Chemical, Aroma and Pro-Health Characteristics of Kaffir Lime Juice—The Approach Using Optimized HS-SPME-GC-TOFMS, MP-OES, 3D-FL and Physiochemical Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Terpenes’ Content
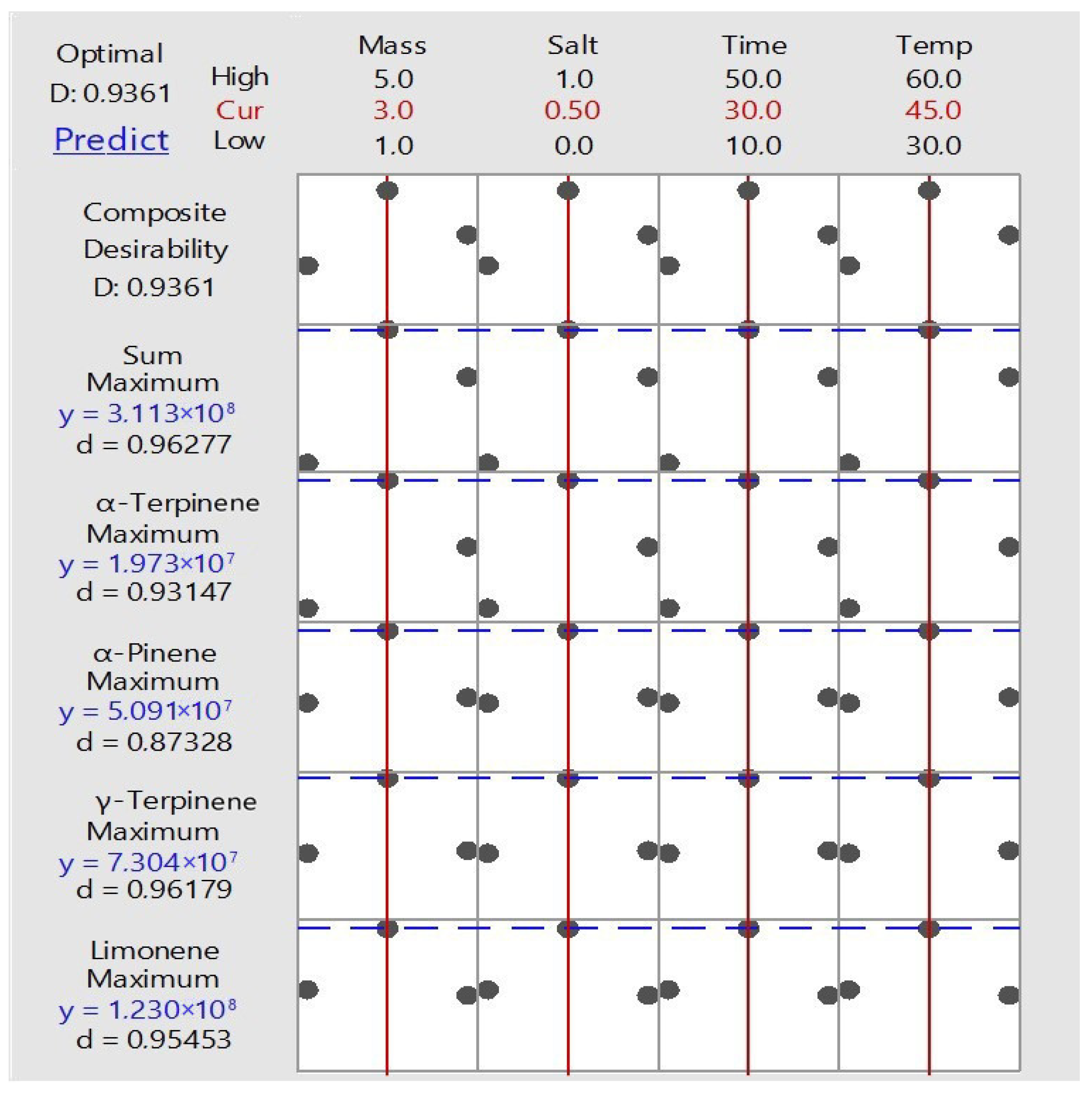
2.1.1. SPME Optimization
2.1.2. Method Validation of HS–SPME GC–TOFMS
2.1.3. Real Samples’ Determination
| Compound | OT µg/g | OAV | Ref. |
|---|---|---|---|
| Camphene | 186 | 0.086545 | [24] |
| Limonene | 0.01 | 54,488.61 | [24] |
| ß-Pinene | 1.5 | 816.8033 | [24] |
| α-Phellandrene | 0.5 | 27.15541 | [25] |
| α-Pinene | 26 | 8.632425 | [26] |
| α-Terpinene | 2.4 | 34.06655 | [27] |
| α-Terpineol | 0.33 | 185.0893 | [28] |
| γ-Terpinene | 1 | 167.8755 | [24] |
| Terpinen-4-ol | 0.34 | 998.0478 | [24] |
| Terpinolene | 0.041 | 272.705 | [24] |
2.2. Physicochemical Characteristics of Kaffir Lime Juice
2.3. Micro- and Macroelements Content in Kaffir Lime Juice
2.4. Fluorescence Properties of Kaffir Lime Juice
3. Materials and Methods
3.1. Samples
3.2. Reagents
3.3. Terpenes’ Analysis
3.3.1. Optimization of Headspace Solid-Phase Microextraction (HS–SPME)
3.3.2. Gas Chromatography
3.4. Physicochemical Characteristics
3.5. Inductively Coupled Plasma—Optical Emission Spectrometry Analysis
- -
- Stage I: 10 min.—temperature rise to 100 °C,
- -
- Stage II: 10 min. at 100 °C,
- -
- Stage III: 10 min.—temperature rise to 180 °C,
- -
- Stage IV: 10 min. at 180 °C,
- -
- Stage V: 10 min.—temperature reduction to 60 °C.
3.6. Three-Dimensional Fluorescence Analysis (3D-FL)
3.7. Data Processing and Presentation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubinska-Szczygeł, M.; Kuczyńska-Łażewska, A.; Rutkowska, M.; Polkowska, Ż.; Katrich, E.; Gorinstein, S. Determination of the Major By-Products of Citrus hystrix Peel and Their Characteristics in the Context of Utilization in the Industry. Molecules 2023, 28, 2596. [Google Scholar] [CrossRef]
- Lim, P.S.M.; Srzednicki, G.; Craske, J.D. Aroma compounds in minor citrus species grown in Australia. Acta Hortic. 2010, 875, 341–350. [Google Scholar]
- Foo-trakul, P.; Watchiradatsatiean, C. Development of Anti-Dandruff Shampoo from Kaffir Lime which is the By-Product of Food Industry. Kasetsart J. (Nat. Sci.) 2005, 39, 725–729. [Google Scholar]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants: From Farm; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030312695. [Google Scholar]
- Lerdau, M.T. The Evolution of Function in Plant Secondary Metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Yuan, M.; Li, S.; Zheng, L.; Zhao, J.; Wang, D.; Wang, C.; Liu, H.; Liu, X.; Fan, M.; Yuan, S.; et al. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Pudlak, D.; Dymerski, T.; Namieśnik, J. Rapid assessment of the authenticity of limequat fruit using the electronic nose and gas chromatography coupled with mass spectrometry. Monatshefte Chem. 2018, 7, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubinska-Szczygieł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T.; Shafreen, R.B.; Weisz, M.; Ezra, A.; Gorinstein, S. Quality of limes juices based on the aroma and antioxidant properties. Food Control 2018, 89, 270–279. [Google Scholar] [CrossRef]
- Beema Shafreen, R.; Dymerski, T.; Namieśnik, J.; Jastrzębski, Z.; Vearasilp, S.; Gorinstein, S. Interaction of human serum albumin with volatiles and polyphenols from some berries. Food Hydrocoll. 2017, 72, 297–303. [Google Scholar] [CrossRef]
- Shafreen, R.B.; Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. Human serum interactions with phenolic and aroma substances of Kaffir (Citrus hystrix) and Key lime (Citrus aurantifolia) juices. J. Lumin. 2018, 201, 115–122. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Karri, V.V.S.R. Experimental design in pesticide extraction methods: A review. Food Chem. 2019, 289, 384–395. [Google Scholar] [CrossRef]
- Kelly, M.; DeSilva, B. Key elements of bioanalytical method validation for macromolecules. AAPS J. 2007, 9, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hoskovec, M.; Grygarová, D.; Cvačka, J.; Streinz, L.; Zima, J.; Verevkin, S.P.; Koutek, B. Determining the vapour pressures of plant volatiles from gas chromatographic retention data. J. Chromatogr. A 2005, 1083, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile Compounds Isolated from Edible Korean Chamchwi (Aster scaber Thunb). J. Agric. Food Chem. 1993, 41, 1693–1697. [Google Scholar] [CrossRef]
- Harangi, J. Retention index calculation without n-alkanes—The virtual carbon number. J. Chromatogr. A 2003, 993, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bäcktorp, C.; Hagvall, L.; Börje, A.; Karlberg, A.T.; Norrby, P.O.; Nyman, G. Mechanism of Air Oxidation of the Fragrance Terpene Geraniol. J. Chem. Theory Comput. 2007, 4, 101–106. [Google Scholar] [CrossRef]
- Taltal, E.L.F.D.E.; Patr, E.L.; Local, D.E.A. Formation of a-terpineol in Citrus Juices, Model and Buffer Solutions. J. Food Sci. 1999, 64, 165–176. [Google Scholar]
- Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Mitchell, P.W.D. Preparation of terpinen-4-ol. European Patent EP0152757B1, 19 October 1988. [Google Scholar]
- Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. A novel analytical approach in the assessment of unprocessed Kaffir lime peel and pulp as potential raw materials for cosmetic applications. Ind. Crop. Prod. 2018, 120, 313–321. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Hebeish, A.; Fouda, M.M.G.; Hamdy, I.A.; EL-Sawy, S.M.; Abdel-Mohdy, F.A. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef] [Green Version]
- Blank, I.; Sen, A.; Grosch, W. Sensory study on the character-impact flavour compounds of dill herb (Anethum graveolens L.). Food Chem. 1992, 43, 337–343. [Google Scholar] [CrossRef]
- ASTM Committee E-18 on Sensory Evaluation of Materials and Products; Fazzalari, F.A. Compilation of Odor and Taste Threshold Values Data; The Society: West Conshohocken, PA, USA, 1978; ISBN 05-048-01036. [Google Scholar]
- Van Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media (Second Enlarged and Revised Edition); Oliemans, Punter & Partners B.V.: Utrecht, The Netherlands, 2011; p. 485. [Google Scholar]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile Constituents of Apricot (Prunus armeniaca). J. Agric. Food Chem. 1990, 38, 471–477. [Google Scholar] [CrossRef]
- Max, B.; Salgado, J.M.; Rodríguez, N.; Cortés, S.; Converti, A.; Domínguez, J.M. Biotechnological production of citric acid. Braz. J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 451–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567. [Google Scholar] [CrossRef]
- Erfando, T.; Cahyani, S.R.; Rita, N. The utilization of citrus hystrix and citrus limon as an organic demulsifier formulation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012145. [Google Scholar] [CrossRef]
- Suciani, N.N.; Said, I.; Diah, A.W.M. Citric Acid Extraction in Citrus hystrix Peels as an Alternative Material for Reducing Water Hardness. J. Akad. Kim. 2021, 10, 53–58. [Google Scholar] [CrossRef]
- Jayasena, V.; Cameron, I. °Brix/Acid ratio as a predictor of consumer acceptability of crimson seedless table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Kimball, D. The Brix/Acid Ratio. In Citrus Processing; Springer: Berlin/Heidelberg, Germany, 1991; pp. 55–65. [Google Scholar] [CrossRef]
- Najwa, F.; Azrina, A.; Fatin Najwa, R.; Azrina, A. Comparison of Vitamin C Content in Citrus Fruits by Titration and High Performance Liquid Chromatography (HPLC ) Methods. Int. Food Res. J. 2017, 24, 726–733. [Google Scholar]
- Prakash, A.; Baskaran, R. Acerola, an untapped functional superfruit: A review on latest frontiers. J. Food Sci. Technol. 2018, 55, 3373. [Google Scholar] [CrossRef]
- Malik, J.; Szakova, J.; Drabek, O.; Balik, J.; Kokoska, L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008, 111, 520–525. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Qin, Z.; Ze, X.; Ali, S.E. Dietary macrominerals: Updated review of their role and orchestration in human nutrition throughout the life cycle with sex differences. Curr. Res. Food Sci. 2023, 6, 100450. [Google Scholar] [CrossRef]
- Grubišić, S.; Kristić, M.; Lisjak, M.; Špoljarić, K.M.; Petrović, S.; Vila, S.; Rebekić, A. Effect of Wheatgrass Juice on Nutritional Quality of Apple, Carrot, Beet, Orange and Lemon Juice. Foods 2022, 11, 445. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164. [Google Scholar] [PubMed]
- Gaiad, J.E.; Hidalgo, M.J.; Villafañe, R.N.; Marchevsky, E.J.; Pellerano, R.G. Tracing the geographical origin of Argentinean lemon juices based on trace element profiles using advanced chemometric techniques. Microchem. J. 2016, 129, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Czech, A.; Zarycka, E.; Yanovych, D.; Zasadna, Z.; Grzegorczyk, I.; Kłys, S. Mineral Content of the Pulp and Peel of Various Citrus Fruit Cultivars. Biol. Trace Elem. Res. 2020, 193, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J. Study of the effect of the hybridisation process on the content of terpenes in oroblanco fruit (Citrus paradisi × Citrus grandis). In Proceedings of the 14th International Students Conference ‘Modern Analytical Chemistry’, Prague, Czech Republic, 20–21 September 2018; Nesměrák, K., Ed.; pp. 236–239. [Google Scholar]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Khan, A. Understanding the interaction between human serum albumin and anti-bacterial/anti-cancer compounds. Curr. Pharm. Des. 2015, 21, 1785–1799. [Google Scholar] [CrossRef]
- Zatón, A.M.L.; Villamor, J.P. Study of heterocycle rings binding to human serum albumin. Chem. Biol. Interact. 2000, 124, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products, Drug Discovery and Therapeutic Medicine; Springer: Berlin/Heidelberg, Germany, 2005; pp. 197–227. [Google Scholar] [CrossRef]
- Daneshgar, P.; Moosavi-Movahedi, A.A.; Norouzi, P.; Ganjali, M.R.; Madadkar-Sobhani, A.; Saboury, A.A. Molecular interaction of human serum albumin with paracetamol: Spectroscopic and molecular modeling studies. Int. J. Biol. Macromol. 2009, 45, 129–134. [Google Scholar] [CrossRef]
- Sun, Y.; Su, B.; Xu, Q.; Liu, R. Insights into the binding of 2-aminobenzothiazole with human serum albumin (HSA): Spectroscopic investigation and molecular modeling studies. Appl. Spectrosc. 2012, 66, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Faisal, Z.; Skaper, R.; Lemli, B.; Bayartsetseg, B.; Hetényi, C.; Gömbös, P.; Szabó, A.; Poór, M. Interaction of the Emerging Mycotoxins Beauvericin, Cyclopiazonic Acid, and Sterigmatocystin with Human Serum Albumin. Biomolecules 2022, 12, 1106. [Google Scholar] [CrossRef]
- Cao, H.; Chen, T.; Shi, Y. Glycation of human serum albumin in diabetes: Impacts on the structure and function. Curr. Med. Chem. 2015, 22, 4–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, B.; Zhang, M.; Shi, J.; Xu, Y. Headspace solid-phase microextraction-gas chromatography-mass spectrometry analysis of the volatile components of longan (Dimocarpus longan Lour.). Eur. Food Res. Technol. 2009, 229, 457–465. [Google Scholar] [CrossRef]
- Dadalı, C.; Elmacı, Y. Optimization of Headspace-Solid Phase Microextraction (HS-SPME) technique for the analysis of volatile compounds of margarine. J. Food Sci. Technol. 2019, 56, 4834. [Google Scholar] [CrossRef]
- Song, N.E.; Lee, J.Y.; Lee, Y.Y.; Park, J.D.; Jang, H.W. Comparison of headspace–SPME and SPME-Arrow–GC–MS methods for the determination of volatile compounds in Korean salt–fermented fish sauce. Appl. Biol. Chem. 2019, 62, 16. [Google Scholar] [CrossRef] [Green Version]
- Šikuten, I.; Štambuk, P.; Kontić, J.K.; Maletić, E.; Tomaz, I.; Preiner, D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. [Google Scholar] [CrossRef]
- Al-Mentafj, H.N. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MD, USA, 2005; pp. 4–5. [Google Scholar]
- Darias-Martín, J.; Lobo-Rodrigo, G.; Hernández-Cordero, J.; Díaz-Díaz, E.; Díaz-Romero, C. Alcoholic beverages obtained from black mulberry. Food Technol. Biotechnol. 2003, 41, 173–176. [Google Scholar]
- Trifunschi, S.; Zugravu, C.A.; Munteanu, M.F.; Borcan, F.; Pogurschi, E.N. Determination of the Ascorbic Acid Content and the Antioxidant Activity of Different Varieties of Vegetables Consumed in Romania, from Farmers and Supermarkets. Sustainability 2022, 14, 13749. [Google Scholar] [CrossRef]
- Kim, Y.M.; Abas, F.; Park, Y.S.; Park, Y.-K.; Ham, K.S.; Kang, S.G.; Lubinska-Szczygeł, M.; Ezra, A.; Gorinstein, S. Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon. Molecules 2021, 26, 4405. [Google Scholar] [CrossRef]




| DOE | Extraction Variables | Extraction Yield (Relative % to Maximum Yield) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ß-Pin | Lim | γ-Ter | α-Pin | α-Terpl | Camp | α-Phell | α-Terpin | Terpin | Sum | |
| 1 | 0 | 0 | 0 | 0 | 11.32 | 94.77 | 95.39 | 89.84 | 27.10 | 73.68 | 3.85 | 100.00 | 62.99 | 97.33 |
| 2 | 0 | 0 | 0 | 0 | 12.51 | 100.00 | 100.00 | 91.41 | 19.48 | 78.41 | 10.09 | 98.58 | 66.52 | 97.76 |
| 3 | 0 | 0 | 0 | 0 | 22.86 | 95.73 | 96.37 | 85.63 | 19.37 | 74.27 | 9.68 | 84.00 | 64.80 | 100.00 |
| 4 | −1 | −1 | 1 | 1 | 54.05 | 44.56 | 65.40 | 27.69 | 24.36 | 36.77 | 7.93 | 27.64 | 54.63 | 67.88 |
| 5 | 1 | 1 | −1 | −1 | 18.30 | 49.33 | 60.59 | 79.08 | 5.99 | 62.09 | 100.00 | 49.61 | 30.03 | 74.78 |
| 6 | 1 | −1 | 1 | −1 | 1.18 | 55.06 | 92.12 | 100.00 | 5.55 | 88.64 | 9.62 | 70.23 | 48.23 | 73.98 |
| 7 | −1 | −1 | −1 | −1 | 7.49 | 73.26 | 72.62 | 57.35 | 1.72 | 50.50 | 5.95 | 28.08 | 29.95 | 65.44 |
| 8 | −1 | 1 | −1 | 1 | 76.39 | 30.37 | 28.10 | 12.88 | 35.95 | 16.46 | 3.94 | 15.24 | 23.17 | 56.01 |
| 9 | 1 | −1 | −1 | 1 | 12.51 | 36.94 | 85.47 | 72.78 | 22.79 | 28.56 | 9.63 | 37.30 | 54.14 | 63.76 |
| 10 | 1 | 1 | 1 | 1 | 5.46 | 72.09 | 73.04 | 60.37 | 100.00 | 100.00 | 14.49 | 59.98 | 100.00 | 87.07 |
| 11 | −1 | 1 | 1 | −1 | 100.00 | 66.73 | 46.91 | 26.80 | 18.09 | 26.92 | 4.98 | 26.80 | 28.73 | 84.90 |
| Sum of Peak Areas of Main Terpenes in Kaffir Lime Juice | |||
|---|---|---|---|
| Mean Square | F-Value | p-Value | |
| Model | 2.92519 × 1015 | 141.91 | 0.007 * |
| Linear | 1.60964 × 1015 | 78.09 | 0.013 * |
| X1 | 8.06023 × 1014 | 39.10 | 0.025 * |
| X2 | 1.25972 × 1015 | 61.11 | 0.016 * |
| X3 | 3.62883 × 1015 | 176.04 | 0.006 * |
| X4 | 7.44009 × 1014 | 36.09 | 0.027 * |
| 2-way interactions | 4.85738 × 1014 | 23.56 | 0.041 * |
| X1X2 | 3.42030 × 1014 | 16.59 | 0.055 |
| X1X3 | 9.73402 × 1013 | 4.72 | 0.162 |
| X1X4 | 1.01785 × 1015 | 49.38 | 0.020 * |
| R2 | 99.82% | ||
| R2 (adjusted) | 99.12% |
| Chemical Compound | R2 |
|---|---|
| ß-Pinene | 98.13% |
| Limonene | 99.74% |
| γ-Terpinene | 99.77% |
| α-Pinene | 99.80% |
| α-Terpineol | 99.45% |
| Camphene | 99.83% |
| α-Phellandrene | 99.69% |
| α-Terpinene | 98.30% |
| Terpinolene | 99.88% |
| Factors | Value | Unit |
|---|---|---|
| X1 sample mass | 3.0 | g |
| X2 mass of salt added | 0.5 | g |
| X3 extraction time | 30 | min |
| X4 extraction temperature | 45 | °C |
| Terpene | RIsample | RIlit. | Ref. | Cond. | a | b | R2 | n | LOQ [µg/g] | LOD [µg/g] | Range | CV | Rec. [%] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | |||||||||||||
| Camphene | 957.91 | 960.64 | [14] | ZB1, 110 °C | 0.0322 | −0.1579 | 0.9956 | 7 | 25 | 8.3 | 25 | 252.5 | 3.07 | 125.88 |
| Limonene | 1031.96 | 1030.3 | [14] | ZB1, 110 °C | 0.0283 | −0.3372 | 0.9954 | 7 | 19 | 6.8 | 19 | 424.8 | 2.85 | 108.92 |
| ß-Pinene | 981.04 | 981.73 | [14] | ZB1, 110 °C | 0.0335 | 0.0125 | 0.9949 | 7 | 39 | 13 | 39 | 1101 | 2.88 | 108.99 |
| α-Phellandrene | 1008.20 | 1006.7 | [14] | ZB1, 110 °C | 0.0324 | −0.1394 | 0.9995 | 7 | 17 | 5.6 | 17 | 213.6 | 6.12 | 46.10 |
| α-Pinene | 942.93 | 941.85 | [14] | ZB1, 110 °C | 0.0284 | −0.1267 | 0.9954 | 7 | 26 | 8.5 | 26 | 433.3 | 0.22 | 94.11 |
| α-Terpinene | 1021.58 | 1020 | [15] | HP-101 | 0.0542 | −0.5572 | 0.9935 | 7 | 31 | 10 | 31 | 211.9 | 9.17 | 93.98 |
| α-Terpineol | 1183.68 | 1179.4 | [16] | DB1, 120 °C | 0.0242 | −0.116 | 0.9913 | 7 | 25 | 8.2 | 25 | 235.9 | 3.33 | 127.02 |
| γ-Terpinene | 1058.39 | 1055.8 | [14] | ZB1, 110 °C | 0.0542 | −0.5572 | 0.9915 | 7 | 23 | 7.8 | 23 | 215.4 | 2.50 | 51.78 |
| Terpinen-4-ol | 1174.64 | 1170.8 | [14] | ZB1, 120 °C | 0.02 | −0.2083 | 0.9944 | 7 | 28 | 9.2 | 28 | 471.21 | 1.97 | 105.45 |
| Terpinolene | 1085.55 | 1079.3 | [14] | ZB1, 120 °C | 0.0264 | 0.0175 | 0.9966 | 7 | 38 | 12.8 | 38 | 172.2 | 3.82 | 38.33 |
| Parameter | Value |
|---|---|
| Total acidity | 7.474 ± 0.050 |
| Ccitric acid | 74.74 ± 0.50 g/kg |
| Brix | 10.35 ± 0.70 |
| pH | 2.406 ± 0.086 |
| °Brix/acidity ratio | 1.385 ± 0.050 |
| Vitamin C | 22.31 ± 0.53 mg/100 mL |
| Peaks | Indices | HSA | KL | Binding to HSA [%] | β-Pinene | Binding to HSA [%] |
|---|---|---|---|---|---|---|
| a | λex/λem (nm/nm) | 227/349 | 231/334 | - | 228/349 | - |
| Int F0 | 765.90 ± 58.14 | 481.24 ± 42.11 | 37.2 ± 3.31 | 497.18 ± 45.71 | 35.09 ± 2.52 | |
| a1 | λex/λem (nm/nm) | - | 233/637 | - | - | - |
| Int F0 | - | 95.40 ± 8.13 | - | - | - | |
| b | λex/λem (nm/nm) | 279/353 | 282/339 | - | 280/354 | - |
| Int F0 | 875.01 ± 79.11 | 723.63 ± 7.63 | 17.3 ± 1.50 | 760.21 ± 68.24 | 13.12 ± 1.21 | |
| b1 | λex/λem (nm/nm) | - | 283/644 | - | - | - |
| Int F0 | - | 129.78 ± 11.2 | - | - | - | |
| c | λex/λem (nm/nm) | - | 347/436 | - | - | - |
| Int F0 | - | 169.44 ± 13.14 | - | - | - |
| Factors | −1 | 0 | 1 | Unit |
|---|---|---|---|---|
| X1 mass of the sample | 1 | 3 | 5 | g |
| X2 mass of salt added | 0 | 0.5 | 1 | g |
| X3 extraction time | 10 | 30 | 50 | min |
| X4 extraction temperature | 30 | 45 | 60 | °C |
| Analyte | Wavelength [nm] | LOD [mg/kg] | LOQ [mg/kg] | Linearity | ||||
|---|---|---|---|---|---|---|---|---|
| Calibration Range [mg/kg] | Number of Meas. Points | Number of Repetitions | R2 | |||||
| min. | max | |||||||
| Na | 568.263 | 1.1 | 3.3 | 10 | 200 | 5 | 4 | 0.9998 |
| K | 766.491 | 0.16 | 0.48 | 2.5 | 20 | 4 | 4 | 0.9997 |
| Fe | 371.993 | 0.33 | 1.0 | 1.0 | 100 | 8 | 4 | 0.9997 |
| Ca | 430.253 | 2.0 | 6.0 | 10 | 250 | 6 | 4 | 0.9995 |
| Pt | 265.945 | 0.075 | 0.23 | 0.40 | 4.0 | 4 | 4 | 0.9994 |
| Zn | 213.857 | 0.19 | 0.58 | 0.58 | 10 | 9 | 4 | 0.9995 |
| Cd | 228.802 | 0.022 | 0.066 | 0.066 | 20 | 8 | 4 | 0.9998 |
| Mg | 279.553 | 0.40 | 1.2 | 1.2 | 40 | 6 | 4 | 0.9996 |
| Pb | 405.781 | 0.012 | 0.035 | 0.050 | 5.0 | 6 | 4 | 0.9999 |
| Cu | 327.395 | 0.026 | 0.077 | 0.30 | 20 | 6 | 4 | 0.9999 |
| Co | 345.351 | 0.012 | 0.035 | 0.050 | 1.0 | 5 | 4 | 0.9999 |
| Ni | 361.939 | 0.0070 | 0.021 | 0.10 | 20 | 7 | 4 | 0.9999 |
| Mo | 386.410 | 0.0060 | 0.018 | 0.018 | 20 | 9 | 4 | 0.9995 |
| Al | 396.152 | 0.088 | 0.26 | 1.0 | 100 | 8 | 4 | 0.9998 |
| Mn | 403.076 | 0.0064 | 0.019 | 0.019 | 1.0 | 5 | 4 | 0.9999 |
| Sr | 421.552 | 0.0045 | 0.013 | 0.013 | 40 | 6 | 4 | 1.0000 |
| Cr | 425.433 | 0.0027 | 0.0082 | 0.01 | 10 | 8 | 4 | 0.9999 |
| Ba | 493.408 | 0.21 | 0.63 | 0.63 | 3.0 | 4 | 4 | 0.9962 |
| V | 437.923 | 0.0057 | 0.017 | 0.017 | 20 | 9 | 4 | 0.9997 |
| Hg | 253.700 | 0.00096 | 0.0029 | 0.0029 | 0.10 | 10 | 3 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubinska-Szczygeł, M.; Polkowska, Ż.; Rutkowska, M.; Gorinstein, S. Chemical, Aroma and Pro-Health Characteristics of Kaffir Lime Juice—The Approach Using Optimized HS-SPME-GC-TOFMS, MP-OES, 3D-FL and Physiochemical Analysis. Int. J. Mol. Sci. 2023, 24, 12410. https://doi.org/10.3390/ijms241512410
Lubinska-Szczygeł M, Polkowska Ż, Rutkowska M, Gorinstein S. Chemical, Aroma and Pro-Health Characteristics of Kaffir Lime Juice—The Approach Using Optimized HS-SPME-GC-TOFMS, MP-OES, 3D-FL and Physiochemical Analysis. International Journal of Molecular Sciences. 2023; 24(15):12410. https://doi.org/10.3390/ijms241512410
Chicago/Turabian StyleLubinska-Szczygeł, Martyna, Żaneta Polkowska, Małgorzata Rutkowska, and Shela Gorinstein. 2023. "Chemical, Aroma and Pro-Health Characteristics of Kaffir Lime Juice—The Approach Using Optimized HS-SPME-GC-TOFMS, MP-OES, 3D-FL and Physiochemical Analysis" International Journal of Molecular Sciences 24, no. 15: 12410. https://doi.org/10.3390/ijms241512410






