The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy
Abstract
1. Introduction
1.1. Endometrial Cancer
1.2. Uterine Sarcoma
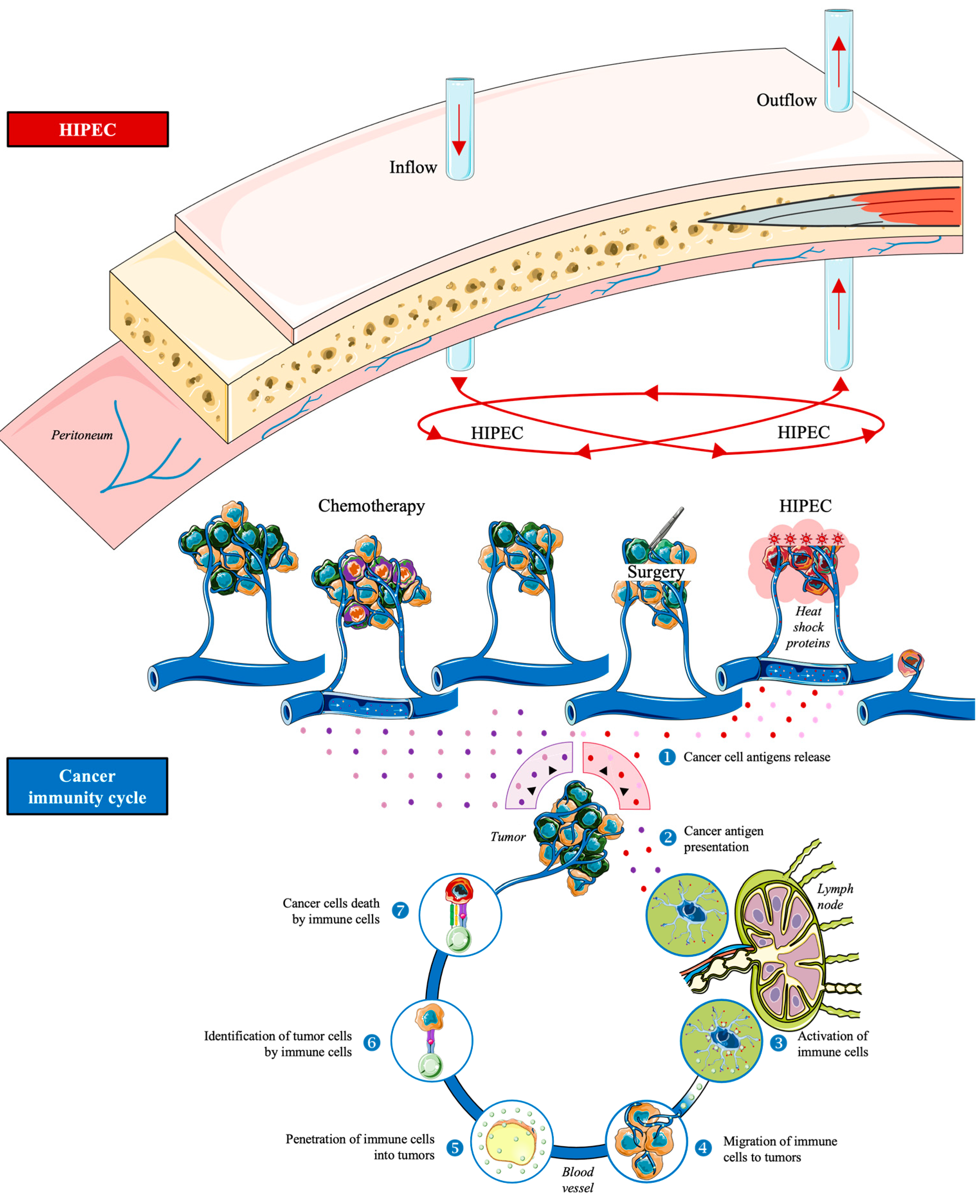
1.3. Hyperthermic Intraperitoneal Chemotherapy
1.4. Aim of the Review
2. Materials and Methods
3. Results
3.1. The Role of HIPEC in Endometrial Cancer Therapy
3.2. The Role of HIPEC in Uterine Sarcoma Therapy
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Americal Cancer Society. Key Statistics for Endometrial Cancer. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statistics.html (accessed on 1 July 2023).
- National Cancer Institute. Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 1 July 2023).
- Sherman, M.E.; Bur, M.E.; Kurman, R.J. p53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum. Pathol. 1995, 26, 1268–1274. [Google Scholar] [CrossRef]
- Takai, N.; Narahara, H. Preclinical studies of chemotherapy using histone deacetylase inhibitors in endometrial cancer. Obstet. Gynecol. Int. 2010, 2010, 923824. [Google Scholar] [CrossRef]
- Jiang, S.; Dowdy, S.C.; Meng, X.W.; Wang, Z.; Jones, M.B.; Podratz, K.C.; Jiang, S.W. Histone deacetylase inhibitors induce apoptosis in both Type I and Type II endometrial cancer cells. Gynecol. Oncol. 2007, 105, 493–500. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Bestvina, C.M.; Fleming, G.F. Chemotherapy for Endometrial Cancer in Adjuvant and Advanced Disease Settings. Oncologist 2016, 21, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takai, N.; Nishida, M.; Nasu, K.; Narahara, H. Apicidin, a novel histone deacetylase inhibitor, has profound anti-growth activity in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2007, 19, 301–308. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Troungos, C.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 9190. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Giaginis, C.; Theocharis, S. Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma. Dis. Markers 2021, 2021, 7850688. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Spartalis, E.; Kalampokas, E.; Kalampokas, T.; Margonis, G.A.; Schizas, D.; Andreatos, N.; Angelou, A.; et al. Targeting histone deacetylases in endometrial cancer: A paradigm-shifting therapeutic strategy? Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 950–960. [Google Scholar] [CrossRef]
- Americal Cancer Society. Survival Rates for Endometrial Cancer. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 July 2023).
- Americal Cancer Society. What Is Uterine Sarcoma? Available online: https://www.cancer.org/cancer/types/uterine-sarcoma/about/what-is-uterine-sarcoma.html (accessed on 1 July 2023).
- American Cancer Society. Key Statistics for Uterine Sarcoma; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- American Cancer Society. Risk Factors for Uterine Sarcoma; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Trope, C.G.; Abeler, V.M.; Kristensen, G.B. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol. 2012, 51, 694–705. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Signs and Symptoms of Uterine Sarcomas; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- American Cancer Society. Tests for Uterine Sarcoma; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Liu, J.; Wang, Z. Advances in the Preoperative Identification of Uterine Sarcoma. Cancers 2022, 14, 3517. [Google Scholar] [CrossRef] [PubMed]
- Americal Cancer Society. Treatment for Uterine Sarcoma, by Type and Stage. Available online: https://www.cancer.org/cancer/types/uterine-sarcoma/treating/by-stage.html (accessed on 1 July 2023).
- Valle, S.J.; Alzahrani, N.A.; Liauw, W.; Sugarbaker, P.H.; Bhatt, A.; Morris, D.L. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Methodology, Drugs and Bidirectional Chemotherapy. Indian J. Surg. Oncol. 2016, 7, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ben Aziz, M.; Di Napoli, R. Hyperthermic Intraperitoneal Chemotherapy; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114. [Google Scholar] [CrossRef]
- Khan, H.; Johnston, F.M. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J. Surg. Oncol. 2022, 125, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Han, E.S. State of the Science: The role of HIPEC in the treatment of ovarian cancer. Gynecol. Oncol. 2021, 160, 364–368. [Google Scholar] [CrossRef]
- van Stein, R.M.; Aalbers, A.G.J.; Sonke, G.S.; van Driel, W.J. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. 2021, 7, 1231–1238. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef]
- Margioula-Siarkou, C.; Almperis, A.; Papanikolaou, A.; Lagana, A.S.; Mavromatidis, G.; Guyon, F.; Dinas, K.; Petousis, S. HIPEC for gynaecological malignancies: A last update (Review). Med. Int. 2023, 3, 25. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Azzam, A.Z.; AlOmar, O.; Salem, H.; Amin, T.; Al-Badawi, I.A. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for managing peritoneal carcinomatosis from endometrial carcinoma: A single-center experience of 6 cases. Ann. Saudi Med. 2014, 34, 159–166. [Google Scholar] [CrossRef][Green Version]
- Bakrin, N.; Cotte, E.; Sayag-Beaujard, A.; Raudrant, D.; Isaac, S.; Mohamed, F.; Gilly, F.N.; Glehen, O. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of recurrent endometrial carcinoma confined to the peritoneal cavity. Int. J. Gynecol. Cancer 2010, 20, 809–814. [Google Scholar] [CrossRef]
- Brind’Amour, A.; Brault, C.; Sideris, L.; De Guerke, L.; Auclair, M.H.; Dube, P.; Fortin, S. Carboplatin Hyperthermic Intraperitoneal Chemotherapy in the Management of Primary Stage IVB Endometrial Cancer. J. Obstet. Gynaecol. Can. 2021, 43, 247–250. [Google Scholar] [CrossRef]
- Chambers, L.M.; Chau, D.; Yao, M.; Costales, A.B.; Rose, P.G.; Michener, C.M.; Debernardo, R.; Vargas, R. Efficacy of hyperthermic intraperitoneal chemotherapy and interval debulking surgery in women with advanced uterine serous carcinoma. Gynecol. Oncol. Rep. 2021, 38, 100876. [Google Scholar] [CrossRef]
- Cornali, T.; Sammartino, P.; Kopanakis, N.; Christopoulou, A.; Framarino Dei Malatesta, M.; Efstathiou, E.; Spagnoli, A.; Ciardi, A.; Biacchi, D.; Spiliotis, J. Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Metastases from Endometrial Cancer. Ann. Surg. Oncol. 2018, 25, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Delotte, J.; Desantis, M.; Frigenza, M.; Quaranta, D.; Bongain, A.; Benchimol, D.; Bereder, J.M. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of endometrial cancer with peritoneal carcinomatosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Gomes David, M.; Bakrin, N.; Salleron, J.; Kaminsky, M.C.; Bereder, J.M.; Tuech, J.J.; Lehmann, K.; Mehta, S.; Glehen, O.; Marchal, F. Cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) vs. CRS alone for treatment of endometrial cancer with peritoneal metastases: A multi-institutional study from PSOGI and BIG RENAPE groups. BMC Surg. 2022, 22, 1. [Google Scholar] [CrossRef]
- Navarro-Barrios, A.; Gil-Martinez, J.; Ramos-Bernardo, I.; Barrios, P.; Munoz-Casares, C.; Torres-Melero, J.; Pereira, F.; Manzanedo, I.; Arjona, A.; Martinez-Regueira, F.; et al. Intraperitoneal hyperthermic chemotherapy after cytoreduction in patients with peritoneal metastases from endometrial cancer. The next frontier? Surg. Oncol. 2020, 33, 19–23. [Google Scholar] [CrossRef]
- Helm, C.W.; Toler, C.R.; Martin, R.S., 3rd; Gordinier, M.E.; Parker, L.P.; Metzinger, D.S.; Edwards, R.P. Cytoreduction and intraperitoneal heated chemotherapy for the treatment of endometrial carcinoma recurrent within the peritoneal cavity. Int. J. Gynecol. Cancer 2007, 17, 204–209. [Google Scholar] [CrossRef]
- Minareci, Y.; Tosun, O.A.; Sozen, H.; Topuz, S.; Salihoglu, M.Y. A Retrospective Clinical Analysis of Hyperthermic Intraperitoneal Chemotherapy in Gynecological Cancers: Technical Details, Tolerability, and Efficacy. Medeni. Med. J. 2020, 35, 202–211. [Google Scholar] [CrossRef]
- Peng, H.H.; Huang, Y.T.; Cheng, Z.X.; Lee, C.L.; Lin, C.T. Immunomodulating Therapy by Picibanil-based Imiquimod and Virotherapy for Advanced Uterine Cancer after Laparoscopic Surgery. Gynecol. Minim. Invasive Ther. 2021, 10, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Rajha, A.; Piso, P.; Halmy, L.; Panczel, I.; Nedelcut, D.S.; Herold, Z.; Szasz, A.M.; Acs, M. Rare Histologies and Infrequent Indications for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Anticancer. Res. 2022, 42, 3681–3692. [Google Scholar] [CrossRef]
- Santeufemia, D.A.; Lumachi, F.; Basso, S.M.; Tumolo, S.; Re, G.L.; Capobianco, G.; Bertozzi, S.; Pasqual, E.M. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy as salvage treatment for a late wound recurrence of endometrial cancer. Anticancer. Res. 2013, 33, 1041–1044. [Google Scholar]
- Yee, F.Z.Y.; Tan, G.H.C.; Chia, C.S.; Soo, K.C.; Teo, M.C.C. Uncommon indications for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Pleura Peritoneum 2017, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Pennacchioli, E.; Kusamura, S.; Fiore, M.; Balestra, M.R.; Colombo, C.; Mingrone, E.; Gronchi, A.; Deraco, M. Peritoneal sarcomatosis: Is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann. Surg. Oncol. 2010, 17, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, S.; Maksymovskyi, V.; Atanasov, D.; Chetverikov, M.; Chetverikova-Ovchynnyk, V. Multiple Interval Debulking Surgery in Recurrent Uterine Sarcoma (Case Report). Georgian Med. News 2021, 3, 37–41. [Google Scholar]
- Diaz-Montes, T.P.; El-Sharkawy, F.; Lynam, S.; Harper, A.; Sittig, M.; MacDonald, R.; Gushchin, V.; Sardi, A. Efficacy of Hyperthermic Intraperitoneal Chemotherapy and Cytoreductive Surgery in the Treatment of Recurrent Uterine Sarcoma. Int. J. Gynecol. Cancer 2018, 28, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Duzgun, O.; Kalin, M. Is There a Role for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Peritoneal Carcinomatosis Due to Uterine Cancer? J. Pers. Med. 2022, 12, 1790. [Google Scholar] [CrossRef]
- Inoue, D.; Yamamoto, M.; Sugita, G.; Kurokawa, T.; Yoshida, Y. Debulking surgery and hyperthermic intraperitoneal chemotherapy in the management of a recurrent aggressive uterine myxoid leiomyosarcoma with peritoneal dissemination. Gynecol. Oncol. Rep. 2015, 13, 60–63. [Google Scholar] [CrossRef]
- Jimenez, W.A.; Sardi, A.; Nieroda, C.; Gushchin, V. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of recurrent high-grade uterine sarcoma with peritoneal dissemination. Am. J. Obstet. Gynecol. 2014, 210, 259.e1–259.e8. [Google Scholar] [CrossRef]
- Kusamura, S.; Raspagliesi, F.; Baratti, D.; Gronchi, A.; Casali, P.; Deraco, M. Uterine sarcoma treated by cytoreductive surgery and intraperitoneal hyperthermic perfusion: A feasiblity study. J. Chemother. 2004, 16 (Suppl. S5), 19–22. [Google Scholar] [CrossRef]
- Sardi, A.; Jimenez, W.; Nieroda, C.; Sittig, M.; Shankar, S.; Gushchin, V. Melphalan: A promising agent in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 908–914. [Google Scholar] [CrossRef]
- Sardi, A.; Sipok, A.; Baratti, D.; Deraco, M.; Sugarbaker, P.; Salti, G.; Yonemura, Y.; Sammartino, P.; Glehen, O.; Bakrin, N.; et al. Multi-institutional study of peritoneal sarcomatosis from uterine sarcoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2017, 43, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Sardi, A.; Munoz-Zuluaga, C.A.; Sittig, M.; Diaz-Montes, T. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in seven patients with peritoneal sarcomatosis from uterine sarcoma. Clin. Case Rep. 2018, 6, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Tentes, A.A.; Vaxevanidou, A.; Korakianitis, O.S.; Rogdakis, A.; Mirelis, C.G.; Datsis, A.C.; Kekelos, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal carcinomatosis. Preliminary results and cost from two centers in Greece. J. BUON 2008, 13, 205–210. [Google Scholar]
- Spiliotis, J.; Vaxevanidou, A.; Halkia, E.; Hadjigeorgiou, G.; Datsis, A. Reoperation combining re-cytoreductive surgery and re-HIPEC for recurrent peritoneal carcinomatosis. J. BUON 2012, 17, 522–525. [Google Scholar] [PubMed]
- Sugarbaker, P.; Ihemelandu, C.; Bijelic, L. Cytoreductive Surgery and HIPEC as a Treatment Option for Laparoscopic Resection of Uterine Leiomyosarcoma with Morcellation: Early Results. Ann. Surg. Oncol. 2016, 23, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Long-term survival is possible using cytoreductive surgery plus HIPEC for sarcomatosis-Case report of 2 patients. Int. J. Surg. Case Rep. 2019, 64, 10–14. [Google Scholar] [CrossRef]
- Yasukawa, M.; Dainty, L.A.; Sugarbaker, P.H. Long-term outcomes after cytoreductive surgery and HIPEC for morcellated uterine leiomyosarcoma; a case series. Gynecol. Oncol. Rep. 2021, 36, 100741. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, T.S.; Padberg, W.; Mann, S.T.W.; Gehron, J.; Sander, M.; Mann, V. Anesthetic Management During Pediatric Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy With Cisplatin in a Small Child: A Case Report and Systematic Literature Review. A A Pract. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Desar, I.M.E.; Ottevanger, P.B.; Benson, C.; van der Graaf, W.T.A. Systemic treatment in adult uterine sarcomas. Crit. Rev. Oncol. Hematol. 2018, 122, 10–20. [Google Scholar] [CrossRef]

| Study | Patient Collective | Post-Treatment Outcomes |
|---|---|---|
| Abu-Zaid et al. [29] | 6 patients with peritoneal carcinomatosis arising from endometrial cancer |
|
| Bakrin et al. [30] | 5 patients with recurrent endometrial cancer |
|
| Brind’Amour et al. [31] | 3 patients with endometrial cancer and synchronous peritoneal metastases |
|
| Chambers et al. [32] | 7 uterine serous carcinoma patients |
|
| Cornali et al. [33] | 33 patients with peritoneal metastases from endometrial cancer |
|
| Delotte et al. [34] | 13 endometrial cancer patients |
|
| Gomes David et al. [35] | 74 patients with peritoneal metastases of endometrial cancer |
|
| Navarro-Barrios et al. [36] | 43 patients with peritoneal metastases and endometrial cancer |
|
| Helm et al. [37] | 5 patients with endometrial carcinoma recurrent within the peritoneal cavity |
|
| Minareci et al. [38] | 2 patients with recurrent endometrial cancer |
|
| Peng et al. [39] | Poorly differentiated grade 3 endometrioid adenocarcinoma patient |
|
| Rahja et al. [40] | 7 patients with peritoneal metastatic endometrial carcinoma |
|
| Santeufemia et al. [41] | Wound recurrence from a surgically removed endometrial cancer |
|
| Yee et al. [42] | 1 patient with endometrioid adenocarcinoma |
|
| Study | Patient Collective | Post-Treatment Outcomes |
|---|---|---|
| Baratti et al. [43] | 11 patients with uterine leiomyosarcoma |
|
| Chetverikov et al. [44] | 1 patient with relapsed uterine sarcoma |
|
| Díaz-Montes et al. [45] | 26 patients with recurrent uterine sarcoma |
|
| Düzgün et al. [46] | 22 cases of uterine-peritoneal carcinomatosis |
|
| Inoue et al. [47] | 1 recurrent aggressive uterine myxoid leiomyosarcoma with peritoneal dissemination |
|
| Jimenez et al. [48] | 3 patients with recurrent high-grade uterine sarcoma |
|
| Kasamura et al. [49] | 10 patients with uterine sarcoma |
|
| Sardi et al. [50,51,52] | 45 patients with peritoneal sarcomatosis from uterine sarcoma |
|
| Spiliotis et al. [53,54] | 3 uterine sarcoma cases |
|
| Sugarbaker et al. [55,56] | 8 uterine leiomyosarcoma patients |
|
| Yasukawa et al. [57] | 6 uterine leiomyosarcoma patients |
|
| Zajonz et al. [58] | Relapse of an alveolar rhabdomyosarcoma of the uterus and peritoneal carcinomatosis in a 2-year-old child |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Damaskos, C.; Garmpis, N.; Vrettou, K.; Garmpi, A.; Sarantis, P.; Koustas, E.; Antoniou, E.A.; Kouraklis, G.; Chionis, A.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 12353. https://doi.org/10.3390/ijms241512353
Psilopatis I, Damaskos C, Garmpis N, Vrettou K, Garmpi A, Sarantis P, Koustas E, Antoniou EA, Kouraklis G, Chionis A, et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(15):12353. https://doi.org/10.3390/ijms241512353
Chicago/Turabian StylePsilopatis, Iason, Christos Damaskos, Nikolaos Garmpis, Kleio Vrettou, Anna Garmpi, Panagiotis Sarantis, Evangelos Koustas, Efstathios A. Antoniou, Gregory Kouraklis, Athanasios Chionis, and et al. 2023. "The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy" International Journal of Molecular Sciences 24, no. 15: 12353. https://doi.org/10.3390/ijms241512353
APA StylePsilopatis, I., Damaskos, C., Garmpis, N., Vrettou, K., Garmpi, A., Sarantis, P., Koustas, E., Antoniou, E. A., Kouraklis, G., Chionis, A., Kontzoglou, K., & Dimitroulis, D. (2023). The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy. International Journal of Molecular Sciences, 24(15), 12353. https://doi.org/10.3390/ijms241512353










