Protaetia Brevitarsis-Derived Protein Hydrolysate Reduces Obesity-Related Colitis Induced by High-Fat Diet in Mice through Anti-Inflammatory Pathways
Abstract
:1. Introduction
2. Results
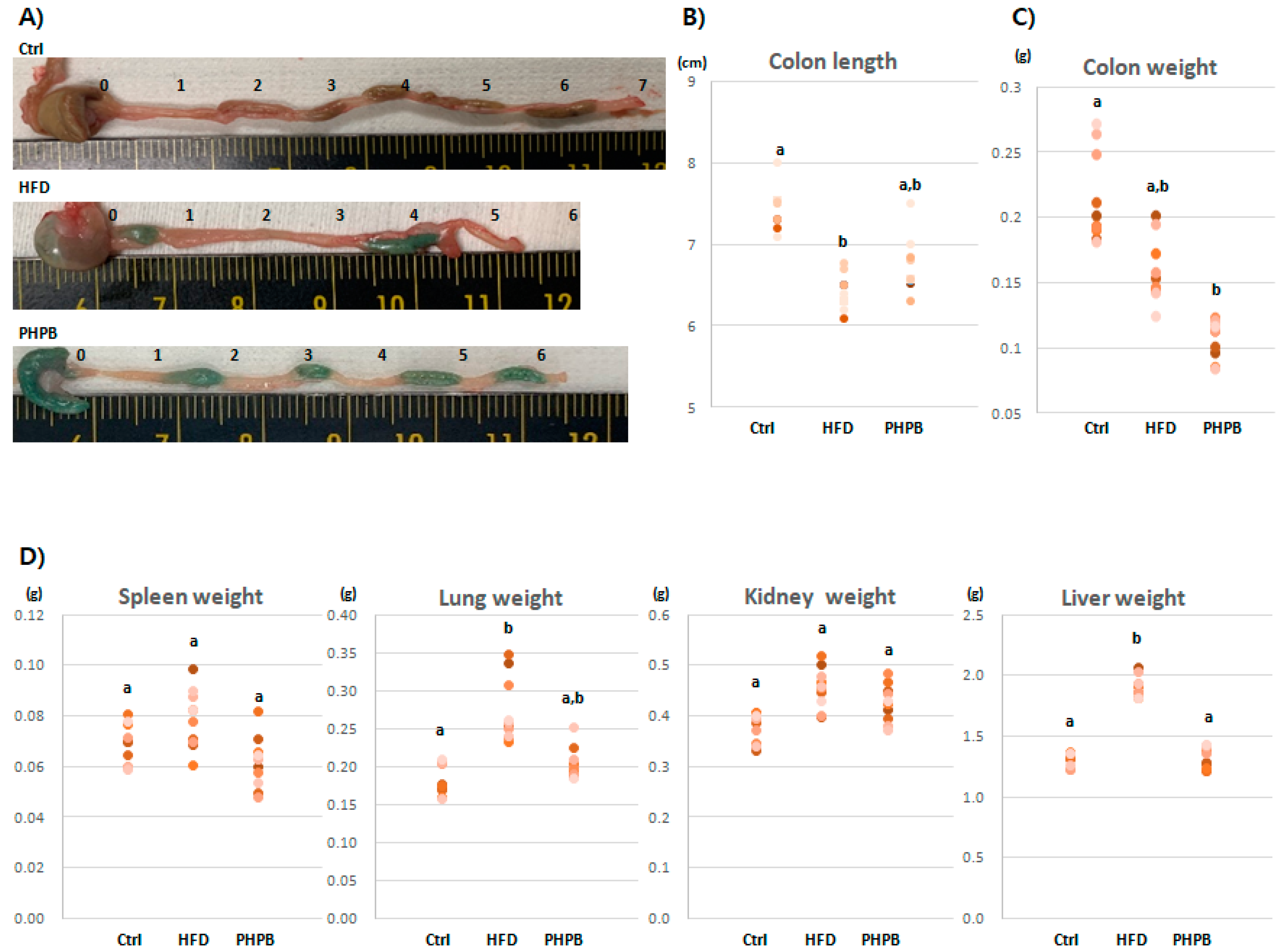
2.1. Effects of PHPB on Stools, Colon Length/Weight, and Immune-Related Organs
2.2. Effects of PHPB on Body Weight and Food Intake
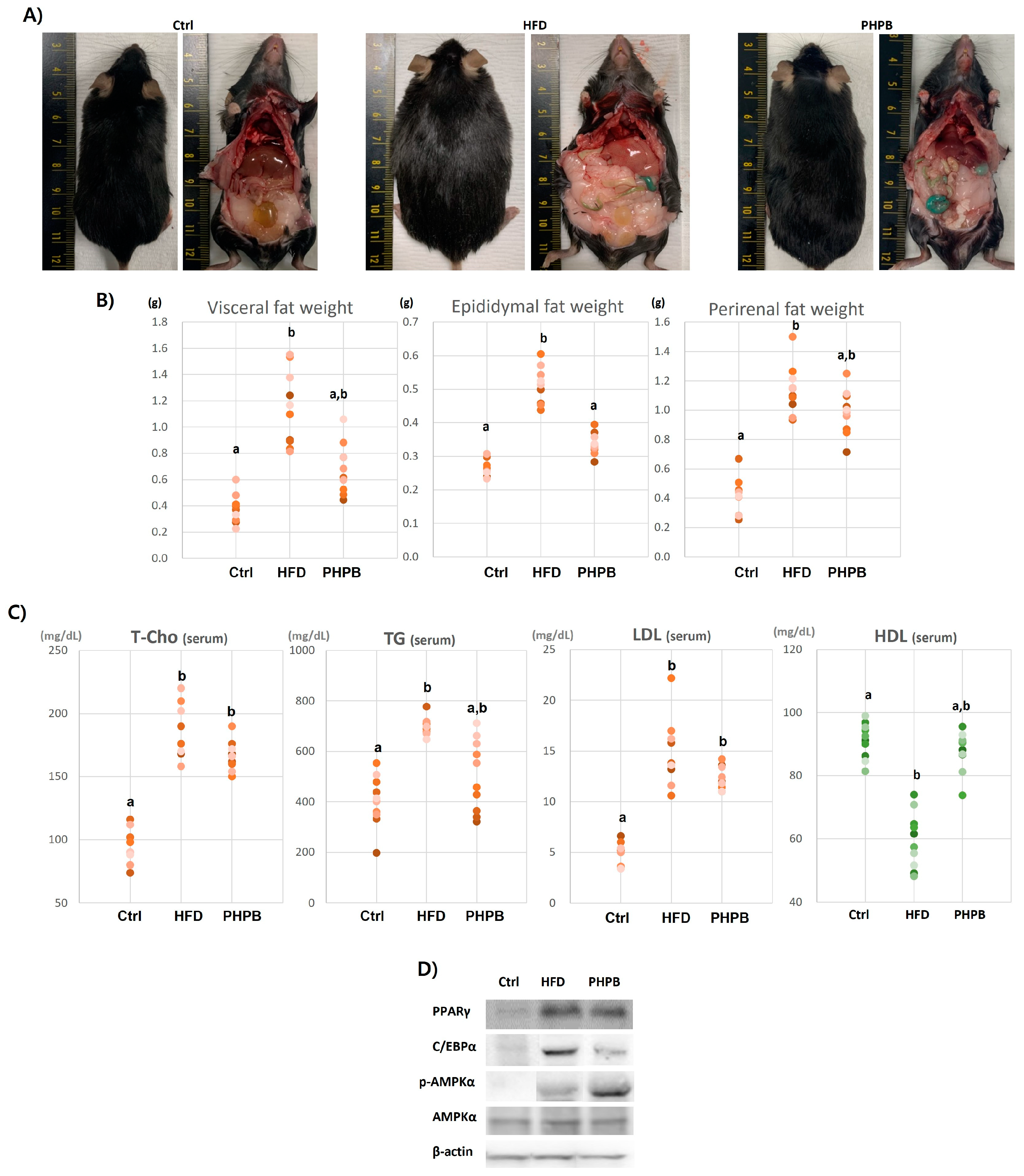
2.3. Effects of PHPB on Fat Accumulation, Lipid Metabolism, and Adipogenesis
2.4. Effects of PHPB on Inflammatory Molecules
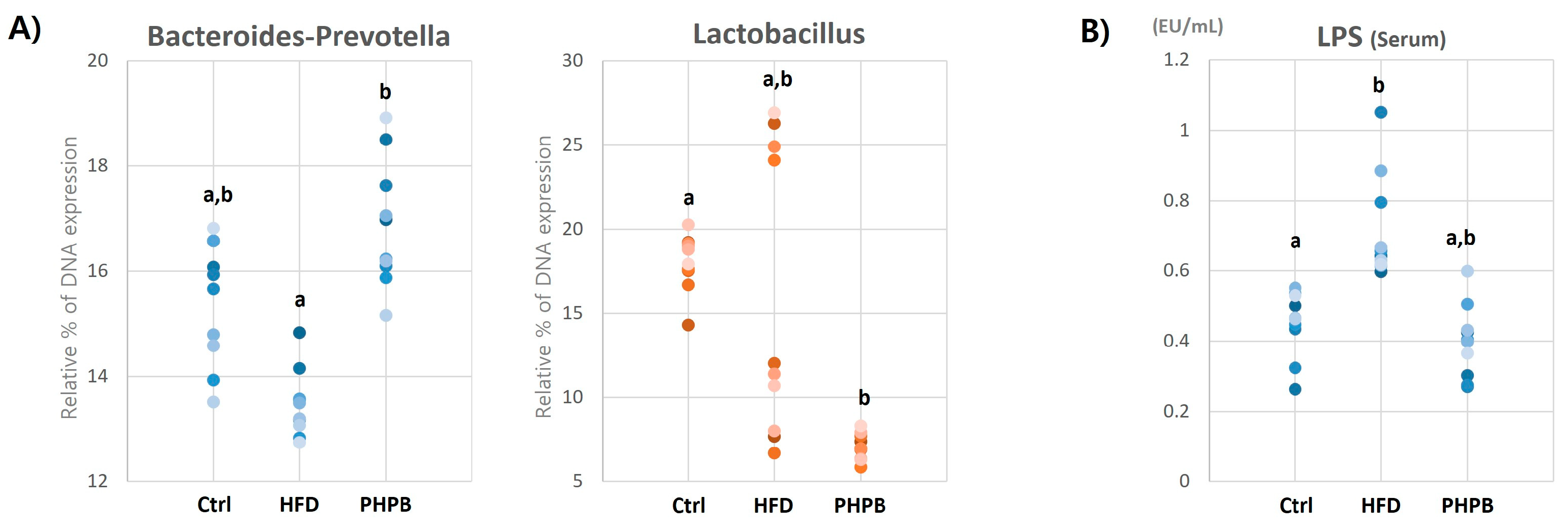
2.5. Effects of PHPB on Gut Microbiota and Serum LPS
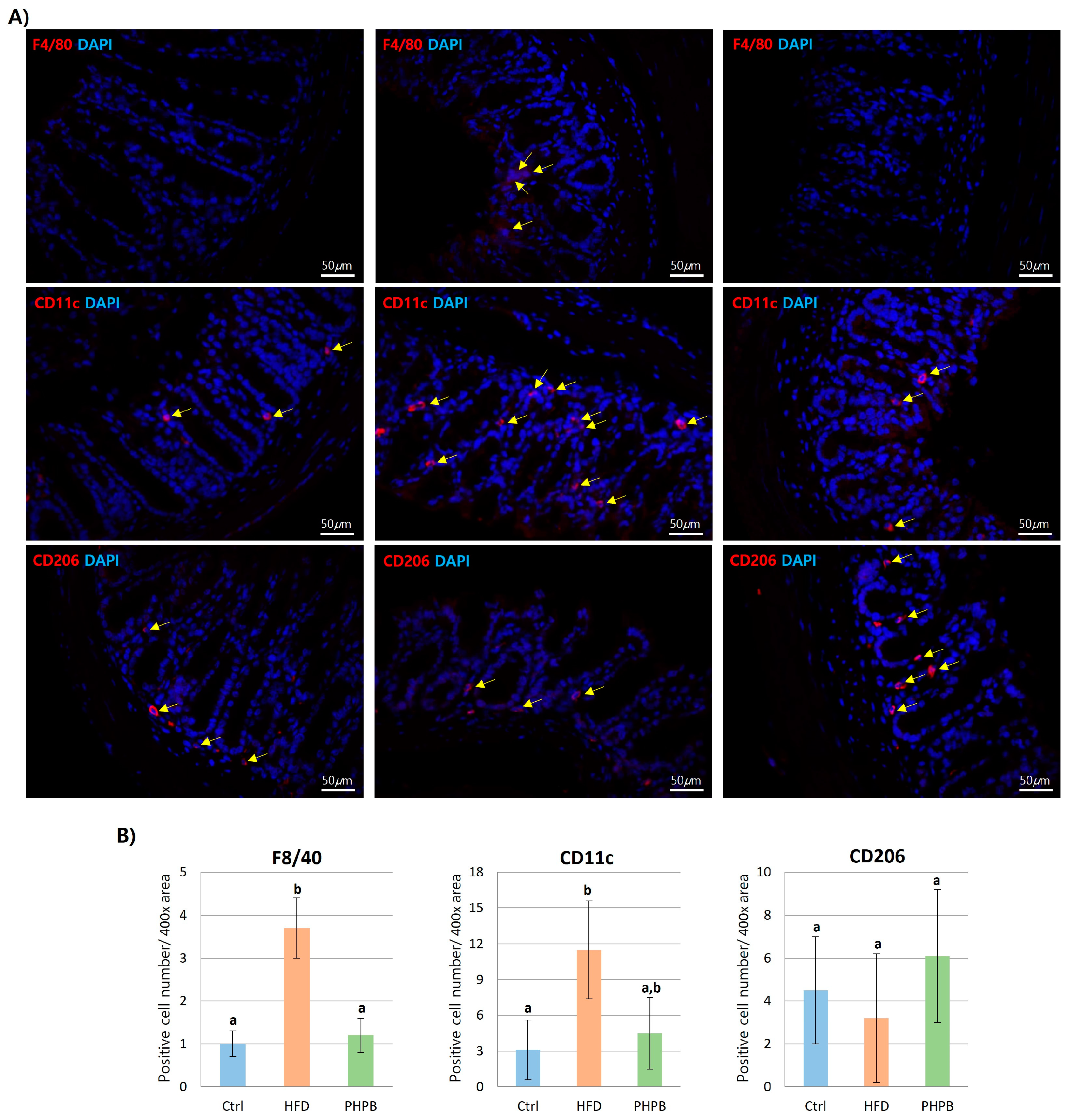
2.6. Effects of PHPB on Macrophage Polarization into M2
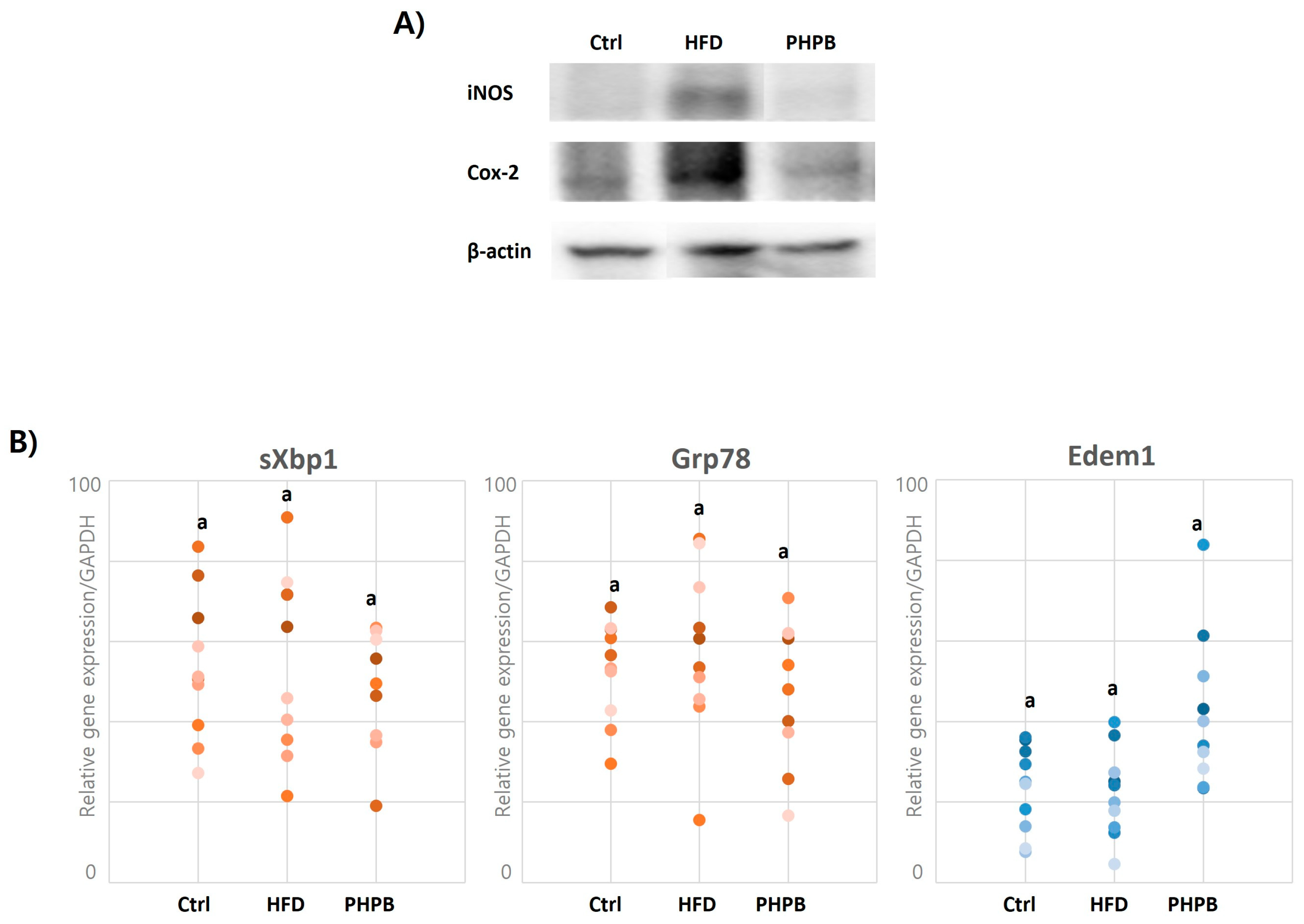
2.7. Effects of PHPB on Oxidative Stress and ER Stress in Epithelial Cells
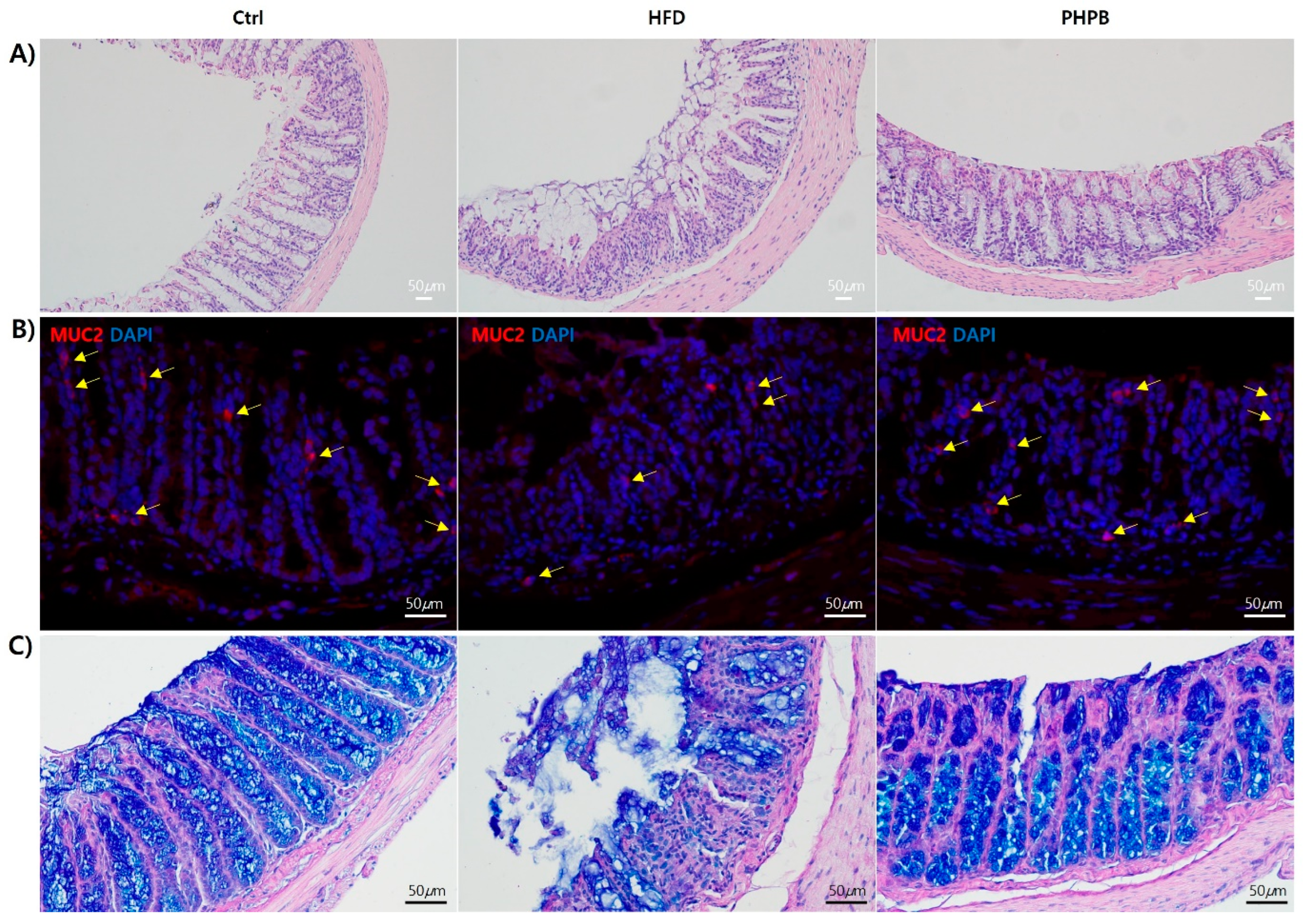
2.8. Effects of PHPB on Goblet Cells
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Diets
4.2. Sampling of Blood, Colon, and Other Target Organs
4.3. Colitis Activity Index
4.4. Serum Chemistry
4.5. Histopathology and Immunohistochemical (IHC) Analysis
4.6. Western Blot Analysis
4.7. Gene Expression Analysis
4.8. Microbial Assessment
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Rijnierse, A.; de Wit, N.; Jonker-Termont, D.; Dekker, J.; Müller, M.; van der Meer, R. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut 2012, 61, 1041–1049. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, X.-F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and Their Role in Intestinal Inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Yang, X.; Yue, W.; Xu, X.; Li, B.; Zou, L.; He, R. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell. Mol. Immunol. 2014, 11, 355–366. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, C.; Yu, Y.; Yi, Y.; Wang, J.; Chen, D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 1464. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Büller, H.A.; Dekker, J.; VAN Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Colucci, R.L.; Pellegrini, C.; Tirotta, E.; Benvenuti, L.; Segnani, C.; Ippolito, C.; Duranti, E.; Virdis, A.; et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS ONE 2018, 13, e0195502. [Google Scholar] [CrossRef]
- Hossain, M.K.; Dayem, A.A.; Han, J.; Yin, Y.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Song, H.-J.; Lee, S.-O. Enzymatic Preparation and Antioxidant Activities of Protein Hy-drolysates from Protaetia brevitarsis Larvae. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1164–1170. [Google Scholar] [CrossRef]
- Yoon, C.-H.; Jeon, S.-H.; Ha, Y.J.; Kim, S.W.; Bang, W.Y.; Bang, K.H.; Gal, S.W.; Kim, I.-S.; Cho, Y.-S. Functional Chemical Components in Protaetia brevitarsis Larvae: Impact of Supplementary Feeds. Korean J. Food Sci. Anim. Resour. 2020, 40, 461–473. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Olivo-Marston, S.E.; Hursting, S.D.; Perkins, S.N.; Schetter, A.; Khan, M.; Croce, C.; Harris, C.C.; Lavigne, J. Efects of calorie restriction and diet-induced obesity on murine colon carcinogenesis, growth and inflammatory factors, and microRNA expresion. PLoS ONE 2014, 9, e94765. [Google Scholar] [CrossRef] [Green Version]
- Carbonnel, F.; Jantchou, P.; Monnet, E.; Cosnes, J. Environmental risk factors in Crohn’s disease and ulcerative colitis: An update. Gastroenterol. Clin. Biol. 2009, 33, S145–S157. [Google Scholar] [CrossRef]
- Song, J.-L.; Choi, J.-H.; Seo, J.-H.; Lim, Y.-I.; Park, K.-Y. Anti-Colitic Effects of Kanjangs (Fermented Soy Sauce and Sesame Sauce) in Dextran Sulfate Sodium-Induced Colitis in Mice. J. Med. Food 2014, 17, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent Effects of Lactobacillus Fermentum HY01 on Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushige, T.; Komiya, M.; Onda, M.; Uchida, K.; Hayamizu, K.; Kabuyama, Y. Fish protein hydrolysate exhibits anti-obesity activity and reduces hypothalamic neuropeptide Y and agouti-related protein mRNA expressions in rats. Biomed. Res. 2017, 38, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Modun, D.; Giustarini, D.; Tsikas, D. Nitric Oxide-Related Oxidative Stress and Redox Status in Health and Disease. Oxidative Med. Cell. Longev. 2014, 2014, 129651. [Google Scholar] [CrossRef]
- Lee, C.G.; Lee, J.K.; Kang, Y.-S.; Shin, S.; Kim, J.H.; Lim, Y.J.; Koh, M.-S.; Lee, J.H.; Kang, H.W. Visceral Abdominal Obesity Is Associated With an Increased Risk of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2015, 110, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J. Esculetin, a coumarin derivative, suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. J. Funct. Foods 2014, 12, 509–515. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef]
- Andersson, C.X.; Gustafson, B.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes/Metab. Res. Rev. 2008, 24, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Brichard, S. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Mudter, J.; Neurath, M.F. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef]
- Obeid, S.; Wankell, M.; Charrez, B.; Sternberg, J.; Kreuter, R.; Esmaili, S.; Ramezani-Moghadam, M.; Devine, C.; Read, S.; Bhathal, P.; et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response. J. Biol. Chem. 2017, 292, 6569–6582. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Song, K. Isolation of an Angiotensin Converting Enzyme Inhibitory Peptide from Irradiated Bovine Blood Plasma Protein Hydrolysates. J. Food Sci. 2006, 68, 2469–2472. [Google Scholar] [CrossRef]
- Gunasekera, D.C.; Ma, J.; Vacharathit, V.; Shah, P.; Ramakrishnan, A.; Uprety, P.; Shen, Z.; Sheh, A.; Brayton, C.F.; Whary, M.T.; et al. The development of colitis in Il10 mice is dependent on IL-22. Mucosal Immunol. 2020, 13, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Khoury, A.; Queiroz, E.L.; MacRae, M. Environmental enrichment rescues the effects of early life inflammation on markers of synaptic transmission and plasticity. Brain Behav. Immun. 2016, 57, 151–160. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [Green Version]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.S. Epithelial ER Stress in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [Green Version]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Raviprakash, V.; Kataria, M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 2007, 15, 188–195. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 Promotes Production of Intestinal Mucus by Suppressing Protein Misfolding and Endoplasmic Reticulum Stress in Goblet Cells. Gastroenterology 2013, 144, 357–368.e359. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Functional Classification | Target Molecules | Detection | Primer Seq. | Company, Dilution |
|---|---|---|---|---|
| Excessive adipogenesis | PPARγ C/EBPα, p-AMPKα AMPKα | WB | - | |
| Pro-inflammatory cytokine | TNF-α | PCR | F: TTCACTGGAGCCTCGAATGT R: ACCTGACCACTCTCCCTTTG | - |
| IL-1β | F: AGGCTCCGAGATGAACAACA R: TCAGCTCATATGGGTCCGAC | |||
| IL-6 | F: AGTTGCCTTCTTGGGACTGA R:TCCACGATTTCCCAGAGAAC | |||
| IL-17α | F: CAGCGATCATCCCTCAAAGC R: GTCGTTGGCCTCAGTGTTTG | |||
| Anti-inflammatory | Adiponectin | PCR | F: GTTGCAAGCTCTCCTGTTCC R: ATCCAACCTGCACAAGTTCC | - |
| IL-10 | F: CCAGGG AGATCCTTTGATGA R: AACTGGCCACAGTTTTCAGG | |||
| Lipin | F: GATGCCGCTAAAGACACTGG R: TGGCTAGGATGCTCACAACA | |||
| defensin | F: CTGCAAAGGAAGAGAACGCA R: TGGCCTCAGTACTCATGCTC | |||
| Gut microbiota | Bacteroides-Prevotella | PCR | F: GAGAGGAAGGTCCCCCAC R: CGCTACTTGGCTGGTTCAG | - |
| Desulfovibrios | F: CCGTAGATATCTGGAGGAACATCAG R: ACATCTAGCATCCATCGTTTACAGC | |||
| Lactobacillus | F: GAGGCAGCAGTAGGGAATCTTC R: GGCCAGTTACTACCTCTATCCTTCTTC | |||
| Bifidobacterium | F: CGCGTCTGGTGTGAAAG R: CCCCACATCCAGCATCCA | |||
| LPS | ELISA | - | R&D Systems | |
| Macrophage polarization | F4/80 CD11c CD206 | IHC | - | |
| Oxidative stress | iNOS Cox2, | WB | - | Abcam, 1:100 |
| ER stress | sXbp1 | PCR | F:GAGTCCGCAGCAGGTGC R:CAAAAGGATATCAGACTCAGAATCTGAA | - |
| Grp78 | F:TGCTGCTAGGCCTGCTCCGA R:CGACCACCGTGCCCACATCC | |||
| Edem1 | F:ATCCTCGGGTGAATCTGAAGACG R:TCATAGAAGGAATCCAGCCCAGC | |||
| Goblet cell dysfunction | Muc2 | IHC PAS/Alcian | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.J.; Chun, S.Y.; Lee, E.H.; Yoon, B.; Han, M.-H.; Chung, J.-W.; Ha, Y.-S.; Lee, J.N.; Kim, H.T.; Kim, D.H.; et al. Protaetia Brevitarsis-Derived Protein Hydrolysate Reduces Obesity-Related Colitis Induced by High-Fat Diet in Mice through Anti-Inflammatory Pathways. Int. J. Mol. Sci. 2023, 24, 12333. https://doi.org/10.3390/ijms241512333
Kwon HJ, Chun SY, Lee EH, Yoon B, Han M-H, Chung J-W, Ha Y-S, Lee JN, Kim HT, Kim DH, et al. Protaetia Brevitarsis-Derived Protein Hydrolysate Reduces Obesity-Related Colitis Induced by High-Fat Diet in Mice through Anti-Inflammatory Pathways. International Journal of Molecular Sciences. 2023; 24(15):12333. https://doi.org/10.3390/ijms241512333
Chicago/Turabian StyleKwon, Hyung Jun, So Young Chun, Eun Hye Lee, BoHyun Yoon, Man-Hoon Han, Jae-Wook Chung, Yun-Sok Ha, Jun Nyung Lee, Hyun Tae Kim, Dae Hwan Kim, and et al. 2023. "Protaetia Brevitarsis-Derived Protein Hydrolysate Reduces Obesity-Related Colitis Induced by High-Fat Diet in Mice through Anti-Inflammatory Pathways" International Journal of Molecular Sciences 24, no. 15: 12333. https://doi.org/10.3390/ijms241512333






