Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Results
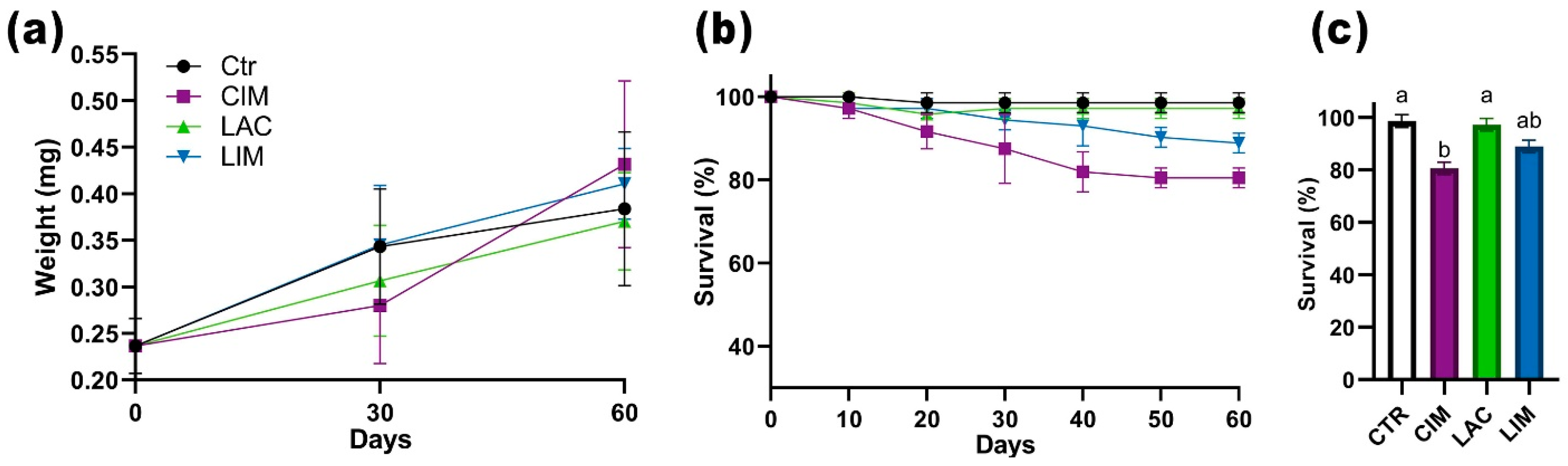
2.1. Survival Rate and Weight Gain
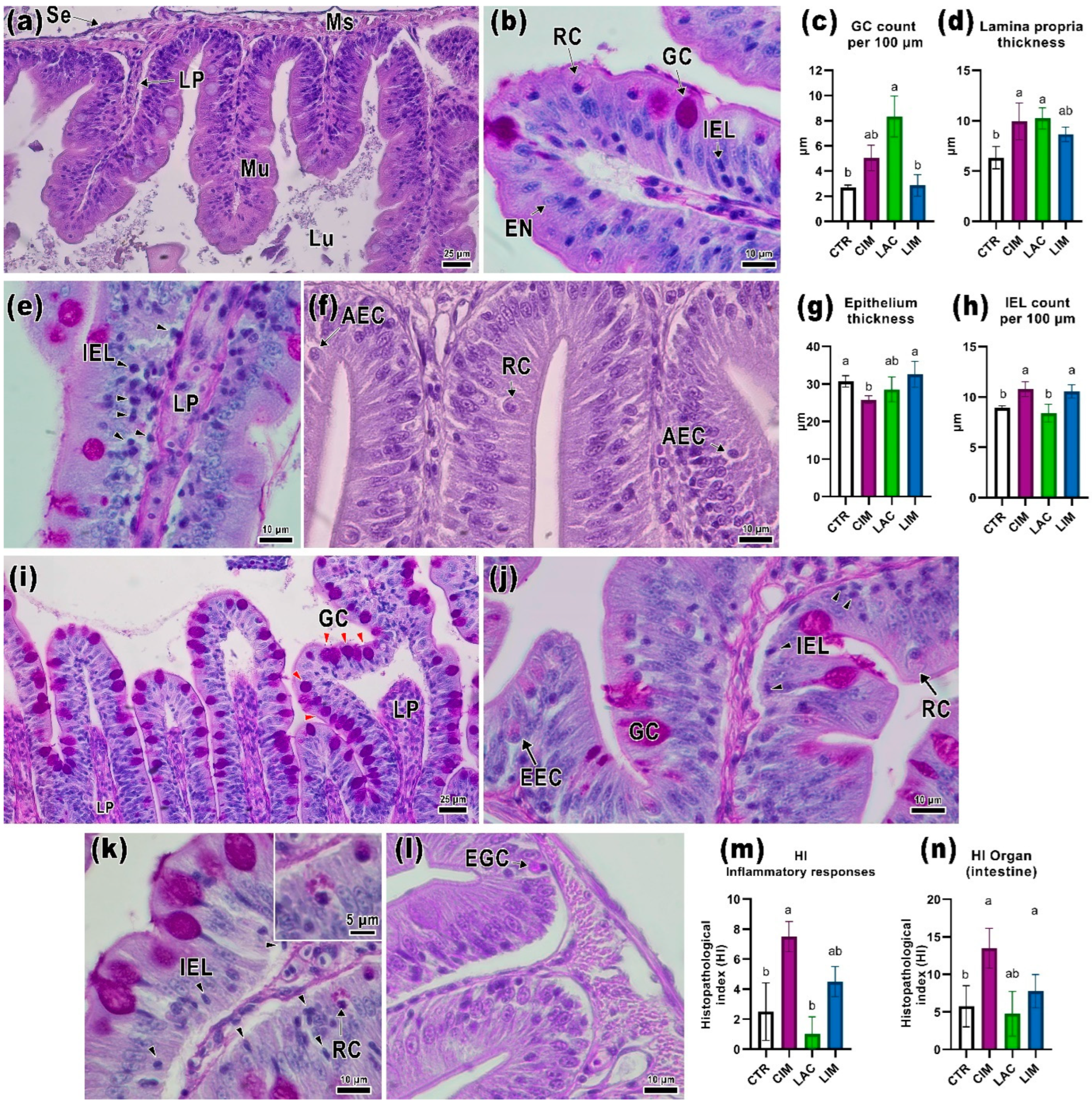
2.2. Intestinal Histology
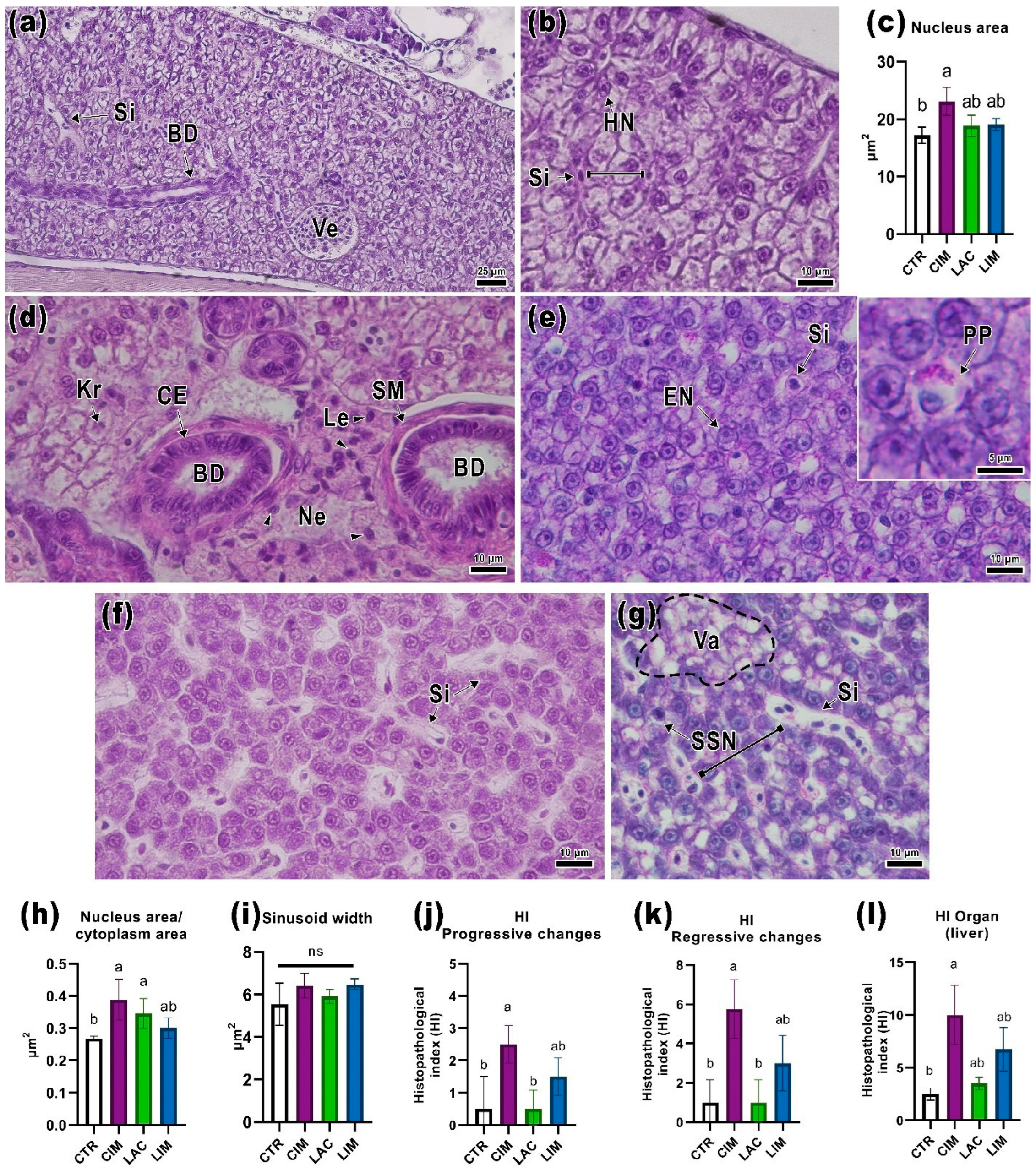
2.3. Liver Histology
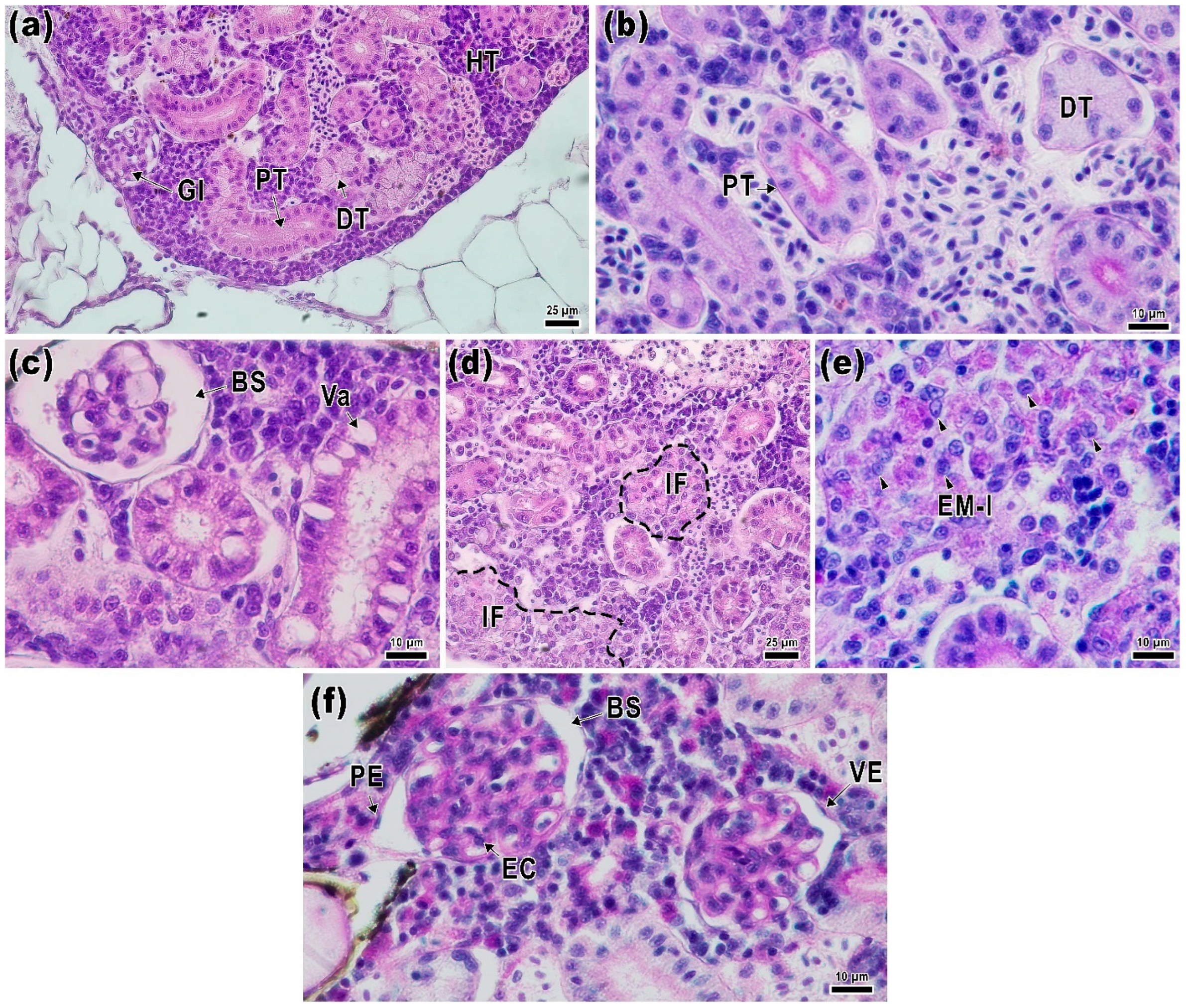
2.4. Kidney Histology
2.5. Principal Component Analysis
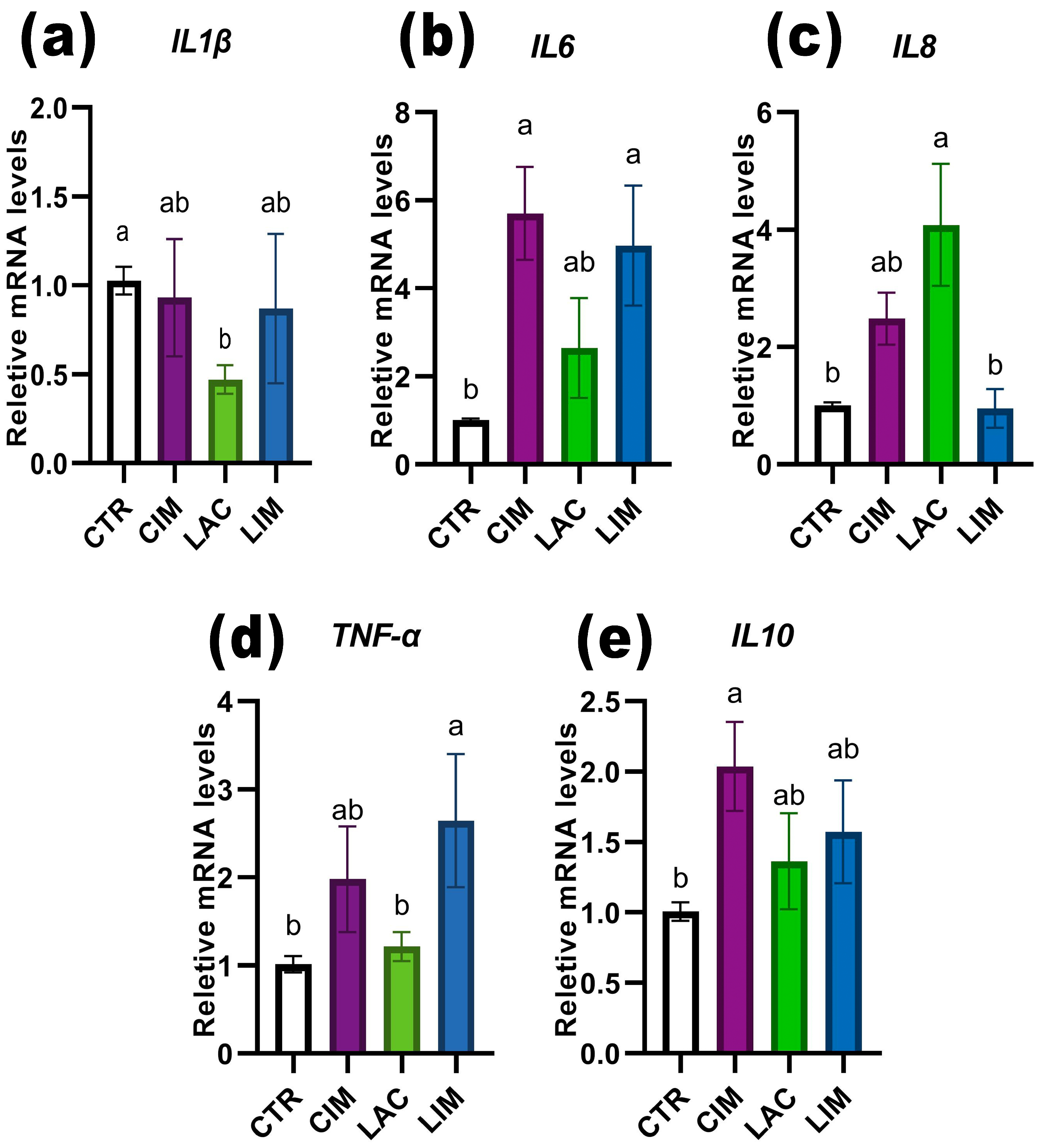
2.6. qRT-PCR Analyzes of Immune-Related Genes in Intestine
3. Discussion
3.1. Toxic Effects of Sublital Concentrations of Imidacloprid
3.2. Histological Parameters of the Intestine
3.3. Histological Parameters of the Liver
3.4. Histological Parameters of the Kidneys
3.5. Expression of Intestinal Pro/Anti-Inflammatory Genes
3.6. Possible Mechanisms of Mitigation of IMI Toxic Effects by Probiotic
4. Materials and Methods
4.1. Animal Collection and Maintenance
4.2. Bacterial Strain
4.3. Culture Media, Growth Conditions and Lyophilization
4.4. Experimental Design
4.5. Feed Preparation
4.6. Histological Preparations
4.7. Histomorphometric Analysis
4.8. Semi-Quantitative Assessment of Histological Lesions
4.9. RNA Extraction
4.10. Purification of RNA and Reverse Transcription Reaction
4.11. Real-Time DNA Amplification
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maldonado Galdeano, C.; Cazorla, S.; Lemme Dumit, J.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Kong, W.; Huang, C.; Tang, Y.; Zhang, D.; Wu, Z.; Chen, X. Effect of Bacillus subtilis on Aeromonas hydrophila-Induced Intestinal Mucosal Barrier Function Damage and Inflammation in Grass Carp (Ctenopharyngodon idella). Sci. Rep. 2017, 7, 1588. [Google Scholar] [CrossRef]
- Aggarwal, J.; Swami, G.; Kumar, M. Probiotics and their effects on metabolic diseases: An update. J. Clin. Diagn. Res. JCDR 2013, 7, 173. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut Microbiota Metagenomics in Aquaculture: Factors Influencing Gut Microbiome and Its Physiological Role in Fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Ibrahem, M.D. Evolution of Probiotics in Aquatic World: Potential Effects, the Current Status in Egypt and Recent Prospectives. J. Adv. Res. 2015, 6, 765–791. [Google Scholar] [CrossRef]
- Beck, B.R.; Kim, D.; Jeon, J.; Lee, S.-M.; Kim, H.K.; Kim, O.-J.; Lee, J.I.; Suh, B.S.; Do, H.K.; Lee, K.H.; et al. The Effects of Combined Dietary Probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on Innate Immunity and Disease Resistance in Olive Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y.; et al. Dietary Administration of Bacillus subtilis HAINUP40 Enhances Growth, Digestive Enzyme Activities, Innate Immune Responses and Disease Resistance of Tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, C.-H.; Wang, S.; Cheng, W. Dietary Administration of the Probiotic, Bacillus subtilis E20, Enhances the Growth, Innate Immune Responses, and Disease Resistance of the Grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef]
- Loghmani, H.; Khalili Hadad, B.; Kazempoor, R.; Sh, A.S. Investigation of the effects of Bifidobacterium bifidum as a probiotic on liver function enzymes due to exposure to E. coli O157H7 in Koi fish (Cyprinus rubrofuscus). J. Surv. Fish. Sci. 2022, 8, 27–33. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Yang, H.-L.; Ma, R.-L.; Song, K.; Lin, W.-Y. Molecular Analysis of Autochthonous Microbiota along the Digestive Tract of Juvenile Grouper Epinephelus coioides following ProbioticBacillus pumilusadministration. J. Appl. Microbiol. 2011, 110, 1093–1103. [Google Scholar] [CrossRef]
- Wu, X.; Teame, T.; Hao, Q.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Zhang, Y.-M.; Zhou, Z.; Duan, M.; et al. Use of a Paraprobiotic and Postbiotic Feed Supplement (HWFTM) Improves the Growth Performance, Composition and Function of Gut Microbiota in Hybrid Sturgeon (Acipenser baerii X Acipenser schrenckii). Fish Shellfish Immunol. 2020, 104, 36–45. [Google Scholar] [CrossRef]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M.Ł. Probiotics as a Biological Detoxification Tool of Food Chemical Contamination: A Review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini-Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Bhattacharya, S. Probiotics against Alleviation of Lead Toxicity: Recent Advances. Interdiscip. Toxicol. 2019, 12, 89–92. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Q.; Zhu, J.; Zhang, C.; Li, T.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Dietary Lactobacillus plantarum Supplementation Enhances Growth Performance and Alleviates Aluminum Toxicity in Tilapia. Ecotoxicol. Environ. Saf. 2017, 143, 307–314. [Google Scholar] [CrossRef]
- Tang, L.; Song, S.; Hu, C.; Lam, J.C.W.; Liu, M.; Zhou, B.; Lam, P.K.S.; Chen, L. Unexpected Observations: Probiotic Administration Greatly Aggravates the Reproductive Toxicity of Perfluorobutanesulfonate in Zebrafish. Chem. Res. Toxicol. 2020, 33, 1605–1608. [Google Scholar] [CrossRef]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei Strain for Zearalenone Binding and Metabolization. Toxicon 2020, 181, 9–18. [Google Scholar] [CrossRef]
- Jang, W.J.; Lee, J.M.; Hasan, M.T.; Lee, B.-J.; Lim, S.G.; Kong, I.-S. Effects of Probiotic Supplementation of a Plant-Based Protein Diet on Intestinal Microbial Diversity, Digestive Enzyme Activity, Intestinal Structure, and Immunity in Olive Flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2019, 92, 719–727. [Google Scholar] [CrossRef]
- Jang, W.S.; Jeon, M.-H.; Lee, S.H.; Park, S.Y.; Lee, Y.H.; Noh, D.-I.; Hur, S.-W.; Lee, S.-H.; Lee, B.-J.; Lee, J.-H.; et al. Dietary Supplementation of Bacillus sp. PM8313 with β-Glucan Modulates the Intestinal Microbiota of Red Sea Bream (Pagrus major) to Increase Growth, Immunity, and Disease Resistance. Front. Immunol. 2022, 13, 960554. [Google Scholar] [CrossRef]
- Vliegenthart, A.D.B.; Tucker, C.S.; Del Pozo, J.; Dear, J.W. Zebrafish as Model Organisms for Studying Drug-Induced Liver Injury. Br. J. Clin. Pharmacol. 2014, 78, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sales, M.; García-Ximénez, F.; Espinós, F.J. Zebrafish as a Possible Bioindicator of Organic Pollutants with Effects on Reproduction in Drinking Waters. J. Environ. Sci. 2015, 33, 254–260. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.; Seng, W.L. Use of Zebrafish Apoptosis Assays for Preclinical Drug Discovery. Expert Opin. Drug Discov. 2013, 8, 1191–1202. [Google Scholar] [CrossRef]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic Modulation of the Microbiota-Gut-Brain Axis and Behaviour in Zebrafish. Sci. Rep. 2016, 6, 30046. [Google Scholar] [CrossRef]
- Hedrera, M.I.; Galdames, J.A.; Jimenez-Reyes, M.F.; Reyes, A.E.; Avendaño-Herrera, R.; Romero, J.; Feijóo, C.G. Soybean Meal Induces Intestinal Inflammation in Zebrafish Larvae. PLoS ONE 2013, 8, e69983. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, Low Concentration Exposure to Pharmaceuticals Impacts Multiple Organ Systems in Zebrafish. Aquat. Toxicol. 2013, 132–133, 200–211. [Google Scholar] [CrossRef]
- Antunes, A.M.; Rocha, T.L.; Pires, F.L.; Alves, M.; Leite, V.S.; Arana, S.; Moreira, P.; Maria, S. Gender-Specific Histopathological Response in Guppies Poecilia reticulata Exposed to Glyphosate or Its Metabolite Aminomethylphosphonic Acid. J. Appl. Toxicol. 2017, 37, 1098–1107. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Montalbano, G.; Germanà, G.; Abbate, F.; Germanà, A. Micro and Nano Plastics Distribution in Fish as Model Organisms: Histopathology, Blood Response and Bioaccumulation in Different Organs. Appl. Sci. 2021, 11, 5768. [Google Scholar] [CrossRef]
- Pilehvar, A.; Town, R.M.; Blust, R. The Effect of Copper on Behaviour, Memory, and Associative Learning Ability of Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109900. [Google Scholar] [CrossRef]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus plantarum ST-III Alleviates the Toxic Effects of Triclosan on Zebrafish (Danio rerio) via Gut Microbiota Modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef]
- Torres, L.; Nilsen, E.; Grove, R.; Patiño, R. Health Status of Largescale Sucker (Catostomus macrocheilus) Collected along an Organic Contaminant Gradient in the Lower Columbia River, Oregon and Washington, USA. Sci. Total Environ. 2014, 484, 353–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, P.; Caeiro, S.; Diniz, M.; Lobo, J.M.; Martins, M.; Ferreira, A.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M. A Description of Chloride Cell and Kidney Tubule Alterations in the Flatfish Solea senegalensis Exposed to Moderately Contaminated Sediments from the Sado Estuary (Portugal). J. Sea Res. 2010, 64, 465–472. [Google Scholar] [CrossRef]
- Au, D.W.T. The Application of Histo-Cytopathological Biomarkers in Marine Pollution Monitoring: A Review. Mar. Pollut. Bull. 2004, 48, 817–834. [Google Scholar] [CrossRef]
- Nikiforov-Nikishin, A.; Smorodinskaya, S.; Kochetkov, N.; Nikiforov-Nikishin, D.; Danilenko, V.; Bugaev, O.; Vatlin, A.; Abrosimova, N.; Antipov, S.; Kudryavtsev, A.; et al. Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio). Animals 2022, 12, 2424. [Google Scholar] [CrossRef] [PubMed]
- Rašković, B.; Čičovački, S.; Ćirić, M.; Marković, Z.; Poleksić, V. Integrative Approach of Histopathology and Histomorphometry of Common Carp (Cyprinus carpio L.) Organs as a Marker of General Fish Health State in Pond Culture. Aquac. Res. 2015, 47, 3455–3463. [Google Scholar] [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A Histology-Based Fish Health Assessment of Farmed Seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375–381. [Google Scholar] [CrossRef]
- Green, J.W.; Springer, T.A.; Saulnier, A.N.; Swintek, J. Statistical Analysis of Histopathological Endpoints. Environ. Toxicol. Chem. 2014, 33, 1108–1116. [Google Scholar] [CrossRef]
- Costa, P.; Caeiro, S.; Costa, P.M. Multi-Organ Histological Observations on Juvenile Senegalese Soles Exposed to Low Concentrations of Waterborne Cadmium. Fish Physiol. Biochem. 2012, 39, 143–158. [Google Scholar] [CrossRef]
- Barišić, J.; Marijić, V.F.; Mijošek, T.; Čož-Rakovac, R.; Dragun, Z.; Krasnići, N.; Ivanković, D.; Kružlicová, D.; Erk, M. Evaluation of Architectural and Histopathological Biomarkers in the Intestine of Brown Trout (Salmo trutta Linnaeus, 1758) Challenged with Environmental Pollution. Sci. Total Environ. 2018, 642, 656–664. [Google Scholar] [CrossRef]
- Kleinow, K.M.; James, M.O. Response of the teleost gastrointestinal system to xenobiotics. In Target Organ Toxicity in Marine and Freshwater Teleosts; Schlenk, D., Benson, W.H., Eds.; Taylor & Francis: Abingdon, UK, 2001; Volume 1, pp. 269–362. [Google Scholar] [CrossRef]
- Van Dijk, T.C.; Van Staalduinen, M.A.; Van der Sluijs, J.P. Macro-Invertebrate Decline in Surface Water Polluted with Imidacloprid. PLoS ONE 2013, 8, e62374. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Gawade, L.; Dadarkar, S.S.; Husain, R.; Gatne, M. A Detailed Study of Developmental Immunotoxicity of Imidacloprid in Wistar Rats. Food Chem. Toxicol. 2013, 51, 61–70. [Google Scholar] [CrossRef]
- Lonare, M.; Kumar, M.; Raut, S.; Badgujar, P.; Doltade, S.; Telang, A. Evaluation of Imidacloprid-Induced Neurotoxicity in Male Rats: A Protective Effect of Curcumin. Neurochem. Int. 2014, 78, 122–129. [Google Scholar] [CrossRef]
- Bagri, P.; Kumar, V.; Sikka, A.K. Assessment of Imidacloprid-Induced Mutagenic Effects in Somatic Cells of Swiss Albino Male Mice. Drug Chem. Toxicol. 2016, 39, 412–417. [Google Scholar] [CrossRef]
- Qadir, S.; Iqbal, F. Effect of Subleathal Concentrtion of Imidacloprid on the Histology of Heart, Liver and Kidney in Labeo rohitr. Pak. J. Pharm. Sci. 2016, 29, 2033–2038. [Google Scholar]
- Priya, B.P.; Vijaya, R.; Maruthi, Y.A. Acute toxicity effect of imidacloprid insecticide on serum biochemical parameters of fresh water teleost Channa punctatus. Int. J. Integr. Sci. Innov. Technol. (IJIIT) 2012, 1, 18–22. [Google Scholar]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Impact of Imidacloprid Exposure on the Biochemical Responses, Transcriptome, Gut Microbiota and Growth Performance of the Pacific White Shrimp Litopenaeus vannamei. J. Hazard. Mater. 2022, 424, 127513. [Google Scholar] [CrossRef]
- Butcherine, P.; Kelaher, B.P.; Taylor, M.D.; Barkla, B.J.; Benkendorff, K. Impact of Imidacloprid on the Nutritional Quality of Adult Black Tiger Shrimp (Penaeus monodon). Ecotoxicol. Environ. Saf. 2020, 198, 110682. [Google Scholar] [CrossRef]
- Vignet, C.; Cappello, T.; Fu, Q.; Lajoie, K.; De Marco, G.; Clérandeau, C.; Mottaz, H.; Maisano, M.; Hollender, J.; Schirmer, K.; et al. Imidacloprid Induces Adverse Effects on Fish Early Life Stages That Are More Severe in Japanese Medaka (Oryzias latipes) than in Zebrafish (Danio rerio). Chemosphere 2019, 225, 470–478. [Google Scholar] [CrossRef]
- Luo, T.; Wang, X.; Jin, Y. Low Concentrations of Imidacloprid Exposure Induced Gut Toxicity in Adult Zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108972. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, G.; Zhu, L.; Wang, J. DNA Damage and Oxidative Stress Induced by Imidacloprid Exposure in the Earthworm Eisenia Fetida. Chemosphere 2016, 144, 510–517. [Google Scholar] [CrossRef]
- Marsova, M.; Abilev, S.K.; Poluektova, E.U.; Danilenko, V.N. A Bioluminescent Test System Reveals Valuable Antioxidant Properties of Lactobacillus Strains from Human Microbiota. World J. Microbiol. Biotechnol. 2018, 34, 27. [Google Scholar] [CrossRef]
- Olekhnovich, E.I.; Batotsyrenova, E.G.; Yunes, R.A.; Kashuro, V.A.; Poluektova, E.U.; Veselovsky, V.V.; Ilina, E.N.; Danilenko, V.N.; Klimina, K.M. The Effects of LeviLactobacillus brevis on the Physiological Parameters and Gut Microbiota Composition of Rats Subjected to Desynchronosis. Microb. Cell Factories 2021, 20, 226. [Google Scholar] [CrossRef]
- Marsova, M.; Odorskaya, M.; Novichkova, M.; Polyakova, V.; Abilev, S.K.; Kalinina, E.A.; Shtil, A.A.; Poluektova, E.U.; Danilenko, V.N. The Lactobacillus brevis 47 F Strain Protects the Murine Intestine from Enteropathy Induced by 5-Fluorouracil. Microorganisms 2020, 8, 876. [Google Scholar] [CrossRef]
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water 2021, 13, 1348. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2019, 28, 16–42. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Wong, W.R.; Riener, R.M.; Schulze, C.J.; Linington, R.G. Examining the Fish Microbiome: Vertebrate-Derived Bacteria as an Environmental Niche for the Discovery of Unique Marine Natural Products. PLoS ONE 2012, 7, e35398. [Google Scholar] [CrossRef]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The Adherence and Colonization of Microorganisms in Fish Gastrointestinal Tract. Rev. Aquac. 2018, 11, 603–618. [Google Scholar] [CrossRef]
- da Cunha, R.L.D.; de Brito-Gitirana, L. Effects of Titanium Dioxide Nanoparticles on the Intestine, Liver, and Kidney of Danio rerio. Ecotoxicol. Environ. Saf. 2020, 203, 111032. [Google Scholar] [CrossRef]
- Moschet, C.; Wittmer, I.; Simovic, J.; Junghans, M.; Piazzoli, A.; Singer, H.; Stamm, C.; Leu, C.; Hollender, J. How a Complete Pesticide Screening Changes the Assessment of Surface Water Quality. Environ. Sci. Technol. 2014, 48, 5423–5432. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A Review of the Direct and Indirect Effects of Neonicotinoids and Fipronil on Vertebrate Wildlife. Environ. Sci. Pollut. Res. 2014, 22, 103–118. [Google Scholar] [CrossRef]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard Identification of Imidacloprid to Aquatic Environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Patel, B.; Upadhyay, A.; Parikh, P. Histological changes in the tissues of Oreochromis mossambicus and Labeo rohita on exposure to imidacloprid and curzate. Int. J. Res. Appl. Nat. Soc. Sci. 2016, 4, 149–160. [Google Scholar]
- Giommi, C.; Habibi, H.R.; Candelma, M.; Carnevali, O.; Maradonna, F. Probiotic Administration Mitigates Bisphenol A Reproductive Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 9314. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, R.; Liu, W.; Fu, Z. Effect of Endocrine Disrupting Chemicals on the Transcription of Genes Related to the Innate Immune System in the Early Developmental Stage of Zebrafish (Danio rerio). Fish Shellfish Immunol. 2010, 28, 854–861. [Google Scholar] [CrossRef]
- Buddington, R.; Krogdahl, A.; Bakke-McKellep, A.M. The Intestines of Carnivorous Fish: Structure and Functions and the Relations with Diet. Acta Physiol. Scandinavica. Suppl. 1997, 638, 67–80. [Google Scholar]
- Kiristioglu, I.; Antony, P.; Fan, Y.; Forbush, B.; Mosley, R.L.; Yang, H.; Teitelbaum, D.H. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Dig. Dis. Sci. 2002, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Liebel, S.; Tomotake, M.E.M.; Oliveira-ribeiro, C.A. Fish Histopathology as Biomarker to Evaluate Water Quality. Ecotoxicol. Environ. Contam. 2013, 8, 9–15. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the Growth Performance, Intestinal Morphology, Hematology and Body Composition of Nile Tilapia, Oreochromis niloticus. Aquac. Int. 2014, 23, 203–217. [Google Scholar] [CrossRef]
- Feng, L.; Ni, P.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Decreased Enteritis Resistance Ability by Dietary Low or Excess Levels of Lipids through Impairing the Intestinal Physical and Immune Barriers Function of Young Grass Carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 67, 493–512. [Google Scholar] [CrossRef]
- Picchietti, S.; Fausto, A.M.; Randelli, E.; Carnevali, O.; Taddei, A.R.; Buonocore, F.; Scapigliati, G.; Abelli, L. Early Treatment with Lactobacillus delbrueckii Strain Induces an Increase in Intestinal T-Cells and Granulocytes and Modulates Immune-Related Genes of Larval Dicentrarchus labrax (L.). Fish Shellfish Immunol. 2009, 26, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of Intestinal Morphology and Immunity in Nile Tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Motta, C.M.; Califano, E.; Scudiero, R.; Avallone, B.; Fogliano, C.; De Bonis, S.; Raggio, A.; Simoniello, P. Effects of Cadmium Exposure on Gut Villi in Danio rerio. Int. J. Mol. Sci. 2022, 23, 1927. [Google Scholar] [CrossRef] [PubMed]
- Elhafeez, H.H.A.; Soliman, S.A. Origin of Rodlet Cells and Mapping Their Distribution in Ruby-Red-Fin Shark (Rainbow Shark) Epalzeorhynchos frenatum (Teleostei: Cyprinidae): Light, Immunohistochemistry and Ultrastructure Study. J. Cytol. Histol. 2016, 7, 435. [Google Scholar] [CrossRef]
- Abd-Elhafeez, H.H.; Abou-Elhamd, A.S.; Abdo, W.; Soliman, S.A. Migratory Activities and Stemness Properties of Rodlet Cells. Microsc. Microanal. 2020, 26, 1035–1052. [Google Scholar] [CrossRef]
- Reite, O.B. The Rodlet Cells of Teleostean Fish: Their Potential Role in Host Defence in Relation to the Role of Mast Cells/Eosinophilic Granule Cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef]
- Vickers, T. A Study of the Intestinal Epithelium of the Goldfish Carassius Auratus: Its Normal Structure, the Dynamics of Cell Replacement, and the Changes Induced by Salts of Cobalt and Manganese. J. Cell Sci. 1962, s3-103, 93–110. [Google Scholar] [CrossRef]
- El-Bakary, N.E.R.; El-Gammal, H.L. Comparative Histological, Histochemical and Ultrastructural Studies on the Liver of Flathead Grey Mullet (Mugil cephalus) and Sea Bream (Sparus aurata). Glob. Vet. 2010, 4, 548–553. [Google Scholar]
- Lukin, A.; Sharova, J.; Belicheva, L.; Camus, L. Assessment of Fish Health Status in the Pechora River: Effects of Contamination. Ecotoxicol. Environ. Saf. 2011, 74, 355–365. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Cellular and Stromal Elements Organization in the Liver of Grass Carp, Ctenopharyngodon idella (Cypriniformes: Cyprinidae). Micron 2018, 112, 1–14. [Google Scholar] [CrossRef]
- Wolf, J.C.; Wolfe, M.J. A Brief Overview of Nonneoplastic Hepatic Toxicity in Fish. Toxicol. Pathol. 2005, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.A.; Wingert, R.A. Zebrafish Renal Pathology: Emerging Models of Acute Kidney Injury. Curr. Pathobiol. Rep. 2015, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Santamaría, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Mechanisms of Renal Apoptosis in Health and Disease. J. Am. Soc. Nephrol. 2008, 19, 1634–1642. [Google Scholar] [CrossRef]
- Sayed, H.; Saleh, M.; Mitani, H. Comparative Histology of Wild-Type and P53-Deficient Medaka (Oryzias latipes): Nephrotoxic Effect of Ultraviolet a Radiation. Photochem. Photobiol. Sci. 2020, 19, 261–273. [Google Scholar] [CrossRef]
- Wolf, J.C.; Baumgartner, W.A.; Blazer, V.S.; Camus, A.C.; Engelhardt, J.A.; Fournie, J.W.; Frasca, S.; Groman, D.B.; Kent, M.L.; Khoo, L.H.; et al. Nonlesions, Misdiagnoses, Missed Diagnoses, and Other Interpretive Challenges in Fish Histopathology Studies. Toxicol. Pathol. 2014, 43, 297–325. [Google Scholar] [CrossRef]
- Castellana, B.; Marín-Juez, R.; Planas, J.V. Transcriptional Regulation of the Gilthead Seabream (Sparus aurata) Interleukin-6 Gene Promoter. Fish Shellfish Immunol. 2013, 35, 71–78. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. Identification and Expression Analysis of Two Fish-Specific IL-6 Cytokine Family Members, the Ciliary Neurotrophic Factor (CNTF)-like and M17 Genes, in Rainbow Trout Oncorhynchus Mykiss. Mol. Immunol. 2009, 46, 2290–2298. [Google Scholar] [CrossRef]
- Hong, X.; Wang, Y.; Tian, X.; Li, J.-S.; Zha, J. Changes of Hematological and Biochemical Parameters Revealed Genotoxicity and Immunotoxicity of Neonicotinoids on Chinese Rare Minnows (Gobiocypris rarus). Environ. Pollut. 2018, 233, 862–871. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.L.; Stolte, E.H.; Metz, J.R.; Chadzinska, M. Chapter 7 Neuroendocrine–Immune Interactions in Teleost Fish. In Fish Neuroendocrinology; Bernier, N.J., Van Der Kraak, G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press Inc.: San Diego, CA, USA, 2009; pp. 313–364. [Google Scholar] [CrossRef]
- Jin, Y.; Pan, X.; Cao, L.; Ma, B.; Fu, Z. Embryonic Exposure to cis-Bifenthrin Enantioselectively Induces the Transcription of Genes Related to Oxidative Stress, Apoptosis and Immunotoxicity in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2013, 34, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and Its Receptors: A Highly Regulated and Dynamic System. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients Mediate Intestinal Bacteria–Mucosal Immune Crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Oehlers, S.H.B.; Flores, M.V.; Hall, C.J.; O’Toole, R.; Swift, S.; Crosier, K.E.; Crosier, P.S. Expression of Zebrafish cxcl8 (Interleukin-8) and Its Receptors during Development and in Response to Immune Stimulation. Dev. Comp. Immunol. 2010, 34, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Nimalan, N.; Sørensen, S.L.; Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Gancarčíková, S.; Vatsos, I.N.; Bisa, S.; Kiron, V.; Sørensen, M. Supplementation of Lactic Acid Bacteria Has Positive Effects on the Mucosal Health of Atlantic Salmon (Salmo salar) Fed Soybean Meal. Aquac. Rep. 2023, 28, 101461. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, Microorganisms and Their Metabolites, and Colon Cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Kazempour, A.; Kazempoor, R. The Effect of Lacticaseibacillus Casei on Inflammatory Cytokine (IL-8) Gene Expression Induced by Exposure to Shigella Sonnei in Zebrafish (Danio rerio). Arq. Bras. De Med. Veterinária E Zootec. 2022, 74, 211–218. [Google Scholar] [CrossRef]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppée, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-Inflammatory Effect of Lactobacillus Caseion Shigella-Infected Human Intestinal Epithelial Cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cao, F.; Li, H.; Teng, M.; Liang, Y.; Qiu, L. The Effects of a Short-Term Exposure to Propiconazole in Zebrafish (Danio rerio) Embryos. Environ. Sci. Pollut. Res. 2020, 27, 38212–38220. [Google Scholar] [CrossRef]
- Li, H.; Song, Y.; Cao, F.; Wang, C.; Zheng, M.; Li, X.; Qiu, L. Developmental Toxicity and Potential Mechanisms of Pyraoxystrobin to Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity Induced by Subchronic Exposure of Fluoride and Chlorpyrifos in Wistar Rats: Mitigating Effect of Ascorbic Acid. Biol. Trace Elem. Res. 2015, 166, 157–162. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as Reproductive Toxicant and Endocrine Disruptor: Investigations in Laboratory Animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103–108. [Google Scholar] [CrossRef]
- Mahajan, L.; Verma, P.K.; Raina, R.; Sood, S. Toxic Effects of Imidacloprid Combined with Arsenic: Oxidative Stress in Rat Liver. Toxicol. Ind. Health 2018, 34, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid Contamination of Global Surface Waters and Associated Risk to Aquatic Invertebrates: A Review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Lu, F.; Zhang, J.; Guo, L. Application of Zebrafish in the Study of the Gut Microbiome. Anim. Models Exp. Med. 2022, 5, 323–336. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, M.; Zhang, M.; Duan, M.; Zheng, J.; Liu, Y.; Qiu, L. Sub-Lethal Concentration of Metamifop Exposure Impair Gut Health of Zebrafish (Danio rerio). Chemosphere 2022, 303, 135081. [Google Scholar] [CrossRef]
- Clarke, G.; Sandhu, K.V.; Griffin, B.T.; Dinan, T.G.; Cryan, J.F.; Hyland, N.P. Gut Reactions: Breaking down Xenobiotic–Microbiome Interactions. Pharmacol. Rev. 2019, 71, 198–224. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of Intestinal Mucins in Innate Host Defense Mechanisms against Pathogens. J. Innate Immun. 2008, 1, 123–135. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. Lactobacillus brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation through the Interplay of MAPK and NF-ΚB Signaling Cascades. Front. Microbiol. 2018, 9, 2425. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Linsalata, M.; Bianco, G.; Notarnicola, M.; D’Attoma, B.; Scavo, M.; Tafaro, A.; Russo, F. Lactobacillus rhamnosus GG Protects the Epithelial Barrier of Wistar Rats from the Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients 2018, 10, 1698. [Google Scholar] [CrossRef]
- Beer, F.; Urbat, F.; Charles; Huch, M.; Kulling, S.E.; Bunzel, M.; Bunzel, D. The Human Fecal Microbiota Metabolizes Foodborne Heterocyclic Aromatic Amines by Reuterin Conjugation and Further Transformations. Mol. Nutr. Food Res. 2019, 63, 1801177. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus Adhesion to Mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Liu, X.; Yang, G.; An, X.; Wang, Q.; Wang, Y. Joint Toxic Effects of Triazophos and Imidacloprid on Zebrafish (Danio rerio). Environ. Pollut. 2018, 235, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Krohn, J.; Hellpointer, E. Environmental Fate of Imidacloprid. Pflanzenschutz-Nachr. Bayer (Spec. Ed.) 2002, 55, 1–26. [Google Scholar]
- Nikiforov-Nikishin, A.; Nikiforov-Nikishin, D.; Kochetkov, N.; Smorodinskaya, S.; Klimov, V. The Influence of Probiotics of Different Microbiological Composition on Histology of the Gastrointestinal Tract of Juvenile Oncorhynchus mykiss. Microsc. Res. Tech. 2021, 85, 538–547. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Hamidian, G.; Zirak, K.; Sheikhzadeh, N.; Oushani, A.K.; Shabanzadeh, S.; Divband, B. Intestinal Histology and Stereology in Rainbow Trout (Oncorhynchus mykiss) Administrated with Nanochitosan/Zeolite and Chitosan/Zeolite Composites. Aquac. Res. 2018, 49, 1803–1815. [Google Scholar] [CrossRef]
- Lai, S.; Yu, W.-M.; Wallace, L.E.; Sigalet, D.L. Intestinal Muscularis Propria Increases in Thickness with Corrected Gestational Age and Is Focally Attenuated in Patients with Isolated Intestinal Perforations. J. Pediatr. Surg. 2014, 49, 114–119. [Google Scholar] [CrossRef]
- Oropesa, A.L.; Jiménez, B.; Fallola, C.; Pula, H.J.; Cuesta, J.M.; Gómez, L. Histological Alterations on the Structure of the Excretory Renal System in Tench (Tinca tinca) after Exposure to 17-Alpha-Ethynylestradiol. Bull. Environ. Contam. Toxicol. 2013, 91, 623–629. [Google Scholar] [CrossRef]
- Escaffre, A.-M.; Kaushik, S.; Mambrini, M. Morphometric Evaluation of Changes in the Digestive Tract of Rainbow Trout (Oncorhynchus mykiss) due to Fish Meal Replacement with Soy Protein Concentrate. Aquaculture 2007, 273, 127–138. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in Fish: Proposal for a Protocol to Assess Aquatic Pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Costa, P.; Diniz, M.; Caeiro, S.; Lobo, J.M.; Martins, M.; Ferreira, A.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M. Histological Biomarkers in Liver and Gills of Juvenile Solea senegalensis Exposed to Contaminated Estuarine Sediments: A Weighted Indices Approach. Aquat. Toxicol. 2009, 92, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Badroo, I.A.; Nandurkar, H.P.; Khanday, A.H. Toxicological Impacts of Herbicide Paraquat Dichloride on Histological Profile (Gills, Liver, and Kidney) of Freshwater Fish Channa punctatus (Bloch). Environ. Sci. Pollut. Res. Int. 2020, 27, 39054–39067. [Google Scholar] [CrossRef] [PubMed]
- Pacorig, V.; Galeotti, M.; Beraldo, P. Multiparametric Semi-quantitative Scoring System for the histological evaluation of marine fish larval and juvenile quality. Aquac. Rep. 2022, 26, 101285. [Google Scholar] [CrossRef]
- Cengiz, E.I.; Unlu, E. Sublethal Effects of Commercial Deltamethrin on the Structure of the Gill, Liver and Gut Tissues of Mosquitofish, Gambusia Affinis: A Microscopic Study. Environ. Toxicol. Pharmacol. 2006, 21, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Haimes, J.; Kelley, M. Demonstration of a ΔΔCq Calculation Method to Compute Relative Gene Expression from QPCR Data. Semantic Scholar. 2015. Available online: https://api.semanticscholar.org/CorpusID:28223471 (accessed on 1 January 2023).
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 May 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 2 June 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochetkov, N.; Smorodinskaya, S.; Vatlin, A.; Nikiforov-Nikishin, D.; Nikiforov-Nikishin, A.; Danilenko, V.; Anastasia, K.; Reznikova, D.; Grishina, Y.; Antipov, S.; et al. Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2023, 24, 12290. https://doi.org/10.3390/ijms241512290
Kochetkov N, Smorodinskaya S, Vatlin A, Nikiforov-Nikishin D, Nikiforov-Nikishin A, Danilenko V, Anastasia K, Reznikova D, Grishina Y, Antipov S, et al. Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2023; 24(15):12290. https://doi.org/10.3390/ijms241512290
Chicago/Turabian StyleKochetkov, Nikita, Svetlana Smorodinskaya, Aleksey Vatlin, Dmitry Nikiforov-Nikishin, Alexei Nikiforov-Nikishin, Valery Danilenko, Klimuk Anastasia, Diana Reznikova, Yelena Grishina, Sergei Antipov, and et al. 2023. "Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio)" International Journal of Molecular Sciences 24, no. 15: 12290. https://doi.org/10.3390/ijms241512290
APA StyleKochetkov, N., Smorodinskaya, S., Vatlin, A., Nikiforov-Nikishin, D., Nikiforov-Nikishin, A., Danilenko, V., Anastasia, K., Reznikova, D., Grishina, Y., Antipov, S., & Marsova, M. (2023). Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio). International Journal of Molecular Sciences, 24(15), 12290. https://doi.org/10.3390/ijms241512290






