Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy
Abstract
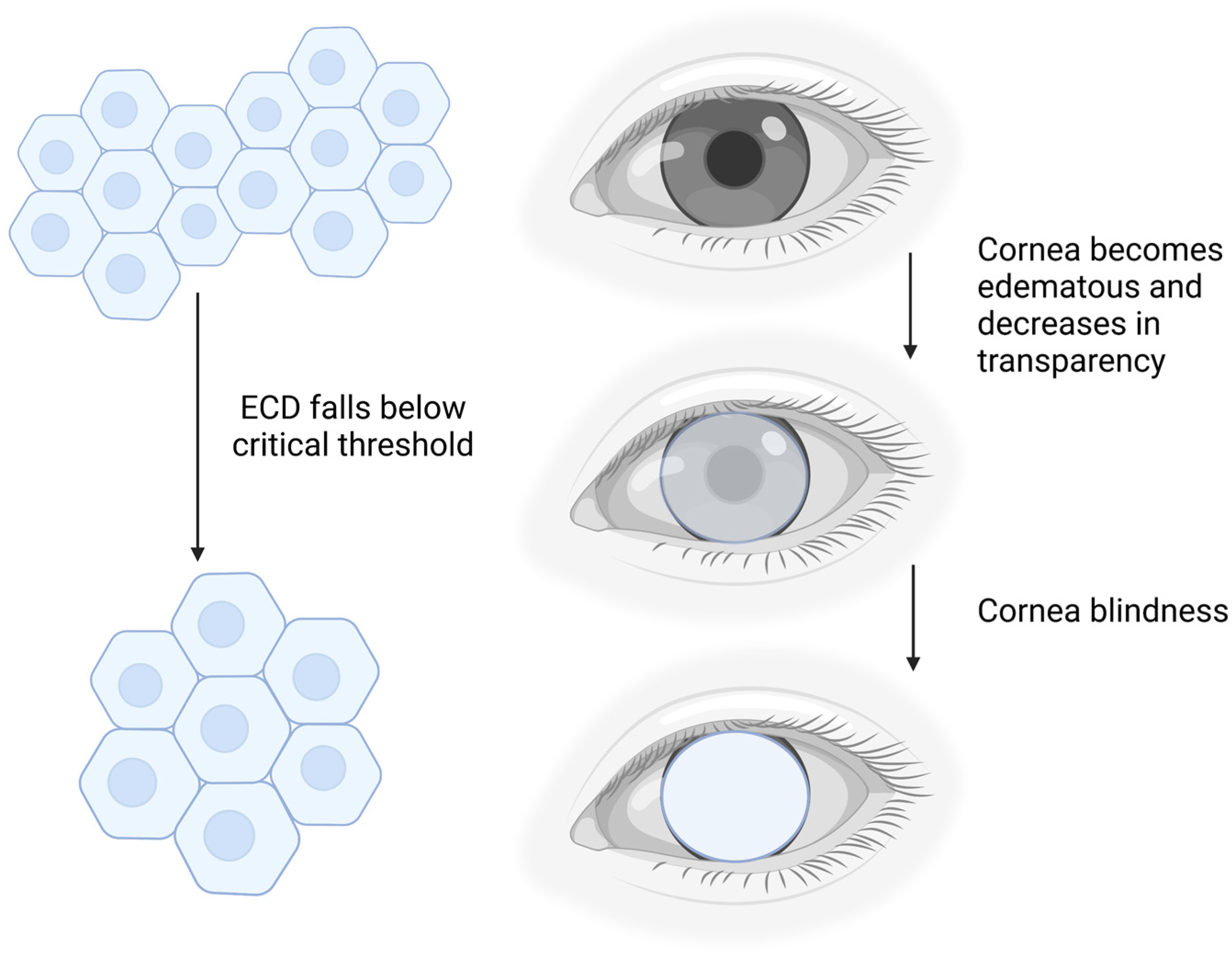
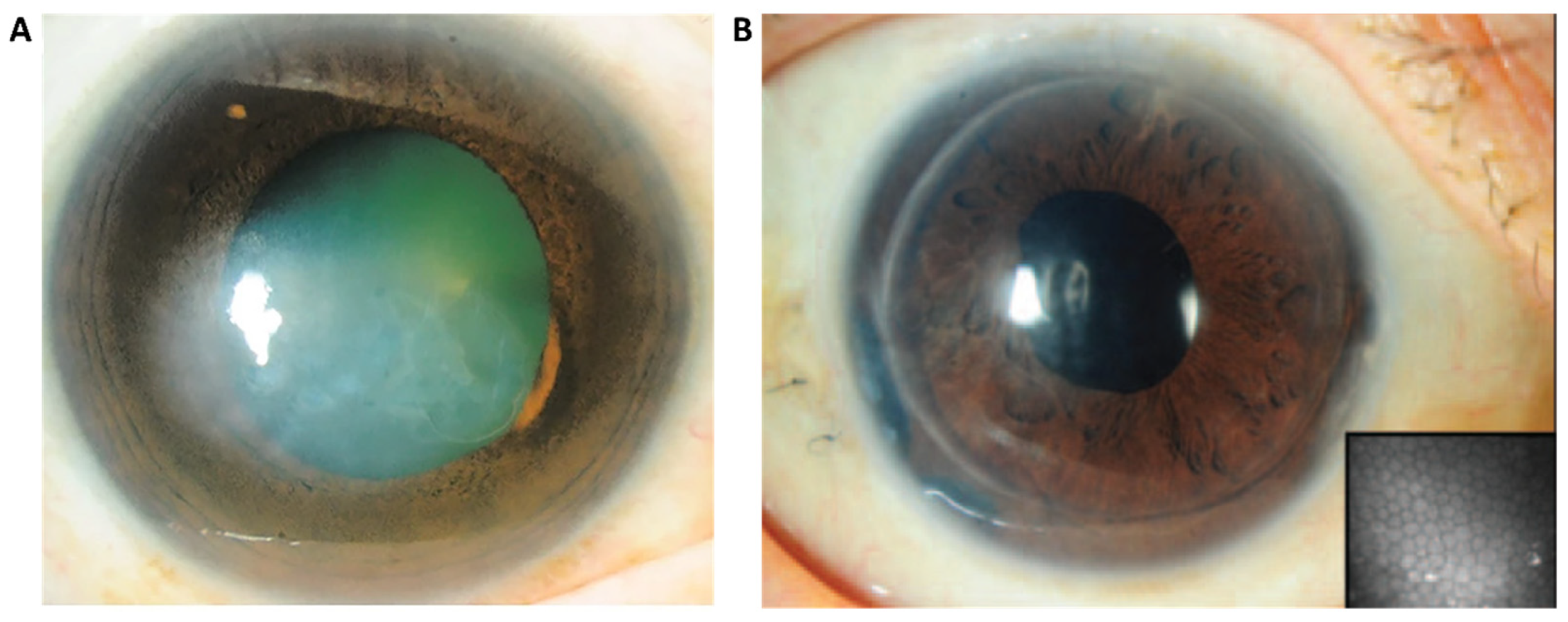
1. Background
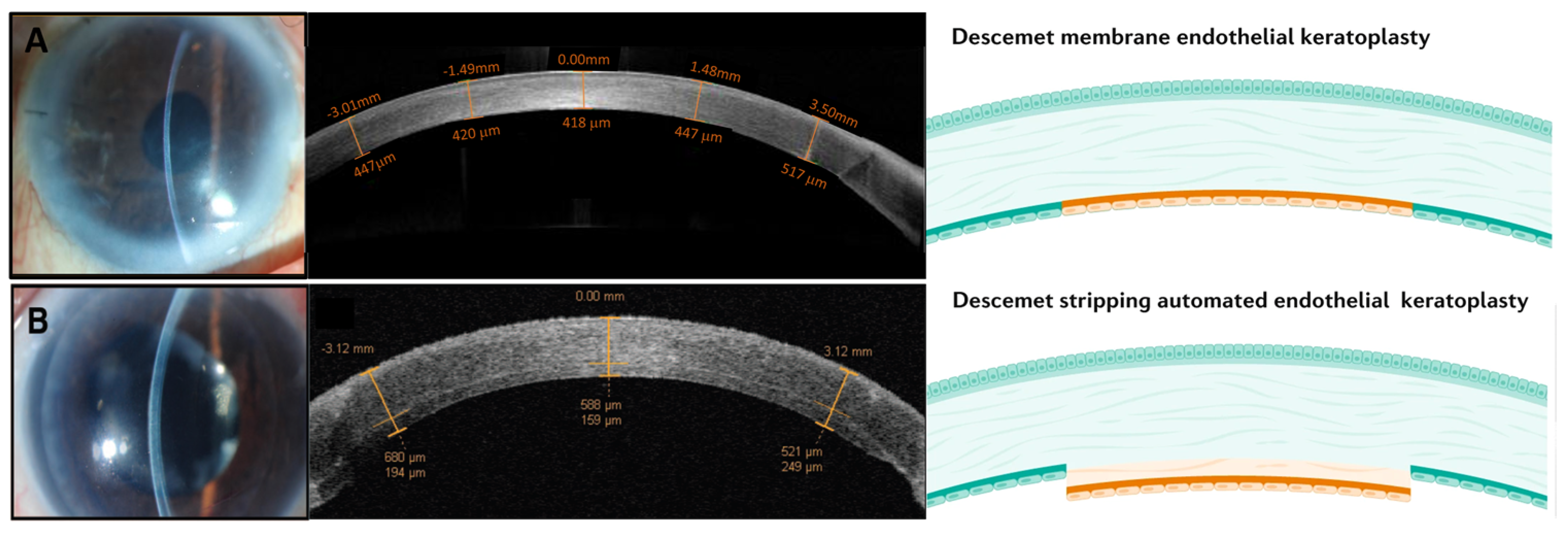
2. Available Methods of Treatment for Corneal Endothelial Dysfunction
3. iPSCs Are Fast Becoming the Holy Grail
4. Progression in the Technology
4.1. Differentiation from iPSCs to NCCs
4.2. Differentiation from NCCs to CECs
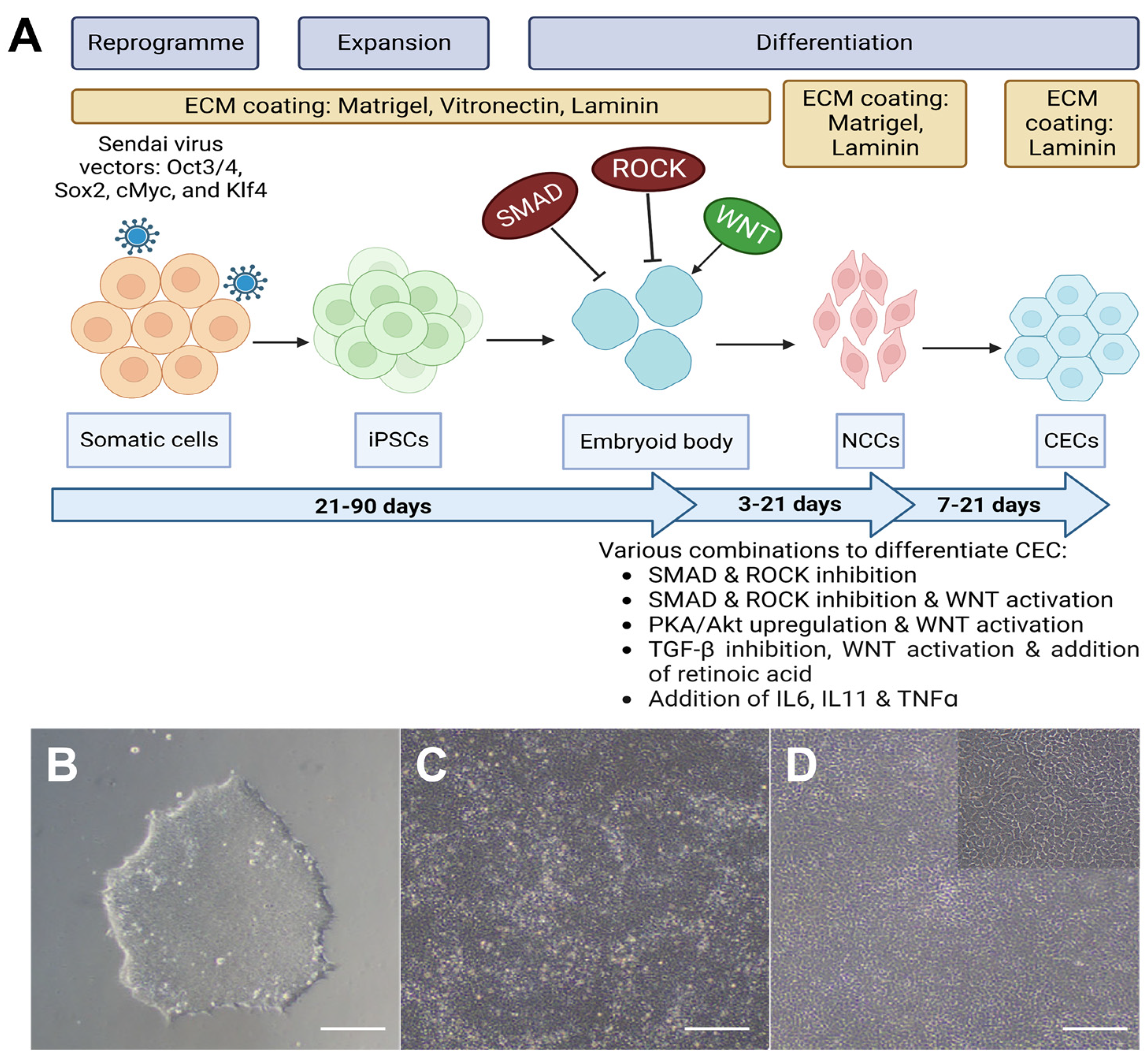
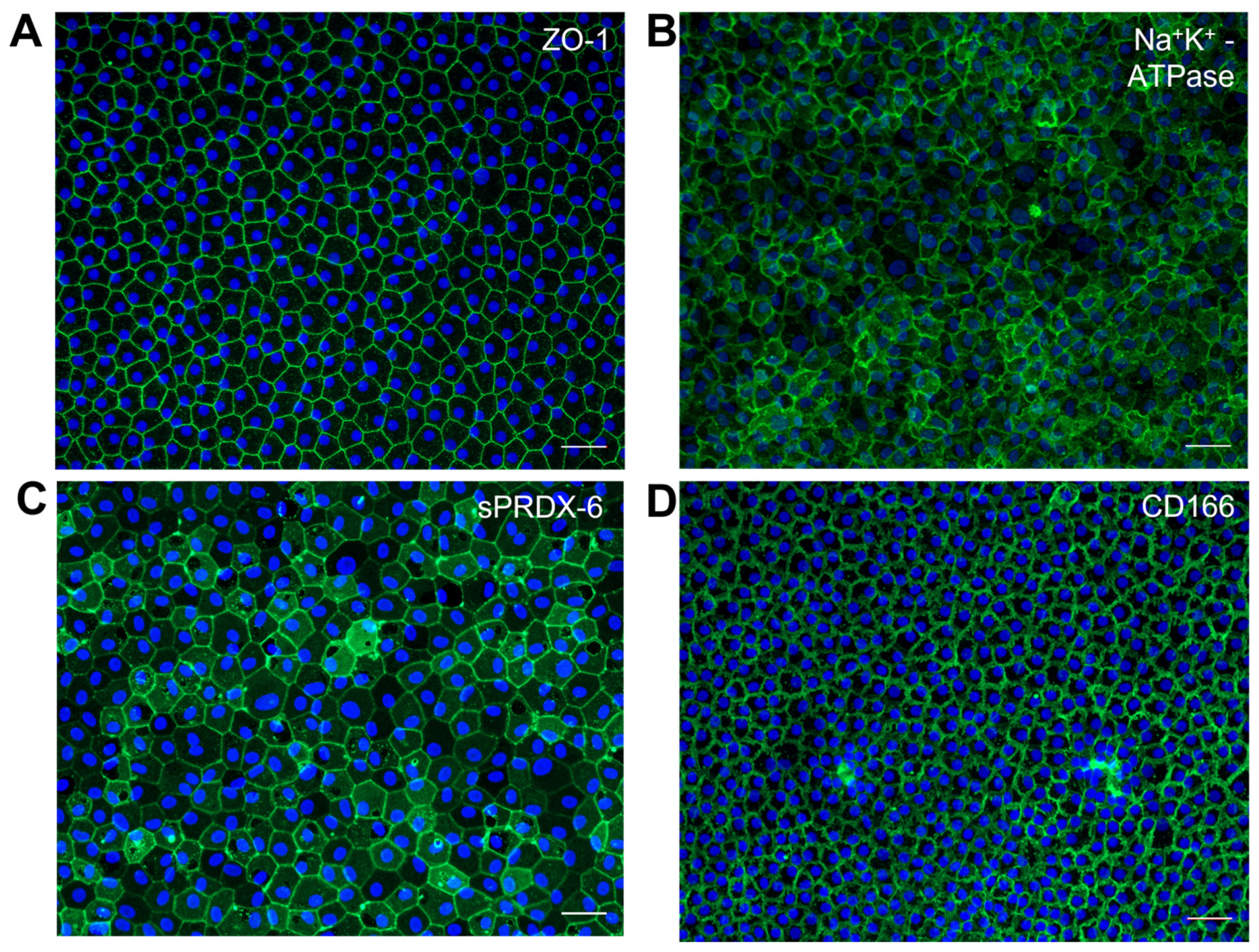
4.3. Characterization of iPSC-Derived CECs
4.4. Evaluating iPSC-Derived CECs in Animal Models
5. Limitations
6. Translational Challenges
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, H.S.; Peh, G.; Neo, D.J.H.; Ang, H.P.; Adnan, K.; Nyein, C.L.; Morales-Wong, F.; Bhogal, M.; Kocaba, V.; Mehta, J.S. A Novel Approach of Harvesting Viable Single Cells from Donor Corneal Endothelium for Cell-Injection Therapy. Cells 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol. 1972, 221, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M. Clinical estimation of corneal endothelial pump function. Trans. Am. Ophthalmol. Soc. 1998, 96, 229–239; discussion 239–242. [Google Scholar]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.P. Dynamic regulation of barrier integrity of the corneal endothelium. Optom. Vis. Sci. 2010, 87, E239–E254. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Srinivas, S.P. Formation and disassembly of adherens and tight junctions in the corneal endothelium: Regulation by actomyosin contraction. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2139–2148. [Google Scholar] [CrossRef]
- Laing, R.A.; Sanstrom, M.M.; Berrospi, A.R.; Leibowitz, H.M. Changes in the corneal endothelium as a function of age. Exp. Eye Res. 1976, 22, 587–594. [Google Scholar] [CrossRef]
- Peh, G.S.; Beuerman, R.W.; Colman, A.; Tan, D.T.; Mehta, J.S. Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 2011, 91, 811–819. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Keane, M.; Coffey, N.; Jones, V.; Lawson, C.; Mills, R.; Williams, K. The Australian Corneal Graft Registry: 2021/22 Report; Flinders University: Bedford Park, Australia, 2022. [Google Scholar]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Kocaba, V.; Weiss, J.S.; Jurkunas, U.V.; Kinoshita, S.; Aldave, A.J.; Mehta, J.S. Corneal dystrophies. Nat. Rev. Dis. Prim. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Peh, G.S.L.; Adnan, K.; Mehta, J.S. Translational and Regulatory Challenges of Corneal Endothelial Cell Therapy: A Global Perspective. Tissue Eng. Part B Rev. 2022, 28, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Hayashi, R.; Koizumi, N. Perspective of Future Potent Therapies for Fuchs Endothelial Corneal Dystrophy. Open Ophthalmol. J. 2018, 12, 154–163. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Peh, G.S.; Chng, Z.; Ang, H.P.; Cheng, T.Y.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transpl. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef] [PubMed]
- EBAA. 2022 Eye Banking Statistical Report. Eye Bank Association of America. Available online: https://restoresight.org/members/publications/statistical-report/ (accessed on 19 May 2023).
- Tan, D.; Ang, M.; Arundhati, A.; Khor, W.B. Development of Selective Lamellar Keratoplasty within an Asian Corneal Transplant Program: The Singapore Corneal Transplant Study (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113, T10. [Google Scholar] [PubMed]
- Peh, G.S.; Adnan, K.; George, B.L.; Ang, H.P.; Seah, X.Y.; Tan, D.T.; Mehta, J.S. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci. Rep. 2015, 5, 9167. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Kubota, H. Development of Corneal-Endothelial Regenerative Medicine Involving Cultivated Human Corneal Endothelial Cell Injection–CHCEC (Identifier: JRCTa050190118); International Clinical Trials Registry Platform: Kyoto, Japan, 2015. [Google Scholar]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Ang, H.P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.Y.; Lin, S.J.; Bhogal, M.; Liu, Y.C.; Tan, D.T.; et al. Regulatory Compliant Tissue-Engineered Human Corneal Endothelial Grafts Restore Corneal Function of Rabbits with Bullous Keratopathy. Sci. Rep. 2017, 7, 14149. [Google Scholar] [CrossRef]
- Toda, M.; Ueno, M.; Hiraga, A.; Asada, K.; Montoya, M.; Sotozono, C.; Kinoshita, S.; Hamuro, J. Production of Homogeneous Cultured Human Corneal Endothelial Cells Indispensable for Innovative Cell Therapy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2011–2020. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef]
- Bandeira, F.; Grottone, G.T.; Covre, J.L.; Cristovam, P.C.; Loureiro, R.R.; Pinheiro, F.I.; Casaroli-Marano, R.P.; Donato, W.; Gomes, J.P. A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach. Int. J. Mol. Sci. 2023, 24, 2982. [Google Scholar] [CrossRef]
- Sanchez-Huerta, V.; Emmecell. Phase 1 Study to Evaluate the Safety and Tolerability of EO1404 in the Treatment of Corneal Edema (Identifier: NCT04191629). ClinicalTrials.gov: México. 2019. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04191629 (accessed on 29 May 2023).
- Arnalich-Montiel, F.; Moratilla, A.; Fuentes-Julián, S.; Aparicio, V.; Cadenas Martin, M.; Peh, G.; Mehta, J.S.; Adnan, K.; Porrua, L.; Pérez-Sarriegui, A.; et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS ONE 2019, 14, e0225480. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef]
- Seow, W.Y.; Kandasamy, K.; Peh, G.S.L.; Mehta, J.S.; Sun, W. Ultrathin, Strong, and Cell-Adhesive Agarose-Based Membranes Engineered as Substrates for Corneal Endothelial Cells. ACS Biomater. Sci. Eng. 2019, 5, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Hsiue, G.H.; Lai, J.Y.; Chen, K.H.; Hsu, W.M. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation 2006, 81, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and functional characterization of corneal endothelial cells during in vitro expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Joyce, N.C. Replication competence and senescence in central and peripheral human corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1387–1396. [Google Scholar] [CrossRef]
- Hatou, S.; Shimmura, S. Review: Corneal endothelial cell derivation methods from ES/iPS cells. Inflamm. Regen. 2019, 39, 19. [Google Scholar] [CrossRef] [PubMed]
- Pappas, J.J.; Yang, P.C. Human ESC vs. iPSC-pros and cons. J. Cardiovasc. Transl. Res. 2008, 1, 96–99. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C.; Noli, L.; Kolundzic, N.; Khalaf, Y. Human embryos from induced pluripotent stem cell-derived gametes: Ethical and quality considerations. Regen. Med. 2017, 12, 681–691. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal cell therapy: With iPSCs, it is no more a far-sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct cell reprogramming: Approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Rao, M.S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Boué, S.; Izpisúa Belmonte, J.C. Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat. Rev. Genet. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Ji, P.; Manupipatpong, S.; Xie, N.; Li, Y. Induced Pluripotent Stem Cells: Generation Strategy and Epigenetic Mystery behind Reprogramming. Stem Cells Int. 2016, 2016, 8415010. [Google Scholar] [CrossRef]
- Rony, I.K.; Baten, A.; Bloomfield, J.A.; Islam, M.E.; Billah, M.M.; Islam, K.D. Inducing pluripotency in vitro: Recent advances and highlights in induced pluripotent stem cells generation and pluripotency reprogramming. Cell Prolif. 2015, 48, 140–156. [Google Scholar] [CrossRef]
- Nakanishi, M.; Otsu, M. Development of Sendai virus vectors and their potential applications in gene therapy and regenerative medicine. Curr. Gene Ther. 2012, 12, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Gunaseeli, I.; Doss, M.X.; Antzelevitch, C.; Hescheler, J.; Sachinidis, A. Induced pluripotent stem cells as a model for accelerated patient- and disease-specific drug discovery. Curr. Med. Chem. 2010, 17, 759–766. [Google Scholar] [CrossRef]
- Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 2018, 557, 619–620. [Google Scholar] [CrossRef]
- Mandai, M.; Kurimoto, Y.; Takahashi, M. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 377, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.M.; Salero, E.; Núñez-Toldrà, R.; Sabater, A.L.; Gil, F.J.; Perez, R.A. Discovering the Potential of Dental Pulp Stem Cells for Corneal Endothelial Cell Production: A Proof of Concept. Front. Bioeng. Biotechnol. 2021, 9, 617724. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.D.; Bohrer, L.R.; Aldrich, B.T.; Greiner, M.A.; Mullins, R.F.; Worthington, K.S.; Tucker, B.A.; Wiley, L.A. Feeder-free differentiation of cells exhibiting characteristics of corneal endothelium from human induced pluripotent stem cells. Biol. Open 2018, 7, bio032102. [Google Scholar] [CrossRef]
- Ali, M.; Khan, S.Y.; Vasanth, S.; Ahmed, M.R.; Chen, R.; Na, C.H.; Thomson, J.J.; Qiu, C.; Gottsch, J.D.; Riazuddin, S.A. Generation and Proteome Profiling of PBMC-Originated, iPSC-Derived Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2437–2444. [Google Scholar] [CrossRef]
- Grönroos, P.; Ilmarinen, T.; Skottman, H. Directed Differentiation of Human Pluripotent Stem Cells towards Corneal Endothelial-Like Cells under Defined Conditions. Cells 2021, 10, 331. [Google Scholar] [CrossRef]
- Hatou, S.; Sayano, T.; Higa, K.; Inagaki, E.; Okano, Y.; Sato, Y.; Okano, H.; Tsubota, K.; Shimmura, S. Transplantation of iPSC-derived corneal endothelial substitutes in a monkey corneal edema model. Stem Cell Res. 2021, 55, 102497. [Google Scholar] [CrossRef]
- Jia, L.; Diao, Y.; Fang, Y.; Yang, K.; Wang, L.; Huang, Y. Methodological study of directed differentiation of pluripotent stem cells into corneal endothelial cells. Ann. Transl. Med. 2022, 10, 482. [Google Scholar] [CrossRef]
- Sun, B.; Bikkuzin, T.; Li, X.; Shi, Y.; Zhang, H. Human-Induced Pluripotent Stem Cells-Derived Corneal Endothelial-Like Cells Promote Corneal Transparency in a Rabbit Model of Bullous Keratopathy. Stem Cells Dev. 2021, 30, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.M.; Sato, Y.; Umekage, M.; Ichisaka, T.; Tsukahara, M.; Takasu, N.; Yamanaka, S. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 2023, 4, 51–66.e10. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Paccola Mesquita, F.C.; Hochman-Mendez, C.; Morrissey, J.; Sampaio, L.C.; Taylor, D.A. Laminin as a Potent Substrate for Large-Scale Expansion of Human Induced Pluripotent Stem Cells in a Closed Cell Expansion System. Stem Cells Int. 2019, 2019, 9704945. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.; Akula, M.; West-Mays, J.A. Corneal development: Role of the periocular mesenchyme and bi-directional signaling. Exp. Eye Res. 2020, 201, 108231. [Google Scholar] [CrossRef]
- Elizabeth, D.H. Development of the Vertebrate Cornea. Int. Rev. Cytol. 1980, 63, 263–322. [Google Scholar]
- Babushkina, A.; Lwigale, P. Periocular neural crest cell differentiation into corneal endothelium is influenced by signals in the nascent corneal environment. Dev. Biol. 2020, 465, 119–129. [Google Scholar] [CrossRef]
- Zhao, J.J.; Afshari, N.A. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6878–6884. [Google Scholar] [CrossRef]
- Zavala, J.; López Jaime, G.R.; Rodríguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef]
- Zacharias, A.L.; Gage, P.J. Canonical Wnt/β-catenin signaling is required for maintenance but not activation of Pitx2 expression in neural crest during eye development. Dev. Dyn. 2010, 239, 3215–3225. [Google Scholar] [CrossRef]
- Bennett, J.L.; Zeiler, S.R.; Jones, K.R. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2996–3005. [Google Scholar]
- Collinson, J.M.; Quinn, J.C.; Hill, R.E.; West, J.D. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev. Biol. 2003, 255, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Gage, P.J.; Zacharias, A.L. Signaling “cross-talk” is integrated by transcription factors in the development of the anterior segment in the eye. Dev. Dyn. 2009, 238, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.A.; Walter, M.A. Genomics and anterior segment dysgenesis: A review. Clin. Exp. Ophthalmol. 2014, 42, 13–24. [Google Scholar] [CrossRef]
- Tokuda, Y.; Okumura, N.; Komori, Y.; Hanada, N.; Tashiro, K.; Koizumi, N.; Nakano, M. Transcriptome dataset of human corneal endothelium based on ribosomal RNA-depleted RNA-Seq data. Sci. Data 2020, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.L.; Kunzevitzky, N.J.; Chiswell, B.P.; Xia, X.; Goldberg, J.L.; Lanza, R. Efficient Generation of Human Embryonic Stem Cell-Derived Corneal Endothelial Cells by Directed Differentiation. PLoS ONE 2015, 10, e0145266. [Google Scholar] [CrossRef]
- Menendez, L.; Kulik, M.J.; Page, A.T.; Park, S.S.; Lauderdale, J.D.; Cunningham, M.L.; Dalton, S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat. Protoc. 2013, 8, 203–212. [Google Scholar] [CrossRef]
- Saika, S.; Liu, C.Y.; Azhar, M.; Sanford, L.P.; Doetschman, T.; Gendron, R.L.; Kao, C.W.; Kao, W.W. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev. Biol. 2001, 240, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ou, Q.; Wang, Z.; Liu, Y.; Hu, S.; Tian, H.; Xu, J.; Gao, F.; Lu, L.; Jin, C.; et al. Small-Molecule Induction Promotes Corneal Endothelial Cell Differentiation From Human iPS Cells. Front. Bioeng. Biotechnol. 2021, 9, 788987. [Google Scholar] [CrossRef]
- Shah, D.D.; Raghani, N.R.; Chorawala, M.R.; Singh, S.; Prajapati, B.G. Harnessing three-dimensional (3D) cell culture models for pulmonary infections: State of the art and future directions. Naunyn Schmiedeberg’s Arch. Pharmacol. 2023, 1–20. [Google Scholar] [CrossRef]
- Wiley, L.A.; Burnight, E.R.; DeLuca, A.P.; Anfinson, K.R.; Cranston, C.M.; Kaalberg, E.E.; Penticoff, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 2016, 6, 30742. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Yanagi, Y.; Usui, T.; Ono, K.; Araie, M.; Amano, S. Sphere therapy for corneal endothelium deficiency in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3128–3135. [Google Scholar] [CrossRef][Green Version]
- Mimura, T.; Yokoo, S.; Araie, M.; Amano, S.; Yamagami, S. Treatment of rabbit bullous keratopathy with precursors derived from cultured human corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3637–3644, Online ahead of print. [Google Scholar] [CrossRef]
- Huang, B.; Blanco, G.; Mercer, R.W.; Fleming, T.; Pepose, J.S. Human corneal endothelial cell expression of Na+,K+-adenosine triphosphatase isoforms. Arch. Ophthalmol. 2003, 121, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Vilas, G.L.; Loganathan, S.K.; Liu, J.; Riau, A.K.; Young, J.D.; Mehta, J.S.; Vithana, E.N.; Casey, J.R. Transmembrane water-flux through SLC4A11: A route defective in genetic corneal diseases. Hum. Mol. Genet. 2013, 22, 4579–4590. [Google Scholar] [CrossRef]
- Loganathan, S.K.; Casey, J.R. Corneal dystrophy-causing SLC4A11 mutants: Suitability for folding-correction therapy. Hum. Mutat. 2014, 35, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Parker, M.D. SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy. Biomed. Res. Int. 2015, 2015, 475392. [Google Scholar] [CrossRef]
- Walckling, M.; Waterstradt, R.; Baltrusch, S. Collagen Remodeling Plays a Pivotal Role in Endothelial Corneal Dystrophies. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Delamere, N.A.; Tamiya, S. Expression, regulation and function of Na,K-ATPase in the lens. Prog. Retin. Eye Res. 2004, 23, 593–615. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Prescott, A.R.; Tholozan, F.M.; Ohno, S.; Quinlan, R.A. Expression and localisation of apical junctional complex proteins in lens epithelial cells. Exp. Eye Res. 2008, 87, 64–70. [Google Scholar] [CrossRef]
- Nepal, N.; Arthur, S.; Haynes, J.; Palaniappan, B.; Sundaram, U. Mechanism of Na-K-ATPase Inhibition by PGE2 in Intestinal Epithelial Cells. Cells 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogerd, B.; Zakaria, N.; Adam, B.; Matthyssen, S.; Koppen, C.; Ní Dhubhghaill, S. Corneal Endothelial Cells Over the Past Decade: Are We Missing the Mark(er)? Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Ding, V.; Chin, A.; Peh, G.; Mehta, J.S.; Choo, A. Generation of novel monoclonal antibodies for the enrichment and characterization of human corneal endothelial cells (hCENC) necessary for the treatment of corneal endothelial blindness. mAbs 2014, 6, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthaei, M.; Ramanan, N.; Grebe, R.; Chakravarti, S.; Speck, C.L.; Kimos, M.; Vij, N.; Eberhart, C.G.; Jun, A.S. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1887–1897. [Google Scholar] [CrossRef]
- Zhang, W.; Ogando, D.G.; Kim, E.T.; Choi, M.J.; Li, H.; Tenessen, J.M.; Bonanno, J.A. Conditionally Immortal Slc4a11-/- Mouse Corneal Endothelial Cell Line Recapitulates Disrupted Glutaminolysis Seen in Slc4a11-/- Mouse Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3723–3731. [Google Scholar] [CrossRef]
- Han, S.B.; Ang, H.P.; Poh, R.; Chaurasia, S.S.; Peh, G.; Liu, J.; Tan, D.T.; Vithana, E.N.; Mehta, J.S. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6179–6189. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Garcia, J.E.; Lozano-Ramirez, J.F.; Zavala, J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res. Notes 2015, 8, 28. [Google Scholar] [CrossRef]
- Doughty, M.J. The cornea and corneal endothelium in the aged rabbit. Optom. Vis. Sci. 1994, 71, 809–818. [Google Scholar] [CrossRef]
- Morita, H. Specular microscopy of corneal endothelial cells in rabbits. J. Vet. Sci. 1995, 57, 273–277. [Google Scholar] [CrossRef][Green Version]
- Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2013.
- Picaud, S.; Dalkara, D.; Marazova, K.; Goureau, O.; Roska, B.; Sahel, J.A. The primate model for understanding and restoring vision. Proc. Natl. Acad. Sci. USA 2019, 116, 26280–26287. [Google Scholar] [CrossRef] [PubMed]
- Kusakawa, S.; Machida, K.; Yasuda, S.; Takada, N.; Kuroda, T.; Sawada, R.; Okura, H.; Tsutsumi, H.; Kawamata, S.; Sato, Y. Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rgnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products. Regen. Ther. 2015, 1, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wesselschmidt, R.L. The teratoma assay: An in vivo assessment of pluripotency. Methods Mol. Biol. 2011, 767, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, E.; Arai, E.; Hatou, S.; Sayano, T.; Taniguchi, H.; Negishi, K.; Kanai, Y.; Sato, Y.; Okano, H.; Tsubota, K.; et al. The Anterior Eye Chamber as a Visible Medium for In Vivo Tumorigenicity Tests. Stem Cells Transl. Med. 2022, 11, 841–849. [Google Scholar] [CrossRef]
- Mikhailova, A.; Ilmarinen, T.; Uusitalo, H.; Skottman, H. Small-molecule induction promotes corneal epithelial cell differentiation from human induced pluripotent stem cells. Stem Cell Rep. 2014, 2, 219–231. [Google Scholar] [CrossRef]
- Fukuta, M.; Nakai, Y.; Kirino, K.; Nakagawa, M.; Sekiguchi, K.; Nagata, S.; Matsumoto, Y.; Yamamoto, T.; Umeda, K.; Heike, T.; et al. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 2014, 9, e112291. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef]
- de Rham, C.; Villard, J. Potential and limitation of HLA-based banking of human pluripotent stem cells for cell therapy. J. Immunol. Res. 2014, 2014, 518135. [Google Scholar] [CrossRef]
- Opelz, G.; Döhler, B. Effect of human leukocyte antigen compatibility on kidney graft survival: Comparative analysis of two decades. Transplantation 2007, 84, 137–143. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Prasad, V.K.; Carter, S.L.; Wagner, J.E.; Baxter-Lowe, L.A.; Wall, D.; Kapoor, N.; Guinan, E.C.; Feig, S.A.; Wagner, E.L.; et al. Results of the Cord Blood Transplantation Study (COBLT): Clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood 2008, 112, 4318–4327. [Google Scholar] [CrossRef]
- Opelz, G.; Döhler, B. Impact of HLA mismatching on incidence of posttransplant non-hodgkin lymphoma after kidney transplantation. Transplantation 2010, 89, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Döhler, B. Pediatric kidney transplantation: Analysis of donor age, HLA match, and posttransplant non-Hodgkin lymphoma: A collaborative transplant study report. Transplantation 2010, 90, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Peacock, S.; Chaudhry, A.N.; Bradley, J.A.; Bolton, E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed]
- McKernan, R.; Watt, F.M. What is the point of large-scale collections of human induced pluripotent stem cells? Nat. Biotechnol. 2013, 31, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef]
- Zhang, W.Y.; de Almeida, P.E.; Wu, J.C. Teratoma formation: A tool for monitoring pluripotency in stem cell research. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Masatoshi, H.; Mieko, S. iPS Cell-Derived Corneal Endothelial Cell Substitutes for Bullous Keratopathy (Identifier: JPRN-jRCTa031210199); International Clinical Trials Registry Platform: Kyoto, Japan, 2021. [Google Scholar]
- Soh, Y.Q.; Peh, G.S.L.; Mehta, J.S. Translational issues for human corneal endothelial tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2425–2442. [Google Scholar] [CrossRef] [PubMed]
- Lo Sardo, V.; Ferguson, W.; Erikson, G.A.; Topol, E.J.; Baldwin, K.K.; Torkamani, A. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 2017, 35, 69–74. [Google Scholar] [CrossRef]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2018, 11, e000043. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.M.; Liu, S.; Figueroa, M.E.; Kulalert, W.; Eminli, S.; Tan, K.Y.; Apostolou, E.; Stadtfeld, M.; Li, Y.; Shioda, T.; et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 2010, 28, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Huo, H.; Loh, Y.H.; Aryee, M.J.; Lensch, M.W.; et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1117–1119. [Google Scholar] [CrossRef]
- Bar-Nur, O.; Russ, H.A.; Efrat, S.; Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 2011, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.J.; Nazor, K.L.; Loring, J.F. Epigenetic regulation of pluripotency and differentiation. Circ. Res. 2014, 115, 311–324. [Google Scholar] [CrossRef]
- Ghosh, Z.; Wilson, K.D.; Wu, Y.; Hu, S.; Quertermous, T.; Wu, J.C. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS ONE 2010, 5, e8975. [Google Scholar] [CrossRef]
- Nishino, K.; Toyoda, M.; Yamazaki-Inoue, M.; Fukawatase, Y.; Chikazawa, E.; Sakaguchi, H.; Akutsu, H.; Umezawa, A. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011, 7, e1002085. [Google Scholar] [CrossRef]
- Garreta, E.; Sanchez, S.; Lajara, J.; Montserrat, N.; Belmonte, J.C.I. Roadblocks in the Path of iPSC to the Clinic. Curr. Transplant. Rep. 2018, 5, 14–18. [Google Scholar] [CrossRef]
- de Almeida, P.E.; Meyer, E.H.; Kooreman, N.G.; Diecke, S.; Dey, D.; Sanchez-Freire, V.; Hu, S.; Ebert, A.; Odegaard, J.; Mordwinkin, N.M.; et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat. Commun. 2014, 5, 3903. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.N.; Westenskow, P.D.; Todorova, D.; Hu, Z.; Lin, T.; Rong, Z.; Kim, J.; He, J.; Wang, M.; et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 353–359. [Google Scholar] [CrossRef]
- Huang, K.; Liu, P.; Li, X.; Chen, S.; Wang, L.; Qin, L.; Su, Z.; Huang, W.; Liu, J.; Jia, B.; et al. Neural progenitor cells from human induced pluripotent stem cells generated less autogenous immune response. Sci. China Life Sci. 2014, 57, 162–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Niederkorn, J.Y. Immunogenicity and immune privilege of corneal allografts. Chem. Immunol. Allergy 2007, 92, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Fujimoto, K.; Okumura, Y.; Zhu, J.; Fujio, K.; Shokirova, H.; Miura, M.; Okano, M.; Funaki, T.; Sung, J.; et al. Novel immunotherapeutic effects of topically administered ripasudil (K-115) on corneal allograft survival. Sci. Rep. 2020, 10, 19817. [Google Scholar] [CrossRef]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. npj Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Kohji, N.; Takeshi, S. First-in-Human Clinical Research of iPS Derived Corneal Epithelial Cell Sheet Transplantation for Patients with Limbal Stem-Cell Deficiency (Identifier: JPRN-jRCTa050190084); International Clinical Trials Registry Platform: Kyoto, Japan, 2019. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, X.Y.; Peh, G.S.L.; Yam, G.H.-F.; Tay, H.G.; Mehta, J.S. Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy. Int. J. Mol. Sci. 2023, 24, 12433. https://doi.org/10.3390/ijms241512433
Ng XY, Peh GSL, Yam GH-F, Tay HG, Mehta JS. Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy. International Journal of Molecular Sciences. 2023; 24(15):12433. https://doi.org/10.3390/ijms241512433
Chicago/Turabian StyleNg, Xiao Yu, Gary S. L. Peh, Gary Hin-Fai Yam, Hwee Goon Tay, and Jodhbir S. Mehta. 2023. "Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy" International Journal of Molecular Sciences 24, no. 15: 12433. https://doi.org/10.3390/ijms241512433
APA StyleNg, X. Y., Peh, G. S. L., Yam, G. H.-F., Tay, H. G., & Mehta, J. S. (2023). Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy. International Journal of Molecular Sciences, 24(15), 12433. https://doi.org/10.3390/ijms241512433






