Expression and Regulatory Mechanisms of MicroRNA in Cholesteatoma: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
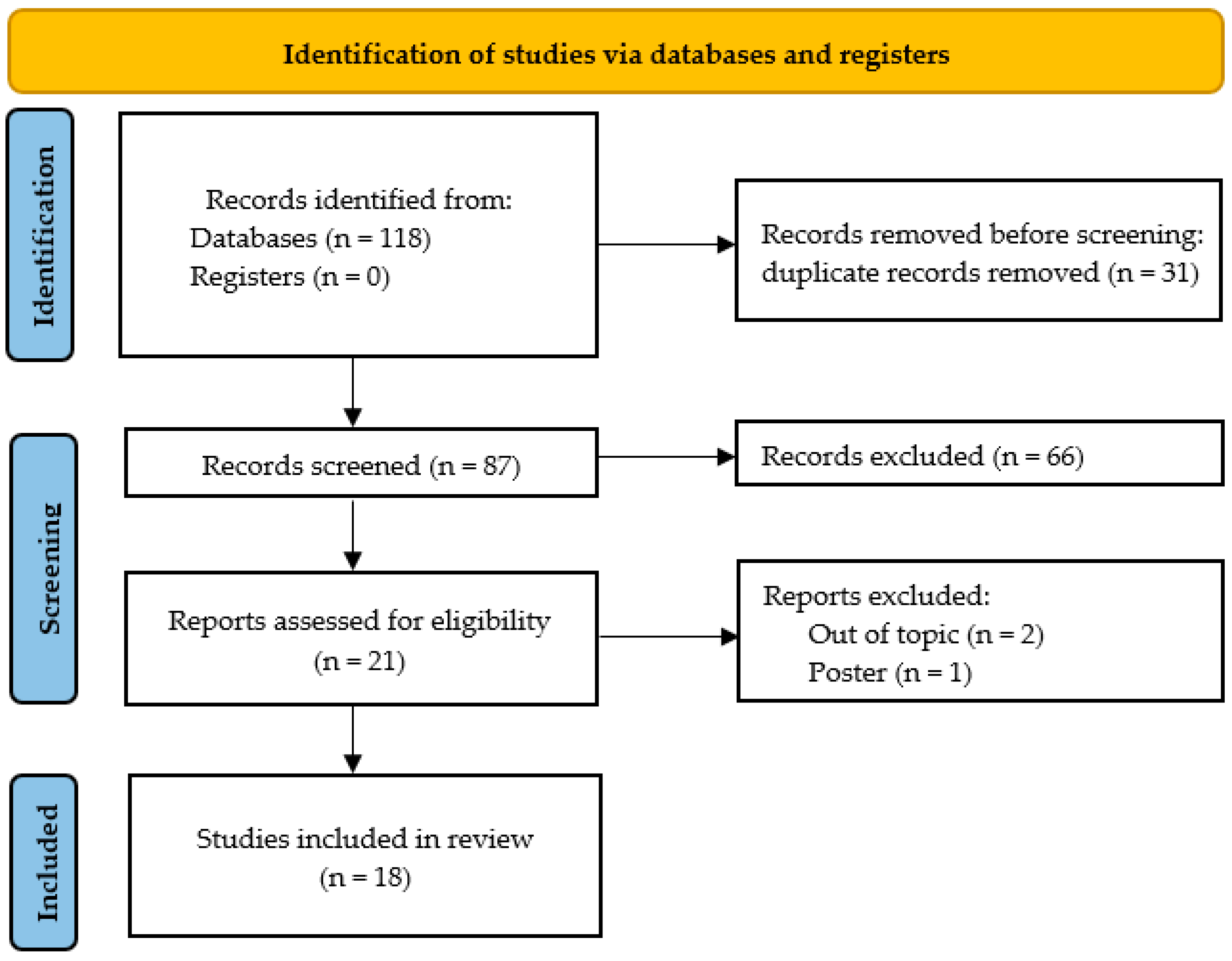
3.1. Search Results, Study Characteristics, and Study Quality
3.2. Differentially Expressed MicroRNAs in Acquired Middle Ear Cholesteatoma
3.3. Role of Exosomal MicroRNAs in Cholesteatoma
3.3.1. MicroRNA-17 Carried by Small Extracellular Vesicles
3.3.2. MicroRNA-106b-5p Carried by Small Extracellular Vesicles
3.4. Other MicroRNAs in Cholesteatoma
3.4.1. MicroRNA-21
3.4.2. MicroRNA-508-3p and Hsa_circ_0000007
3.4.3. Let-7a MicroRNA
3.4.4. MicroRNA-125 and Circ_0074491
3.4.5. MicroRNA-10a-5p
3.4.6. MicroRNA-802
3.4.7. MicroRNA-1297, MicroRNA-26a-5p, and MicroRNA-203a
3.4.8. MicroRNA-199a
3.4.9. MicroRNA-142-5p
3.4.10. Micro-RNA-34
3.5. Strengths and Limitations of the Included Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.J.; Tinling, S.P.; Chole, R.A. Expression patterns of cytokeratins in cholesteatomas: Evidence of increased migration and proliferation. J. Korean Med. Sci. 2002, 17, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jufas, N.; Forer, M.; Patel, N. Incidence and trends of middle ear cholesteatoma surgery and mastoidectomy in Australia-A national hospital morbidity database analysis. Laryngoscope Investig. Otolaryngol. 2022, 7, 210–218. [Google Scholar] [CrossRef]
- Britze, A.; Møller, M.L.; Ovesen, T. Incidence, 10-year recidivism rate and prognostic factors for cholesteatoma. J. Laryngol. Otol. 2017, 131, 319–328. [Google Scholar] [CrossRef]
- Shibata, S.; Murakami, K.; Umeno, Y.; Komune, S. Epidemiological study of cholesteatoma in Fukuoka City. J. Laryngol. Otol. 2015, 129 (Suppl. 2), S6–S11. [Google Scholar] [CrossRef]
- Kemppainen, H.O.; Puhakka, H.J.; Laippala, P.J.; Sipilä, M.M.; Manninen, M.P.; Karma, P.H. Epidemiology and aetiology of middle ear cholesteatoma. Acta Otolaryngol. 1999, 119, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Homøe, P.; Rosborg, J. Family cluster of cholesteatoma. J. Laryngol. Otol. 2007, 121, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.A.; Prinsley, P.; Philpott, C.; Willis, G.; Bhutta, M.F. The genetics of cholesteatoma. A systematic review using narrative synthesis. Clin. Otolaryngol. 2018, 43, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, R.; Ta, N.H.; Jennings, B.A.; Prinsley, P.; Philpott, C.M.; Steel, N.; Clark, A. Cholesteatoma and family history: An international survey. Clin. Otolaryngol. 2020, 45, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnard, Å.; Engmér Berglin, C.; Wincent, J.; Eriksson, P.O.; Westman, E.; Feychting, M.; Mogensen, H. The Risk of Cholesteatoma in Individuals With First-degree Relatives Surgically Treated for the Disease. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 390–396. [Google Scholar] [CrossRef]
- Poliner, A.; Mahomva, C.; Williams, C.; Alfonso, K.; Anne, S.; Musso, M.; Liu, Y.C. Prevalence and surgical management of cholesteatoma in Down Syndrome children. Int. J. Pediatr. Otorhinolaryngol. 2022, 157, 111126. [Google Scholar] [CrossRef]
- Spinner, A.; Munjuluru, A.; Wootten, C.T. Prevalence of Cholesteatoma in Children With Down Syndrome Receiving Treatment at Pediatric Health Care Facilities. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, D.; Di Lella, F.; Negri, M.; Vincenti, V. Surgical management of middle ear cholesteatoma in children with Turner syndrome: A multicenter experience. Acta Biomed. 2018, 89, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Dorney, I.; Otteson, T.; Kaelber, D.C. Middle ear cholesteatoma prevalence in over 3,600 children with Turner Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2022, 161, 111289. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, W.L.; Truelson, J. Cholesteatoma staging. Laryngoscope 1986, 96, 935–939. [Google Scholar] [CrossRef]
- Rosito, L.S.; Netto, L.F.; Teixeira, A.R.; da Costa, S.S. Classification of Cholesteatoma According to Growth Patterns. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.L. Etiopathogenesis of Acquired Cholesteatoma: Prominent Theories and Recent Advances in Biomolecular Research. Laryngoscope 2015, 125, 234–240. [Google Scholar] [CrossRef]
- Liu, W.; Ren, H.; Ren, J.; Yin, T.; Hu, B.; Xie, S.; Dai, Y.; Wu, W.; Xiao, Z.; Yang, X.; et al. The role of EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear cholesteatoma. Mediat. Inflamm. 2013, 2013, 651207. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Fukuda, T.; Takahashi, H.; Koji, T. Expression of keratinocyte growth factor (KGF) and its receptor in a middle-ear cavity problem. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Fukuda, T.; Akiyama, N. Keratinocyte growth factor signaling promotes stem/progenitor cell proliferation under p63 expression during middle ear cholesteatoma formation. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 291–295. [Google Scholar] [CrossRef]
- Kuczkowski, J.; Sakowicz-Burkiewicz, M.; Iżycka-Świeszewska, E.; Mikaszewski, B.; Pawełczyk, T. Expression of tumor necrosis factor-α, interleukin-1α, interleukin-6 and interleukin-10 in chronic otitis media with bone osteolysis. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 93–99. [Google Scholar] [CrossRef]
- Mulazimoglu, S.; Meco, C. Endoscopic diving technique for hearing preservation in managing labyrinth-invading cholesteatomas. Eur. Arch. Otorhinolaryngol. 2023, 280, 1639–1646. [Google Scholar] [CrossRef]
- Salem, J.; Bakundukize, J.; Milinis, K.; Sharma, S.D. Mastoid obliteration versus canal wall down or canal wall up mastoidectomy for cholesteatoma: Systematic review and meta-analysis. Am. J. Otolaryngol. 2023, 44, 103751. [Google Scholar] [CrossRef] [PubMed]
- Shakya, D.; Nepal, A. Transcanal Endoscopic Retrograde Mastoidectomy for Cholesteatoma: A Prospective Study. Ear Nose Throat J. 2023, 102, Np269–Np276. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.L.; Connolly, K.M.; Albert, C.L.; Goldman, J.L.; Cash, E.D.; Severtson, M.A. Postoperative Recurrent Cholesteatoma in Rural Versus Urban Populations. Otol. Neurotol. 2021, 42, e459–e463. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudian-Sani, M.R.; Mehri-Ghahfarrokhi, A.; Ahmadinejad, F.; Hashemzadeh-Chaleshtori, M.; Saidijam, M.; Jami, M.S. MicroRNAs: Effective elements in ear-related diseases and hearing loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 2373–2380. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing risk of bias in human environmental epidemiology studies using three tools: Different conclusions from different tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Qin, Z. MicroRNA-21 promotes the proliferation and invasion of cholesteatoma keratinocytes. Acta Oto-Laryngol. 2016, 136, 1261–1266. [Google Scholar] [CrossRef]
- Chen, X.; Qin, Z. Post-transcriptional regulation by microRNA-21 and let-7a microRNA in paediatric cholesteatoma. J. Int. Med. Res. 2011, 39, 2110–2118. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, F.; Dong, S.; Chen, Y.; Wang, J. MiR-21 inhibits the proliferation of childhood cholesteatoma glioma cells by negatively regulating the expressions of PTEN and PDCD4. Trop. J. Pharm. Res. 2021, 20, 1119–1124. [Google Scholar] [CrossRef]
- Friedland, D.R.; Eernisse, R.; Erbe, C.; Gupta, N.; Cioffi, J.A. Cholesteatoma growth and proliferation: Posttranscriptional regulation by microRNA-21. Otol. Neurotol. 2009, 30, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Zhu, W.; Xu, R.; Teng, Z.; Deng, C.; Zhou, H.; Xia, M.; Zhao, M. Keratinocytes-derived exosomal miRNA regulates osteoclast differentiation in middle ear cholesteatoma. Biochem. Biophys. Res. Commun. 2020, 525, 341–347. [Google Scholar] [CrossRef]
- Hu, Y.; Qian, X. Hsa_circ_0074491 regulates the malignance of cholesteatoma keratinocytes by modulating the PI3K/Akt pathway by binding to miR-22-3p and miR-125a-5p: An observational study. Medicine 2021, 100, e27122. [Google Scholar] [CrossRef]
- Li, N.; Qin, Z.B. Inflammation-induced miR-802 promotes cell proliferation in cholesteatoma. Biotechnol. Lett. 2014, 36, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Hu, J.; Ren, X.; Sheng, Y. Down-regulation of exosomal miR-106b-5p derived from cholesteatoma perimatrix fibroblasts promotes angiogenesis in endothelial cells by overexpression of Angiopoietin 2. Cell Biol. Int. 2018, 42, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, X. Mir-508-3p promotes proliferation and inhibits apoptosis of middle ear cholesteatoma cells by targeting pten/pi3k/akt pathway. Int. J. Med. Sci. 2021, 18, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.; Shi, W.; Han, S.; Fan, X.; Zhang, X.; Wang, N.; Zhang, H.; Xu, A.; Liu, C. MiR-142-5p directly targets cyclin-dependent kinase 5-mediated upregulation of the inflammatory process in acquired middle ear cholesteatoma. Mol. Immunol. 2022, 141, 236–245. [Google Scholar] [CrossRef]
- Xie, S.; Liu, X.; Pan, Z.; Chen, X.; Peng, A.; Yin, T.; Ren, J.; Liu, W. Microarray analysis of differentially-expressed microRNAs in acquired middle ear cholesteatoma. Int. J. Med. Sci. 2018, 15, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yan, W.; Tang, S.; Huang, Z.; Ye, M.; Lu, Z.; Liu, Q. Expression and Correlation Research of MicroRNA10a-5p and PIK3CA in Middle Ear Cholesteatoma. J. Int. Adv. Otol. 2023, 19, 212–216. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, W.; Zheng, J.; Lu, X.; Zhang, F. MiR-199a Targeting PNRC1 to Promote Keratinocyte Proliferation and Invasion in Cholesteatoma. BioMed Res. Int. 2021, 2021, 1442093. [Google Scholar] [CrossRef]
- Zang, J.; Hui, L.; Yang, N.; Yang, B.; Jiang, X. Downregulation of MiR-203a disinhibits bmi1 and promotes growth and proliferation of keratinocytes in cholesteatoma. Int. J. Med. Sci. 2018, 15, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Zang, J.; Yang, B.; Feng, S.; Jiang, X. Low expression of microRNA-125b enhances the expression of STAT3 and contributes to cholesteatoma growth. Arch. Med. Sci. 2022, 18, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.; Qin, Z. MicroRNA let-7a suppresses the growth and invasion of cholesteatoma keratinocytes. Mol. Med. Rep. 2015, 11, 2097–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Wang, W.; Li, S.; Han, L. The Effect of Zbxz23ir-21 NANO(nanomaterials) Delivery Vector on Apoptosis and PTEN(phosphatase and tensin homolog deleted on chromosome ten)/PI3K(Intracellular phosphatidylinositol kinase)/AKT(related to the A and C kinase) in Children with CHOLESTEATOMA in Middle Ear. Bioengineered 2021, 12, 8809–8821. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, F.; Hao, S.; Yu, Q.; Wang, Y.; Lou, W.; Zhao, K.; Li, H. MiR-1297 and MiR-26a-5p Inhibit Cell Progression of Keratinocytes in Cholesteatoma Depending on the Regulation of BMI1. Biotechnol. Bioprocess Eng. 2022, 27, 79–88. [Google Scholar] [CrossRef]
- Doghish, A.S.; Elballal, M.S.; Elazazy, O.; Elesawy, A.E.; Shahin, R.K.; Midan, H.M.; Sallam, A.M.; Elbadry, A.M.M.; Mohamed, A.K.I.; Ishak, N.W.; et al. miRNAs as potential game-changers in bone diseases: Future medicinal and clinical uses. Pathol. Res. Pract. 2023, 245, 154440. [Google Scholar] [CrossRef]
- Kuczkowski, J.; Brzoznowski, W.; Nowicki, T. Bone Damage in Chronic Otitis Media. Ear Nose Throat J. 2022, 101, 428–429. [Google Scholar] [CrossRef]
- Imai, R.; Sato, T.; Iwamoto, Y.; Hanada, Y.; Terao, M.; Ohta, Y.; Osaki, Y.; Imai, T.; Morihana, T.; Okazaki, S.; et al. Osteoclasts Modulate Bone Erosion in Cholesteatoma via RANKL Signaling. J. Assoc. Res. Otolaryngol. 2019, 20, 449–459. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Nishikawa, K.; Imai, R.; Furuya, M.; Uenaka, M.; Ohta, Y.; Morihana, T.; Itoi-Ochi, S.; Penninger, J.M.; Katayama, I.; et al. Intercellular Communication between Keratinocytes and Fibroblasts Induces Local Osteoclast Differentiation: A Mechanism Underlying Cholesteatoma-Induced Bone Destruction. Mol. Cell Biol. 2016, 36, 1610–1620. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.J.; Kim, K.N.; Heo, S.J.; Han, S.C.; Kim, J.; Ko, Y.J.; Kang, H.K.; Yoo, E.S. Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-κB and MAPKs in RANKL-induced RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2013, 434, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Bujía, J.; Holly, A.; Stammberger, M.; Sudhoff, H. Angiogenesis in cholesteatoma of the middle ear. Acta Otorrinolaringol. Esp. 1996, 47, 187–192. [Google Scholar] [PubMed]
- Sudhoff, H.; Dazert, S.; Gonzales, A.M.; Borkowski, G.; Park, S.Y.; Baird, A.; Hildmann, H.; Ryan, A.F. Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am. J. Otol. 2000, 21, 793–798. [Google Scholar]
- Olszewska, E.; Chodynicki, S.; Chyczewski, L. Apoptosis in the pathogenesis of cholesteatoma in adults. Eur. Arch. Oto-Rhino-Laryngol. 2006, 263, 409–413. [Google Scholar] [CrossRef]
- Fukudome, S.; Wang, C.; Hamajima, Y.; Ye, S.; Zheng, Y.; Narita, N.; Sunaga, H.; Fujieda, S.; Hu, X.; Feng, L.; et al. Regulation of the angiogenesis of acquired middle ear cholesteatomas by inhibitor of DNA binding transcription factor. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Starke, R.D.; Ferraro, F.; Paschalaki, K.E.; Dryden, N.H.; McKinnon, T.A.; Sutton, R.E.; Payne, E.M.; Haskard, D.O.; Hughes, A.D.; Cutler, D.F.; et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011, 117, 1071–1080. [Google Scholar] [CrossRef]
- Samuelson Bannow, B.; Recht, M.; Négrier, C.; Hermans, C.; Berntorp, E.; Eichler, H.; Mancuso, M.E.; Klamroth, R.; O’Hara, J.; Santagostino, E.; et al. Factor VIII: Long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019, 35, 43–50. [Google Scholar] [CrossRef]
- Acharya, S.S.; Kaplan, R.N.; Macdonald, D.; Fabiyi, O.T.; DiMichele, D.; Lyden, D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood 2011, 117, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.R.; Rego, A.R.; Soares, T.; Sousa, C.A.E.; Coutinho, M.B. Changes in Coagulation Study and Risk of Developing Cholesteatoma: Is There a Link? J. Audiol. Otol. 2023, 27, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Reis Rego, Â.; Santos, M.; Coutinho, M.; Feliciano, T.; Almeida e Sousa, C. Is von Willebrand disease linked to cholesteatoma aetiology? Med. Hypotheses 2017, 100, 43–45. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kojima, H.; Yaguchi, Y.; Okada, N.; Saito, H.; Moriyama, H. Cholesteatoma Fibroblasts Promote Epithelial Cell Proliferation through Overexpression of Epiregulin. PLoS ONE 2013, 8, e66725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Balkom, B.W.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Liu, L.; Cui, Y.; Liu, H.; Yi, J.; Bing, W.; Liu, C.; Jiang, D.; Bi, Y. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res. Ther. 2022, 13, 352. [Google Scholar] [CrossRef]
- Cabello, P.; Torres-Ruiz, S.; Adam-Artigues, A.; Forés-Martos, J.; Martínez, M.T.; Hernando, C.; Zazo, S.; Madoz-Gúrpide, J.; Rovira, A.; Burgués, O.; et al. miR-146a-5p Promotes Angiogenesis and Confers Trastuzumab Resistance in HER2+ Breast Cancer. Cancers 2023, 15, 2138. [Google Scholar] [CrossRef]
- Dżaman, K.; Czerwaty, K. Roles of Exosomes in Chronic Rhinosinusitis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 1284. [Google Scholar] [CrossRef]
- Surina, S.; Fontanella, R.A.; Scisciola, L.; Marfella, R.; Paolisso, G.; Barbieri, M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 767064. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Xiong, Y.; Wang, X.; Xu, K.; Han, B.; Bai, Y.; Li, L.; Zhang, Y.; Zhou, L. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell Physiol. Biochem. 2017, 43, 945–958. [Google Scholar] [CrossRef]
- Yune, T.Y.; Byun, J.Y. Expression of PTEN and phosphorylated Akt in human cholesteatoma epithelium. Acta Otolaryngol. 2009, 129, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, X. Expression and significance of PTEN, P-ERK and P-AKT in the middle ear cholesteatoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014, 28, 238–242, 245. [Google Scholar]
- Cai, Q.; Yang, H.S.; Li, Y.C.; Zhu, J. Dissecting the Roles of PDCD4 in Breast Cancer. Front. Oncol. 2022, 12, 855807. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.H.; Chen, M.; Wang, Y.; Cui, P.G.; Liu, S.B.; Xu, Z.Y. MicroRNA-21 regulates the ERK/NF-κB signaling pathway to affect the proliferation, migration, and apoptosis of human melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol. Carcinog. 2017, 56, 886–894. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.; Kim, E.K.; Lee, W.M.; Hong, Y.O.; Hong, S.A. Low PDCD4 Expression Is Associated With Poor Prognosis of Colorectal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xiang, Y.; Wang, X.; Ren, H.; Yin, T.; Ren, J.; Liu, W. Acquired cholesteatoma epithelial hyperproliferation: Roles of cell proliferation signal pathways. Laryngoscope 2016, 126, 1923–1930. [Google Scholar] [CrossRef]
- Friedland, D.R.; Eernisse, R.; Erbe, C.; Gupta, N.; Cioffi, J.A. MicroRNA regulation of cholesteatoma growth microrna regulation of cholesteatoma growth. Laryngoscope 2009, 119, S114. [Google Scholar] [CrossRef]
- Orobello, N.; Harrington, C.; Reilly, B.K. Updates in paediatric cholesteatoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, D.; Pu, W.; Wang, J.; Peng, Y. Circular RNAs in Cancer: Biogenesis, Function, and Clinical Significance. Trends Cancer 2020, 6, 319–336. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.Q.; Chen, M.; Xu, J.; Zhu, D. Circular RNA in cancer development and immune regulation. J. Cell Mol. Med. 2022, 26, 1785–1798. [Google Scholar] [CrossRef]
- Altesha, M.A.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef]
- Jin, J.; Sun, H.; Shi, C.; Yang, H.; Wu, Y.; Li, W.; Dong, Y.H.; Cai, L.; Meng, X.M. Circular RNA in renal diseases. J. Cell Mol. Med. 2020, 24, 6523–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Xiao, Y.; Ma, J.; Wang, A. Circular RNA: A novel potential biomarker for skin diseases. Pharmacol. Res. 2020, 158, 104841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, S.Y.; Hu, J.; Zhang, H.H. Circular RNA in Diabetes and its Complications. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2022, 44, 521–528. [Google Scholar] [CrossRef]
- Li, T.R.; Jia, Y.J.; Wang, Q.; Shao, X.Q.; Lv, R.J. Circular RNA: A new star in neurological diseases. Int. J. Neurosci. 2017, 127, 726–734. [Google Scholar] [CrossRef]
- Singh, M.; Dwibedy, S.L.L.; Biswal, S.R.; Muthuswamy, S.; Kumar, A.; Kumar, S. Circular RNA: A novel and potential regulator in pathophysiology of schizophrenia. Metab. Brain Dis. 2022, 37, 1309–1316. [Google Scholar] [CrossRef]
- Gao, J.; Tang, Q.; Xue, R.; Zhu, X.; Wang, S.; Zhang, Y.; Liu, W.; Gao, Z.; Yang, H. Comprehensive circular RNA expression profiling with associated ceRNA network reveals their therapeutic potential in cholesteatoma. Oncol. Rep. 2020, 43, 1234–1244. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, S.; Ionita-Radu, F.; Stefani, C.; Miricescu, D.; Stanescu, S., II; Greabu, M.; Ripszky Totan, A.; Jinga, M. Targeting PI3K/AKT/mTOR Signaling Pathway in Pancreatic Cancer: From Molecular to Clinical Aspects. Int. J. Mol. Sci. 2022, 23, 132. [Google Scholar] [CrossRef]
- Mao, X.B.; Sheng, T.; Zhuang, L.P.; Wu, C.K.; Zhao, G.G. Expression of miRNA let-7a and HMGA2 and Diagnostic Value of Serum miRNA let-7a Level in Pancreatic Cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 2020, 51, 540–545. [Google Scholar] [CrossRef]
- Motoyama, K.; Inoue, H.; Nakamura, Y.; Uetake, H.; Sugihara, K.; Mori, M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin. Cancer Res. 2008, 14, 2334–2340. [Google Scholar] [CrossRef] [Green Version]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, D.L.; Jiang, X.; Rom, S. let-7 microRNAs: Their Role in Cerebral and Cardiovascular Diseases, Inflammation, Cancer, and Their Regulation. Biomedicines 2021, 9, 606. [Google Scholar] [CrossRef]
- Yin, H.; Sun, Y.; Wang, X.; Park, J.; Zhang, Y.; Li, M.; Yin, J.; Liu, Q.; Wei, M. Progress on the relationship between miR-125 family and tumorigenesis. Exp. Cell Res. 2015, 339, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Sun, Y.N.; Tan, L.J.; Zhao, J.N.; Zhou, X.J.; Yu, T.J.; Liu, J.T. MiR-125 family improves the radiosensitivity of head and neck squamous cell carcinoma. Mol. Biol. Rep. 2023, 50, 5307–5317. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Xu, Y.Y.; Huang, R.Y.; Chen, X.M.; Chen, H.M.; Han, L.; Yan, Y.H.; Lu, C.J. Role of an imbalanced miRNAs axis in pathogenesis of psoriasis: Novel perspectives based on review of the literature. Oncotarget 2017, 8, 5498–5507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Brodin, P.; Wei, T.; Meisgen, F.; Eidsmo, L.; Nagy, N.; Kemeny, L.; Ståhle, M.; Sonkoly, E.; Pivarcsi, A. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J. Investig. Dermatol. 2011, 131, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Sano, S.; Kiguchi, K.; Anders, J.; Komazawa, N.; Takeda, J.; DiGiovanni, J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J. Clin. Investig. 2004, 114, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.A. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007, 251, 199–210. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Gao, X.; Chen, H.; Geng, L. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int. Immunopharmacol. 2014, 19, 327–333. [Google Scholar] [CrossRef]
- Ho, K.Y.; Huang, H.H.; Hung, K.F.; Chen, J.C.; Chai, C.Y.; Chen, W.T.; Tsai, S.M.; Chien, C.Y.; Wang, H.M.; Wu, Y.J. Cholesteatoma growth and proliferation: Relevance with serpin B3. Laryngoscope 2012, 122, 2818–2823. [Google Scholar] [CrossRef]
- Liu, W.; Xie, S.; Chen, X.; Rao, X.; Ren, H.; Hu, B.; Yin, T.; Xiang, Y.; Ren, J. Activation of the IL-6/JAK/STAT3 signaling pathway in human middle ear cholesteatoma epithelium. Int. J. Clin. Exp. Pathol. 2014, 7, 709–715. [Google Scholar] [PubMed]
- Park, H.R.; Min, S.K.; Min, K.; Jun, S.Y.; Seo, J.; Kim, H.J. Increased expression of p63 and survivin in cholesteatomas. Acta Otolaryngol. 2009, 129, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, Y.; Komori, M.; Preciado, D.A.; Choo, D.I.; Moribe, K.; Murakami, S.; Ondrey, F.G.; Lin, J. The role of inhibitor of DNA-binding (Id1) in hyperproliferation of keratinocytes: The pathological basis for middle ear cholesteatoma from chronic otitis media. Cell Prolif. 2010, 43, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Zhao, H.Y.; Gu, A.D.; Wu, Y.W.; Weng, Y.H.; Li, S.J.; Song, J.Y.; Gu, X.F.; Qiu, J.; Zhao, W. miRNA-10a-5p inhibits cell metastasis in hepatocellular carcinoma via targeting SKA1. Kaohsiung J. Med. Sci. 2021, 37, 784–794. [Google Scholar] [CrossRef]
- Zhu, H.; Kang, M.; Bai, X. TCF21 regulates miR-10a-5p/LIN28B signaling to block the proliferation and invasion of melanoma cells. PLoS ONE 2021, 16, e0255971. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Sun, X.Y.; Yang, C.X.; Zou, X.Y. MiR-10a-5p restrains the aggressive phenotypes of ovarian cancer cells by inhibiting HOXA1. Kaohsiung J. Med. Sci. 2021, 37, 276–285. [Google Scholar] [CrossRef]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Gao, T.; Zou, M.; Shen, T.; Duan, S. Dysfunction of miR-802 in tumors. J. Clin. Lab. Anal. 2021, 35, e23989. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Wu, W.; Tang, J.; Luo, L.; Zhang, K.; Jin, L.; Lin, S.; Gao, Y.; Yan, X.; et al. MiR-802 causes nephropathy by suppressing NF-κB-repressing factor in obese mice and human. J. Cell Mol. Med. 2019, 23, 2863–2871. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Ma, D.; Zhao, W.; Wang, D.; Liu, T.; Liu, Y.; Yang, Y.; Liu, Y.; Mu, J.; Li, B.; et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat. Commun. 2020, 11, 1822. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, G.; Li, J.; Chen, W. Long noncoding RNA TRG-AS1 protects against glucocorticoid-induced osteoporosis in a rat model by regulating miR-802-mediated CAB39/AMPK/SIRT-1/NF-κB axis. Hum. Cell 2022, 35, 1424–1439. [Google Scholar] [CrossRef]
- Yao, J.; Gao, R.; Luo, M.; Li, D.; Guo, L.; Yu, Z.; Xiong, F.; Wei, C.; Wu, B.; Xu, Z.; et al. miR-802 participates in the inflammatory process of inflammatory bowel disease by suppressing SOCS5. Biosci. Rep. 2020, 40, BSR20192257. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, X.; Wang, L.; Zhang, Z.; Li, Z.; Li, M. Circ_PGPEP1 Serves as a Sponge of miR-1297 to Promote Gastric Cancer Progression via Regulating E2F3. Dig. Dis. Sci. 2021, 66, 4302–4313. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Guo, Q.; Hu, X.; Zhao, Z.; Ni, L.; Liu, L.; Wang, X.; Wang, Z.; Tong, D.; et al. MicroRNA-1297 inhibits proliferation and promotes apoptosis in gastric cancer cells by downregulating CDC6 expression. Anticancer Drugs 2019, 30, 803–811. [Google Scholar] [CrossRef]
- Liang, L.; Feng, L.; Wei, B. microRNA-1297 involves in the progression of oral squamous cell carcinoma through PTEN. Saudi J. Biol. Sci. 2018, 25, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, Y.; Shu, R.; Wang, S. MicroRNA-1297 regulates hepatocellular carcinoma cell proliferation and apoptosis by targeting EZH2. Int. J. Clin. Exp. Pathol. 2015, 8, 4972–4980. [Google Scholar]
- Wang, Y.; Xue, J.; Kuang, H.; Zhou, X.; Liao, L.; Yin, F. microRNA-1297 Inhibits the Growth and Metastasis of Colorectal Cancer by Suppressing Cyclin D2 Expression. DNA Cell Biol. 2017, 36, 991–999. [Google Scholar] [CrossRef]
- Park, C.R.; Lee, M.; Lee, S.Y.; Kang, D.; Park, S.J.; Lee, D.C.; Koo, H.; Park, Y.G.; Yu, S.L.; Jeong, I.B.; et al. Regulating POLR3G by MicroRNA-26a-5p as a promising therapeutic target of lung cancer stemness and chemosensitivity. Noncoding RNA Res. 2023, 8, 273–281. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Liu, M.; Ning, Q.; Xiang, Z.; Zheng, X.; Tang, S.; Mo, Z. MiR-26a-5p regulates proliferation, apoptosis, migration and invasion via inhibiting hydroxysteroid dehydrogenase like-2 in cervical cancer cell. BMC Cancer 2022, 22, 876. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Qing, J.; Li, C.; Chen, X.; Shen, J. LINC00240 knockdown inhibits nasopharyngeal carcinoma progress by targeting miR-26a-5p. J. Clin. Lab. Anal. 2022, 36, e24424. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Qu, X.; Kan, D.; Luo, Y. miR-26a-5p suppresses nasopharyngeal carcinoma progression by inhibiting PTGS2 expression. Cell Cycle 2022, 21, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cheng, Y.T.; Kao, Y.H.; Tsai, W.C.; Huang, G.K.; Chen, Y.T.; Shen, Y.C.; Tai, M.H.; Chiang, P.H. MiR-26a-5p as a useful therapeutic target for upper tract urothelial carcinoma by regulating WNT5A/β-catenin signaling. Sci. Rep. 2022, 12, 6955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, S.; Guo, P.; Guo, X.; Zheng, J. MicroRNA-1297 inhibits metastasis and epithelial-mesenchymal transition by targeting AEG-1 in cervical cancer. Oncol. Rep. 2017, 38, 3121–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Li, H.; Tan, J.; Weng, X.; Zhou, L.; Weng, Y.; Cao, X. miR-1297 Suppresses Osteosarcoma Proliferation and Aerobic Glycolysis by Regulating PFKFB2. Onco. Targets Ther. 2020, 13, 11265–11275. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Q.; Xu, C.; Jia, Q.; Wang, H.; Xue, W.; Wang, Y.; Zhu, Z.; Tian, L. MiR-26a-5p from HucMSC-derived extracellular vesicles inhibits epithelial mesenchymal transition by targeting Adam17 in silica-induced lung fibrosis. Ecotoxicol. Environ. Saf. 2023, 257, 114950. [Google Scholar] [CrossRef]
- Xie, T.; Pei, Y.; Shan, P.; Xiao, Q.; Zhou, F.; Huang, L.; Wang, S. Identification of miRNA-mRNA Pairs in the Alzheimer’s Disease Expression Profile and Explore the Effect of miR-26a-5p/PTGS2 on Amyloid-β Induced Neurotoxicity in Alzheimer’s Disease Cell Model. Front. Aging Neurosci. 2022, 14, 909222. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Adhikary, G.; Eckert, R.L. The Bmi-1 polycomb protein antagonizes the (-)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis 2010, 31, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Zhao, S.; Yang, W.; Wang, Y. B-cell-specific Moloney murine leukemia virus integration site 1 knockdown impairs adriamycin resistance of gastric cancer cells. Arab J. Gastroenterol. 2023. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jiang, Y.N.; Huang, H.; Xu, J.F.; Wu, Y.H.; Wang, Q.; Zhu, Y.; Zheng, B.; Shen, C.; Qian, W.F.; et al. BMI-1 promotes breast cancer proliferation and metastasis through different mechanisms in different subtypes. Cancer Sci. 2023, 114, 449–462. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Chen, L.; Liu, D.; Dong, H. MicroRNA-154 functions as a tumor suppressor in non-small cell lung cancer through directly targeting B-cell-specific Moloney murine leukemia virus insertion site 1. Oncol. Lett. 2018, 15, 10098–10104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Y.; Ge, J.; Li, W.; Yin, L.; Zhao, Z.; Liu, S.; Qin, H.; Yang, J.; Wang, L.; et al. Doublecortin-Like Kinase 1 (DCLK1) Regulates B Cell-Specific Moloney Murine Leukemia Virus Insertion Site 1 (Bmi-1) and is Associated with Metastasis and Prognosis in Pancreatic Cancer. Cell Physiol. Biochem. 2018, 51, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Espersen, M.L.; Linnemann, D.; Christensen, I.J.; Alamili, M.; Troelsen, J.T.; Høgdall, E. The prognostic value of polycomb group protein B-cell-specific moloney murine leukemia virus insertion site 1 in stage II colon cancer patients. Apmis 2016, 124, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abobaker, S.; Kulbe, H.; Taube, E.T.; Darb-Esfahani, S.; Richter, R.; Denkert, C.; Jank, P.; Sehouli, J.; Braicu, E.I. Polycomb Protein BMI-1 as a Potential Therapeutic Target in Mucinous Ovarian Cancer. Anticancer Res. 2022, 42, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Adhikary, G.; Balasubramanian, S.; Gopalakrishnan, R.; McCormick, T.; Dimri, G.P.; Eckert, R.L.; Rorke, E.A. Expression of Bmi-1 in epidermis enhances cell survival by altering cell cycle regulatory protein expression and inhibiting apoptosis. J. Invest. Dermatol. 2008, 128, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, C.M.; Uthman, A.; Erovic, B.M.; Pammer, J. Expression of BMI-1 in normal skin and inflammatory and neoplastic skin lesions. J. Cutan Pathol. 2007, 34, 174–180. [Google Scholar] [CrossRef]
- Wang, S.L.; Dong, X.W.; Zhao, F.; Li, C.X. MiR-203 inhibits cell proliferation, invasion, and migration of ovarian cancer through regulating RGS17. J. Biol. Regul. Homeost. Agents 2021, 35, 1109–1115. [Google Scholar] [CrossRef]
- Song, S.; Johnson, K.S.; Lujan, H.; Pradhan, S.H.; Sayes, C.M.; Taube, J.H. Nanoliposomal Delivery of MicroRNA-203 Suppresses Migration of Triple-Negative Breast Cancer through Distinct Target Suppression. Noncoding. RNA 2021, 7, 45. [Google Scholar] [CrossRef]
- Altan, Z.; Sahin, Y. miR-203 suppresses pancreatic cancer cell proliferation and migration by modulating DUSP5 expression. Mol. Cell Probes. 2022, 66, 101866. [Google Scholar] [CrossRef]
- Yi, R.; Poy, M.N.; Stoffel, M.; Fuchs, E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008, 452, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, S.B.; Lee, H.B. Oleic acid enhances keratinocytes differentiation via the upregulation of miR-203 in human epidermal keratinocytes. J. Cosmet. Dermatol. 2019, 18, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yin, T.; Ren, J.; Li, L.; Xiao, Z.; Chen, X.; Xie, D. Activation of the EGFR/Akt/NF-κB/cyclinD1 survival signaling pathway in human cholesteatoma epithelium. Eur. Arch. Otorhinolaryngol. 2014, 271, 265–273. [Google Scholar] [CrossRef]
- Huisman, M.A.; De Heer, E.; Grote, J.J. Survival signaling and terminal differentiation in cholesteatoma epithelium. Acta Otolaryngol. 2007, 127, 424–429. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, B.; Wang, P.; Yao, F.; Zhang, C.; Yu, G. Overview of microRNA-199a Regulation in Cancer. Cancer Manag. Res. 2019, 11, 10327–10335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahra, M.A.; Kamha, E.S.; Abdelaziz, H.K.; Nounou, H.A.; Deeb, H.M.E. Aberrant Expression of Serum MicroRNA-153 and -199a in Generalized Epilepsy and its Correlation with Drug Resistance. Ann. Neurosci. 2022, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yu, H.; Wang, Y.; Duan, G.; Wang, B.; Li, W.; Zhu, Z. microRNA-199a counteracts glucocorticoid inhibition of bone marrow mesenchymal stem cell osteogenic differentiation through regulation of Klotho expression in vitro. Cell Biol. Int. 2020, 44, 2532–2540. [Google Scholar] [CrossRef]
- Sharma, S. Immunomodulation: A definitive role of microRNA-142. Dev. Comp. Immunol. 2017, 77, 150–156. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Sun, Y.; Neal, C.; Ireland, A.; Trissal, M.C.; Sullivan, R.P.; Wagner, J.A.; Leong, J.W.; Wong, P.; Mah-Som, A.Y.; et al. MicroRNA-142 Is Critical for the Homeostasis and Function of Type 1 Innate Lymphoid Cells. Immunity 2019, 51, 479–490.e476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, P.; Lei, L.; Huang, Y.; Wu, Y. Overexpression of miR-142-5p inhibits the progression of nonalcoholic steatohepatitis by targeting TSLP and inhibiting JAK-STAT signaling pathway. Aging 2020, 12, 9066–9084. [Google Scholar] [CrossRef]
- Shrestha, A.; Mukhametshina, R.T.; Taghizadeh, S.; Vásquez-Pacheco, E.; Cabrera-Fuentes, H.; Rizvanov, A.; Mari, B.; Carraro, G.; Bellusci, S. MicroRNA-142 is a multifaceted regulator in organogenesis, homeostasis, and disease. Dev. Dyn. 2017, 246, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Xu, L.; Wei, B.; Qian, Z.; Wang, J.; Hui, H.; Sun, Y. miR-142-5p regulates pancreatic cancer cell proliferation and apoptosis by regulation of RAP1A. Pathol. Res. Pract. 2019, 215, 152416. [Google Scholar] [CrossRef]
- Yu, W.; Li, D.; Zhang, Y.; Li, C.; Zhang, C.; Wang, L. MiR-142-5p Acts as a Significant Regulator Through Promoting Proliferation, Invasion, and Migration in Breast Cancer Modulated by Targeting SORBS1. Technol. Cancer Res. Treat 2019, 18, 1533033819892264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Yang, B.; Lin, S.; Xing, R.; Lu, Y. Downregulation of miR-142-5p promotes tumor metastasis through directly regulating CYR61 expression in gastric cancer. Gastric. Cancer 2019, 22, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Li, J.; Zhang, L.; Hu, L. miR-142-5p suppresses proliferation and promotes apoptosis of human osteosarcoma cell line, HOS, by targeting PLA2G16 through the ERK1/2 signaling pathway. Oncol. Lett. 2019, 17, 1363–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Yuan, Y.; He, X.; Jin, L.; Jin, D. Enhanced plasma miR-142-5p promotes the progression of intrahepatic cholangiocarcinoma via targeting PTEN. Exp. Ther. Med. 2019, 17, 4190–4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. Loss of Cdk5 in breast cancer cells promotes ROS-mediated cell death through dysregulation of the mitochondrial permeability transition pore. Oncogene 2018, 37, 1788–1804. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yin, R.; Chen, X.; Hu, B.; Jiang, B.; Tang, W.; Zhang, X.; Jin, X.; Ying, M.; Fu, J. Higher Levels of Tumour-Infiltrating Lymphocytes (TILs) are Associated with a Better Prognosis, While CDK5 Plays a Different Role Between Nonmetastatic and Metastatic Colonic Carcinoma. Cancer Control 2023, 30, 10732748231169396. [Google Scholar] [CrossRef]
- Ling, R.; Sheng, Y.; Hu, Y.; Wang, D.; Zhou, Y.; Shu, Y. Comprehensive analysis of CDK5 as a novel biomarker for progression in esophageal cancer. Esophagus 2023, 20, 502–514. [Google Scholar] [CrossRef]
- Shao, X.; Yang, Y.; Chen, J.; Zhao, R.; Xu, L.; Guo, X.; Feng, Y.; Qin, L. Identification of Two CDK5R1-Related Subtypes and Characterization of Immune Infiltrates in Alzheimer’s Disease Based on an Integrated Bioinformatics Analysis. Comput. Math Methods Med. 2022, 2022, 6766460. [Google Scholar] [CrossRef]
- Batra, S.; Jahan, S.; Ashraf, A.; Alharby, B.; Jawaid, T.; Islam, A.; Hassan, I. A review on cyclin-dependent kinase 5: An emerging drug target for neurodegenerative diseases. Int. J. Biol. Macromol. 2023, 230, 123259. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhang, H.L.; Zhi, L.L.; Jin, P.; Zhao, L.; Li, T.; Zhou, X.M.; Sun, D.S.; Cheng, G.H.; Xin, Q.; et al. CDK5 Regulates PD-L1 Expression and Cell Maturation in Dendritic Cells of CRSwNP. Inflammation 2019, 42, 135–144. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Groot, M.; Sharma, L.; Dela Cruz, C.S.; Jin, Y. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax 2019, 74, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fang, Q.Y.; Wang, S.N.; Zhang, Z.W.; Hou, Z.J.; Li, J.N.; Fu, S.Q. Lnc-RNA BLACAT1 regulates differentiation of bone marrow stromal stem cells by targeting miR-142-5p in osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Huang, Z.; Hermeking, H. Meta-analysis of miR-34 target mRNAs using an integrative online application. Comput. Struct. Biotechnol. J. 2023, 21, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, X.; He, J.; Cao, Q.; Du, D.; Zhan, X.; Zeng, Y.; Yuan, S.; Sun, L. The comprehensive landscape of miR-34a in cancer research. Cancer Metastasis Rev. 2021, 40, 925–948. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Daudia, A.; Birchall, J.P.; Banerjee, A.R. The localization of matrix metalloproteinases-8 and -13 in cholesteatoma, deep-meatal and post-auricular skin: A comparative analysis. Acta Otolaryngol. 2007, 127, 138–142. [Google Scholar] [CrossRef]
- Lee, R.J.; Mackenzie, I.C.; Hall, B.K.; Gantz, B.J. The nature of the epithelium in acquired cholesteatoma. Clin. Otolaryngol. Allied Sci. 1991, 16, 168–173. [Google Scholar] [CrossRef]
- Ferlito, S.; Fadda, G.; Lechien, J.R.; Cammaroto, G.; Bartel, R.; Borello, A.; Cavallo, G.; Piccinini, F.; La Mantia, I.; Cocuzza, S.; et al. Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study. J. Clin. Med. 2022, 11, 7000. [Google Scholar] [CrossRef]
- Ferlito, S.; La Mantia, I.; Merlino, F.; Cocuzza, S.; Di Stadio, A.; Cammaroto, G.; Bartel, R.; Fadda, G.; Iannella, G.; Mat, Q.; et al. Long-Term Anatomical and Hearing Outcomes of Canal Wall down Tympanoplasty for Tympano-Mastoid Cholesteatoma: A 20-Year Retrospective Study. Life 2022, 12, 1745. [Google Scholar] [CrossRef]
| Databases | Number of Hits | Search Lines |
|---|---|---|
| Scopus | 83 | TITLE-ABS-KEY ((microRNA) OR (miRNA)) AND (cholesteatoma) Limited to 1. Document type: Article 2. Language: English |
| Web of Science | 18 | (AB = ((microRNA) OR (miRNA))) AND (AB = (cholesteatoma)) |
| Pubmed/Medline | 17 | (“Cholesteatoma” [Mesh]) AND “MicroRNAs” [Mesh] |
| Cochrane | 0 | #1 “cholesteatoma” [Mesh] #2 “microRNAs” [Mesh] #1 AND #2 |
| Inclusion Criteria | Exclusion Criteria | |
| Topic | Studies concerning miRNA/cholesteatoma | Studies not related to miRNA/cholesteatoma |
| Study type | Original articles | Reviews, case reports, book chapters, expert opinions, letters to the editor, conference reports, posters |
| Study status | Completed, published | Unfinished, unpublished |
| Language | Full text available in English | Language other than English or only abstract available in English |
| Quality | Good-quality research studies | Poor-quality research studies |
| Study | miRNA | Expression in Cholesteatoma Tissue |
|---|---|---|
| Yang, J. [41] | miR-10a-5p | downregulated |
| Zhu, X. [47] | miR-1297, miR-26a-5p | downregulated |
| Zang, J. [44] | miR-125b | downregulated |
| Sui, R. [39] | miR-142-5p | downregulated |
| Yao, L. [42] | miR-199a | upregulated |
| Liu, D. [38] | miR-508-3p | upregulated |
| Hu, Y. [35] | miR-22-3p, miR-125a-5p | upregulated |
| Gong, N. [34] | exosomal miR-17 | downregulated |
| Zang, J. [43] | miR-203a | downregulated |
| Xie, S. [40] | miR-21-3p, miR-584-5p, miR-16-1-3p, miR-338-5p, miR-320b, miR-181a-3p, miR-181a-5p, miR-181b-5p, miR-335-3p, miR-155-5p, miR-224-3p, etc. | upregulated |
| miR-10a-5p, miR-152-5p, miR-203b-5p, miR-30a-5p, miR-1297, miR-539-3p, miR-9-3p, miR-769-3p, etc. | downregulated | |
| Li, Y. [37] | exosomal miR-106b-5p | downregulated |
| Chen, X. [31] | miR-21, let-7a miRNA | upregulated |
| Friedland, D.R. [33] | miR-21 | upregulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżaman, K.; Czerwaty, K.; Reichert, T.E.; Szczepański, M.J.; Ludwig, N. Expression and Regulatory Mechanisms of MicroRNA in Cholesteatoma: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12277. https://doi.org/10.3390/ijms241512277
Dżaman K, Czerwaty K, Reichert TE, Szczepański MJ, Ludwig N. Expression and Regulatory Mechanisms of MicroRNA in Cholesteatoma: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(15):12277. https://doi.org/10.3390/ijms241512277
Chicago/Turabian StyleDżaman, Karolina, Katarzyna Czerwaty, Torsten E. Reichert, Mirosław J. Szczepański, and Nils Ludwig. 2023. "Expression and Regulatory Mechanisms of MicroRNA in Cholesteatoma: A Systematic Review" International Journal of Molecular Sciences 24, no. 15: 12277. https://doi.org/10.3390/ijms241512277
APA StyleDżaman, K., Czerwaty, K., Reichert, T. E., Szczepański, M. J., & Ludwig, N. (2023). Expression and Regulatory Mechanisms of MicroRNA in Cholesteatoma: A Systematic Review. International Journal of Molecular Sciences, 24(15), 12277. https://doi.org/10.3390/ijms241512277