Potential Harm of IQOS Smoke to Rat Liver
Abstract
1. Introduction
2. Results
2.1. IQOS Does Not Affect Liver Weight
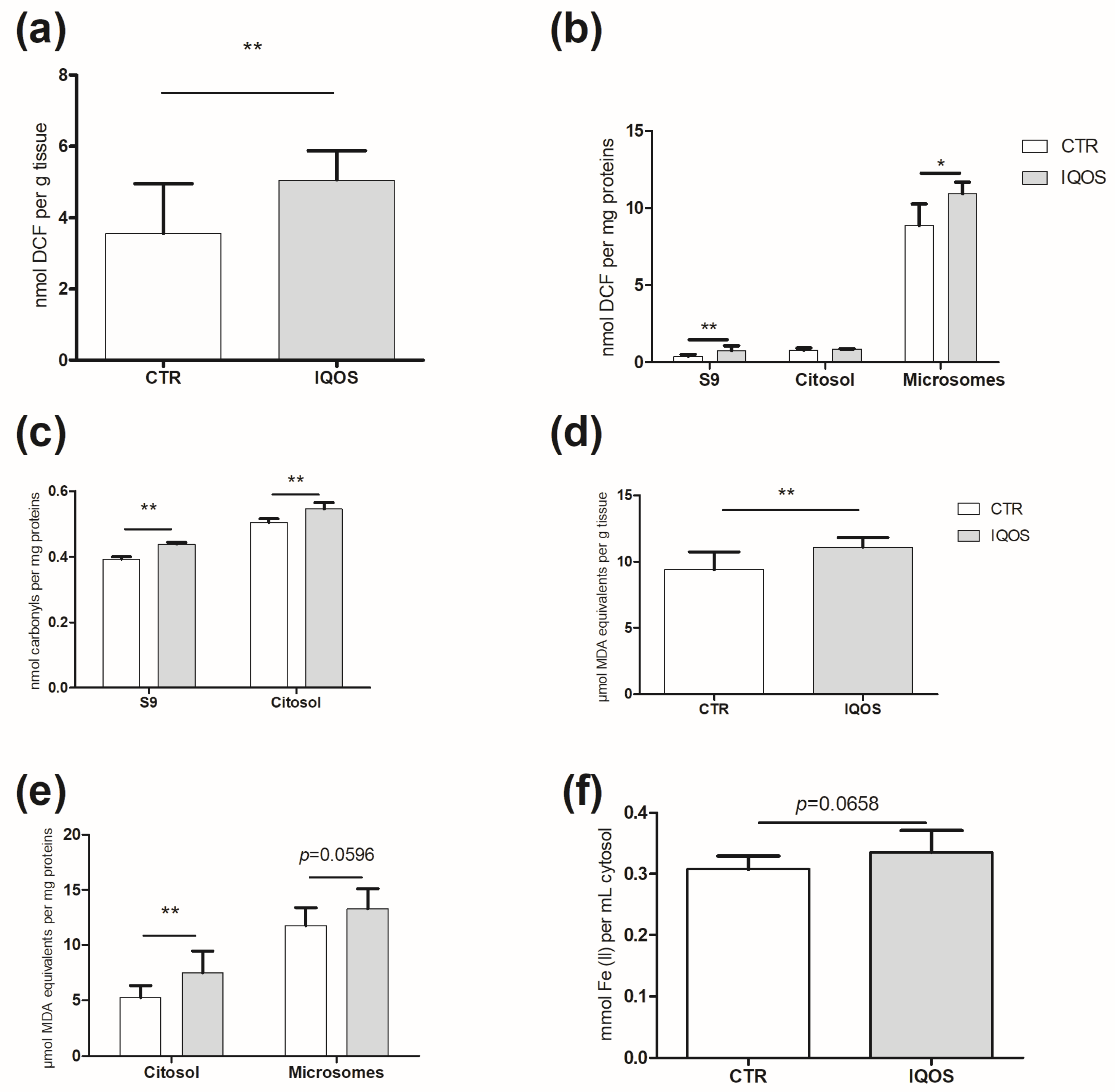
2.2. IQOS Increases Radical Oxygen Species (ROS) and Induces Oxidative Stress in the Liver
2.3. GSH and Antioxidant Enzymatic System
2.4. Phase I Enzymes Are Upregulated by IQOS
2.5. Phase II Enzymes
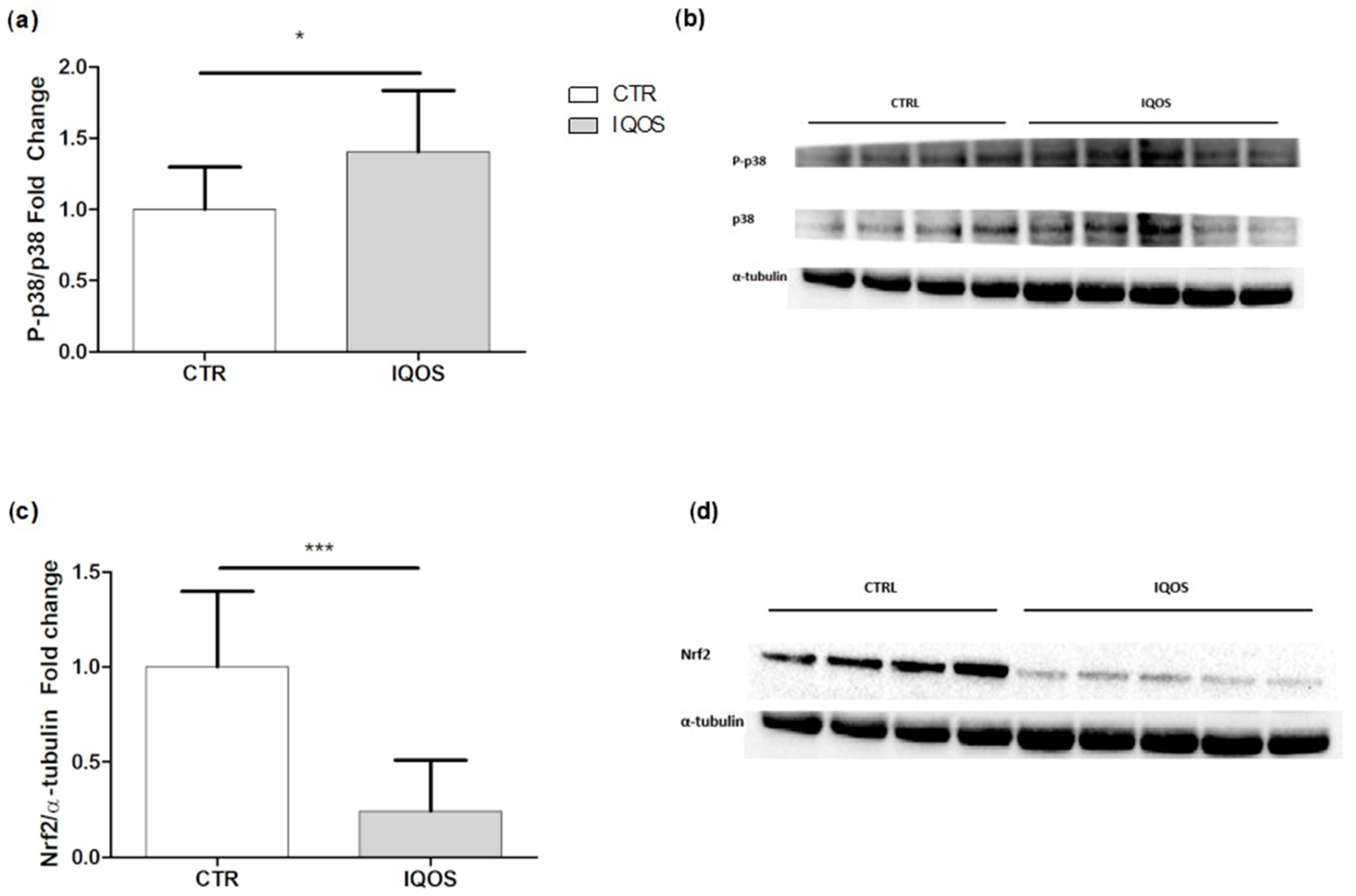
2.6. IQOS Affects Mitogen-Activated Protein Kinase (MAPK) p38 and Erythroid Nuclear Transcription Factor 2 (Nrf2)
2.7. Respiratory Chain Activity and Mitochondrial Content
2.8. IQOS Alters Lipid Profile
2.9. Principal Component Analysis (PCA) Reveals IQOS Impact on Liver Function
3. Discussion
4. Materials and Methods
4.1. Chemical Analysis of IQOS Mainstream Aerosol
4.2. Animal Exposure
4.3. Tissue Collection and Sub-Cellular Fraction Isolation
4.4. Protein Concentration
4.5. DCHF-DA Assay for Reactive Oxygen Species (ROS) Estimation in Tissue Homogenate and Subcellular Fractions
4.6. Ferric Reducing Antioxidant Power (FRAP)
4.7. Thiobarbituric Acid Reactive Substance (TBARs)
4.8. Carbonylated Proteins (CP)
4.9. Antioxidants
4.10. Cyclooxygenase (COX)
4.11. Phase I Enzymes
4.12. Phase II Enzymes
4.13. Western Blot
4.14. Respiration Rate Measurement
4.15. Citrate Synthase and Protein Assay
4.16. Lipid Analysis
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patil, S.; Arakeri, G.; Patil, S.; Ali Baeshen, H.; Raj, T.; Sarode, S.C.; Sarode, G.S.; Awan, K.H.; Gomez, R.; Brennan, P.A. Are electronic nicotine delivery systems (ENDs) helping cigarette smokers quit?-Current evidence. J. Oral Pathol. Med. 2020, 49, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Vivarelli, F.; Turrini, E.; Fimognari, C.; Burattini, S.; Falcieri, E.; Rocchi, M.B.L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M.; et al. The Customizable E-cigarette Resistance Influences Toxicological Outcomes: Lung Degeneration, Inflammation, and Oxidative Stress-Induced in a Rat Model. Toxicol. Sci. 2019, 172, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, F.; Granata, S.; Rullo, L.; Mussoni, M.; Candeletti, S.; Romualdi, P.; Fimognari, C.; Cruz-Chamorro, I.; Carrillo-Vico, A.; Paolini, M.; et al. On the toxicity of e-cigarettes consumption: Focus on pathological cellular mechanisms. Pharmacol. Res. 2022, 182, 106315. [Google Scholar] [CrossRef]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Babot Marquillas, C.; Buschini, A.; Lazzaretti, M.; Marchi, L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Lodovici, M.; et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017, 7, 2028. [Google Scholar] [CrossRef]
- Jaklevic, M.C. First Tobacco Product Receives “Reduced Exposure” Authorization. JAMA 2020, 324, 622. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Miwa, S. Comparison of cytotoxicity of cigarette smoke extract derived from heat-not-burn and combustion cigarettes in human vascular endothelial cells. J. Pharmacol. Sci. 2021, 147, 223–233. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Elias, R.J.; Granata, S.; Mussoni, M.; Burattini, S.; Falcieri, E.; Turrini, E.; Fimognari, C.; et al. Unburned Tobacco Cigarette Smoke Alters Rat Ultrastructural Lung Airways and DNA. Nicotine Tob. Res. 2021, 23, 2127–2134. [Google Scholar] [CrossRef]
- Davis, B.; To, V.; Talbot, P. Comparison of cytotoxicity of IQOS aerosols to smoke from Marlboro Red and 3R4F reference cigarettes. Toxicol. Vitr. 2019, 61, 104652. [Google Scholar] [CrossRef]
- Lüdicke, F.; Picavet, P.; Baker, G.; Haziza, C.; Poux, V.; Lama, N.; Weitkunat, R. Effects of Switching to the Tobacco Heating System 2.2 Menthol, Smoking Abstinence, or Continued Cigarette Smoking on Biomarkers of Exposure: A Randomized, Controlled, Open-Label, Multicenter Study in Sequential Confinement and Ambulatory Settings (Part 1). Nicotine Tob Res. 2018, 20, 161–172. [Google Scholar] [CrossRef]
- Chun, L.; Moazed, F.; Matthay, M.; Calfee, C.; Gotts, J. Possible hepatotoxicity of IQOS. Tob. Control 2018, 27 (Suppl. S1), s39–s40. [Google Scholar] [CrossRef]
- Salman, R.; Talih, S.; El-Hage, R.; Haddad, C.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.A.; Shihadeh, A. Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product. Nicotine Tob. Res. 2019, 21, 1285–1288. [Google Scholar] [CrossRef]
- Katz, M.H. No Smoke-Just Cancer-Causing Chemicals. JAMA Intern. Med. 2017, 177, 1052. [Google Scholar] [CrossRef][Green Version]
- Tran, C.T.; Bosilkovska, M.; de La Bourdonnaye, G.; Blanc, N.; Haziza, C. Reduced levels of biomarkers of exposure in smokers switching to the Carbon-Heated Tobacco Product 1.0: A controlled, randomized, open-label 5-day exposure trial. Sci. Rep. 2020, 10, 19227. [Google Scholar] [CrossRef]
- Kim, K.M.; Ki, S.H. Chapter 28: Nrf2: A Key Regulator of Redox Signaling in Liver Diseases. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 355–374. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Cantelli-Forti, G.; Perocco, P.; Pedulli, G.F.; Abdel-Rahman, S.Z.; Legator, M.S. Co-carcinogenic effect of beta-carotene. Nature 1999, 398, 760–761. [Google Scholar] [CrossRef]
- Paolini, M.; Sapone, A.; Gonzalez, F.J. Parkinson’s disease, pesticides and individual vulnerability. Trends Pharmacol. Sci. 2004, 25, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Pozzetti, L.; Pedulli, G.F.; Cipollone, M.; Mesirca, R.; Cantelli-Forti, G. Paramagnetic resonance in detecting carcinogenic risk from cytochrome P450 overexpression. J. Investig. Med. 1996, 44, 470–473. [Google Scholar]
- Sapone, A.; Canistro, D.; Melega, S.; Moles, R.; Vivarelli, F.; Paolini, M. On enzyme-based anticancer molecular dietary manipulations. J. Biom. Biotechnol. 2012, 2012, 790987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schein, J.R. Cigarette smoking and clinically significant drug interactions. Ann. Pharmacother. 1995, 29, 1139–1148. [Google Scholar] [CrossRef]
- Wong, E.T.; Kogel, U.; Veljkovic, E.; Martin, F.; Xiang, Y.; Boue, S.; Vuillaume, G.; Leroy, P.; Guedj, E.; Rodrigo, G.; et al. Evaluation of the Tobacco Heating System 2.2. Part 4: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects compared with cigarette smoke. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. S2), S59–S81. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations, and Applications. In Measurement of Antioxidant Activity Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Loguercio, C.; De Girolamo, V.; Federico, A.; Feng, S.L.; Crafa, E.; Cataldi, V.; Gialanella, G.; Moro, R.; Del Vecchio Blanco, C. Relationship of blood trace elements to liver damage, nutritional status, and oxidative stress in chronic nonalcoholic liver disease. Biol. Trace Elem. Res. 2001, 81, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Lee, C.S.; Debinski, H.S.; Desmond, P.V.; Mackenzie, P.; Henniker, J.; Rode, J. Human liver injuries and the effects on udp glucuronosyltransferase. Pathology 1992, 24 (Suppl. S1), 21. [Google Scholar] [CrossRef]
- Garbin, U.; Fratta Pasini, A.; Stranieri, C.; Cominacini, M.; Pasini, A.; Manfro, S.; Lugoboni, F.; Mozzini, C.; Guidi, G.; Faccini, G.; et al. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS ONE 2009, 4, e8225. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Kong, A.N. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, J.; Wang, X.; Chen, J.; Geng, S.; Wu, J.; Zhong, C.; Li, X. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 39. [Google Scholar] [CrossRef]
- Masliah-Planchon, J.; Garinet, S.; Pasmant, E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget 2016, 7, 38892–38907. [Google Scholar] [CrossRef]
- Miller, E.; Walczak, A.; Saluk, J.; Ponczek, M.B.; Majsterek, I. Oxidative modification of patient’s plasma proteins and its role in pathogenesis of multiple sclerosis. Clin. Biochem. 2012, 45, 26–30. [Google Scholar] [CrossRef]
- Del Ben, M.; Polimeni, L.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Baratta, F.; Loffredo, L.; Pignatelli, P.; Violi, F.; Angelico, F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014, 14, 81. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.Á.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef]
- Serviddio, G.; Blonda, M.; Bellanti, F.; Villani, R.; Iuliano, L.; Vendemiale, G. Oxysterols and redox signaling in the pathogenesis of non-alcoholic fatty liver disease. Free Radic. Res. 2013, 47, 881–893. [Google Scholar] [CrossRef]
- Garenc, C.; Julien, P.; Levy, E. Oxysterols in biological systems: The gastrointestinal tract, liver, vascular wall and central nervous system. Free Radic. Res. 2010, 44, 47–73. [Google Scholar] [CrossRef]
- Noel, A.; Campen, M.; McKinney, W. The Importance of Conventional Toxicological Metrics of Aerosol Characterization. Toxicol. Sci. 2022, 189, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, P.; Liu, J.; Havel, C.M.; Ibrahim, S.; Derakhshandeh, R.; Jacob Iii, P.; Springer, M.L. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob. Control 2018, 27 (Suppl. S1), s13–s19. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.M.; Xu, B.; Liang, J.; Xie, X.S.; Zhu, Y.; Feldman, J.L. Nicotine delivery to rats via lung alveolar region-targeted aerosol technology produces blood pharmacokinetics resembling human smoking. Nicotine Tob. Res. 2013, 15, 1248–1258. [Google Scholar] [CrossRef]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Costa, G.; Andreotti, C.; Paolini, M. Comparison between in toto peach (Prunus persica L. Batsch) supplementation and its polyphenolic extract on rat liver xenobiotic metabolizing enzymes. Food Chem. Toxicol. 2016, 97, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Barogi, S.; Baracca, A.; Parenti Castelli, G.; Bovina, C.; Formiggini, G.; Marchetti, M.; Solaini, G.; Lenaz, G. Lack of major changes in ATPase activity in mitochondria from liver, heart, and skeletal muscle of rats upon ageing. Mech. Ageing Dev. 1995, 84, 139–150. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Papi, A.; Spisni, E.; Vornoli, A.; Croce, C.M.D.; Longo, V.; Franchi, P.; Filippi, S.; et al. Co-carcinogenic effects of vitamin E in prostate. Sci. Rep. 2019, 9, 11636. [Google Scholar] [CrossRef]
- Cirillo, S.; Urena, J.F.; Lambert, J.D.; Vivarelli, F.; Canistro, D.; Paolini, M.; Cardenia, V.; Rodriguez-Estrada, M.T.; Richie, J.P., Jr.; Elias, R.J. Impact of electronic cigarette heating coil resistance on the production of reactive carbonyls, reactive oxygen species and induction of cytotoxicity in human lung cancer cells in vitro. Regul. Toxicol. Pharmacol. 2019, 109, 104500. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Babot Marquillas, C.; Cirillo, S.; De Nicola, G.R.; Iori, R.; Biagi, G.; Pinna, C.; Gentilini, F.; Pozzo, L.; et al. The combined effect of Sango sprout juice and caloric restriction on metabolic disorders and gut microbiota composition in an obesity model. Int. J. Food Sci. Nutr. 2018, 69, 192–204. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-free conditions. Life Sci. 2019, 228, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Budriesi, R.; Vivarelli, F.; Canistro, D.; Aldini, R.; Babot Marquillas, C.; Corazza, I.; Fato, R.; Cirillo, S.; Bergamini, C.; D’Errico, A.; et al. Liver and intestinal protective effects of Castanea sativa Mill. bark extract in high-fat diet rats. PLoS ONE. 2018, 13, e0201540. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.I.; Hänninen, O. A sensitive kinetic assay for UDPglucuronosyltransferase using 1-naphthol as substrate. Anal. Biochem. 1980, 109, 362–368. [Google Scholar] [CrossRef]
- Barbato, S.; Sgarbi, G.; Gorini, G.; Baracca, A.; Solaini, G. The inhibitor protein (IF1) of the F1F0-ATPase modulates human osteosarcoma cell bioenergetics. J. Biol. Chem. 2015, 290, 6338–6348. [Google Scholar] [CrossRef] [PubMed]
- Baracca, A.; Sgarbi, G.; Mattiazzi, M.; Casalena, G.; Pagnotta, E.; Valentino, M.L.; Moggio, M.; Lenaz, G.; Carelli, V.; Solaini, G. Biochemical phenotypes associated with the mitochondrial ATP6 gene mutations at nt8993. Biochim. Biophys. Acta 2007, 1767, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Solaini, G.; Baracca, A.; Gabellieri, E.; Lenaz, G. Modification of the mitochondrial F1-ATPase epsilon subunit, enhancement of the ATPase activity of the IF1-F1 complex and IF1-binding dependence of the conformation of the epsilon subunit. Biochem. J. 1997, 327 Pt 2, 443–448. [Google Scholar] [CrossRef]
- Bosetti, F.; Baracca, A.; Lenaz, G.; Solaini, G. Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett. 2004, 563, 161–164. [Google Scholar] [CrossRef]
- Aleardi, A.M.; Benard, G.; Augereau, O.; Malgat, M.; Talbot, J.C.; Mazat, J.P.; Letellier, T.; Dachary-Prigent, J.; Solaini, G.C.; Rossignol, R. Gradual alteration of mitochondrial structure and function by beta-amyloids: Importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 2005, 37, 207–225. [Google Scholar] [CrossRef]
- Sgarbi, G.; Liuzzi, F.; Baracca, A.; Solaini, G. Resveratrol preserves mitochondrial function in a human post-mitotic cell model. J. Nutr. Biochem. 2018, 62, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Melega, S.; Canistro, D.; Pagnotta, E.; Iori, R.; Sapone, A.; Paolini, M. Effect of sprout extract from Tuscan black cabbage on xenobiotic-metabolizing and antioxidant enzymes in rat liver. Mutat. Res. 2013, 751, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol Pharmacokinetics, Bioavailability and Its Multiple Effects on the Liver Antioxidant and Xenobiotic-Metabolizing Enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L. DT Diaphorase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1967; Volume 10, pp. 309–317. [Google Scholar] [CrossRef]
- Kumar, K.A.; Reddy, T.C.; Reddy, G.V.; Reddy, D.B.; Mahipal, S.V.; Sinha, S.; Gaikwad, A.N.; Reddanna, P. High-throughput screening assays for cyclooxygenase-2 and 5-lipoxygenase, the targets for inflammatory disorders. Indian J. Biochem. Biophys. 2011, 48, 256–261. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Shintani, H. Determination of Xanthine Oxidase. Pharm. Anal. Acta 2013, S7, 004. [Google Scholar] [CrossRef]
- Aitio, A. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal. Biochem. 1978, 85, 488–491. [Google Scholar] [CrossRef]
- Mazel, P. Experiments Illustrating drug Metabolism In Vitro. In Fundamentals of Drug Metabolism and Drug Disposition; Williams & wilkins: Philadelphia, PA, USA, 1971; pp. 546–582. [Google Scholar]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Cardenia, V.; Zappaterra, M.; Motta, V.; Bosi, P.; Rodriguez-Estrada, M.T.; Trevisi, P. Evaluation of Breed and Parity Order Effects on the Lipid Composition of Porcine Colostrum. J. Agric. Food Chem. 2018, 66, 12911–12920. [Google Scholar] [CrossRef]
- Fieser, L.F.; Fieser, M. Reagents for Organic Chemistry; Wiley: New York, NY, USA, 1967; pp. 191–192. [Google Scholar]
- European Commision (EC). Allegate X. B. Regulation 796/02. Off. J. Eur. Communities 2002, L128, 14–18. [Google Scholar]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Baldacci, E.; Lercker, G. Health-related lipids components of sardine muscle as affected by photooxidation. Food Chem. Toxicol. 2013, 57, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Cardenia, V.; Massimini, M.; Poerio, A.; Venturini, M.C.; Rodriguez-Estrada, M.T.; Vecchia, P.; Lercker, G. Effect of dietary supplementation on lipid photooxidation in beef meat, during storage under commercial retail conditions. Meat Sci. 2015, 105, 126–135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, S.; Canistro, D.; Vivarelli, F.; Morosini, C.; Rullo, L.; Mercatante, D.; Rodriguez-Estrada, M.T.; Baracca, A.; Sgarbi, G.; Solaini, G.; et al. Potential Harm of IQOS Smoke to Rat Liver. Int. J. Mol. Sci. 2023, 24, 12462. https://doi.org/10.3390/ijms241512462
Granata S, Canistro D, Vivarelli F, Morosini C, Rullo L, Mercatante D, Rodriguez-Estrada MT, Baracca A, Sgarbi G, Solaini G, et al. Potential Harm of IQOS Smoke to Rat Liver. International Journal of Molecular Sciences. 2023; 24(15):12462. https://doi.org/10.3390/ijms241512462
Chicago/Turabian StyleGranata, Silvia, Donatella Canistro, Fabio Vivarelli, Camilla Morosini, Laura Rullo, Dario Mercatante, Maria Teresa Rodriguez-Estrada, Alessandra Baracca, Gianluca Sgarbi, Giancarlo Solaini, and et al. 2023. "Potential Harm of IQOS Smoke to Rat Liver" International Journal of Molecular Sciences 24, no. 15: 12462. https://doi.org/10.3390/ijms241512462
APA StyleGranata, S., Canistro, D., Vivarelli, F., Morosini, C., Rullo, L., Mercatante, D., Rodriguez-Estrada, M. T., Baracca, A., Sgarbi, G., Solaini, G., Ghini, S., Fagiolino, I., Sangiorgi, S., & Paolini, M. (2023). Potential Harm of IQOS Smoke to Rat Liver. International Journal of Molecular Sciences, 24(15), 12462. https://doi.org/10.3390/ijms241512462








