Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies
Abstract
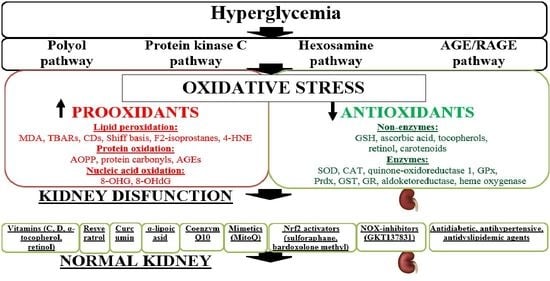
:1. Introduction
2. Diabetic Nephropathy (DN): Pathogenesis and Existing Correction Methods
3. OS
OS Biomarkers
4. DN: The Significance of Determining OS
5. DN and OS: Experimental Studies
6. DN and OS: Clinical Studies
7. Opportunities for Antioxidant Therapies
7.1. Vitamins
7.1.1. Vitamin E
7.1.2. Vitamin D
7.1.3. Vitamin C (Ascorbate)
7.2. Resveratrol
7.3. Curcumin
7.4. α-Lipoic Acid
7.5. Coenzyme Q10
7.6. Other Methods of Protection against ROS
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Gopalan, H.; Jayawardena, R.; Hills, A.P.; Soares, M.; Reza-Albarrán, A.A.; Ramaiya, K.L. Diabetes in developing countries. J. Diabetes 2019, 11, 522–539. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative stress: Pathogenetic role in the development of diabetes mellitus and its complications, therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021, 171, 136–149. [Google Scholar] [CrossRef]
- Mauricio, D.; Alonso, N.; Gratacòs, M. Chronic diabetes complications: The need to move beyond classical concepts. Trends Endocrinol. Metab. 2020, 31, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Li, K.X.; Ji, M.J.; Sun, H.J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef]
- Thomas, B. The Global Burden of Diabetic Kidney Disease: Time Trends and Gender Gaps. Curr. Diab. Rep. 2019, 19, 18. [Google Scholar] [CrossRef]
- González-Pérez, A.; Saez, M.; Vizcaya, D.; Lind, M.; Rodriguez, L.G. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: A population-based cohort study in the UK. BMJ Open Diabetes Res. Care 2021, 9, e002146. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: An overview. Diabet. Nephropathy. Methods Mol. Biol. 2020, 2067, 3–7. [Google Scholar] [CrossRef]
- Seshan, S.V.; Reddi, A.S. Albuminuria and Proteinuria. In Diabetes and Kidney Disease; Lerma, E.V., Batuman, V., Eds.; Springer: Cham, Switzerland, 2022; pp. 243–262. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Adeva-Contreras, L.; Fernández-Fernández, C.; Carneiro-Freire, N.; Domínguez-Montero, A. Histological Manifestations of Diabetic Kidney Disease and its Relationship with Insulin Resistance. Curr. Diabetes Rev. 2023, 19, 50–70. [Google Scholar] [CrossRef]
- Pelle, M.C.; Provenzano, M.; Busutti, M.; Porcu, C.V.; Zaffina, I.; Stanga, L.; Arturi, F. Up-Date on Diabetic Nephropathy. Life 2022, 12, 1202. [Google Scholar] [CrossRef]
- Warren, A.M.; Knudsen, S.T.; Cooper, M.E. Diabetic nephropathy: An insight into molecular mechanisms and emerging therapies. Expert. Opin. Ther. Targets 2019, 23, 579–591. [Google Scholar] [CrossRef]
- Sulaiman, M.K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019, 11, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Jaisser, F. Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes Obes. Metab. 2020, 22, 16–31. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic kidney disease: Challenges, advances, and opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Chugunova, E.V.; Kolesnikov, S.I.; Grebenkina, L.A.; Semenova, N.V.; Nikitina, O.A.; Kolesnikova, L.I. Content of carbonyl compounds and parameters of glutathione metabolism in men with type 1 diabetes mellitus at preclinical stages of diabetic nephropathy. Bull. Exp. Biol. Med. 2021, 171, 592–595. [Google Scholar] [CrossRef]
- Semenova, N.V.; Rychkova, L.V.; Darenskaya, M.A.; Kolesnikov, S.I.; Nikitina, O.A.; Petrova, A.G.; Vyrupaeva, E.V.; Kolesnikova, L.I. Superoxide dismutase activity in male and female patients of different age with moderate COVID-19. Bull. Exp. Biol. Med. 2022, 173, 51–53. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxidative Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.P.; Penjweini, R.; Knutson, J.R.; Wang, P.Y.; Hwang, P.M. Mitochondria and oxygen homeostasis. FEBS J. 2022, 289, 6959–6968. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R. Cellular and Molecular Mechanisms in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8017. [Google Scholar] [CrossRef]
- Darenskaya, M.; Chugunova, E.; Kolesnikov, S.; Semenova, N.; Michalevich, I.; Nikitina, O.; Lesnaya, A.; Kolesnikova, L. Receiver Operator Characteristic (ROC) Analysis of Lipids, Proteins, DNA Oxidative Damage, and Antioxidant Defense in Plasma and Erythrocytes of Young Reproductive-Age Men with Early Stages of Type 1 Diabetes Mellitus (T1DM) Nephropathy in the Irkutsk Region, Russia. Metabolites 2022, 12, 1282. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Rychkova, L.V.; Kolesnikov, S.I.; Kravtsova, O.V.; Semenova, N.V.; Brichagina, A.; Bliznyuk, A.; Yuzvak, N.; Rashidova, M.A.; Kolesnikova, L.I. Oxidative stress index levels in asian adolescents with exogenous-constitutional obesity. Int. J. Biomed. 2022, 12, 142–146. [Google Scholar] [CrossRef]
- Darenskaya, M.; Kolesnikova, L.; Kolesnikov, S. The association of respiratory viruses with oxidative stress and antioxidants. implications for the COVID-19 pandemic. Curr. Pharm. Design. 2021, 27, 1618–1627. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef]
- Ito, M.; Gurumani, M.Z.; Merscher, S.; Fornoni, A. Glucose- and Non-Glucose-Induced Mitochondrial Dysfunction in Diabetic Kidney Disease. Biomolecules 2022, 12, 351. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Halliwell, B. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.S.I.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábcková, J.; Tóthová, L’. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Tang, C.; Cai, J.; Yin, X.M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Thongboonkerd, V. Mitochondria-derived vesicles and their potential roles in kidney stone disease. J. Transl. Med. 2023, 21, 294. [Google Scholar] [CrossRef]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2021, 10, 18. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Y.; Kang, Y.; Lu, H.; Niu, H.; Liu, W.; Li, Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019, 234, 5033–5043. [Google Scholar] [CrossRef]
- Casalena, G.A.; Yu, L.; Gil, R.; Rodriguez, S.; Sosa, S.; Janssen, W.; Daehn, I.S. The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes. Cell Commun. Signal. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-T.; Wan, C.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Mitochondrial Oxidative Stress and Cell Death in Podocytopathies. Biomolecules 2022, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Xiaoju, M.A.; Jingru, M.A.; Tian, L.; Zhongzhu, Y.; Tingting, H.; Qiuyan, L.; Tao, S. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef]
- Li, S.; Zheng, L.; Zhang, J.; Liu, X.; Wu, Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic. Biol. Med. 2021, 162, 435–449. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, Y.; Deng, X.; Li, Y.; Ma, X.; Zeng, J.; Zhao, Y. Diabetic nephropathy: Focusing on pathological signals, clinical treatment, and dietary regulation. Biomed. Pharmacother. 2023, 159, 114252. [Google Scholar] [CrossRef]
- Kushwaha, K.; Sharma, S.; Gupta, J. Metabolic memory and diabetic nephropathy: Beneficial effects of natural epigenetic modifiers. Biochimie 2020, 170, 140–151. [Google Scholar] [CrossRef]
- Pathomthongtaweechai, N.; Chutipongtanate, S. AGE/RAGE signaling-mediated endoplasmic reticulum stress and future prospects in non-coding RNA therapeutics for diabetic nephropathy. Biomed. Pharmacother. 2020, 131, 110655. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Q.; Wang, J.; Chen, P.; Jiang, S. Ellagic acid attenuates streptozocin induced diabetic nephropathy via the regulation of oxidative stress and inflammatory signaling. Food Chem. Toxicol. 2019, 123, 16–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Draves, S.O.; Rosca, M. Mitochondria in Diabetic Kidney Disease. Cells 2021, 10, 2945. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular pathways that drive diabetic kidney disease. J. Clin. Investig. 2023, 133, e165654. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Yoo, T.H. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab. J. 2022, 46, 181–197. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, J.Y.; Ahn, E.; Hyeon, J.S.; Kim, G.H.; Park, K.J.; Kim, W.H. Associations between local acidosis induced by renal LDHA and renal fibrosis and mitochondrial abnormalities in patients with diabetic kidney disease. Transl. Res. 2022, 249, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Kapucu, A. Crocin ameliorates oxidative stress and suppresses renal damage in streptozotocin induced diabetic male rats. Biotech. Histochem. 2021, 96, 153–160. [Google Scholar] [CrossRef]
- Kausar, M.A.; Parveen, K.; Siddiqui, W.A.; Anwar, S.; Zahra, A.; Ali, A.; Saeed, M. Nephroprotective effects of polyherbal extract via attenuation of the severity of kidney dysfunction and oxidative damage in the diabetic experimental model. Cell. Mol. Biol. 2021, 67, 42–55. [Google Scholar] [CrossRef]
- Biswas, S.; Chen, S.; Liang, G.; Feng, B.; Cai, L.; Khan, Z.A.; Chakrabarti, S. Curcumin analogs reduce stress and inflammation indices in experimental models of diabetes. Front. Endocrinol. 2019, 10, 887. [Google Scholar] [CrossRef] [Green Version]
- Antar, S.A.; Abdo, W.; Taha, R.S.; Farage, A.E.; El-Moselhy, L.E.; Amer, M.E.; Mahmoud, A.M. Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci. 2022, 291, 120260. [Google Scholar] [CrossRef]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef]
- Yang, S.; Fei, X.; Lu, Y.; Xu, B.; Ma, Y.; Wan, H. miRNA-214 suppresses oxidative stress in diabetic nephropathy via the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp. Ther. Med. 2019, 17, 3530–3538. [Google Scholar] [CrossRef] [Green Version]
- Hussain Lodhi, A.; Ahmad, F.U.D.; Furwa, K.; Madni, A. Role of oxidative stress and reduced endogenous hydrogen sulfide in diabetic nephropathy. Drug Des. Dev. Ther. 2021, 15, 1031–1043. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Upasani, C.D.; Upaganlawar, A.B.; Gulecha, V.S. Renoprotective effect of co-enzyme Q10 and N-acetylcysteine on streptozotocin-induced diabetic nephropathy in rats. Int. J. Diabetes Clin. Res. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Anwar, S.; Kausar, M.A.; Parveen, K.; Siddiqui, W.A.; Zahra, A.; Ali, A.; Saeed, M. A vegetable oil blend administration mitigates the hyperglycemia-induced redox imbalance, renal histopathology, and function in diabetic nephropathy. J. King Saud Univ. Sci. 2022, 34, 102018. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, M.Y.; Hung, T.W.; Chang, Y.C.; Wang, C.J. Nelumbo nucifera leaves extract attenuate the pathological progression of diabetic nephropathy in high-fat diet-fed and streptozotocin-induced diabetic rats. J. Food Drug Anal. 2019, 27, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Shalayel, M.H. Galangin protects against oxidative damage and attenuates inflammation and apoptosis via modulation of NF-κB p65 and caspase-3 signaling molecules in a rat model of diabetic nephropathy. J. Physiol. Pharmacol. 2021, 72, 35–44. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Shahraki, J. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef]
- Casanova, A.G.; López-Hernández, F.J.; Vicente-Vicente, L.; Morales, A.I. Are antioxidants useful in preventing the progression of chronic kidney disease? Antioxidants 2021, 10, 1669. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell Longev. 2019, 2019, 30857569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denova, L.D.; Ivanov, D.D. Influence of oxidative, carbonyl, and nitrosative stresses on the course of chronic kidney disease (analytical review). KIDNEYS 2022, 11, 53–61. [Google Scholar] [CrossRef]
- Bigagli, E.; Lodovici, M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxidative Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghela, B.N.; Vaidya, F.U.; Agrawal, Y.; Santra, M.K.; Mishra, V.; Pathak, C. Molecular insights of NADPH oxidases and its pathological consequences. Cell Biochem. Funct. 2021, 39, 218–234. [Google Scholar] [CrossRef]
- Ricciardi, C.A.; Gnudi, L. Kidney disease in diabetes: From mechanisms to clinical presentation and treatment strategies. Metabolism 2021, 124, 154890. [Google Scholar] [CrossRef] [PubMed]
- Martinusen, D.; Marin, J.G.; Cheng, E.; Lau, W. Chronic kidney disease and end stage renal disease. In Renal Medicine and Clinical Pharmacy; Braund, R., Ed.; Springer: Cham, Switzerland, 2020; Volume 1. [Google Scholar]
- Sun, Y.; Jin, D.; Zhang, Z.; Zhang, Y.; Zhang, Y.; Kang, X.; Jiang, L.; Tong, X.; Lian, F. Effects of antioxidants on diabetic kidney diseases: Mechanistic interpretations and clinical assessment. Chin. Med. 2023, 18, 3. [Google Scholar] [CrossRef]
- Pastukhova, Y.; Luzza, F.; Shevel, S.; Savchuk, O.; Ostapchenko, L.; Falalyeyeva, T.; Kobyliak, N. Changes in Metabolic Parameters in Patients with Diabetic Kidney Disease Depending on the Status of D3. Rev. Recent Clin. Trials 2022, 17, 280–290. [Google Scholar] [CrossRef]
- Abd-Alsalam, A.; Zainal, I.G.; Taqa, G.A. Estimation of protein oxidation parameters in patients with diabetic nephropathy. AIP Conf. Proc. 2022, 2394, 20035. [Google Scholar] [CrossRef]
- Popykhova, E.B.; Ivanov, A.N.; Stepanova, T.V.; Lagutina, D.D.; Savkina, A.A. Diabetic nephropathy—Possibilities of early laboratory diagnostics and course prediction. Klin. Labor. Diagn. 2021, 66, 593–602. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Chugunova, E.V.; Kolesnikov, S.I.; Grebenkina, L.A.; Semenova, N.V.; Nikitina, O.A.; Kolesnikova, L.I. Markers of oxidative damage lipids and dna in men with type 1 diabetes mellitus and different levels of albuminuria. Diabetes Mellit. 2022, 25, 120–127. [Google Scholar] [CrossRef]
- Chugunova, E.V.; Darenskaya, M.A.; Semenova, N.V.; Kolesnikova, L.I.; Kolesnikov, S.I. Some oxidative stress indicators changes in type 1 diabetes mellitus patients at the preclinical stage of diabetic nephropathy. Diabetes Technol. Ther. 2021, 23, 235. [Google Scholar] [CrossRef]
- Fotheringham, A.K.; Gallo, L.A.; Borg, D.J.; Forbes, J.M. Advanced glycation end products (AGEs) and chronic kidney disease: Does the modern diet AGE the kidney? Nutrients 2022, 14, 2675. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhang, D.D.; Wang, Y.N.; Tan, Y.Q.; Yu, X.Y.; Zhao, Y.Y. AGE/RAGE in diabetic kidney disease and ageing kidney. Free Radic. Biol. Med. 2021, 171, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.T.; Lopez Gonzalez, E.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair, and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef] [PubMed]
- Steeneke, M.; Speeckaert, R.; Desmedt, S.; Glorieux, G.; Delanghe, J.R.; Speeckaert, M.M. The role of advanced glycation end products and its soluble receptor in kidney diseases. Int. J. Mol. Sci. 2022, 23, 3439. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Chugunova, E.V.; Kolesnikov, S.I.; Grebenkina, L.A.; Semenova, N.V.; Nikitina, O.A.; Kolesnikova, L.I. Markers of kidney injury, lipid metabolism, and carbonyl stress in patients with type 1 diabetes and different levels of albuminuria. Bull. Sib. Med. 2022, 21, 33–40. [Google Scholar] [CrossRef]
- Atef, S.; Abd El-Alim, B.A.; Galal, R.E.E.; Rashed, L.A.; Hassan, M.M. Diagnostic Implications of Urine 8-Hydroxy-2-Deoxyguanosine (8-Ohdg) As A Sensitive Biomarker For Early Prediction Of Diabetic Kidney Disease Among Adolescents With Type 1 Diabetes Mellitus. J. Pharm. Negat. Results 2023, 14, 185–195. [Google Scholar] [CrossRef]
- Rico-Fontalvo, J.; Aroca-Martínez, G.; Daza-Arnedo, R.; Cabrales, J.; Rodríguez-Yanez, T.; Cardona-Blanco, M.; Montejo-Hernández, J.; Rodelo Barrios, D.; Patiño-Patiño, J.; Osorio Rodríguez, E. Novel Biomarkers of Diabetic Kidney Disease. Biomolecules 2023, 13, 633. [Google Scholar] [CrossRef]
- Soliman, A.M.; Awad, E.T.; Abd-Elghffar, A.R.B.; Emara, I.A.; Abd, E.l.; Azeem, E.M. Biochemical and molecular studies of different parameters as markers for nephropathy in type 1 diabetic patients. Series Endo. Diab. Met. 2022, 4, 1–11. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Del Campo, G.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef] [Green Version]
- Ramachandrayya, S.A.; Jacob, J.; Mala, M. A correlative study of copper, ceruloplasmin, iron, total iron binding capacity and total antioxidant capacity in diabetic nephropathy. Biomedicine 2022, 42, 469–473. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Kong, D.; Yang, C.; Yu, H.; Pan, Q.; Yang, C.; Yu, H.; Pan, Q.; Liu, W.; et al. The effect of antioxidant vitamins on patients with diabetes and albuminuria: A meta-analysis of randomized controlled trials. J. Ren. Nutr. 2020, 30, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Rajeshwari, A.; Divija, D.A.; Somshekhar, G.N. Study of serum sialic acid, microalbuminuria, oxidative stress and antioxidant status in diabetic nephropathy. IJBB 2019, 15, 17–25. [Google Scholar]
- Vrzhesinskaya, O.A.; Leonenko, S.N.; Kodentsova, V.M.; Beketova, N.A.; Kosheleva, O.V.; Pilipenko, V.V.; Plotnikova, O.A.; Alekseeva, R.I.; Sharafetdinov, K.K. Vitamin supply of patients with type 2 diabetes mellitus complicated by nephropathy. Vopr. Pitan. 2022, 91, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bonomini, M.; Bartolini, D.; Zatini, L.; Reboldi, G.; Marcantonini, G.; Gentile, G.; Sirolli, V.; Di Pietro, N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants 2022, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, A.; Tana, C.; El Hadi, H.; Pagano, C.; Vettor, R.; Rossato, M. Antioxidant, anti-inflammatory, and metabolic properties of tocopherols and tocotrienols: Clinical implications for vitamin E supplementation in diabetic kidney disease. Int. J. Mol. Sci. 2019, 20, 5101. [Google Scholar] [CrossRef] [Green Version]
- Suresh, V.; Reddy, A.; Muthukumar, P.; Selvam, T. Antioxidants: Pharmacothearapeutic boon for diabetes. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/77150 (accessed on 31 May 2023).
- Altuhafi, A.; Altun, M.; Hadwan, M.H. The correlation between selenium-dependent glutathione peroxidase activity and oxidant/antioxidant balance in sera of diabetic patients with nephropathy. Rep. Biochem. Mol. Biol. 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Yanowsky-Escatell, F.G.; Andrade-Sierra, J.; Pazarín-Villaseñor, L.; Santana-Arciniega, C.; Torres-Vázquez, E.D.J.; Chávez-Iñiguez, J.S.; Preciado-Figueroa, F.M. The Role of Dietary Antioxidants on Oxidative Stress in Diabetic Nephropathy. Iran. J. Kidney Dis. 2020, 14, 81–94. [Google Scholar]
- Okdahl, T.; Brock, C. Molecular aspects in the potential of vitamins and supplements for treating diabetic neuropathy. Curr. Diabetes Rep. 2021, 21, 31. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Trejo, M.H.; Robles-Osorio, M.L.; Sabath, E. Liposoluble vitamins A and E in kidney disease. World J. Nephrol. 2022, 11, 96–104. [Google Scholar] [CrossRef]
- Pirhadi-Tavandashti, N.; Imani, H.; Ebrahimpour-Koujan, S.; Samavat, S.; Hakemi, M.S. The effect of vitamin E supplementation on biomarkers of endothelial function and inflammation among hemodialysis patients: A double-blinded randomized clinical trial. Complement. Ther. Med. 2020, 49, 102357. [Google Scholar] [CrossRef]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.-I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A Phase IIb Randomized Controlled Trial Investigating the Effects of Tocotrienol-Rich Vitamin E on Diabetic Kidney Disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef]
- Zafar, H.; Mirza, I.A.; Hussain, W.; Fayyaz, M. Comparative Efficacy of Tocotrienol and Tocopherol for Their Anti Diabetic Effects. Biomed. J. Sci. Tech. Res. 2021, 38, 30835–30840. [Google Scholar] [CrossRef]
- Zainal, Z.; Khaza’ai, H.; Radhakrishnan, A.K.; Chang, S.K. Therapeutic potential of palm oil vitamin E-derived tocotrienols in inflammation and chronic diseases: Evidence from preclinical and clinical studies. Food Res. Int. 2022, 156, 111175. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Nazarian, B.; Yousefi, M.; Anjom-Shoae, J.; Rasekhi, H.; Sadeghi, O. Effect of vitamin E intake on glycemic control and insulin resistance in diabetic patients: An updated systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2023, 22, 1–22. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, T.-W.; Hong, Z.-X.; Lim, L.-M. Vitamin D and Diabetic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 3751. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Zhou, Q.; Zhang, H.; Yi, B. Effects of vitamin D supplementation on renal function, inflammation and glycemic control in patients with diabetic nephropathy: A systematic review and meta-analysis. Kidney Blood Press. Res. 2019, 44, 72–87. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, Y.B.; Choi, H.S.; Jeong, T.D.; Kim, J.T.; Sung, Y.A. Association of vitamin D deficiency with diabetic nephropathy. Endocrinol. Metab. 2021, 36, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Xia, X.Y.; Yin, J. Relationship of serum vitamin D levels with diabetic microvascular complications in patients with type 2 diabetes mellitus. Chin. Med. J. 2021, 134, 814–820. [Google Scholar] [CrossRef]
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. 2019, 13, 542–547. [Google Scholar] [CrossRef]
- Nakhoul, N.; Thawko, T.; Farber, E.; Dahan, I.; Tadmor, H.; Nakhoul, R.; Hanut, A.; Salameh, G.; Shagrawy, I.; Nakhoul, F. The Therapeutic Effect of Active Vitamin D Supplementation in Preventing the Progression of Diabetic Nephropathy in a Diabetic Mouse Model. J. Diabetes Res. 2020, 2020, 7907605. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, X.; Zhao, Y.; Zhou, M.; Yang, Y.; Zhang, X. Calcitriol attenuates renal tubular epithelial cells apoptosis via inhibiting p38MAPK signaling in diabetic nephropathy. Acta Diabetol. 2020, 57, 1327–1335. [Google Scholar] [CrossRef]
- Derakhshanian, H.; Djazayery, A.; Javanbakht, M.H.; Eshraghian, M.R.; Mirshafiey, A.; Zarei, M.; Alvandi, E.; Djalali, E.; Djalali, M. The Effect of Vitamin D on Cellular Pathways of Diabetic Nephropathy. Rep. Biochem. Mol. Biol. 2019, 7, 217–222. [Google Scholar]
- He, L.; Zhou, L.; Zhao, T.Y.; Witherspoon, A.T.; Ouyang, L. Effect of Vitamin D on Urinary Albumin Excretion in Diabetic Nephropathy Patients: A Meta-analysis of Randomized Controlled Trials. Iran. J. Kidney Dis. 2022, 16, 273–279. [Google Scholar] [PubMed]
- Tareke, A.A.; Hadgu, A.A. The effect of vitamin C supplementation on lipid profile of type 2 diabetic patients: A systematic review and meta-analysis of clinical trials. Diabetol. Metab. Syndr. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Chen, Z.Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Kang, Q.; Wang, Q.; Wang, Q.; Chen, F.; Cheng, K.W. Resveratrol: Evidence for its nephroprotective effect in diabetic nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef]
- Salami, M.; Salami, R.; Mafi, A.; Aarabi, M.H.; Vakili, O.; Asemi, Z. Therapeutic potential of resveratrol in diabetic nephropathy according to molecular signaling. Curr. Mol. Pharmacol. 2022, 15, 716–735. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn-Schmiedeberg Arch. Pharmacol. 2020, 393, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, L.; Wang, B.; Wang, J.; Hao, M.; Chen, Y.B.; Li, X.Z.; Li, Y.; Jiang, Y.F.; Li, C.C.; et al. A novel compound AB38b attenuates oxidative stress and ECM protein accumulation in kidneys of diabetic mice through modulation of Keap1/Nrf2 signaling. Acta Pharmacol. 2020, 41, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Okulicz, M.; Hertig, I.; Szkudelski, T. Resveratrol ameliorates inflammatory and oxidative stress in type 2 diabetic Goto-Kakizaki rats. Biomed. Pharmacother. 2020, 125, 110026. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef]
- Peng, X.; Su, H.; Liang, D.; Li, J.; Ting, W.J.; Liao, S.C.; Huang, C.Y. Ramipril and resveratrol co-treatment attenuates RhoA/ROCK pathway-regulated early-stage diabetic nephropathy-associated glomerulosclerosis in streptozotocin-induced diabetic rats. Environ. Toxicol. 2019, 34, 861–868. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [PubMed]
- Wang, H.; Pan, L.; Xu, R.; Si, L.; Zhang, X. The molecular mechanism of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by BaP in the scallop Chlamys farreri. Fish. Shellfish. Immunol. 2019, 92, 489–499. [Google Scholar] [CrossRef]
- Bashir, S.O. Concomitant administration of resveratrol and insulin protects against diabetes mellitus type-1-induced renal damage and impaired function via an antioxidant-mediated mechanism and up-regulation of Na+/K+-ATPase. Arch. Physiol. Biochem. 2019, 125, 104–113. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Yeganeh, B.S.; Omrani, G.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, X.; Du, C.; Su, Y.; Yin, L.; Tan, X.; Xu, X. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Biomed. Pharmacother. 2022, 153, 113438. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, S.; Goodarzi, M.T.; Karimi, J.; Hashemnia, M.; Khodadadi, I. Does curcumin or metformin attenuate oxidative stress and diabetic nephropathy in rats? J. Nephropathol. 2019, 8, e08. [Google Scholar] [CrossRef]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and safety of curcumin supplement on improvement of insulin resistance in people with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2021, 2021, 4471944. [Google Scholar] [CrossRef]
- Al-Tamimi, J.Z.; Al-Faris, N.A.; Al-Farga, A.M.; Alshammari, G.M.; Bin Mowyna, M.N.; Yahya, M.A. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p66Shc axis and activation of FOXO-3a. J. Nutr. Biochem. 2021, 87, 108515. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacology 2020, 173, 113820. [Google Scholar] [CrossRef]
- Gao, L.; Lv, Q.; Wang, Y.; Zhang, D.; Ding, W.; Cao, L.; Ou, S. Research on Mechanism of Curcumin with Chitosan Nanoparticles in Regulating the Activity of Podocytes in Diabetic Nephropathy Through Alleviating Oxidative Stress and Inflammation. Sci. Adv. Mater. 2022, 14, 752–759. [Google Scholar] [CrossRef]
- Machado, D.I.; de Oliveira Silva, E.; Ventura, S.; Vattimo, M.D.F.F. The effect of curcumin on renal ischemia/reperfusion injury in diabetic rats. Nutrients 2022, 14, 2798. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Quispe, C.; Herrera-Bravo, J.; Javed, Z.; Khan, K.; Raza, S.; Gulsunoglu-Konuskan, Z.; Calina, D. Therapeutic applications of curcumin in diabetes: A review and perspective. BioMed Res. Int. 2022, 2022, 1375892. [Google Scholar] [CrossRef]
- Hassanizadeh, S.; Shojaei, M.; Bagherniya, M.; Orekhov, A.N.; Sahebkar, A. Effect of nano-curcumin on various diseases: A comprehensive review of clinical trials. BioFactors 2023, 49, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z.; Reiner, Ž.; Soleimani, A.; Aghadavod, E.; Bahmani, F. The effects of nano-curcumin on metabolic status in patients with diabetes on hemodialysis, a randomized, double blind, placebo-controlled trial. Iran. J. Kidney Dis. 2020, 14, 290–299. [Google Scholar]
- Macena, M.D.L.; Nunes, L.F.D.S.; da Silva, A.F.; Pureza, I.R.O.M.; Praxedes, D.R.S.; Santos, J.C.D.F.; Bueno, N.B. Effects of dietary polyphenols in the glycemic, renal, inflammatory, and oxidative stress biomarkers in diabetic nephropathy: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2022, 80, 2237–2259. [Google Scholar] [CrossRef]
- Ganugula, R.; Nuthalapati, N.K.; Dwivedi, S.; Zou, D.; Arora, M.; Friend, R.; Kumar, M.R. Nanocurcumin combined with insulin alleviates diabetic kidney disease through P38/P53 signaling axis. J. Control. Release 2023, 353, 621–633. [Google Scholar] [CrossRef] [PubMed]
- D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Sharifi-Rad, J. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamt, S.F.; Liu, J.; Yan, L.J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Grdović, N.; Rajić, J.; Arambašić Jovanović, J.; Dinić, S.; Tolić, A.; Đorđević, M.; Mihailović, M. α-Lipoic Acid Increases Collagen Synthesis and Deposition in Nondiabetic and Diabetic Rat Kidneys. Oxidative Med. Cell. Longev. 2021, 2021, 6669352. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; N’guessan, B.B.; Atule, S.; Mensah, S.D.; Adjei, S. Supplementation of conventional anti-diabetic therapy with alpha-lipoic acid prevents early development and progression of diabetic nephropathy. Biomed. Pharmacother. 2022, 149, 112818. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, J.; Du, J.; Feng, Y.; Xu, W.; Bai, M.; Zhang, H. Alpha-lipoic acid could attenuate the effect of chemerin-induced diabetic nephropathy progression. Iran. J. Basic Med. Sci. 2021, 24, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.; Samraj, P.I.; Raj, B.S. The Role of Alpha-lipoic Acid Supplementation in the Prevention of Diabetes Complications: A Comprehensive Review of Clinical Trials. Curr. Diabetes Rev. 2021, 17, e011821190404. [Google Scholar] [CrossRef]
- Vakali, E.; Rigopoulos, D.; Carrillo, A.E.; Flouris, A.D.; Dinas, P.C. Effects of Alpha-lipoic Acid Supplementation on Human Diabetic Nephropathy: A Systematic Review and Meta-analysis. Curr. Diabetes Rev. 2022, 18, e140921196457. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Jiang, D.; Cai, J. Effects of valsartan combined with α-lipoic acid on renal function in patients with diabetic nephropathy: A systematic review and meta-analysis. BMC Endocr. Disord. 2021, 21, 178. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Chugunova, E.V.; Kolesnikov, S.I.; Semenova, N.V.; Nikitina, O.A.; Kolesnikova, L.I. Lipid peroxidation processes in men with type 1 diabetes mellitus following α-lipoic acid treatment. AIMS Med. Sci. 2021, 8, 291–300. [Google Scholar] [CrossRef]
- Kayhan Kuştepe, E.; Bahar, L.; Zayman, E.; Sucu, N.; Gül, S.; Gül, M. A light microscopic investigation of the renoprotective effects of α-lipoic acid and α-tocopherol in an experimental diabetic rat model. Biotech. Histochem. 2020, 95, 305–316. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, H.M.; Xiang, J.Y.; Zhou, X.C.; Wang, D.; Chen, R.Y.; Tan, W.L.; Liang, L.Q.; Liu, L.L.; Shi, M.J.; et al. Alpha lipoamide inhibits diabetic kidney fibrosis via improving mitochondrial function and regulating RXRα expression and activation. Acta Pharmacol. Sin. 2023, 44, 1051–1065. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I. Coenzyme Q10 and Degenerative Disorders Affecting Longevity: An Overview. Antioxidants 2019, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, Z.; Liu, Q.; Quan, H.; Cheng, X. Effects of coenzyme Q10 intervention on diabetic kidney disease: A systematic review and meta-analysis. Medicine 2019, 98, e15850. [Google Scholar] [CrossRef]
- Salman, M.I.; Rashied, R.M.; Hamad, H.M.; Hamad, H.S. The protective effect of coenzyme Q10 on experimental diabetic nephropathy in male rats. Eurasia J. Biosci. 2020, 14, 6883–6888. [Google Scholar]
- Rasal, P.B.; Kasar, G.N.; Mahajan, M.S.; Upaganlawar, A.B.; Upasani, C.D. Ameliorative effect of lycopene alone and in combination with co-enzyme Q10 in streptozotocin-induced diabetic nephropathy in experimental rats. Int. J. Plant Based Pharm. 2023, 3, 123–130. [Google Scholar] [CrossRef]
- Fallah, M.; Askari, G.; Soleimani, A.; Feizi, A.; Asemi, Z. Clinical Trial of the Effects of Coenzyme Q10 Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Hemodialysis Patients. Int. J. Prev. Med. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.S.; Shalini, M.; Giridharan, R.; Basha, K.S.; Udhaya, B.; Prince, S.E. Lavinya & Sabina Evan Prince Administration of coenzyme Q10 to a diabetic rat model: Changes in biochemical, antioxidant, and histopathological indicators. Int. J. Diabetes Dev. Ctries 2020, 40, 143–152. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Shams, H.A.; Al-Mamorri, F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. J. Lab. Physicians 2019, 11, 317–322. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Tippairote, T.; Bjørklund, G.; Gasmi, A.; Semenova, Y.; Peana, M.; Chirumbolo, S.; Hangan, T. Combined Supplementation of Coenzyme Q10 and Other Nutrients in Specific Medical Conditions. Nutrients 2022, 14, 4383. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Bosnjak, Z.J. Clinical Relevance of lncRNA and Mitochondrial Targeted Antioxidants as Therapeutic Options in Regulating Oxidative Stress and Mitochondrial Function in Vascular Complications of Diabetes. Antioxidants 2023, 12, 898. [Google Scholar] [CrossRef]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef]
- Akpoveso, O.-O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant Phytochemicals as Potential Therapy for Diabetic Complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef]
- Braga, P.C.; Alves, M.G.; Rodrigues, A.S.; Oliveira, P.F. Mitochondrial Pathophysiology on Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 1776. [Google Scholar] [CrossRef]
- Kusirisin, P.; Chattipakorn, S.C.; Chattipakorn, N. Contrast-induced nephropathy and oxidative stress: Mechanistic insights for better interventional approaches. J. Transl. Med. 2020, 18, 1–35. [Google Scholar] [CrossRef]
- Danta, C.C.; Boab, A.N.; Bhandari, S.; Sathyapalan, T.; Xu, S.Z. Recent advances in drug discovery for diabetic kidney disease. Expert Opin. Drug Discov. 2021, 16, 447–461. [Google Scholar] [CrossRef]
- Shreya, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of apocynin: A natural acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Rezus, C. Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney disease (DKD): New perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH oxidase inhibition: Preclinical and clinical studies in diabetic complications. Antioxid. Redox Signal. 2020, 33, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Narasimhan, K.K.S.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Periandavan, K. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, L.; Jin, R.; Liu, J.; Luo, R.; Zhang, Y.; Zhu, L.; Peng, X. Diosgenin Inhibits ROS Generation by Modulating NOX4 and Mitochondrial Respiratory Chain and Suppresses Apoptosis in Diabetic Nephropathy. Nutrients 2023, 15, 2164. [Google Scholar] [CrossRef]
- Ho, C.C.; Yang, Y.S.; Huang, C.N.; Lo, S.C.; Wang, Y.H.; Kornelius, E. The efficacy of pioglitazone for renal protection in diabetic kidney disease. PLoS ONE 2022, 17, e0264129. [Google Scholar] [CrossRef]
- Winiarska, A.; Knysak, M.; Nabrdalik, K.; Gumprecht, J.; Stompór, T. Inflammation and oxidative stress in diabetic kidney disease: The targets for SGLT2 inhibitors and GLP-1 receptor agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darenskaya, M.; Kolesnikov, S.; Semenova, N.; Kolesnikova, L. Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 12378. https://doi.org/10.3390/ijms241512378
Darenskaya M, Kolesnikov S, Semenova N, Kolesnikova L. Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies. International Journal of Molecular Sciences. 2023; 24(15):12378. https://doi.org/10.3390/ijms241512378
Chicago/Turabian StyleDarenskaya, Marina, Sergey Kolesnikov, Natalya Semenova, and Lyubov Kolesnikova. 2023. "Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies" International Journal of Molecular Sciences 24, no. 15: 12378. https://doi.org/10.3390/ijms241512378
APA StyleDarenskaya, M., Kolesnikov, S., Semenova, N., & Kolesnikova, L. (2023). Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies. International Journal of Molecular Sciences, 24(15), 12378. https://doi.org/10.3390/ijms241512378