Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types
Abstract
:1. Introduction
2. Results
2.1. Activation of Neutrophils by A23187 and PMA (The Question Is, What Are the Statistical Data on Cell Morphology and the Evolution of Cell Activation?)
2.2. Control Neutrophil Response to Exposure to Activators (The Question Is, How Does a Neutrophil Change from Intact to Activated?)
2.3. Decondensation of Chromatin and Release of NETS (The Questions Are, What Causes Changes in the Membrane and How Does the Cell and Nuclear Volume Change?)
2.3.1. 30 min after Activation
2.3.2. 60 min after Activation
2.3.3. 120 min after Activation
2.3.4. 120–180/240 min after Activation
2.3.5. Patterns of Neutrophil NETosis Imaging
3. Discussion
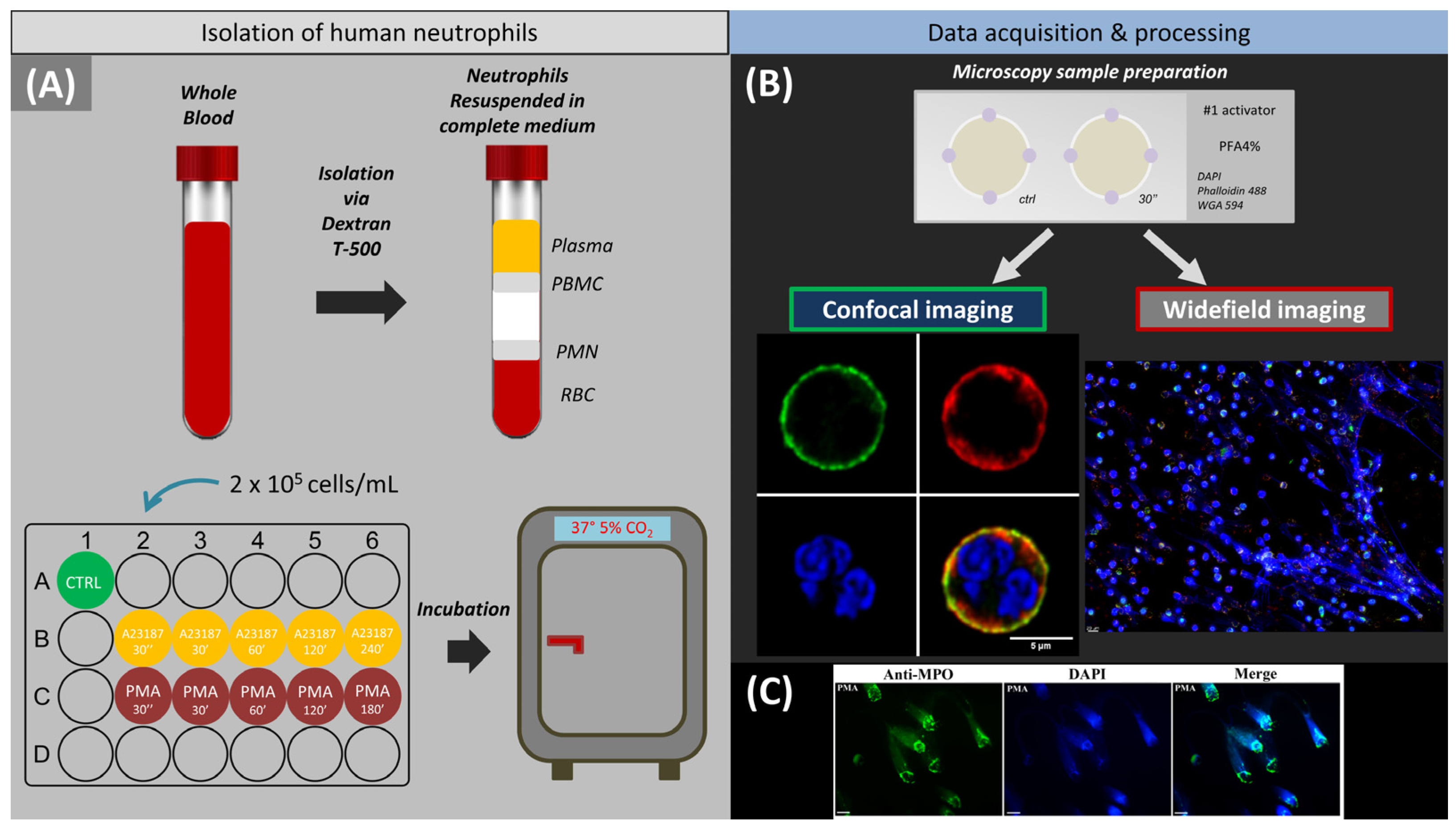
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Primary Human Neutrophils
4.3. Induction and Detection of Neutrophil Extracellular Traps
4.4. Staining Procedure
4.5. Widefield Fluorescence Microscopy
4.6. Confocal Laser-Scanning Microscope (CLSM)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun. Rev. 2015, 14, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Opneja, A.; Nayak, L. The role of neutrophils in thrombosis. Thromb. Res. 2018, 170, 87–96. [Google Scholar] [CrossRef]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, H.; Qu, M.; Nan, K.; Cao, H.; Cata, J.P.; Chen, W.; Miao, C. Review: The Emerging Role of Neutrophil Extracellular Traps in Sepsis and Sepsis-Associated Thrombosis. Front. Cell. Infect. Microbiol. 2021, 11, 189. [Google Scholar] [CrossRef]
- Grebenchikov, O.A.; Kasatkina, I.S.; Kadantseva, K.K.; Meshkov, M.A.; Bayeva, A.A. The Effect of Lithium Chloride on Neutrophil Activation on Exposure to Serum of Patients with Septic Shock. Gen. Reanimatol. 2020, 16, 45–55. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, J. Neutrophil extracellular traps: New players in cancer research. Front. Immunol. 2022, 13, 937565. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many Ways to Die. Front. Immunol. 2021, 12, 631821. [Google Scholar] [CrossRef]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.; Albrett, A.M.; Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 2011, 91, 369–376. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165664. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Dagil, Y.; Pashenkov, M.; Pinegin, B.; Chernyak, B. Protein kinase C isoforms mediate the formation of neutrophil extracellular traps. Int. Immunopharmacol. 2023, 114, 109448. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chan, D.W.; Zaal, K.J.; Kaplan, M.J. A High-Throughput Real-Time Imaging Technique To Quantify NETosis and Distinguish Mechanisms of Cell Death in Human Neutrophils. J. Immunol. 2018, 200, 869–879. [Google Scholar] [CrossRef] [Green Version]
- Neubert, E.; Meyer, D.; Rocca, F.; Günay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schön, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 3767. [Google Scholar] [CrossRef]
- Neubert, E.; Meyer, D.; Kruss, S.; Erpenbeck, L. The power from within—Understanding the driving forces of neutrophil extracellular trap formation. J. Cell Sci. 2020, 133, jcs241075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V. Actin powers the neutrophil traps. Blood 2022, 139, 3104–3105. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev. Cell 2017, 43, 449–462.e5. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Qiu, R.; Kittisopikul, M.; Vahabikashi, A.; Goldman, A.E.; Goldman, R.D.; Wagner, D.D.; Waterman, C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. USA 2020, 117, 7326–7337. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Du, F.; Rönnow, C.-F.; Wang, Y.; Rahman, M.; Thorlacius, H. Actin-related protein 2/3 complex regulates neutrophil extracellular trap expulsion and lung damage in abdominal sepsis. Am. J. Physiol. Cell. Mol. Physiol. 2022, 322, L662–L672. [Google Scholar] [CrossRef]
- Watts, R.G.; Crispens, M.A.; Howard, T.H. A quantitative study of the role of F-actin in producing neutrophil shape. Cell Motil. Cytoskelet. 1991, 19, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Aranda-Espinoza, H.; Smith, L.; Janmey, P.; Hammer, D. Spreading of Neutrophils: From Activation to Migration. Biophys. J. 2006, 91, 4638–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Chi, H.; Sun, L. Neutrophil Extracellular Traps of Cynoglossus semilaevis: Production Characteristics and Antibacterial Effect. Front. Immunol. 2017, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Miller, D.; Unkel, R.; Shaman, M.; Jacques, S.M.; Panaitescu, B.; Garcia-Flores, V.; Hassan, S.S. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod. Sci. 2017, 24, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.; Harman, M.; Hazlett, L.; Odzer, J.; Witt, H.; Franck, C.; Reichner, J.; Lefort, C. Vinculin in Neutrophil Adhesion, Motility and Trafficking. FASEB J. 2018, 32, 280.11. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Olivares-Navarrete, R. Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials 2021, 271, 120715. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Fazzi, R.; Montali, M.; Carnicelli, V.; Lazzarini, E.; Petrini, M. Specific Integrin Expression Is Associated with Podosome-Like Structures on Mesodermal Progenitor Cells. Stem. Cells Dev. 2013, 22, 1830–1838. [Google Scholar] [CrossRef]
- Tokuhiro, T.; Ishikawa, A.; Sato, H.; Takita, S.; Yoshikawa, A.; Anzai, R.; Sato, S.; Aoyagi, R.; Arita, M.; Shibuya, T.; et al. Oxidized Phospholipids and Neutrophil Elastase Coordinately Play Critical Roles in NET Formation. Front. Cell Dev. Biol. 2021, 9, 718586. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inozemtsev, V.; Sergunova, V.; Vorobjeva, N.; Kozlova, E.; Sherstyukova, E.; Lyapunova, S.; Chernysh, A. Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types. Int. J. Mol. Sci. 2023, 24, 12355. https://doi.org/10.3390/ijms241512355
Inozemtsev V, Sergunova V, Vorobjeva N, Kozlova E, Sherstyukova E, Lyapunova S, Chernysh A. Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types. International Journal of Molecular Sciences. 2023; 24(15):12355. https://doi.org/10.3390/ijms241512355
Chicago/Turabian StyleInozemtsev, Vladimir, Viktoria Sergunova, Nina Vorobjeva, Elena Kozlova, Ekaterina Sherstyukova, Snezhanna Lyapunova, and Aleksandr Chernysh. 2023. "Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types" International Journal of Molecular Sciences 24, no. 15: 12355. https://doi.org/10.3390/ijms241512355
APA StyleInozemtsev, V., Sergunova, V., Vorobjeva, N., Kozlova, E., Sherstyukova, E., Lyapunova, S., & Chernysh, A. (2023). Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types. International Journal of Molecular Sciences, 24(15), 12355. https://doi.org/10.3390/ijms241512355






