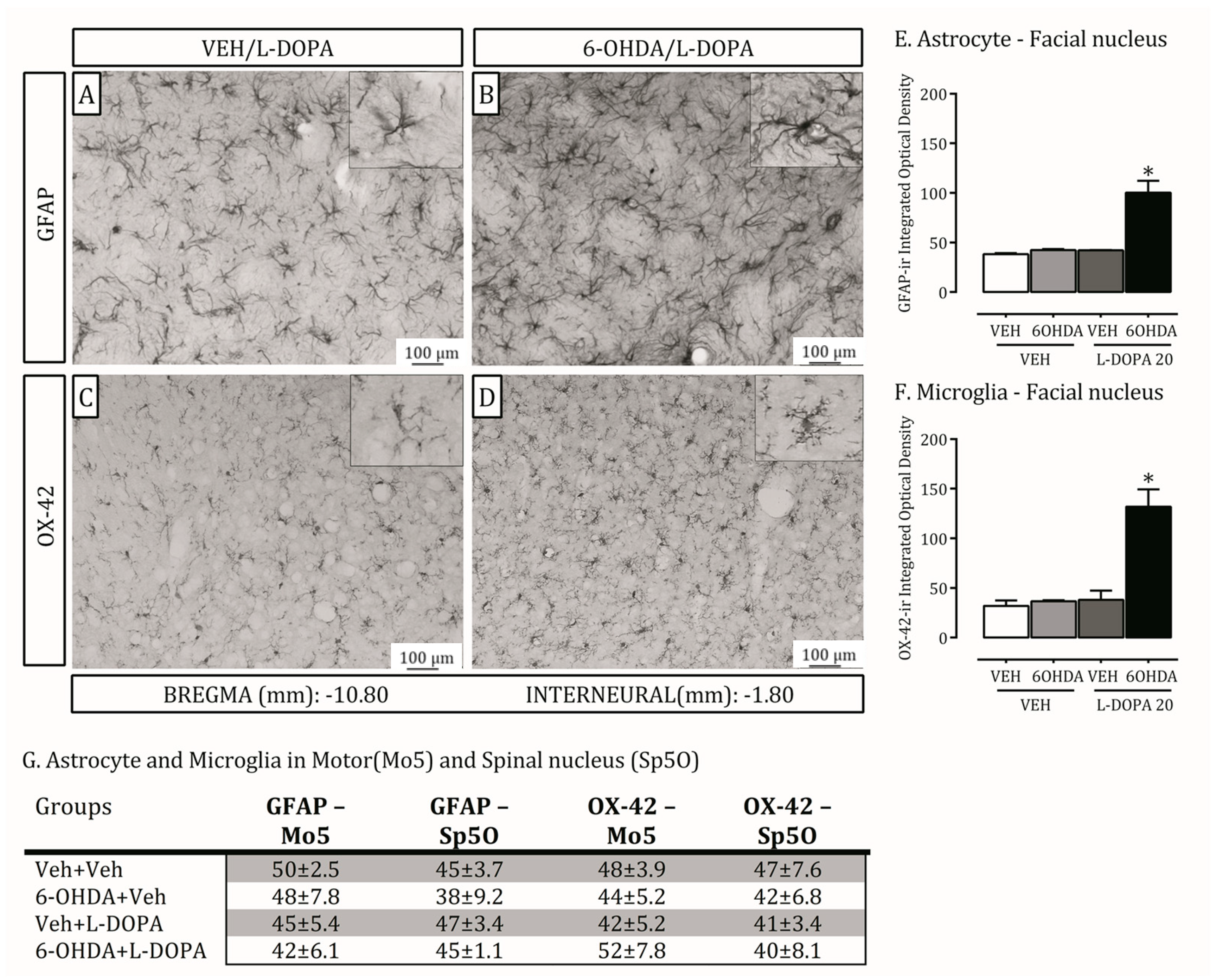
2.4. Is the Trigeminal System Mobilized Due to Orofacial Changes Induced by 6-OHDA Lesion and LID?
3. Discussion
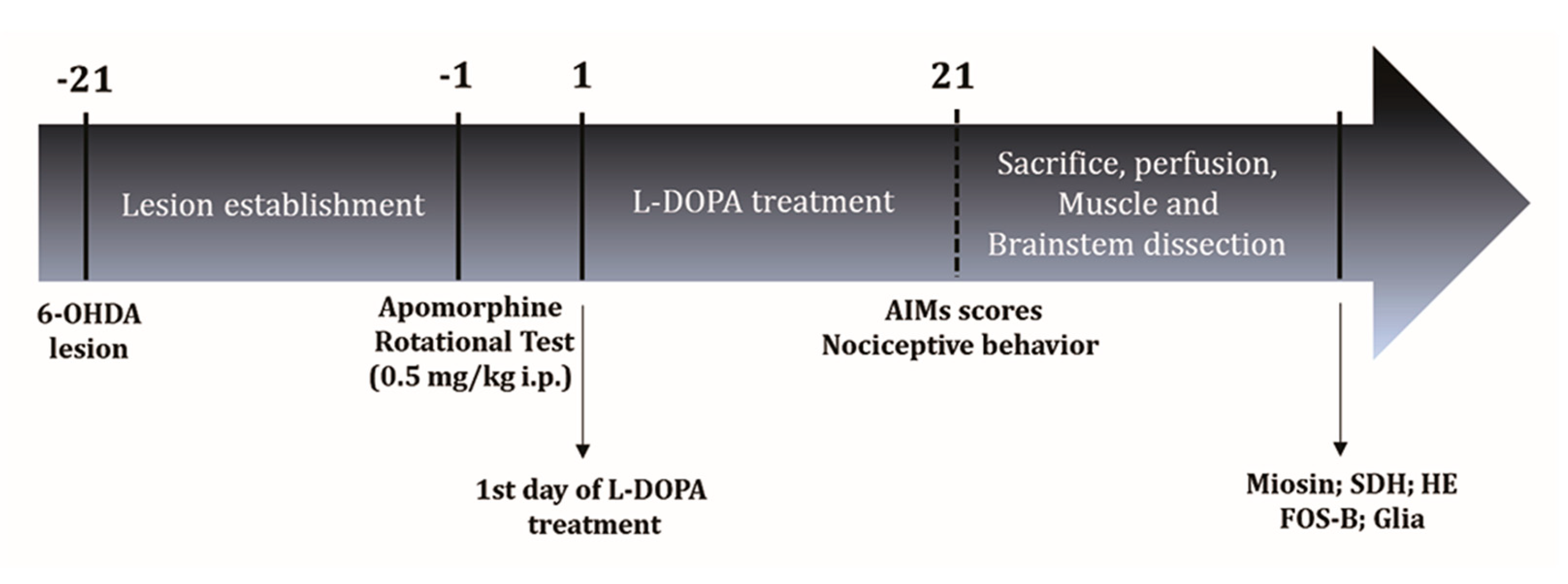
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. A Dopaminergic Lesion with the Neurotoxin 6-Hydroxydopamine
4.4. L-DOPA-Induced Abnormal Involuntary Movements (AIMs)
4.5. Nociceptive Analysis—Orofacial Formalin Test
4.6. Animal Euthanasia
4.7. Immunohistochemistry
4.8. Metabolism Profile—Succinate Dehydrogenase Activity (SDH)
4.9. Morphometric Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelhamid, R.E.; Sluka, K.A. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology 2015, 30, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Adachi, K.; Fujita, S.; Lee, J.; Gionhaku, N.; Cools, A.R.; Koshikawa, N. Role of GABA(A) receptors in the retrorubral field and ventral pallidum in rat jaw movementselicited by dopaminergic stimulation of the nucleus accumbens shell. Eur. J. Pharmacol. 2005, 510, 39–47. [Google Scholar] [CrossRef]
- van Stiphout, M.A.E.; Marinus, J.; van Hilten, J.J.; Lobbezoo, F.; de Baat, C. Oral Health of Parkinson’s Disease Patients: A Case-Control Study. Park. Dis. 2018, 2018, 9315285. [Google Scholar] [CrossRef] [Green Version]
- Fahn, S. How do you treat motor complications in Parkinson’s disease: Medicine, surgery, or both? Ann. Neurol. 2008, 64, 56–64. [Google Scholar] [CrossRef]
- Koshikawa, N.; Fujita, S.; Adachi, K. Behavioral pharmacology of orofacial movement disorders. Int. Rev. Neurobiol. 2011, 97, 1–38. [Google Scholar]
- Shimizu, M.; Miyazaki, I.; Higashi, Y.; Eslava-Alva, M.J.; Diaz-Corrales, F.J.; Asanuma, M.; Ogawa, N. Specific induction of PAG608 in cranial and spinal motor neurons of L-DOPA-treated parkinsonian rats. Neurosci. Res. 2008, 60, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Koshikawa, N.; Tomiyama, K.; Omiya, K.; de Beltran, K.K.; Kobayashi, M. Dopamine D-1 but not D-2 receptor stimulation of the dorsal striatum potentiates apomorphineinduced jaw movements in rats. Eur. J. Pharmacol. 1990, 178, 189–194. [Google Scholar] [CrossRef]
- Lee, J.; Adachi, K.; Gionhaku, N.; Fujita, S.; Uchida, T.; and Koshikawa, N. Measurement of dopamine receptor-mediated jaw movements by a magnet-sensing system in freely moving rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 525–530. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Abraham, W.C. Glutamate receptors and synaptic plasticity: The impact of Evans and Watkins. Neuropharmacology 2022, 206, 108922. [Google Scholar] [CrossRef]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Ebralidze, A.K.; Rossi, D.J.; Tonegawa, S.; and Slater, N.T. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J. Neurosci. 1996, 16, 5014–5025. [Google Scholar] [CrossRef] [Green Version]
- Tassorelli, C.; Armentero, M.T.; Greco, R.; Fancellu, R.; Sandrini, G.; Nappi, G.; Blandini, F. Behavioral responses and Fos activation following painful stimuli in a rodent model of Parkinson’s disease. Brain Res. 2007, 1176, 53–61. [Google Scholar] [CrossRef]
- Maegawa, H.; Morimoto, Y.; Kudo, C.; Hanamoto, H.; Boku, A.; Sugimura, M.; Kato, T.; Yoshida, A.; Niwa, H. Neural mechanism underlying hyperalgesic response to orofacial pain in Parkinson’s disease model rats. Neurosci. Res. 2015, 96, 59–68. [Google Scholar] [CrossRef]
- Dieb, W.; Alvarez, P.; Hafidi, A. PKCγ-positive neurons gate light tactile inputs to pain pathway through pERK1/2 neuronal network in trigeminal neuropathic pain model. J. Oral Facial Pain Headache 2015, 29, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.Z.; Del Bel, E.A. Effects of electrolytic and 6-hydroxydopamine lesions of rat nigrostriatal pathway on nitric oxide synthase and nicotinamide adenine dinucleotide phosphate diaphorase. Brain Res. Bull. 2003, 62, 107–115. [Google Scholar] [CrossRef]
- Padovan-Neto, F.E.; Cavalcanti-Kiwiatkoviski, R.; Carolino, R.O.; Anselmo-Franci, J.; Del Bel, E. Effects of prolonged neuronal nitric oxide synthase inhibition on the development and expression of L-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Neuropharmacology 2015, 89, 87–99. [Google Scholar] [CrossRef]
- Cenci, M.A.; Lee, C.S.; Björklund. A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prody-norphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 1998, 10, 2694–2706. [Google Scholar] [CrossRef]
- Dieb, W.; Ouachikh, O.; Durif, F.; Hafidi, A. Lesion of the dopaminergic nigrostriatal pathway induces trigeminal dynamic me-chanical allodynia. Brain Behav. 2014, 4, 368–380. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tétreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Haghparast, A.; Ghalandari-Shamami, M.; Hassanpour-Ezatti, M. Blockade of D1/D2 dopamine receptors within the nucleus accumbens attenuated the antinociceptive effect of cannabinoid receptor agonist in the basolateral amygdala. Brain Res. 2012, 1471, 23–32. [Google Scholar] [CrossRef]
- Nascimento, G.C.; Bariotto-Dos-Santos, K.; Leite-Panissi, C.R.A.; Del-Bel, E.A.; Bortolanza, M. Nociceptive Response to L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats. Neurotox Res. 2018, 34, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Crivelaro do Nascimento, G.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira-Junior, N.C. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology 2020, 163, 107808. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Del Vesco, C.; Fincati, E.; Ottaviani, S.; Smania, N.; Moretto, G.; Fiaschi, A.; Martino, D.; Defazio, G. Pain and motor complications in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 822–825. [Google Scholar] [CrossRef] [Green Version]
- Billet, F.; Costentin, J.; Dourmap, N. Influence of corticostriatal δ-opioid receptors on abnormal involuntary movements induced by L-DOPA in hemiparkinsonian rats. Exp. Neurol. 2012, 236, 339–350. [Google Scholar] [CrossRef]
- Carta, A.R.; Frau, L.; Pinna, A.; Pontis, S.; Simola, N.; Schintu, N.; Morelli, M. Behavioral and biochemical correlates of the dyskinetic potential of dopaminergic agonists in the 6-OHDA lesioned rat. Synapse 2008, 62, 524–533. [Google Scholar] [CrossRef]
- Hanrieder, J.; Ljungdahl, A.; Fälth, M.; Mammo, S.E.; Bergquist, J.; Andersson, M. L-DOPA-induced dyskinesia is associated with regional increase of striatal dynorphin peptides as elucidated by imaging mass spectrometry. Mol. Cell Proteom. 2011, 10, 111.009308. [Google Scholar] [CrossRef] [Green Version]
- Coura, C.O.; Chaves, H.V.; do Val, D.R.; Vieira, L.V.; Silveira, F.D.; Dos Santos Lopes, F.M.; Gomes, F.I.; Frota, A.F.; Souza, R.B.; Clemente-Napimoga, J.T.; et al. Mechanisms involved in antinociception induced by a polysulfated fraction from seaweed Gracilaria cornea in the temporomandibular joint of rats. Int. J. Biol. Macromol. 2017, 97, 76–84. [Google Scholar] [CrossRef]
- Baram, S.; Thomsen, C.E.; Øzhayat, E.B.; Karlsborg, M.; Bakke, M. Orofacial function and temporomandibular disorders in Parkinson’s Disease: A case-controlled study. BMC Oral Health 2023, 23, 381. [Google Scholar] [CrossRef]
- Wall, C.E.; Holmes, M.; Soderblom, E.J.; Taylor, A.B. Proteomics and immunohistochemistry identify the expression of α-cardiac myosin heavy chain in the jaw-closing muscles of sooty mangabeys (order Primates). Arch. Oral Biol. 2018, 91, 103–108. [Google Scholar] [CrossRef]
- Lowey, S.; Waller, G.S.; Trybus, K.M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature 1993, 365, 454–456. [Google Scholar] [CrossRef]
- Allen, D.L.; Harrison, B.C.; Leinwand, L.A. Inactivation of myosin heavy chain genes in the mouse: Diverse and unexpected phenotypes. Microsc. Res. Tech. 2000, 50, 492–499. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients withParkinson’s disease. J. Electromyogr. Kinesiol. 2017, 33, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Bouillaud, F. Inhibition of Succinate Dehydrogenase by Pesticides (SDHIs) and Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 4045. [Google Scholar] [CrossRef]
- Kim, E.; Rath, E.M.; Tsang, V.H.; Duff, A.P.; Robinson, B.G.; Church, W.B.; Benn, D.E.; Dwight, T.; Clifton-Bligh, R.J. Structural and functional consequences of succinate dehydrogenase subunit B mutations. Endocr. Relat. Cancer 2015, 22, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Beyer, I.; Njemini, R.; Bautmans, I.; Demanet, C.; Bergmann, P.; Mets, T. Inflammation-related muscle weakness and fatigue in geriatric patients. Exp. Gerontol. 2012, 47, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bastide, M.F.; Dovero, S.; Charron, G.; Porras, G.; Gross, C.E.; Fernagut, P.O.; Bézard, E. Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol. Dis. 2014, 62, 179–192. [Google Scholar] [CrossRef]
- Iwata, S.; Shimizu, T.; Nomoto, M.; Fukuda, T. Characteristic upregulation of dopamine D1-receptor in rat striatum after 6-hydroxydopamine treatment. Jpn. J. Pharmacol. 1996, 71, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Yasui, Y.; Tsumori, T.; Ando, A.; Domoto, T.; Kayahara, T.; Nakano, K. Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res. 1994, 656, 420–426. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Unno, S.; Ando, H.; Masuda, Y.; Kitagawa, J. Neuron-Glia Crosstalk and Neuropathic Pain: Involvement in the Modulation of Motor Activity in the Orofacial Region. Int. J. Mol. Sci. 2017, 18, 2051. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.Y.; Wang, S.M.; Tseng, G.F.; Liu, P.H. Differential regulation of glial reactions in the central facial tract and the facial nucleus after facialneurorrhaphy. J. Chem. Neuroanat. 2017, 79, 38–50. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Mahler, M.; Norman, K.M.; Ettinger, R.L. Parkinson disease: Systemic and orofacial manifestations, medical and dental management. J. Am. Dent. Assoc. 2009, 140, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, J. Parkinson & disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar]
- Bortolanza, M. Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol. Dis. 2015, 73, 377–387. [Google Scholar] [CrossRef]
- Bortolanza, M.; Nascimento, G.C.D.; Raisman-Vozari, R.; Del-Bel, E. Doxycycline and its derivative, COL-3, decrease dyskinesia induced by L-DOPA in hemiparkinsonian rats. Br. J. Pharmacol. 2021, 178, 2595–2616. [Google Scholar] [CrossRef]
- Winkler, C.; Kirik, D.; Björklund, A.; Cenci, M.A. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2002, 10, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Lundblad, M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6OHDA lesion model of Parkinson’s disease in rats and mice. Curr. Protoc. Neurosci. 2007, 41, 9.25.21–9.25.23. [Google Scholar] [CrossRef]
- do Nascimento, G.C.; Leite-Panissi, C.R. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammaton of the temporomandibular joint. Physiol. Behav. 2014, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grabow, T.S.; Dougherty, P.M. Gabapentin produces dose-dependent antinociception in the orofacial formalin test in the rat. Reg. Anesth Pain Med. 2002, 27, 277–283. [Google Scholar] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Loyola, B.M.; Nascimento, G.C.; Fernández, R.A.; Iyomasa, D.M.; Pereira, Y.C.; Leite-Panissi, C.R.; Issa, J.P.; Iyomasa, M.M. Chronic stress effects in contralateral medial pterygoid muscle of rats with occlusion alteration. Physiol. Behav. 2016, 164, 369–375. [Google Scholar] [CrossRef]
- Mandarim-de-lacerda, C.A. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 2003, 75, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Peter, J.B.; Barnard, R.J.; Edgerton, V.R.; Gillespie, C.A.; Stempel, K.E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 1972, 11, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, G.C.; Jacob, G.; Milan, B.A.; Leal-Luiz, G.; Malzone, B.L.; Vivanco-Estela, A.N.; Escobar-Espinal, D.; Dias, F.J.; Del-Bel, E. Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions. Int. J. Mol. Sci. 2023, 24, 12270. https://doi.org/10.3390/ijms241512270
Nascimento GC, Jacob G, Milan BA, Leal-Luiz G, Malzone BL, Vivanco-Estela AN, Escobar-Espinal D, Dias FJ, Del-Bel E. Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions. International Journal of Molecular Sciences. 2023; 24(15):12270. https://doi.org/10.3390/ijms241512270
Chicago/Turabian StyleNascimento, Glauce Crivelaro, Gabrielle Jacob, Bruna Araujo Milan, Gabrielli Leal-Luiz, Bruno Lima Malzone, Airam Nicole Vivanco-Estela, Daniela Escobar-Espinal, Fernando José Dias, and Elaine Del-Bel. 2023. "Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions" International Journal of Molecular Sciences 24, no. 15: 12270. https://doi.org/10.3390/ijms241512270





