Targeting Epithelium Dysfunction and Impaired Nasal Biofilms to Treat Immunological, Functional, and Structural Abnormalities of Chronic Rhinosinusitis
Abstract
:1. Introduction
2. Comorbidities and Atopic Status
3. Pathophysiology
3.1. Genetics-Epigenetics
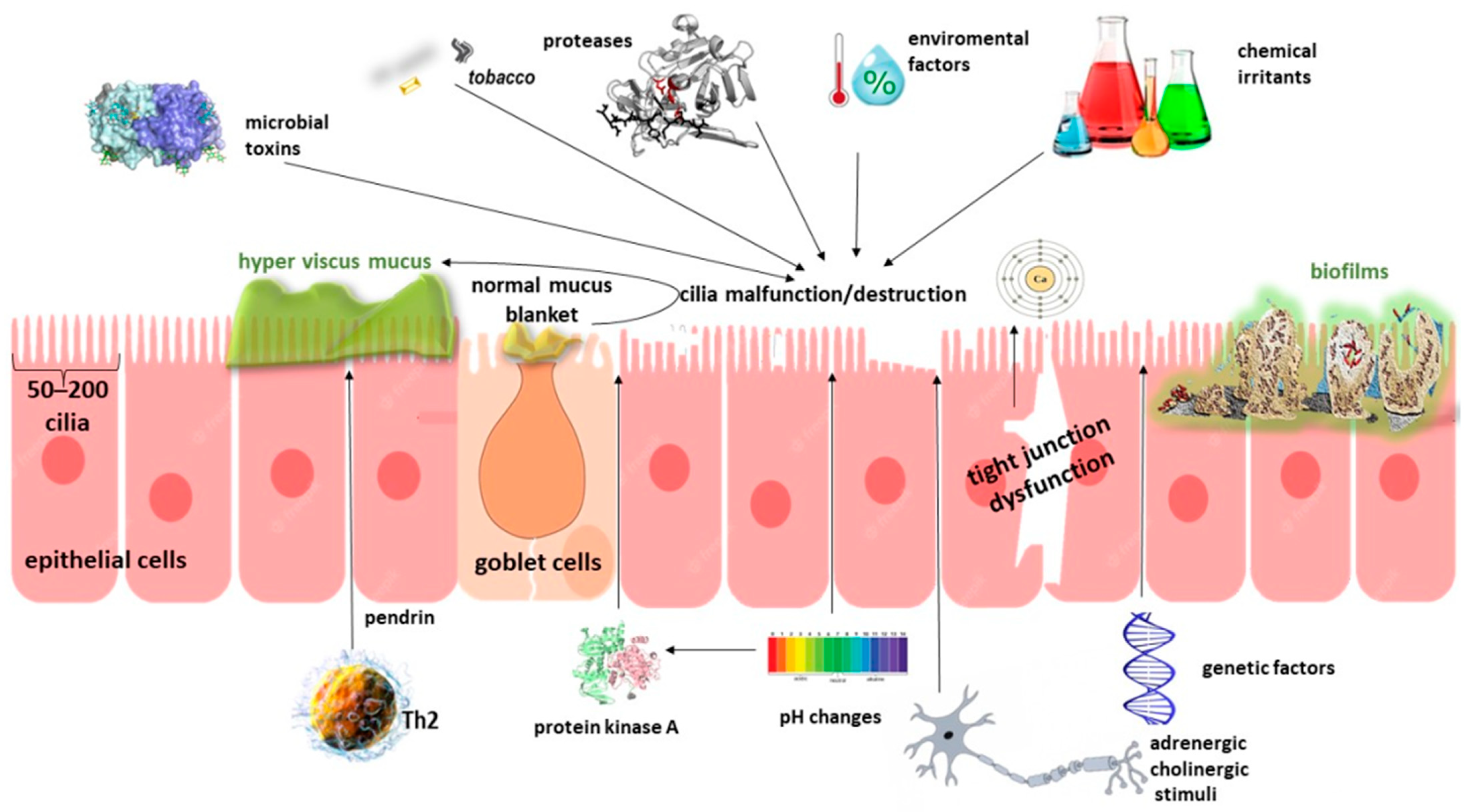
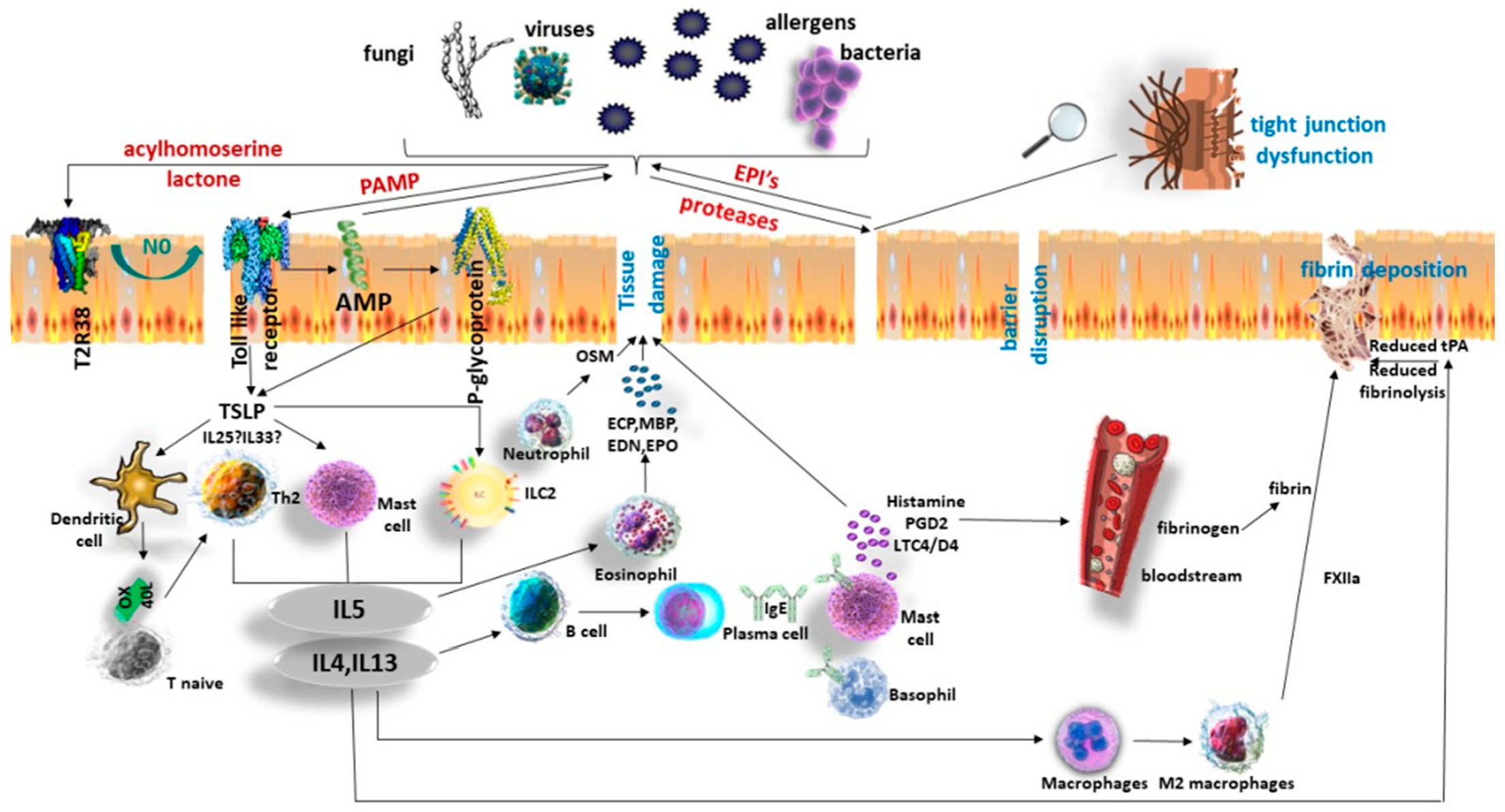
3.2. Barrier Disruption—Role of the Epithelium
4. Inflammatory Endotypes in Chronic Rhinosinusitis
4.1. Inflammation in CRSwNP
4.2. Inflammation in CRSsNP
5. Diagnosis of CRS
- endoscopic signs of: nasal polyps (NP), and/or mucopurulent discharge primarily from middle meatus and/or oedema/mucosal obstruction primarily in middle meatus, and/or
- CT-scan changes: mucosal changes within the ostiomeatal complex and/or sinuses
5.1. CT-Scan Findings
5.2. Biomarkers
6. Chronic Rhinosinusitis Management
6.1. Nasal Irrigation
6.2. Nasal Steroids
6.3. Systemic Steroids
6.4. Systemic Antibiotics
6.5. Biologics
6.6. Targeting Janus Kinases
6.7. Surgical Procedures
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Huynh, T.M.T.; Vandeplas, G.; Joish, V.N.; Mannent, L.P.; Tomassen, P.; van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; et al. The GALEN rhinosinusitis cohort: Chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology 2019, 57, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beule, A. Epidemiology of chronic rhinosinusitis, selected risk factors, comorbidities, and economic burden. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2015, 14, Doc11. [Google Scholar] [PubMed]
- Khan, A.; Vandeplas, G.; Huynh, T.M.T.; Joish, V.N.; Mannent, L.; Tomassen, P.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; et al. The Global Allergy and Asthma European Network (GALEN rhinosinusitis cohort: A large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology 2019, 57, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, N. The economic burden and symptom manifestations of chronic rhinosinusitis. Am. J. Rhinol. 2003, 17, 27–32. [Google Scholar] [CrossRef]
- Hirsch, A.G.; Stewart, W.F.; Sundaresan, A.S.; Young, A.J.; Kennedy, T.L.; Scott Greene, J.; Feng, W.; Tan, B.K.; Schleimer, R.P.; Kern, R.C.; et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy 2017, 72, 274–281. [Google Scholar]
- Hedman, J.; Kaprio, J.; Poussa, T.; Nieminen, M.M. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int. J. Epidemiol. 1999, 28, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Klossek, J.M.; Neukirch, F.; Pribil, C.; Jankowski, R.; Serrano, E.; Chanal, I.; El Hasnaoui, A. Prevalence of nasal polyposis in France: A cross-sectional, case-control study. Allergy 2005, 60, 233–237. [Google Scholar] [CrossRef]
- Settipane, G.A.; Chafee, F.H. Nasal polyps in asthma and rhinitis. A review of 6037 patients. J. Allergy Clin. Immunol. 1977, 59, 17–21. [Google Scholar] [CrossRef]
- We, J.; Lee, W.H.; Tan, K.L.; Wee, J.H.; Rhee, C.S.; Lee, C.H.; Ahn, S.; Lee, J.H.; Kim, J.W. Prevalence of nasal polyps and its risk factors: Korean National Health and Nutrition Examination Survey 2009–2011. Am. J. Rhinol. Allergy 2015, 29, e24–e28. [Google Scholar] [CrossRef]
- Shi, J.B.; Fu, Q.L.; Zhang, H.; Cheng, L.; Wang, Y.J.; Zhu, D.D.; Lv, W.; Liu, S.X.; Li, P.Z.; Ou, C.Q.; et al. Epidemiology of chronic rhinosinusitis: Results from a cross-sectional survey in seven Chinese cities. Allergy 2015, 70, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gevaert, E.; Lou, H.; Wang, X.; Zhang, L.; Bachert, C.; Zhang, N. Chronic rhinosinusitis in Asia. J. Allergy Clin. Immunol. 2017, 140, 1230–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, T.; Levin, M.; Snidvongs, K.; Banglawala, S.M.; Sommer, D.D. Comorbidities associated with eosinophilic chronic rhinosinusitis: A systematic review and meta-analysis. Clin. Otolaryngol. 2020, 45, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Gutman, M.; Torres, A.; Keen, K.J.; Houser, S.M. Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2004, 130, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.; Roland, L.T.; DelGaudio, J.M.; Wise, S.K. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2019, 4, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, J.P.; Jawad, B.A.; McCoul, E.D. Polypoid Change of the Middle Turbinate and Paranasal Sinus Polyposis Are Distinct Entities. Otolaryngol. Head Neck Surg. 2017, 157, 519–523. [Google Scholar] [CrossRef]
- DelGaudio, J.M.; Loftus, P.A.; Hamizan, A.W.; Harvey, R.J.; Wise, S.K. Central compartment atopic disease. Am. J. Rhinol. Allergy 2017, 31, 228–234. [Google Scholar] [CrossRef]
- Hamizan, A.W.; Loftus, P.A.; Alvarado, R.; Ho, J.; Kalish, L.; Sacks, R.; DelGaudio, J.M.; Harvey, R.J. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope 2018, 128, 2015–2021. [Google Scholar] [CrossRef]
- Lam, K.; Kern, R.C.; Luong, A. Is there a future for biologics in the management of chronic rhinosinusitis? Int. Forum Allergy Rhinol. 2016, 6, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.A.; Widelitz, J.S.; Chiu, A.G.; Palmer, J.N.; Kennedy, D.W. Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol. Head Neck Surg. 2006, 134, 601–604. [Google Scholar] [CrossRef]
- Delagrand, A.; Gilbert-Dussardier, B.; Burg, S.; Allano, G.; Gohler-Desmonts, C.; Lebreton, J.P.; Dufour, X.; Klossek, J.M. Nasal polyposis: Is there an inheritance pattern? A single family study. Rhinology 2008, 46, 125–130. [Google Scholar] [PubMed]
- Bohman, A.; Oscarsson, M.; Holmberg, K.; Johansson, L.; Millqvist, E.; Nasic, S.; Torinsson-Naluai, A.; Bende, M. Heredity of nasal polyps. Rhinology 2015, 53, 25–28. [Google Scholar] [CrossRef]
- Oakley, G.M.; Curtin, K.; Orb, Q.; Schaefer, C.; Orlandi, R.R.; Alt, J.A. Familial risk of chronic rhinosinusitis with and without nasal polyposis: Genetics or environment. Int. Forum Allergy Rhinol. 2015, 5, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H. New insights into the pathogenesis of cystic fibrosis sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Rollin, M.; Seymour, K.; Hariri, M.; Harcourt, J. Rhinosinusitis, symptomatology & absence of polyposis in children with primary ciliary dyskinesia. Rhinology 2009, 47, 75–78. [Google Scholar]
- Horani, A.; Ferkol, T.W. Understanding Primary Ciliary Dyskinesia and Other Ciliopathies. J. Pediatr. 2021, 230, 15–22.e1. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2014, 4, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Purnell, P.R.; Addicks, B.L.; Zalzal, H.G.; Shapiro, S.; Wen, S.; Ramadan, H.H.; Setola, V.; Siderovski, D.P. Single Nucleotide Polymorphisms in Chemosensory Pathway Genes GNB3, TAS2R19, and TAS2R38 Are Associated with Chronic Rhinosinusitis. Int. Arch. Allergy Immunol. 2019, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D.; Vangelista, L. Staphylococcus aureus Infection and Persistence in Chronic Rhinosinusitis: Focus on Leukocidin ED. Toxins 2020, 12, 678. [Google Scholar] [CrossRef]
- Cormier, C.; Mfuna Endam, L.; Filali-Mouhim, A.; Boisvert, P.; Boulet, L.P.; Boulay, M.E.; Vallee-Smedja, S.; Bosse, Y.; Desrosiers, M. A pooling-based genomewide association study identifies genetic variants associated with Staphylococcus aureus colonization in chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2014, 4, 207–215. [Google Scholar] [CrossRef]
- Kidoguchi, M.; Noguchi, E.; Nakamura, T.; Ninomiya, T.; Morii, W.; Yoshida, K.; Morikawa, T.; Kato, Y.; Imoto, Y.; Sakashita, M.; et al. DNA Methylation of Proximal PLAT Promoter in Chronic Rhinosinusitis with Nasal Polyps. Am. J. Rhinol. Allergy 2018, 32, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, D.K.; Yu, M.S.; Cha, M.J.; Yu, S.L.; Kang, J. Role of epigenetics in the pathogenesis of chronic rhinosinusitis with nasal polyps. Mol. Med. Rep. 2018, 17, 1219–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitts, K.B.; Chang, E.H. Genetics of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2020, 145, 777–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial barrier repair and prevention of allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [PubMed] [Green Version]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, H.G.; Koo, T.W.; Chung, P.S. Localization of ZO-1 and E-cadherin in the nasal polyp epithelium. Eur. Arch. Otorhinolaryngol. 2002, 259, 465–469. [Google Scholar] [CrossRef]
- Rogers, G.A.; Den Beste, K.; Parkos, C.A.; Nusrat, A.; Delgaudio, J.M.; Wise, S.K. Epithelial tight junction alterations in nasal polyposis. Int. Forum Allergy Rhinol. 2011, 1, 50–54. [Google Scholar] [CrossRef]
- Cookson, W. The immunogenetics of asthma and eczema: A new focus on the epithelium. Nat. Rev. Immunol. 2004, 4, 978–988. [Google Scholar] [CrossRef]
- Wang, J.; Shen, S.; Yan, B.; He, Y.; Zhang, G.; Shan, C.; Yang, Q.; Qin, L.; Duan, Z.; Jiang, L.; et al. Individual exposure of ambient particulate matters and eosinophilic chronic rhinosinusitis with nasal Polyps: Dose-Response, mediation effects and recurrence prediction. Environ. Int. 2023, 177, 108031. [Google Scholar] [CrossRef]
- Chen, B.; Shaari, J.; Claire, S.E.; Palmer, J.N.; Chiu, A.G.; Kennedy, D.W.; Cohen, N.A. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am. J. Rhinol. 2006, 20, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Gudis, D.; Zhao, K.Q.; Cohen, N.A. Acquired cilia dysfunction in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2012, 26, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieu, D.D.; Peters, A.T.; Carter, R.G.; Suh, L.; Conley, D.B.; Chandra, R.; Norton, J.; Grammer, L.C.; Harris, K.E.; Kato, A.; et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2010, 125, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, S.; Lin, D.C.; Rosati, M.; Carter, R.G.; Norton, J.E.; Suh, L.; Kato, A.; Chandra, R.K.; Harris, K.E.; Chu, H.W.; et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy 2012, 67, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Schleimer, R.P. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007, 19, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar]
- Hupin, C.; Gohy, S.; Bouzin, C.; Lecocq, M.; Polette, M.; Pilette, C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy 2014, 69, 1540–1549. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhou, P.; Liu, Y.; Liu, F.; Yi, X.; Liu, S.; Holtappels, G.; Bachert, C.; Zhang, N. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS ONE 2013, 8, e82373. [Google Scholar] [CrossRef] [Green Version]
- Mihalj, M.; Bujak, M.; Butkovic, J.; Zubcic, Z.; Tolusic Levak, M.; Ces, J.; Kopic, V.; Baus Loncar, M.; Mihalj, H. Differential Expression of TFF1 and TFF3 in Patients Suffering from Chronic Rhinosinusitis with Nasal Polyposis. Int. J. Mol. Sci. 2019, 20, 5461. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Zhao, Y. Eupatilin alleviates inflammation and epithelial-to-mesenchymal transition in chronic rhinosinusitis with nasal polyps by upregulating TFF1 and inhibiting the Wnt/beta-catenin signaling pathway. Histol. Histopathol. 2023, 2013, 18638. [Google Scholar]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Forster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziaber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Shiozawa, A.; Ono, N.; Kusunoki, T.; Hirotsu, M.; Homma, H.; Saitoh, T.; Murata, J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013, 123, E1–E9. [Google Scholar] [CrossRef]
- Poposki, J.A.; Klingler, A.I.; Tan, B.K.; Soroosh, P.; Banie, H.; Lewis, G.; Hulse, K.E.; Stevens, W.W.; Peters, A.T.; Grammer, L.C.; et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun. Inflamm. Dis. 2017, 5, 233–243. [Google Scholar] [CrossRef]
- Nagarkar, D.R.; Poposki, J.A.; Tan, B.K.; Comeau, M.R.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Norton, J.; Harris, K.E.; Grammer, L.C.; et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2013, 132, 593–600.e12. [Google Scholar] [CrossRef] [Green Version]
- Brusilovsky, M.; Rochman, M.; Rochman, Y.; Caldwell, J.M.; Mack, L.E.; Felton, J.M.; Habel, J.E.; Porollo, A.; Pasare, C.; Rothenberg, M.E. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat. Immunol. 2021, 22, 1316–1326. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Workman, A.D.; Patel, N.N.; Hung, L.Y.; Shtraks, J.P.; Chen, B.; Blasetti, M.; Doghramji, L.; Kennedy, D.W.; Adappa, N.D.; et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2018, 142, 460–469.e7. [Google Scholar] [CrossRef]
- Murphy, R.C.; Altman, M.C. Ignition sequence start: Epithelial allergen sensing and regulation of the allergic inflammatory response. Nat. Immunol. 2021, 22, 1207–1209. [Google Scholar] [CrossRef]
- Kato, A. Group 2 Innate Lymphoid Cells in Airway Diseases. Chest 2019, 156, 141–149. [Google Scholar] [CrossRef]
- Stevens, W.W.; Kato, A. Group 2 innate lymphoid cells in nasal polyposis. Ann. Allergy Asthma Immunol. 2021, 126, 110–117. [Google Scholar] [CrossRef]
- Ogasawara, N.; Klingler, A.I.; Tan, B.K.; Poposki, J.A.; Hulse, K.E.; Stevens, W.W.; Peters, A.T.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; et al. Epithelial activators of type 2 inflammation: Elevation of thymic stromal lymphopoietin, but not IL-25 or IL-33, in chronic rhinosinusitis with nasal polyps in Chicago, Illinois. Allergy 2018, 73, 2251–2254. [Google Scholar] [CrossRef]
- Ogasawara, N.; Poposki, J.A.; Klingler, A.I.; Tan, B.K.; Hulse, K.E.; Stevens, W.W.; Peters, A.T.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; et al. Role of RANK-L as a potential inducer of ILC2-mediated type 2 inflammation in chronic rhinosinusitis with nasal polyps. Mucosal Immunol. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Perez-Novo, C.A.; Watelet, J.B.; Claeys, C.; Van Cauwenberge, P.; Bachert, C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J. Allergy Clin. Immunol. 2005, 115, 1189–1196. [Google Scholar] [CrossRef]
- Derycke, L.; Eyerich, S.; Van Crombruggen, K.; Perez-Novo, C.; Holtappels, G.; Deruyck, N.; Gevaert, P.; Bachert, C. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS ONE 2014, 9, e97581. [Google Scholar]
- Lam, E.P.; Kariyawasam, H.H.; Rana, B.M.; Durham, S.R.; McKenzie, A.N.; Powell, N.; Orban, N.; Lennartz-Walker, M.; Hopkins, C.; Ying, S.; et al. IL-25/IL-33-responsive TH2 cells characterize nasal polyps with a default TH17 signature in nasal mucosa. J. Allergy Clin. Immunol. 2016, 137, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.L.; Song, J.; Xiong, P.; Cao, P.P.; Liao, B.; Ma, J.; Zhang, Y.N.; Zeng, M.; Liu, Y.; Wang, H.; et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2014, 190, 628–638. [Google Scholar] [CrossRef]
- Miljkovic, D.; Bassiouni, A.; Cooksley, C.; Ou, J.; Hauben, E.; Wormald, P.J.; Vreugde, S. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy 2014, 69, 1154–1161. [Google Scholar] [CrossRef]
- Ma, J.; Tibbitt, C.A.; Georen, S.K.; Christian, M.; Murrell, B.; Cardell, L.O.; Bachert, C.; Coquet, J.M. Single-cell analysis pinpoints distinct populations of cytotoxic CD4(+) T cells and an IL-10(+)CD109(+) T(H)2 cell population in nasal polyps. Sci. Immunol. 2021, 6, eabg6356. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.; Kasjanski, R.; Poposki, J.; Hernandez, D.; Chen, J.N.; Norton, J.E.; Suh, L.; Carter, R.G.; Stevens, W.W.; Peters, A.T.; et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin. Exp. Allergy 2017, 47, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Bachert, C.; Zhang, N.; Holtappels, G.; De Lobel, L.; van Cauwenberge, P.; Liu, S.; Lin, P.; Bousquet, J.; Van Steen, K. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 2010, 126, 962–968.e6. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, D.F.; Ordovas-Montanes, J.; Allon, S.J.; Buchheit, K.M.; Vukovic, M.; Derakhshan, T.; Feng, C.; Lai, J.; Hughes, T.K.; Nyquist, S.K.; et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci. Immunol. 2021, 6, eabb7221. [Google Scholar] [CrossRef]
- Johansson, S.G.O.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.E.; Ring, J.; et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef]
- Powe, D.G.; Jagger, C.; Kleinjan, A.; Carney, A.S.; Jenkins, D.; Jones, N.S. ‘Entopy’: Localized mucosal allergic disease in the absence of systemic responses for atopy. Clin. Exp. Allergy 2003, 33, 1374–1379. [Google Scholar] [CrossRef]
- Valenta, R.; Seiberler, S.; Natter, S.; Mahler, V.; Mossabeb, R.; Ring, J.; Stingl, G. Autoallergy: A pathogenetic factor in atopic dermatitis? J. Allergy Clin. Immunol. 2000, 105, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N.; Riedl, M.A.; Valent, P.; Podder, I.; Maurer, M. Urticaria and Angioedema: Understanding Complex Pathomechanisms to Facilitate Patient Communication, Disease Management, and Future Treatment. J. Allergy Clin. Immunol. Pract. 2023, 11, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Holtappels, G.; Zhang, N.; Kubica, M.; Deswarte, K.; Derycke, L.; Claeys, S.; Hammad, H.; Brusselle, G.G.; Vandenabeele, P.; et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy 2011, 66, 396–403. [Google Scholar] [CrossRef]
- Peterson, S.; Poposki, J.A.; Nagarkar, D.R.; Chustz, R.T.; Peters, A.T.; Suh, L.A.; Carter, R.; Norton, J.; Harris, K.E.; Grammer, L.C.; et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2012, 129, 119–127.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Tan, B.K.; Chandra, R.K.; et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2013, 132, 584–592.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochner, B.S.; Stevens, W.W. Biology and Function of Eosinophils in Chronic Rhinosinusitis with or without Nasal Polyps. Allergy Asthma Immunol. Res. 2021, 13, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Watanabe, T.; Saitoh, A.; Hirosaki, T.; Araki, Y.; Kikawada, T.; Betsuyaku, T.; Ohara, O.; et al. Dysregulated fatty acid metabolism in nasal polyp-derived eosinophils from patients with chronic rhinosinusitis. Allergy 2019, 74, 1113–1124. [Google Scholar] [CrossRef]
- Delemarre, T.; Bochner, B.S.; Simon, H.U.; Bachert, C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2021, 148, 327–335. [Google Scholar] [CrossRef]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Gevaert, E.; Zhang, N.; Krysko, O.; Lan, F.; Holtappels, G.; De Ruyck, N.; Nauwynck, H.; Yousefi, S.; Simon, H.U.; Bachert, C. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J. Allergy Clin. Immunol. 2017, 139, 1849–1860.e6. [Google Scholar] [CrossRef] [Green Version]
- Gevaert, E.; Delemarre, T.; De Volder, J.; Zhang, N.; Holtappels, G.; De Ruyck, N.; Persson, E.; Heyndrickx, I.; Verstraete, K.; Aegerter, H.; et al. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J. Allergy Clin. Immunol. 2020, 145, 427–430.e4. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Stevens, W.W.; Peters, A.T.; Suh, L.A.; Norton, J.; Carter, R.G.; Hulse, K.E.; Harris, K.E.; et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J. Allergy Clin. Immunol. 2017, 139, 699–703.e7. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of T(H) cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Eun, K.M.; Roh, E.Y.; Shin, S.; Kim, D.K. Chronic Rhinosinusitis without Nasal Polyps in Asian Patients Shows Mixed Inflammatory Patterns and Neutrophil-Related Disease Severity. Mediat. Inflamm. 2019, 2019, 7138643. [Google Scholar] [CrossRef]
- Klingler, A.I.; Stevens, W.W.; Tan, B.K.; Peters, A.T.; Poposki, J.A.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; Kern, R.C.; et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar]
- Hurgin, V.; Novick, D.; Werman, A.; Dinarello, C.A.; Rubinstein, M. Antiviral and immunoregulatory activities of IFN-gamma depend on constitutively expressed IL-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 5044–5049. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [PubMed]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Singhania, A.; Wallington, J.C.; Smith, C.G.; Horowitz, D.; Staples, K.J.; Howarth, P.H.; Gadola, S.D.; Djukanovic, R.; Woelk, C.H.; Hinks, T.S.C. Multitissue Transcriptomics Delineates the Diversity of Airway T Cell Functions in Asthma. Am. J. Respir. Cell Mol. Biol. 2018, 58, 261–270. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.D.; Cohen, N.A.; Palmer, J.N. Biofilms in chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 27–31. [Google Scholar] [CrossRef]
- Tan, N.C.; Foreman, A.; Jardeleza, C.; Douglas, R.; Tran, H.; Wormald, P.J. The multiplicity of Staphylococcus aureus in chronic rhinosinusitis: Correlating surface biofilm and intracellular residence. Laryngoscope 2012, 122, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Adappa, N.D.; Chiu, A.G.; Doghramji, L.J.; Cohen, N.A.; Palmer, J.N. Biofilm-forming bacteria and quality of life improvement after sinus surgery. Int. Forum Allergy Rhinol. 2015, 5, 643–649. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Bachert, C.; Baraniuk, J.; Baroody, F.M.; Benninger, M.S.; et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol. Head Neck Surg. 2004, 131 (Suppl. S6), S1–S62. [Google Scholar]
- Aalokken, T.M.; Hagtvedt, T.; Dalen, I.; Kolbenstvedt, A. Conventional sinus radiography compared with CT in the diagnosis of acute sinusitis. Dentomaxillofac. Radiol. 2003, 32, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Neurologic Imaging; Kirsch, C.F.E.; Bykowski, J.; Aulino, J.M.; Berger, K.L.; Choudhri, A.F.; Conley, D.B.; Luttrull, M.D.; Nunez, D., Jr.; Shah, L.M.; et al. ACR Appropriateness Criteria® Sinonasal Disease. J. Am. Coll Radiol. 2017, 14, S550–S559. [Google Scholar] [CrossRef] [Green Version]
- Dillon, W.P.; Som, P.M.; Fullerton, G.D. Hypointense MR signal in chronically inspissated sinonasal secretions. Radiology 1990, 174, 73–78. [Google Scholar] [CrossRef]
- Metson, R.; Gliklich, R.E.; Stankiewicz, J.A.; Kennedy, D.W.; Duncavage, J.A.; Hoffman, S.R.; Ohnishi, T.; Terrell, J.E.; White, P.S. Comparison of sinus computed tomography staging systems. Otolaryngol. Head Neck Surg. 1997, 117, 372–379. [Google Scholar] [CrossRef]
- Oluwole, M.; Russell, N.; Tan, L.; Gardiner, Q.; White, P. A comparison of computerized tomographic staging systems in chronic sinusitis. Clin. Otolaryngol. Allied Sci. 1996, 21, 91–95. [Google Scholar]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar]
- Dietz de Loos, D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkens, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef]
- Scadding, G.; Lund, V. Investigative Rhinology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Leung, N.; Mawby, T.A.; Turner, H.; Qureishi, A. Osteitis and chronic rhinosinusitis: A review of the current literature. Eur. Arch. Otorhinolaryngol. 2016, 273, 2917–2923. [Google Scholar] [CrossRef]
- Snidvongs, K.; Lam, M.; Sacks, R.; Earls, P.; Kalish, L.; Phillips, P.S.; Pratt, E.; Harvey, R.J. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int. Forum Allergy Rhinol. 2012, 2, 376–385. [Google Scholar] [CrossRef]
- Soler, Z.M.; Sauer, D.; Mace, J.; Smith, T.L. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol. Head Neck Surg. 2010, 142, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Zuo, K.; Guo, J.; Chen, F.; Xu, R.; Xu, G.; Shi, J.; Li, H. Clinical characteristics and surrogate markers of eosinophilic chronic rhinosinusitis in Southern China. Eur. Arch. Otorhinolaryngol. 2014, 271, 2461–2468. [Google Scholar] [CrossRef]
- Ho, J.; Hamizan, A.W.; Alvarado, R.; Rimmer, J.; Sewell, W.A.; Harvey, R.J. Systemic Predictors of Eosinophilic Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2018, 32, 252–257. [Google Scholar] [CrossRef]
- Ho, J.; Li, W.; Grayson, J.W.; Alvarado, R.; Rimmer, J.; Sewell, W.A.; Harvey, R.J. Systemic medication requirement in post-surgical patients with eosinophilic chronic rhinosinusitis. Rhinology 2021, 59, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Gevaert, P.; Holtappels, G.; Johansson, S.G.; van Cauwenberge, P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J. Allergy Clin. Immunol. 2001, 107, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Calus, L.; Van Bruaene, N.; Bosteels, C.; Dejonckheere, S.; Van Zele, T.; Holtappels, G.; Bachert, C.; Gevaert, P. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin. Transl. Allergy 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, A.D.; Kohanski, M.A.; Cohen, N.A. Biomarkers in Chronic Rhinosinusitis with Nasal Polyps. Immunol. Allergy Clin. N. Am. 2018, 38, 679–692. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, N.; Wang, H.; Li, Y.; Fan, E.; Zhang, L.; Zhang, L.; Bachert, C. Association of periostin expression with eosinophilic inflammation in nasal polyps. J. Allergy Clin. Immunol. 2015, 136, 1700–1703.e9. [Google Scholar] [CrossRef] [Green Version]
- Feldman, R.E.; Lam, A.C.; Sadow, P.M.; Bleier, B.S. P-glycoprotein is a marker of tissue eosinophilia and radiographic inflammation in chronic rhinosinusitis without nasal polyps. Int. Forum Allergy Rhinol. 2013, 3, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef]
- Kim, K.S.; Won, H.R.; Park, C.Y.; Hong, J.H.; Lee, J.H.; Lee, K.E.; Cho, H.S.; Kim, H.J. Analyzing serum eosinophil cationic protein in the clinical assessment of chronic rhinosinusitis. Am. J. Rhinol. Allergy 2013, 27, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Van Zele, T.; Claeys, S.; Gevaert, P.; Van Maele, G.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006, 61, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, M.; He, F.; Wang, X.; Zhang, L. Role of exhaled nasal nitric oxide in distinguishing between chronic rhinosinusitis with and without nasal polyps. Am. J. Rhinol. Allergy 2017, 31, 389–394. [Google Scholar] [CrossRef]
- Delclaux, C.; Malinvaud, D.; Chevalier-Bidaud, B.; Callens, E.; Mahut, B.; Bonfils, P. Nitric oxide evaluation in upper and lower respiratory tracts in nasal polyposis. Clin. Exp. Allergy 2008, 38, 1140–1147. [Google Scholar] [CrossRef]
- Rudmik, L.; Hopkins, C.; Peters, A.; Smith, T.L.; Schlosser, R.J.; Soler, Z.M. Patient-reported outcome measures for adult chronic rhinosinusitis: A systematic review and quality assessment. J. Allergy Clin. Immunol. 2015, 136, 1532–1540.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachert, C.; Han, J.K.; Wagenmann, M.; Hosemann, W.; Lee, S.E.; Backer, V.; Mullol, J.; Gevaert, P.; Klimek, L.; Prokopakis, E.; et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J. Allergy Clin. Immunol. 2021, 147, 29–36. [Google Scholar] [CrossRef]
- Rudmik, L.; Soler, Z.M.; Hopkins, C.; Schlosser, R.J.; Peters, A.; White, A.A.; Orlandi, R.R.; Fokkens, W.J.; Douglas, R.; Smith, T.L. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: A RAND/UCLA appropriateness study. Rhinology 2016, 54, 117–128. [Google Scholar]
- Luk, L.J.; DelGaudio, J.M. Topical Drug Therapies for Chronic Rhinosinusitis. Otolaryngol. Clin. N. Am. 2017, 50, 533–543. [Google Scholar] [CrossRef]
- Pynnonen, M.A.; Mukerji, S.S.; Kim, H.M.; Adams, M.E.; Terrell, J.E. Nasal saline for chronic sinonasal symptoms: A randomized controlled trial. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 1115–1120. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Pan, M.; Li, Y.; Tan, G.; Yang, Y. Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: Systematic review and meta-analysis. Braz. J. Otorhinolaryngol. 2020, 86, 639–646. [Google Scholar] [CrossRef]
- Chitsuthipakorn, W.; Kanjanawasee, D.; Hoang, M.P.; Seresirikachorn, K.; Snidvongs, K. Optimal Device and Regimen of Nasal Saline Treatment for Sinonasal Diseases: Systematic Review. OTO Open 2022, 6, 2473974X221105277. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.S. Topical Irrigations for Chronic Rhinosinusitis. Immunol. Allergy Clin. N. Am. 2020, 40, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Rudmik, L.; Soler, Z.M. Medical Therapies for Adult Chronic Sinusitis: A Systematic Review. JAMA 2015, 314, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Fandino, M.; Macdonald, K.I.; Lee, J.; Witterick, I.J. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: A systematic review and meta-analysis. Am. J. Rhinol. Allergy 2013, 27, e146–e157. [Google Scholar]
- Kalish, L.; Snidvongs, K.; Sivasubramaniam, R.; Cope, D.; Harvey, R.J. Topical steroids for nasal polyps. Cochrane Database Syst. Rev. 2012, 12, CD006549. [Google Scholar]
- Rudmik, L.; Schlosser, R.J.; Smith, T.L.; Soler, Z.M. Impact of topical nasal steroid therapy on symptoms of nasal polyposis: A meta-analysis. Laryngoscope 2012, 122, 1431–1437. [Google Scholar] [CrossRef]
- Harvey, R.J.; Snidvongs, K.; Kalish, L.H.; Oakley, G.M.; Sacks, R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 461–470. [Google Scholar] [CrossRef]
- Luz-Matsumoto, G.R.; Cabernite-Marchetti, E.; Sasaki, L.S.K.; Marquez, G.J.; Lacerda, L.S.; Almeida, T.R.; Kosugi, E.M. Nasal irrigation with corticosteroids in Brazil: The clinical response of 1% compounded budesonide drops and betamethasone cream. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S5), S32–S41. [Google Scholar] [CrossRef]
- Harvey, R.; Hannan, S.A.; Badia, L.; Scadding, G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst. Rev. 2007, 3, CD006394. [Google Scholar]
- Verkerk, M.M.; Bhatia, D.; Rimmer, J.; Earls, P.; Sacks, R.; Harvey, R.J. Intranasal steroids and the myth of mucosal atrophy: A systematic review of original histological assessments. Am. J. Rhinol. Allergy 2015, 29, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Hwang, P.H. Oral corticosteroid therapy in chronic rhinosinusitis without polyposis: A systematic review. Int. Forum Allergy Rhinol. 2011, 1, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Devesa, P.; Patiar, S. Oral steroids for nasal polyps. Cochrane Database Syst. Rev. 2011, 7, CD005232. [Google Scholar]
- Poetker, D.M.; Jakubowski, L.A.; Lal, D.; Hwang, P.H.; Wright, E.D.; Smith, T.L. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2013, 3, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Jie, J.L.Z.; Tran, T.N. Adverse outcomes from initiation of systemic corticosteroids for asthma: Long-term observational study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Head, K.; Chong, L.Y.; Piromchai, P.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 4, CD011994. [Google Scholar] [CrossRef] [Green Version]
- Van Zele, T.; Gevaert, P.; Holtappels, G.; Beule, A.; Wormald, P.J.; Mayr, S.; Hens, G.; Hellings, P.; Ebbens, F.A.; Fokkens, W.; et al. Oral steroids and doxycycline: Two different approaches to treat nasal polyps. J. Allergy Clin. Immunol. 2010, 125, 1069–1076.e4. [Google Scholar] [CrossRef]
- Jiang, R.S.; Wu, S.H.; Tsai, C.C.; Li, Y.H.; Liang, K.L. Efficacy of Chinese herbal medicine compared with a macrolide in the treatment of chronic rhinosinusitis without nasal polyps. Am. J. Rhinol. Allergy 2012, 26, 293–297. [Google Scholar] [CrossRef]
- Videler, W.J.; Badia, L.; Harvey, R.J.; Gane, S.; Georgalas, C.; van der Meulen, F.W.; Menger, D.J.; Lehtonen, M.T.; Toppila-Salmi, S.K.; Vento, S.I.; et al. Lack of efficacy of long-term, low-dose azithromycin in chronic rhinosinusitis: A randomized controlled trial. Allergy 2011, 66, 1457–1468. [Google Scholar] [CrossRef]
- Wallwork, B.; Coman, W.; Mackay-Sim, A.; Greiff, L.; Cervin, A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006, 116, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, F.; Lai, Y.; Luo, Q.; Xu, R.; Ou, C.; Fu, Q.; Shi, J. Lack of additional effects of long-term, low-dose clarithromycin combined treatment compared with topical steroids alone for chronic rhinosinusitis in China: A randomized, controlled trial. Int. Forum Allergy Rhinol. 2018, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Majima, Y.; Kurono, Y.; Hirakawa, K.; Ichimura, K.; Haruna, S.; Suzaki, H.; Kawauchi, H.; Takeuchi, K.; Naito, K.; Kase, Y.; et al. Efficacy of combined treatment with S-carboxymethylcysteine (carbocisteine) and clarithromycin in chronic rhinosinusitis patients without nasal polyp or with small nasal polyp. Auris Nasus Larynx 2012, 39, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cervin, A.; Wallwork, B. Efficacy and safety of long-term antibiotics (macrolides) for the treatment of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2014, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Malhotra-Kumar, S.; Lammens, C.; Coenen, S.; Van Herck, K.; Goossens, H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: A randomised, double-blind, placebo-controlled study. Lancet 2007, 369, 482–490. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.S.; Hoffmans, R.; Georgalas, C.; Fokkens, W.J. Complications of acute rhinosinusitis in The Netherlands. Fam. Pract. 2012, 29, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Casanova, J.L.; Holland, S.M.; Notarangelo, L.D. Inborn errors of human JAKs and STATs. Immunity 2012, 36, 515–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Damania, A.; Mair, M.L.; Otukoya, E.; Li, Y.D.; Polsky, K.; Zeng, Y.; Alt, J.A.; Citardi, M.J.; Corry, D.B.; et al. STAT6 Blockade Abrogates Aspergillus-Induced Eosinophilic Chronic Rhinosinusitis and Asthma, A Model of Unified Airway Disease. Front. Immunol. 2022, 13, 818017. [Google Scholar] [CrossRef]
- Wei, H.; Xu, L.; Sun, P.; Xing, H.; Zhu, Z.; Liu, J. Activation of STAT6 by intranasal allergens correlated with the development of eosinophilic chronic rhinosinusitis in a mouse model. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221109529. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Satoh, T.; Yamamoto, Y.; Saeki, K.; Igawa, K.; Okano, M.; Moriya, T.; Imamura, O.; Nemoto, Y.; Yokozeki, H. Gene silencing of STAT6 with siRNA ameliorates contact hypersensitivity and allergic rhinitis. Allergy 2011, 66, 124–131. [Google Scholar] [CrossRef]
- Darcan-Nicolaisen, Y.; Meinicke, H.; Fels, G.; Hegend, O.; Haberland, A.; Kuhl, A.; Loddenkemper, C.; Witzenrath, M.; Kube, S.; Henke, W.; et al. Small interfering RNA against transcription factor STAT6 inhibits allergic airway inflammation and hyperreactivity in mice. J. Immunol. 2009, 182, 7501–7508. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Shen, Y.; Li, C.; Liu, C.; Wang, Z.H.; Li, Y.S.; Ke, X.; Hu, G.H. IL-37 attenuates allergic process via STAT6/STAT3 pathways in murine allergic rhinitis. Int. Immunopharmacol. 2019, 69, 27–33. [Google Scholar] [CrossRef]
- Joo, Y.H.; Cho, H.J.; Jeon, Y.J.; Kim, J.H.; Jung, M.H.; Jeon, S.Y.; Suh, Y.S.; Park, J.J.; Kim, S.W. Therapeutic Effects of Intranasal Tofacitinib on Chronic Rhinosinusitis with Nasal Polyps in Mice. Laryngoscope 2021, 131, E1400–E1407. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.H.; Price, C.P.E.; Schauer, J.M.; Suh, L.A.; Harmon, R.; Conley, D.B.; Welch, K.C.; Kern, R.C.; Shintani-Smith, S.; et al. Prognostic factors for polyp recurrence in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2022, 150, 352–361.e7. [Google Scholar] [CrossRef]
- Morrissey, D.K.; Bassiouni, A.; Psaltis, A.J.; Naidoo, Y.; Wormald, P.J. Outcomes of modified endoscopic Lothrop in aspirin-exacerbated respiratory disease with nasal polyposis. Int. Forum Allergy Rhinol. 2016, 6, 820–825. [Google Scholar] [CrossRef]
- Gomes, S.C.; Cavaliere, C.; Masieri, S.; Van Zele, T.; Gevaert, P.; Holtappels, G.; Zhang, N.; Ramasamy, P.; Voegels, R.L.; Bachert, C. Reboot surgery for chronic rhinosinusitis with nasal polyposis: Recurrence and smell kinetics. Eur. Arch. Otorhinolaryngol. 2022, 279, 5691–5699. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petalas, K.; Goudakos, J.; Konstantinou, G.N. Targeting Epithelium Dysfunction and Impaired Nasal Biofilms to Treat Immunological, Functional, and Structural Abnormalities of Chronic Rhinosinusitis. Int. J. Mol. Sci. 2023, 24, 12379. https://doi.org/10.3390/ijms241512379
Petalas K, Goudakos J, Konstantinou GN. Targeting Epithelium Dysfunction and Impaired Nasal Biofilms to Treat Immunological, Functional, and Structural Abnormalities of Chronic Rhinosinusitis. International Journal of Molecular Sciences. 2023; 24(15):12379. https://doi.org/10.3390/ijms241512379
Chicago/Turabian StylePetalas, Konstantinos, John Goudakos, and George N. Konstantinou. 2023. "Targeting Epithelium Dysfunction and Impaired Nasal Biofilms to Treat Immunological, Functional, and Structural Abnormalities of Chronic Rhinosinusitis" International Journal of Molecular Sciences 24, no. 15: 12379. https://doi.org/10.3390/ijms241512379




