Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain
Abstract
:1. Introduction
2. Results
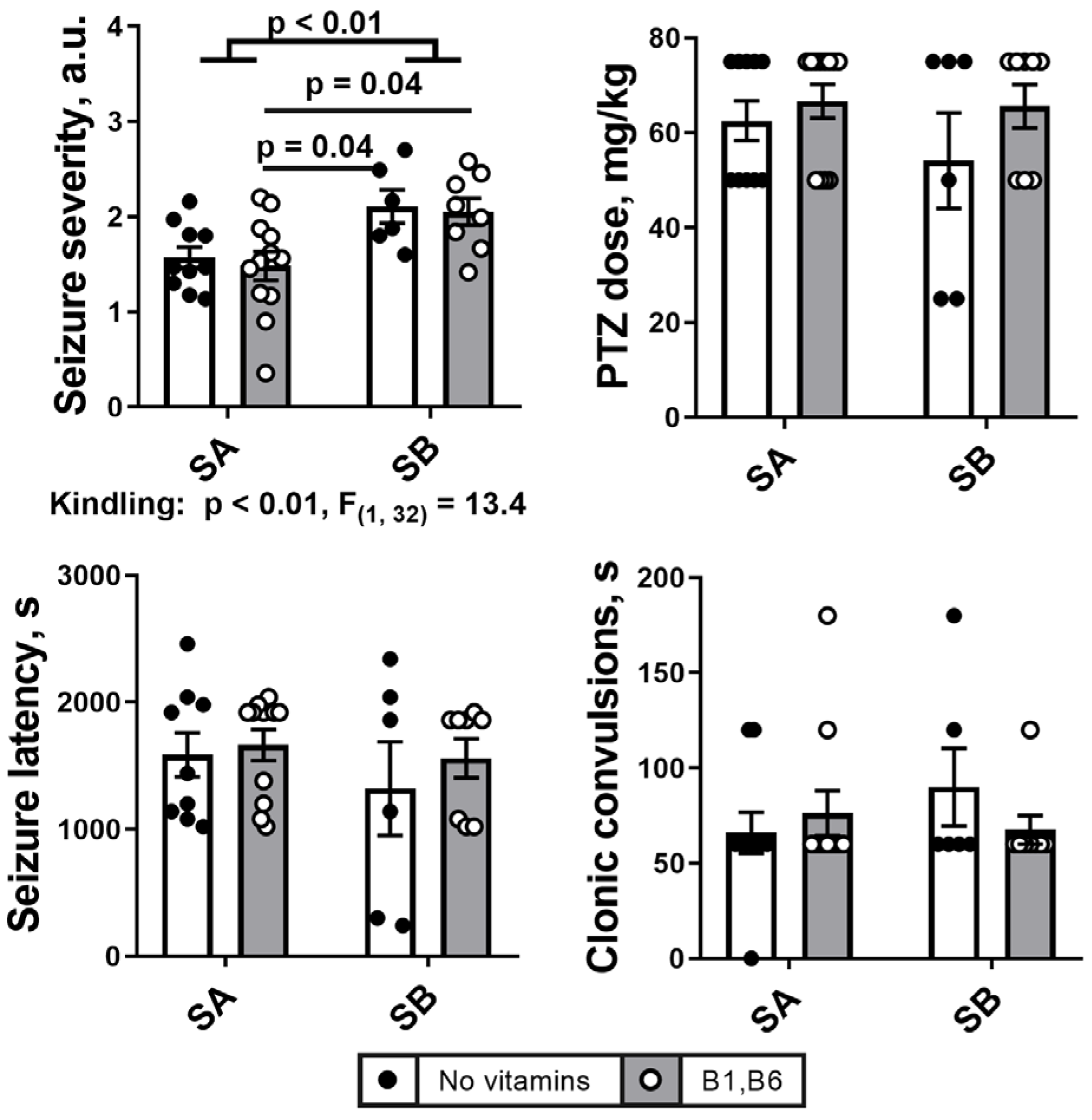
2.1. PTZ Kindling Increases Severety of Seizures
2.2. Effects of the PTZ Kindling and the Administration of Vitamins B1 and B6 on Behavioral and ECG Parameters after the Seizures
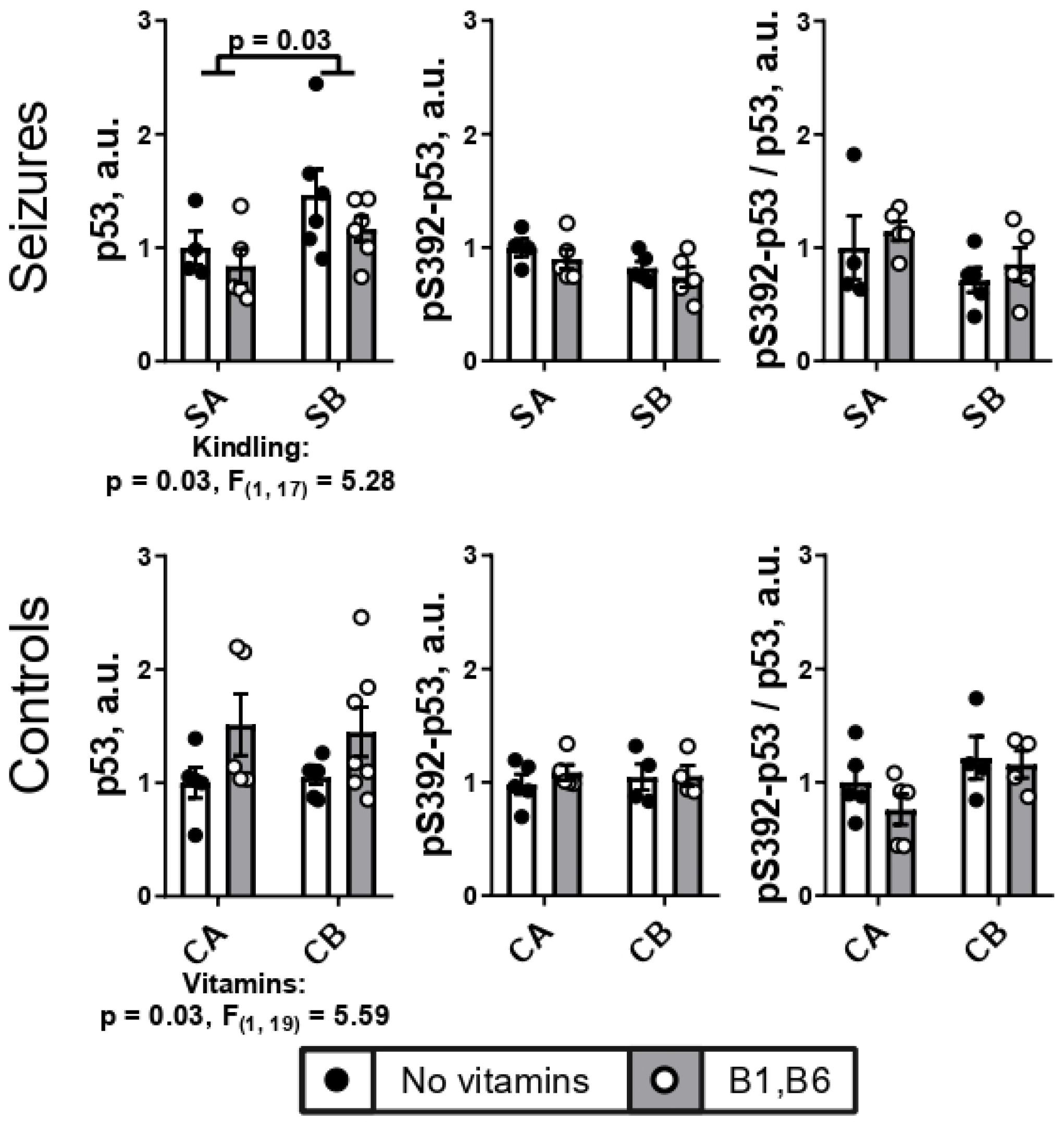
2.3. PTZ Kindling Increases Expression of Transcriptional Factor p53 in the Brain
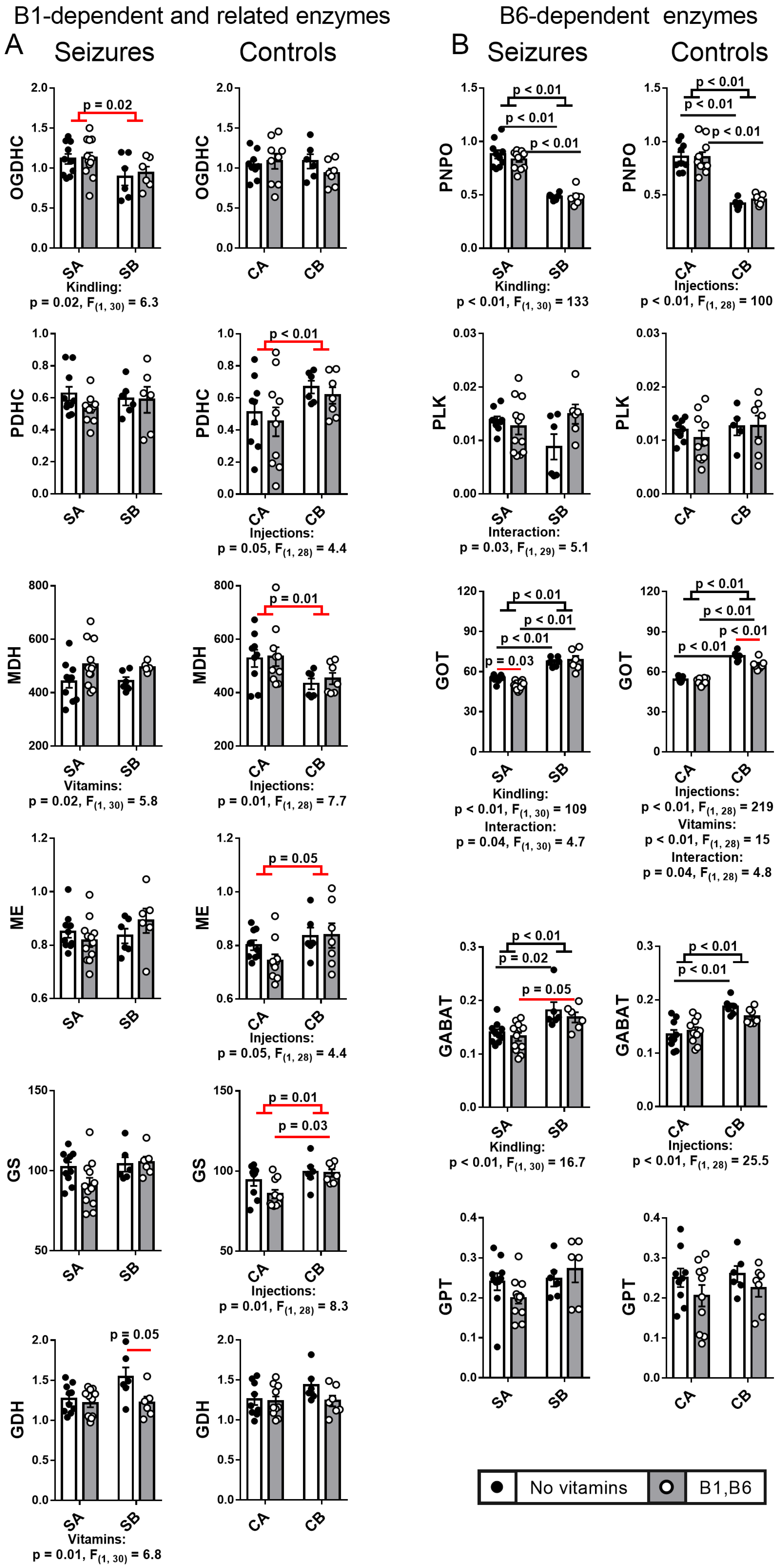
2.4. Effect of PTZ Kindling and Administration of Vitamins B1 and B6 on the Activities of the B1- and B6-Dependent and Related Enzymes in the Animal Brain after Seizures
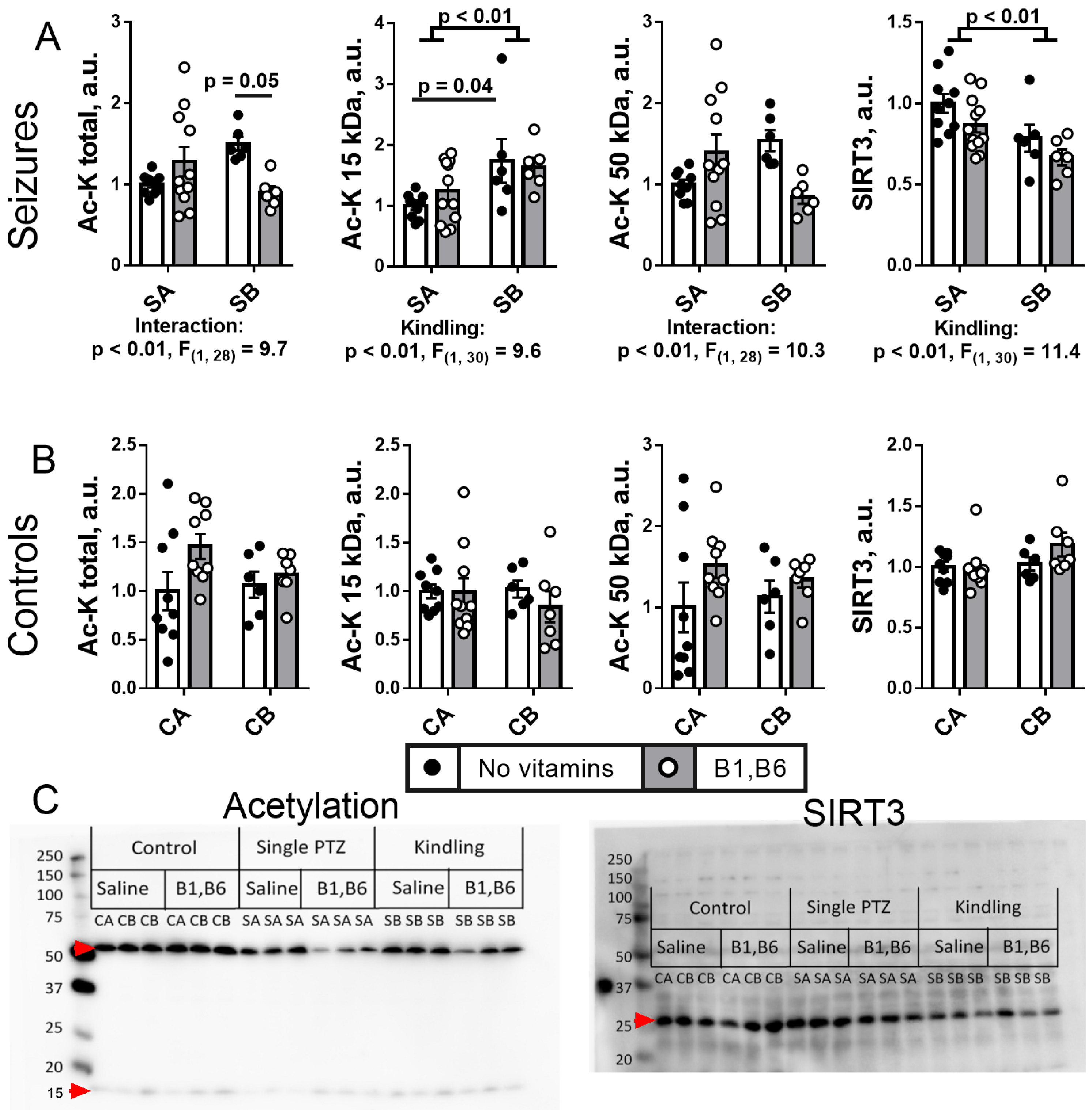
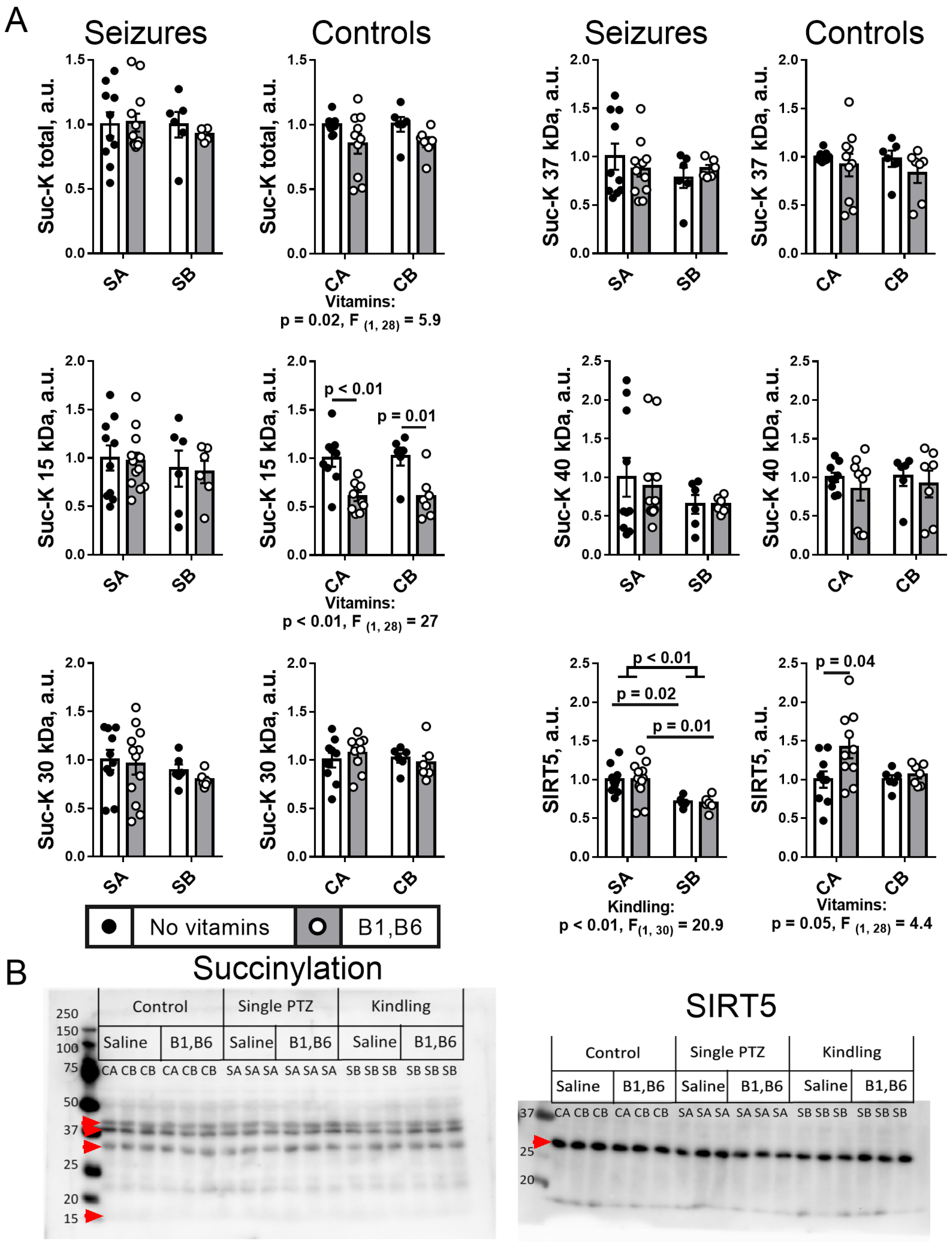
2.5. Effect of PTZ Kindling and Administration of Vitamins B1 and B6 on the Brain Protein Acylation System
2.5.1. Brain Protein Acetylation
2.5.2. Brain Protein Succinylation
2.6. Effects of PTZ Kindling on the Levels of Metabolites
2.6.1. Redox Indicators
2.6.2. Free Amino Acids and Related Compounds
2.7. Administration of Vitamins B1 and B6 Affects Correlations between the Assessed Parameters in the Post-Seizures and Control Rats
2.7.1. Vitamins Regulate the Seizures-Induced Metabolic and Physiological Changes
2.7.2. Regulation of Metabolism and Physiology by the Vitamins Administration in the Control Rats
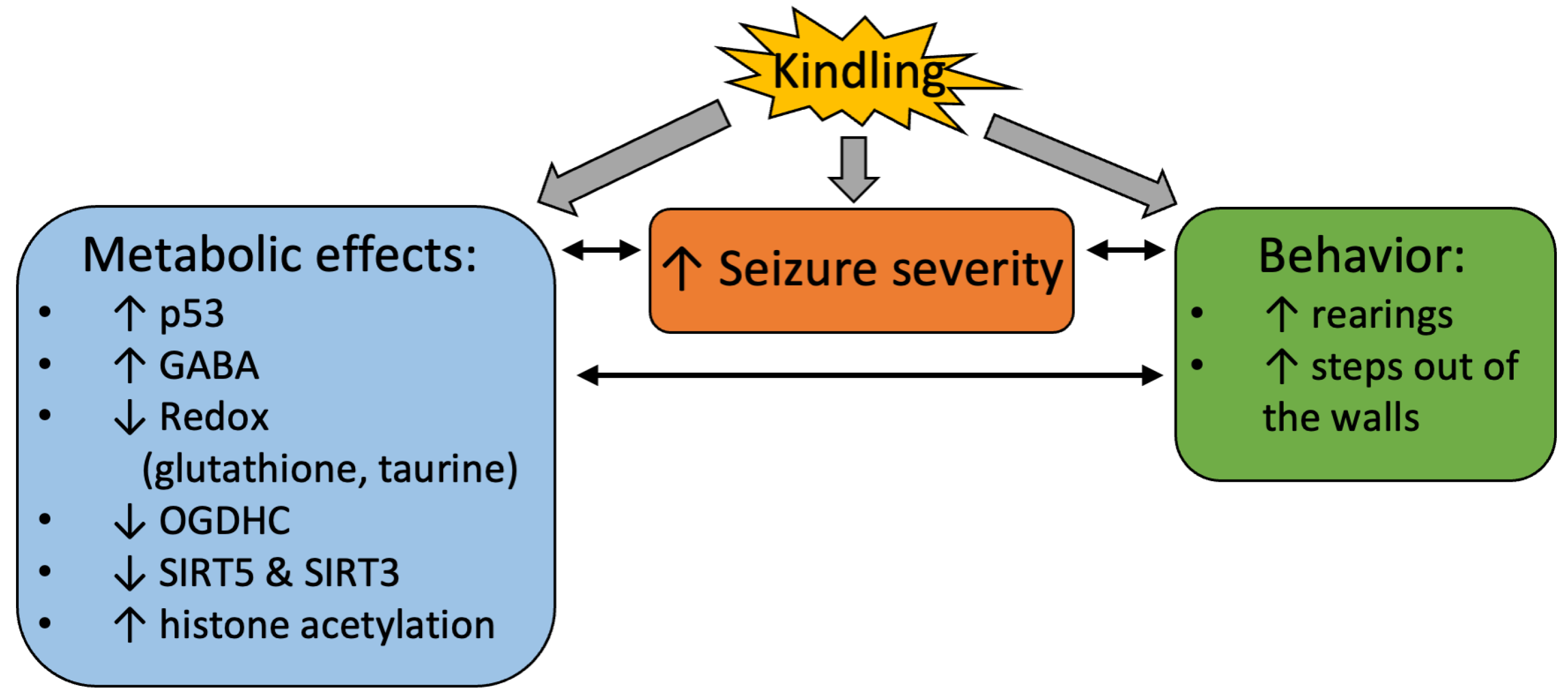
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.1.1. Model of Single Episode of PTZ-Induced Seizures
4.1.2. Model of PTZ-Induced Seizures after the PTZ Kindling
4.1.3. Administration of Vitamins
4.1.4. Animal Survival
4.1.5. Control Animals to Reveal the Injections-Related Stress Effects
4.2. Assessment of Physiological Parmeters after Seizures and in the Corresponding Control Groups
4.3. Preparation of Homogenates of the Rat Cerebral Cortex
4.4. Measurement of Enzymatic Activities
4.5. Western Blotting
4.6. Preparation of Tissue Extracts and Quantifications of Metabolites
4.7. Statistical Analysis and Data Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTZ | pentylenetetrazole |
| OGDHC | 2-oxoglutarate dehydrogenase complex |
| PDHC | pyruvate dehydrogenase complex |
| GDH | glutamate dehydrogenase |
| MDH | malate dehydrogenase |
| PLK | pyridoxal kinase |
| PNPO | pyridoxal 5′-phosphate oxidase; |
| GS | glutamine synthetase |
| ME | malic enzyme |
| GOT | glutamate-oxaloacetate transaminase |
| GPT | glutamate-pyruvate transaminase |
| GABA | gamma-aminobutyric acid |
| GABAT | GABA transaminase |
| NOS | nitric oxide synthase |
References
- Treiman, D.M. GABAergic Mechanisms in Epilepsy. Epilepsia 2001, 42, 8–12. [Google Scholar] [CrossRef]
- Kim, A.Y.; Baik, E.J. Glutamate Dehydrogenase as a Neuroprotective Target Against Neurodegeneration. Neurochem. Res. 2018, 44, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Tu, N.; Lee, T.-S.W.; Lai, J.C.K. Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem. Int. 2013, 63, 670–681. [Google Scholar] [CrossRef] [Green Version]
- Storici, P.; Capitani, G.; De Biase, D.; Moser, M.; John, R.A.; Jansonius, J.N.; Schirmer, T. Crystal Structure of GABA-Aminotransferase, a Target for Antiepileptic Drug Therapy. Biochemistry 1999, 38, 8628–8634. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liang, X.-J.; Li, W.-J.; Wu, D.; Liu, M.; Cao, B.-Y.; Chen, J.-J.; Qin, M.; Meng, X.; Gong, C.-X. Clinical and Molecular Spectrum of Glutamate Dehydrogenase Gene Defects in 26 Chinese Congenital Hyperinsulinemia Patients. J. Diabetes Res. 2018, 2018, 2802540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 136, e56573. [Google Scholar] [CrossRef] [Green Version]
- Dhir, A. Pentylenetetrazol (PTZ) Kindling Model of Epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9–37. [Google Scholar] [CrossRef]
- Schmoll, H.; Badan, I.; Grecksch, G.; Walker, L.; Kessler, C.; Popa-Wagner, A. Kindling Status in Sprague-Dawley Rats Induced by Pentylenetetrazole. Am. J. Pathol. 2003, 162, 1027–1034. [Google Scholar] [CrossRef]
- Hoeller, A.; de Carvalho, C.; Franco, P.; Formolo, D.; Imthon, A.; dos Santos, H.; Eidt, I.; Souza, G.; Constantino, L.; Ferreira, C.; et al. Behavioral and Neurochemical Consequences of Pentylenetetrazol-Induced Kindling in Young and Middle-Aged Rats. Pharmaceuticals 2017, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Postma, T.; Krupp, E.; Li, X.L.; Post, R.M.; Weiss, S.R. Lamotrigine treatment during amygdala-kindled seizure development fails to inhibit seizures and diminishes subsequent anticonvulsant efficacy. Epilepsia 2000, 41, 1514–1521. [Google Scholar] [CrossRef]
- Kelly, M.E.; McIntyre, D.C. Hippocampal kindling protects several structures from the neuronal damage resulting from kainic acid-induced status epilepticus. Brain Res. 1994, 634, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Velisek, L.; Kubova, H.; Pohl, M.; Stankova, L.; Mareš, P.; Schickerova, R. Pentylenetetrazol-induced seizures in rats: An ontogenetic study. Naunyn-Schmiedebergs Arch. Pharmacol. 1992, 346, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.F.; Saederup, E.; Crawley, J.N.; Skolnick, P.; Paul, S.M. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984, 35, 1439–1444. [Google Scholar] [CrossRef]
- Huang, R.Q.; Bell-Horner, C.L.; Dibas, M.I.; Covey, D.F.; Drewe, J.A.; Dillon, G.H. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J. Pharmacol. Exp. Ther. 2001, 298, 986–995. [Google Scholar]
- Kalueff, A.V. Mapping convulsants’ binding to the GABA-A receptor chloride ionophore: A proposed model for channel binding sites. Neurochem. Int. 2007, 50, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavazos, J.E.; Sutula, T.P. Progressive neuronal loss induced by kindling: A possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990, 527, 1–6. [Google Scholar] [CrossRef]
- Inan, S.; Buyukafsar, K. Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br. J. Pharmacol. 2008, 155, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Angelatou, F.; Pagonopoulou, O.; Kostopoulos, G. Alterations of A1 adenosine receptors in different mouse brain areas after pentylentetrazol-induced seizures, but not in the epileptic mutant mouse ‘tottering’. Brain Res. 1990, 534, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.M.; Palomero-Gallagher, N.; Bidmon, H.J.; Schleicher, A.; Speckmann, E.J.; Zilles, K. Pentylenetetrazole-induced seizures affect binding site densities for GABA, glutamate and adenosine receptors in the rat brain. Neuroscience 2009, 163, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bidmon, H.J.; Gorg, B.; Palomero-Gallagher, N.; Schliess, F.; Gorji, A.; Speckmann, E.J.; Zilles, K. Bilateral, vascular and perivascular glial upregulation of heat shock protein-27 after repeated epileptic seizures. J. Chem. Neuroanat. 2005, 30, 1–16. [Google Scholar] [CrossRef]
- Rauca, C.; Zerbe, R.; Jantze, H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999, 847, 347–351. [Google Scholar] [CrossRef]
- de Souza, A.G.; Chaves Filho, A.J.M.; Souza Oliveira, J.V.; de Souza, D.A.A.; Lopes, I.S.; de Carvalho, M.A.J.; de Lima, K.A.; Florenco Sousa, F.C.; Mendes Vasconcelos, S.M.; Macedo, D.; et al. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomed. Pharmacother. 2019, 109, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Ngoupaye, G.T.; Adassi, M.B.; Foutsop, A.F.; Yassi, F.B.; Ngo Bum, E. Pentylenetetrazole kindling-induced epilepsy rat models: Insight on the severity state, a comparative study. IBRO Neurosci. Rep. 2022, 13, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, L.; Pfluger, P.; Regner, G.G.; Santos, F.M.; Aguirre, D.G.; Brito, V.B.; Moura, D.J.; Dos Santos, N.M.; Picada, J.N.; Parmeggiani, B.; et al. Lacosamide improves biochemical, genotoxic, and mitochondrial parameters after PTZ-kindling model in mice. Fundam. Clin. Pharmacol. 2021, 35, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Kumar, A. Neuroprotective mechanism of Coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: Possible role of microglia inhibition. Pharmacol. Rep. 2016, 68, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal Nitric Oxide Synthase Contributes to PTZ Kindling Epilepsy-Induced Hippocampal Endoplasmic Reticulum Stress and Oxidative Damage. Front. Cell Neurosci. 2017, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Erakovic, V.; Zupan, G.; Varljen, J.; Laginja, J.; Simonic, A. Altered activities of rat brain metabolic enzymes caused by pentylenetetrazol kindling and pentylenetetrazol--induced seizures. Epilepsy Res. 2001, 43, 165–173. [Google Scholar] [CrossRef]
- Bonan, C.D.; Amaral, O.B.; Rockenbach, I.C.; Walz, R.; Battastini, A.M.; Izquierdo, I.; Sarkis, J.J. Altered ATP hydrolysis induced by pentylenetetrazol kindling in rat brain synaptosomes. Neurochem. Res. 2000, 25, 775–779. [Google Scholar] [CrossRef]
- Zavileyskiy, L.G.; Aleshin, V.A.; Kaehne, T.; Karlina, I.S.; Artiukhov, A.V.; Maslova, M.V.; Graf, A.V.; Bunik, V.I. The Brain Protein Acylation System Responds to Seizures in the Rat Model of PTZ-Induced Epilepsy. Int. J. Mol. Sci. 2022, 23, 12302. [Google Scholar] [CrossRef]
- Jewett, K.A.; Christian, C.A.; Bacos, J.T.; Lee, K.Y.; Zhu, J.; Tsai, N.P. Feedback modulation of neural network synchrony and seizure susceptibility by Mdm2-p53-Nedd4-2 signaling. Mol. Brain 2016, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Engel, T.; Murphy, B.M.; Schindler, C.K.; Henshall, D.C. Elevated p53 and lower MDM2 expression in hippocampus from patients with intractable temporal lobe epilepsy. Epilepsy Res. 2007, 77, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, R.S.; Kinoshita, Y. The role of p53 in neuronal cell death. Cell Death Differ. 2000, 7, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.X.; Tian, F.F.; Guo, J.L.; Li, K.; He, J.X.; Song, M.Y.; Li, L.; Huang, X. Dynamic expression patterns of ATF3 and p53 in the hippocampus of a pentylenetetrazole-induced kindling model. Mol. Med. Rep. 2014, 10, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Engel, T.; Sanz-Rodgriguez, A.; Jimenez-Mateos, E.M.; Concannon, C.G.; Jimenez-Pacheco, A.; Moran, C.; Mesuret, G.; Petit, E.; Delanty, N.; Farrell, M.A.; et al. CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain J. Neurol. 2013, 136, 577–592. [Google Scholar] [CrossRef] [Green Version]
- Ghate, N.B.; Kim, S.; Mehmood, R.; Shin, Y.; Kim, K.; An, W. VprBP/DCAF1 regulates p53 function and stability through site-specific phosphorylation. Oncogene 2023, 42, 1405–1416. [Google Scholar] [CrossRef]
- Zavileyskiy, L.; Bunik, V. Regulation of p53 Function by Formation of Non-Nuclear Heterologous Protein Complexes. Biomolecules 2022, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 2004, 4, 793–805. [Google Scholar] [CrossRef]
- Ashcroft, M.; Kubbutat, M.H.; Vousden, K.H. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 1999, 19, 1751–1758. [Google Scholar] [CrossRef] [Green Version]
- Accorsi, P.; Cellini, E.; Paolantonio, C.D.; Panzarino, G.; Verrotti, A.; Giordano, L. Pyridoxine responsiveness in pyridox(am)ine-5-phosphate oxidase deficiency: The importance of early treatment. Clin. Neurol. Neurosurg. 2017, 163, 90–93. [Google Scholar] [CrossRef]
- Barile, A.; Mills, P.; di Salvo, M.L.; Graziani, C.; Bunik, V.; Clayton, P.; Contestabile, R.; Tramonti, A. Characterization of Novel Pathogenic Variants Causing Pyridox(am)ine 5′-Phosphate Oxidase-Dependent Epilepsy. Int. J. Mol. Sci. 2021, 22, 12013. [Google Scholar] [CrossRef]
- Barile, A.; Nogues, I.; di Salvo, M.L.; Bunik, V.; Contestabile, R.; Tramonti, A. Molecular characterization of pyridoxine 5′-phosphate oxidase and its pathogenic forms associated with neonatal epileptic encephalopathy. Sci. Rep. 2020, 10, 13621. [Google Scholar] [CrossRef]
- Keyser, A.; De Bruijn, S.F. Epileptic manifestations and vitamin B1 deficiency. Eur. Neurol. 1991, 31, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.O.; Kubota, M.; Terashima, H.; Ishiguro, A.; Kashii, H. Early administration of vitamins B1 and B6 and l-carnitine prevents a second attack of acute encephalopathy with biphasic seizures and late reduced diffusion: A case control study. Brain Dev. 2019, 41, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, A.A.; Cock, H.R. Medical management of status epilepticus: Emergency room to intensive care unit. Seizure 2020, 75, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiao, Y.; Meng, F.; Li, Y.; Shi, Z.; Qian, K. Functions and Mechanisms of Lysine Glutarylation in Eukaryotes. Front. Cell Dev. Biol. 2021, 9, 667684. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Graf, A.V.; Artiukhov, A.V.; Boyko, A.I.; Ksenofontov, A.L.; Maslova, M.V.; Nogués, I.; di Salvo, M.L.; Bunik, V.I. Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharmaceuticals 2021, 14, 737. [Google Scholar] [CrossRef]
- Yang, M.T.; Chou, I.C.; Wang, H.S. Role of vitamins in epilepsy. Epilepsy Behav. 2023, 139, 109062. [Google Scholar] [CrossRef]
- Bunik, V. The Therapeutic Potential of Vitamins B1, B3 and B6 in Charcot–Marie–Tooth Disease with the Compromised Status of Vitamin-Dependent Processes. Biology 2023, 12, 897. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A.; Zhou, X.; Krishnan, S.; Karlsson, A. Regulation of Thiamine (Vitamin B1)-Dependent Metabolism in Mammals by p53. Biochem. Biokhimiia 2020, 85, 801–807. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A.; Zhou, X.; Tabakov, V.Y.; Karlsson, A. Activation of Mitochondrial 2-Oxoglutarate Dehydrogenase by Cocarboxylase in Human Lung Adenocarcinoma Cells A549 Is p53/p21-Dependent and Impairs Cellular Redox State, Mimicking the Cisplatin Action. Int. J. Mol. Sci. 2020, 21, 3759. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Sibiryakina, D.A.; Kazantsev, A.V.; Graf, A.V.; Bunik, V.I. Acylation of the Rat Brain Proteins is Affected by the Inhibition of Pyruvate Dehydrogenase in vivo. Biochemistry 2023, 88, 105–118. [Google Scholar] [CrossRef]
- Graf, A.V.; Maslova, M.V.; Artiukhov, A.V.; Ksenofontov, A.L.; Aleshin, V.A.; Bunik, V.I. Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age. Int. J. Mol. Sci. 2022, 23, 2579. [Google Scholar] [CrossRef]
- Sengupta, A.; Haldar, D. Human sirtuin 3 (SIRT3) deacetylates histone H3 lysine 56 to promote nonhomologous end joining repair. DNA Repair 2018, 61, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.B.; Vaquero, A.; Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes. Dev. 2007, 21, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2019, 63, e1800569. [Google Scholar] [CrossRef]
- Bunik, V.; Artiukhov, A.; Aleshin, V.; Mkrtchyan, G. Multiple Forms of Glutamate Dehydrogenase in Animals: Structural Determinants and Physiological Implications. Biology 2016, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mkrtchyan, G.V.; Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Positive correlation between rat brain glutamate concentrations and mitochondrial 2-oxoglutarate dehydrogenase activity. Anal. Biochem. 2018, 552, 100–109. [Google Scholar] [CrossRef]
- Bunik, V.I.; Fernie, A.R. Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: A cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation. Biochem. J. 2009, 422, 405–421. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.E.; Park, L.C.; Sheu, K.F.; Blass, J.P.; Calingasan, N.Y. The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem. Int. 2000, 36, 97–112. [Google Scholar] [CrossRef]
- Kabysheva, M.S.; Storozhevykh, T.P.; Pinelis, V.G.; Bunik, V.I. Synthetic regulators of the 2-oxoglutarate oxidative decarboxylation alleviate the glutamate excitotoxicity in cerebellar granule neurons. Biochem. Pharmacol. 2009, 77, 1531–1540. [Google Scholar] [CrossRef]
- Zundorf, G.; Kahlert, S.; Bunik, V.I.; Reiser, G. alpha-Ketoglutarate dehydrogenase contributes to production of reactive oxygen species in glutamate-stimulated hippocampal neurons in situ. Neuroscience 2009, 158, 610–616. [Google Scholar] [CrossRef]
- Weidinger, A.; Milivojev, N.; Hosmann, A.; Duvigneau, J.C.; Szabo, C.; Toro, G.; Rauter, L.; Vaglio-Garro, A.; Mkrtchyan, G.V.; Trofimova, L.; et al. Oxoglutarate dehydrogenase complex controls glutamate-mediated neuronal death. Redox Biol. 2023, 62, 102669. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Zhou, X.; Krishnan, S.; Karlsson, A.; Bunik, V.I. Interplay between Thiamine and p53/p21 Axes Affects Antiproliferative Action of Cisplatin in Lung Adenocarcinoma Cells by Changing Metabolism of 2-Oxoglutarate/Glutamate. Front. Genet. 2021, 12, 658446. [Google Scholar] [CrossRef]
- Boyko, A.; Tsepkova, P.; Aleshin, V.; Artiukhov, A.; Mkrtchyan, G.; Ksenofontov, A.; Baratova, L.; Ryabov, S.; Graf, A.; Bunik, V. Severe Spinal Cord Injury in Rats Induces Chronic Changes in the Spinal Cord and Cerebral Cortex Metabolism, Adjusted by Thiamine That Improves Locomotor Performance. Front. Mol. Neurosci. 2021, 14, 620593. [Google Scholar] [CrossRef]
- Chornyy, S.; Parkhomenko, Y.; Chorna, N. Thiamine antagonists trigger p53-dependent apoptosis in differentiated SH-SY5Y cells. Sci. Rep. 2017, 7, 10632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Rong, F.; Tang, J.; Zhu, C.; Chen, X.; Jia, S.; Wang, Z.; Sun, X.; Deng, H.; Zha, H.; et al. Repression of p53 function by SIRT5-mediated desuccinylation at Lysine 120 in response to DNA damage. Cell Death Differ. 2022, 29, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Morris-Blanco, K.C.; Dave, K.R.; Saul, I.; Koronowski, K.B.; Stradecki, H.M.; Perez-Pinzon, M.A. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci. Rep. 2016, 6, 29790. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, A.; Chen, Q. SirT3 and p53 Deacetylation in Aging and Cancer. J. Cell Physiol. 2017, 232, 2308–2311. [Google Scholar] [CrossRef]
- Jin, Y.; Gu, W.; Chen, W. Sirt3 is critical for p53-mediated ferroptosis upon ROS-induced stress. J. Mol. Cell Biol. 2021, 13, 151–154. [Google Scholar] [CrossRef]
- Tsepkova, P.M.; Artiukhov, A.V.; Boyko, A.I.; Aleshin, V.A.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine Induces Long-Term Changes in Amino Acid Profiles and Activities of 2-Oxoglutarate and 2-Oxoadipate Dehydrogenases in Rat Brain. Biochemistry 2017, 82, 723–736. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Aleshin, V.A.; Karlina, I.S.; Kazantsev, A.V.; Sibiryakina, D.A.; Ksenofontov, A.L.; Lukashev, N.V.; Graf, A.V.; Bunik, V.I. Phosphonate Inhibitors of Pyruvate Dehydrogenase Perturb Homeostasis of Amino Acids and Protein Succinylation in the Brain. Int. J. Mol. Sci. 2022, 23, 13186. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kumar, A. Neuroprotective Effect of Lycopene Against PTZ-induced Kindling Seizures in Mice: Possible Behavioural, Biochemical and Mitochondrial Dysfunction. Phytother. Res. 2016, 30, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Watanabe, M.; Yoshikawa, K.; Kanaho, Y.; Berliner, L.J.; Fujii, H. Magnetic resonance and biochemical studies during pentylenetetrazole-kindling development: The relationship between nitric oxide, neuronal nitric oxide synthase and seizures. Neuroscience 2004, 129, 757–766. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, G.; Naidu, P.S.; Kulkarni, S.K. Protective effect of FK506 (tacrolimus) in pentylenetetrazol-induced kindling in mice. Pharmacol. Biochem. Behav. 2003, 75, 853–860. [Google Scholar] [CrossRef]
- Endoh, M.; Maiese, K.; Wagner, J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994, 651, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garthwaite, J.; Boulton, C.L. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995, 57, 683–706. [Google Scholar] [CrossRef]
- Boyko, A.; Ksenofontov, A.; Ryabov, S.; Baratova, L.; Graf, A.; Bunik, V. Delayed Influence of Spinal Cord Injury on the Amino Acids of NO(*) Metabolism in Rat Cerebral Cortex Is Attenuated by Thiamine. Front. Med. 2017, 4, 249. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Lopez, A.; Sierra-Paredes, G.; Sierra-Marcuno, G. Seizures induced by microperfusion of glutamate and glycine in the hippocampus of rats pretreated with latrunculin A. Neurosci. Lett. 2005, 388, 81–85. [Google Scholar] [CrossRef]
- Loscher, W.; Horstermann, D.; Honack, D.; Rundfeldt, C.; Wahnschaffe, U. Transmitter amino acid levels in rat brain regions after amygdala-kindling or chronic electrode implantation without kindling: Evidence for a pro-kindling effect of prolonged electrode implantation. Neurochem. Res. 1993, 18, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Yeh, G.C.; Bonhaus, D.W.; Nadler, J.V.; McNamara, J.O. N-methyl-D-aspartate receptor plasticity in kindling: Quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proc. Natl. Acad. Sci. USA 1989, 86, 8157–8160. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Morimoto, K.; Yamada, N.; Otsuki, S. Antiepileptogenic action of 7-chlorokynurenic acid on amygdala kindling of rats. Pharmacol. Biochem. Behav. 1993, 46, 275–281. [Google Scholar] [CrossRef]
- Shen, H.Y.; van Vliet, E.A.; Bright, K.A.; Hanthorn, M.; Lytle, N.K.; Gorter, J.; Aronica, E.; Boison, D. Glycine transporter 1 is a target for the treatment of epilepsy. Neuropharmacology 2015, 99, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azize, N.A.; Ngah, W.Z.; Othman, Z.; Md Desa, N.; Chin, C.B.; Md Yunus, Z.; Mohan, A.; Hean, T.S.; Syed Zakaria, S.Z.; Lock-Hock, N. Mutation analysis of glycine decarboxylase, aminomethyltransferase and glycine cleavage system protein-H genes in 13 unrelated families with glycine encephalopathy. J. Hum. Genet. 2014, 59, 593–597. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine andN-ethylmaleimide. Toxicol. Mech. Methods 2015, 25, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Carmody, S.; Brennan, L. Effects of pentylenetetrazole-induced seizures on metabolomic profiles of rat brain. Neurochem. Int. 2010, 56, 340–344. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, H.; Kim, H.; Kim, K. Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience 2006, 140, 673–684. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Shen, K.; Bai, Y.; Chao, J.; Yao, H. Neuronal nitric oxide synthase contributes to pentylenetetrazole-kindling-induced hippocampal neurogenesis. Brain Res. Bull. 2016, 121, 138–147. [Google Scholar] [CrossRef]
- Shahpari, M.; Aligholi, H.; Namavar, M.R.; Vafaee, F.; Emamghoreishi, M. Improved Stage Categorization of PTZ-Induced Kindling and Late Enhanced Neurogenesis in PTZ Kindled Mice. Galen. Med. J. 2019, 8, e1511. [Google Scholar] [CrossRef]
- Barile, A.; Tramonti, A.; di Salvo, M.L.; Nogués, I.; Nardella, C.; Malatesta, F.; Contestabile, R. Allosteric feedback inhibition of pyridoxine 5′-phosphate oxidase from Escherichia coli. J. Biol. Chem. 2019, 294, 15593–15603. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef]
- Zybina, A.; Anshakova, A.; Malinovskaya, J.; Melnikov, P.; Baklaushev, V.; Chekhonin, V.; Maksimenko, O.; Titov, S.; Balabanyan, V.; Kreuter, J.; et al. Nanoparticle-based delivery of carbamazepine: A promising approach for the treatment of refractory epilepsy. Int. J. Pharm. 2018, 547, 10–23. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration. J. Neurochem. 2020, 153, 80–102. [Google Scholar] [CrossRef]
- Mkrtchyan, G.V.; Ucal, M.; Mullebner, A.; Dumitrescu, S.; Kames, M.; Moldzio, R.; Molcanyi, M.; Schaefer, S.; Weidinger, A.; Schaefer, U.; et al. Thiamine preserves mitochondrial function in a rat model of traumatic brain injury, preventing inactivation of the 2-oxoglutarate dehydrogenase complex. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, G.; Graf, A.; Bettendorff, L.; Bunik, V. Cellular thiamine status is coupled to function of mitochondrial 2-oxoglutarate dehydrogenase. Neurochem. Int. 2016, 101, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; Gonzalez-Gross, M. Vitamin B6 status, deficiency and its consequences—An overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar] [PubMed]
- Hawk, P.B.; Oser, B.L.; Summerson, W.H. Practical physiological chemistry. By Philip B. Hawk, Bernard L. Oser, and William H. Summerson. The Blakiston Co., Inc. New York, 1954. 13th ed. xvi + 1439 pp. 23.5 × 16 cm. Price $12. J. Am. Pharm. Assoc. 1955, 44, 62–63. [Google Scholar] [CrossRef]
- Galvin, R.; Brathen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Graf, A.V.; Kazantsev, A.V.; Boyko, A.I.; Aleshin, V.A.; Ksenofontov, A.L.; Bunik, V.I. Increasing Inhibition of the Rat Brain 2-Oxoglutarate Dehydrogenase Decreases Glutathione Redox State, Elevating Anxiety and Perturbing Stress Adaptation. Pharmaceuticals 2022, 15, 182. [Google Scholar] [CrossRef]
- Boer, T.D.; Bruinvels, J. Assay and Properties of 4-Aminobutyric-2-Oxoglutaric Acid Transaminase and Succinic Semialdehyde Dehydrogenase in Rat Brain Tissue. J. Neurochem. 1977, 28, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, L.; Ksenofontov, A.; Mkrtchyan, G.; Graf, A.; Baratova, L.; Bunik, V. Quantification of Rat Brain Amino Acids: Analysis of the Data Consistency. Curr. Anal. Chem. 2016, 12, 349–356. [Google Scholar] [CrossRef]
- Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells 2020, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Artiukhov, A.V.; Pometun, A.A.; Zubanova, S.A.; Tishkov, V.I.; Bunik, V.I. Advantages of formate dehydrogenase reaction for efficient NAD(+) quantification in biological samples. Anal. Biochem. 2020, 603, 113797. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef] [PubMed]




| No Vitamins | GDH | OGDHC | p53 | Glu | GABA | Grooming Acts | Rearing Acts | Steps Out | RMSSD | SI | Seizure Latency | Seizure Severity | Seizure Duration | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamins | ||||||||||||||
| GDH | −0.42 0.10 | −0.26 0.47 | 0.26 0.42 | 0.34 0.28 | 0.31 0.23 | 0.20 0.45 | 0.80 0.00 | 0.08 0.78 | −0.38 0.16 | −0.11 0.69 | 0.30 0.26 | 0.58 0.02 | ||
| OGDHC | 0.11 0.66 | −0.42 0.23 | −0.34 0.28 | −0.32 0.31 | −0.04 0.89 | 0.11 0.68 | −0.40 0.13 | 0.28 0.31 | −0.11 0.69 | 0.18 0.51 | −0.23 0.39 | −0.21 0.45 | ||
| p53 | −0.21 0.54 | −0.26 0.43 | 0.79 0.05 | 0.18 0.71 | −0.08 0.83 | −0.63 0.06 | −0.46 0.18 | −0.80 0.01 | 0.77 0.02 | 0.52 0.13 | 0.41 0.25 | −0.36 0.30 | ||
| Glu | −0.07 0.84 | 0.37 0.26 | 0.75 0.07 | 0.38 0.22 | 0.19 0.54 | −0.17 0.59 | 0.04 0.90 | −0.45 0.17 | 0.37 0.26 | 0.45 0.15 | 0.36 0.25 | −0.03 0.94 | ||
| GABA | 0.33 0.33 | −0.41 0.21 | 0.43 0.35 | 0.33 0.33 | 0.48 0.11 | 0.35 0.27 | 0.55 0.07 | 0.15 0.67 | −0.11 0.75 | −0.16 0.61 | 0.59 0.05 | 0.23 0.49 | ||
| Grooming acts | 0.27 0.29 | −0.33 0.18 | 0.01 0.98 | −0.13 0.72 | 0.15 0.65 | 0.53 0.04 | 0.44 0.09 | 0.20 0.46 | −0.12 0.67 | 0.14 0.61 | 0.07 0.81 | 0.57 0.02 | ||
| Rearing acts | 0.33 0.18 | −0.34 0.17 | 0.27 0.42 | −0.26 0.44 | 0.57 0.07 | 0.19 0.44 | 0.42 0.11 | 0.34 0.22 | −0.33 0.22 | −0.28 0.29 | −0.12 0.67 | 0.53 0.03 | ||
| Steps out | 0.35 0.15 | −0.30 0.22 | 0.20 0.55 | −0.36 0.27 | 0.40 0.22 | 0.45 0.06 | 0.66 0.00 | 0.37 0.18 | −0.37 0.17 | −0.25 0.35 | 0.16 0.54 | 0.59 0.01 | ||
| RMSSD | −0.33 0.18 | −0.21 0.41 | 0.15 0.67 | 0.14 0.69 | 0.25 0.47 | −0.16 0.53 | 0.27 0.27 | 0.25 0.32 | −0.70 0.00 | −0.17 0.53 | −0.28 0.32 | 0.23 0.41 | ||
| SI | 0.25 0.31 | 0.37 0.13 | −0.36 0.27 | −0.10 0.78 | −0.40 0.22 | 0.22 0.38 | −0.27 0.28 | −0.23 0.36 | −0.70 0.00 | 0.32 0.25 | −0.05 0.85 | −0.59 0.02 | ||
| Seizure latency | 0.29 0.24 | 0.69 0.00 | −0.22 0.51 | 0.24 0.47 | −0.07 0.83 | −0.32 0.19 | −0.05 0.84 | −0.22 0.38 | −0.05 0.83 | 0.19 0.46 | 0.37 0.15 | −0.27 0.32 | ||
| Seizure severity | −0.16 0.53 | −0.20 0.42 | 0.16 0.63 | −0.49 0.13 | 0.33 0.33 | −0.09 0.72 | 0.53 0.02 | 0.45 0.06 | 0.41 0.09 | −0.51 0.03 | 0.20 0.42 | 0.15 0.60 | ||
| Seizure duration | −0.43 0.08 | −0.21 0.40 | −0.20 0.73 | −0.42 0.20 | −0.33 0.33 | 0.28 0.27 | −0.23 0.35 | 0.07 0.77 | −0.05 0.83 | 0.30 0.23 | −0.34 0.17 | −0.08 0.75 | ||
| Score | Behavioral Manifestations of Seizures |
|---|---|
| 0 | Normal behavior, no abnormality |
| 1 | immobilization, lying on belly |
| 2 | head nodding, facial, forelimb or hindlimb myoclonus |
| 3 | myoclonic twitches, continuous whole-body myoclonus, tail held up stiffly |
| 4 | clonic rearing, bilateral clonic seizure, falling down on a side |
| 5 | tonic-clonic seizure, falling down on back, wild rushing and jumping |
| Seizure Model | Group | n0 | n | Survival, % |
|---|---|---|---|---|
| A. Single seizure episode (up to 7 injections) | Seizure induction | 10 | 10 | 100 |
| Seizure induction with B1,B6 | 12 | 12 | 100 | |
| B1,B6 (saline and vitamin injections) | 10 | 10 | 100 | |
| Control (saline injections) | 9 | 9 | 100 | |
| B. Seizure episode after PTZ kindling (up to 16 injections) | Kindling + seizure induction | 6 * | 6 | 100 |
| Kindling + seizure induction with B1,B6 | 8 * | 6 | 75 | |
| B1,B6 (saline and vitamin injections) | 7 | 7 | 100 | |
| Control (saline injections) | 6 | 6 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleshin, V.A.; Graf, A.V.; Artiukhov, A.V.; Ksenofontov, A.L.; Zavileyskiy, L.G.; Maslova, M.V.; Bunik, V.I. Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain. Int. J. Mol. Sci. 2023, 24, 12405. https://doi.org/10.3390/ijms241512405
Aleshin VA, Graf AV, Artiukhov AV, Ksenofontov AL, Zavileyskiy LG, Maslova MV, Bunik VI. Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain. International Journal of Molecular Sciences. 2023; 24(15):12405. https://doi.org/10.3390/ijms241512405
Chicago/Turabian StyleAleshin, Vasily A., Anastasia V. Graf, Artem V. Artiukhov, Alexander L. Ksenofontov, Lev G. Zavileyskiy, Maria V. Maslova, and Victoria I. Bunik. 2023. "Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain" International Journal of Molecular Sciences 24, no. 15: 12405. https://doi.org/10.3390/ijms241512405
APA StyleAleshin, V. A., Graf, A. V., Artiukhov, A. V., Ksenofontov, A. L., Zavileyskiy, L. G., Maslova, M. V., & Bunik, V. I. (2023). Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain. International Journal of Molecular Sciences, 24(15), 12405. https://doi.org/10.3390/ijms241512405







