Cytokine-Induced Killer Cells in Combination with Heat Shock Protein 90 Inhibitors Functioning via the Fas/FasL Axis Provides Rationale for a Potential Clinical Benefit in Burkitt’s lymphoma
Abstract
:1. Introduction
2. Results
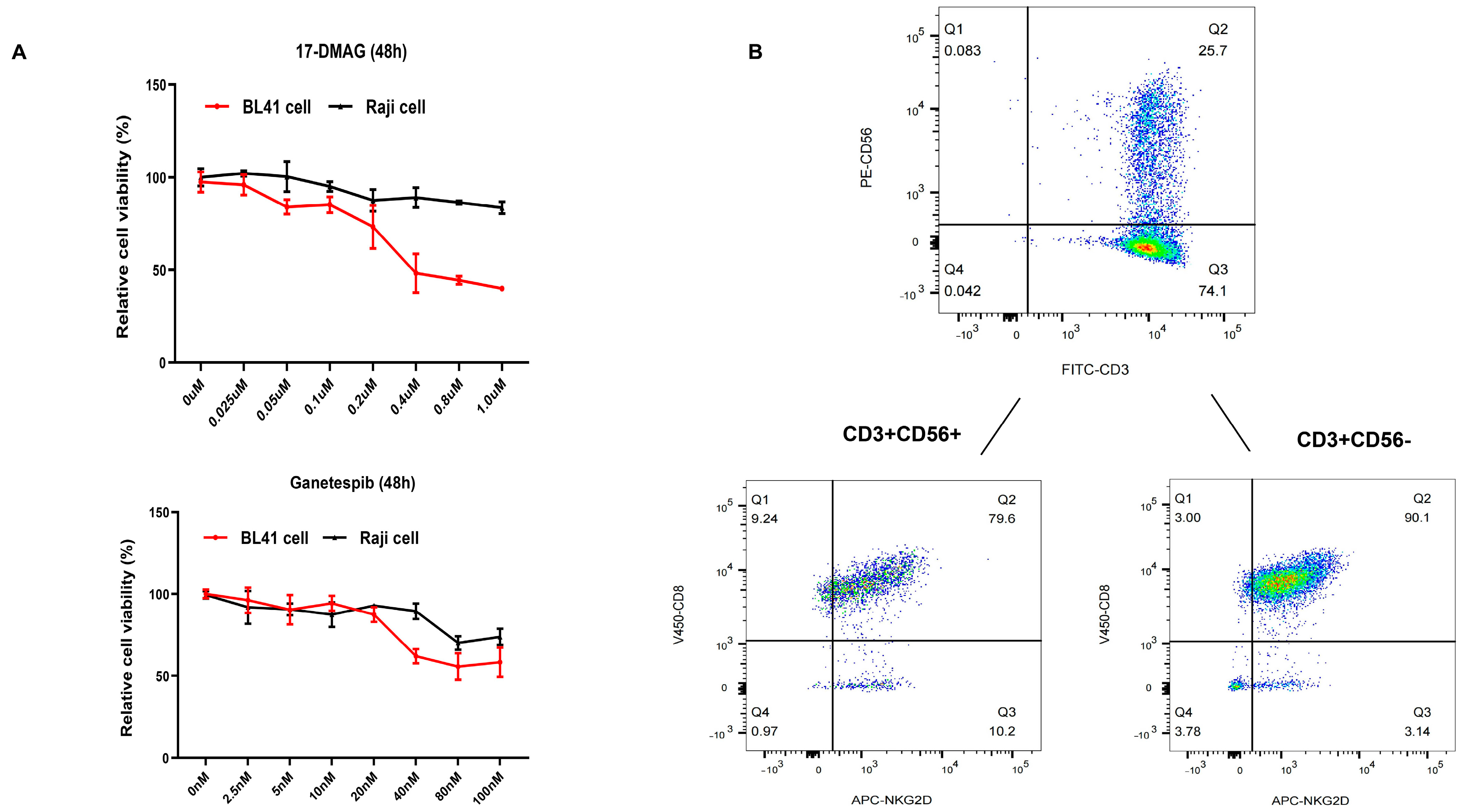
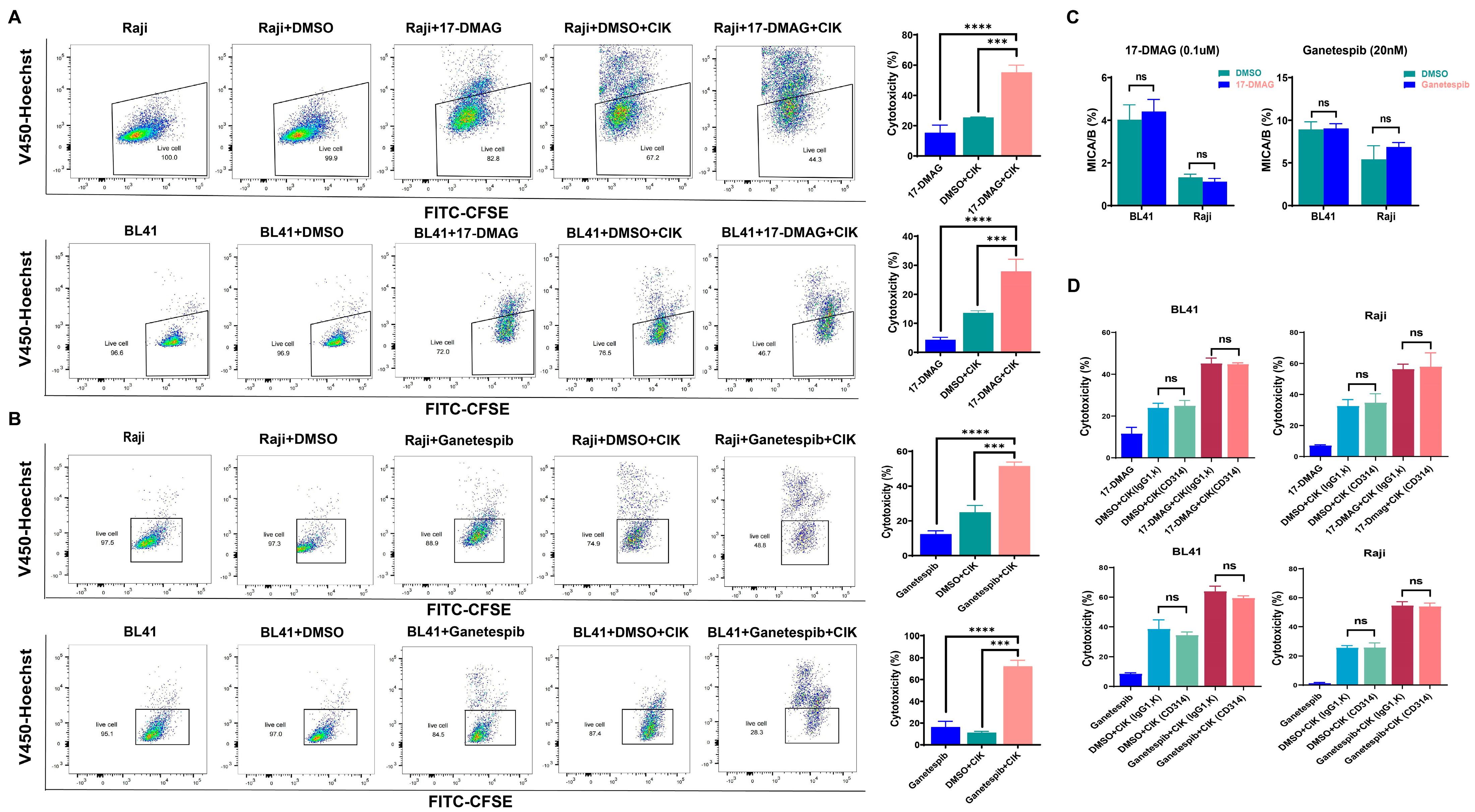
2.1. HSP90 Inhibitors Showed Synergistic Effect with CIK Cells in Burkitt’s Lymphoma Cells
2.2. Cytotoxic Effect of CIK Cells Synergizing with HSP90 Is Independent of the NKD2D/NKG2DL Axis
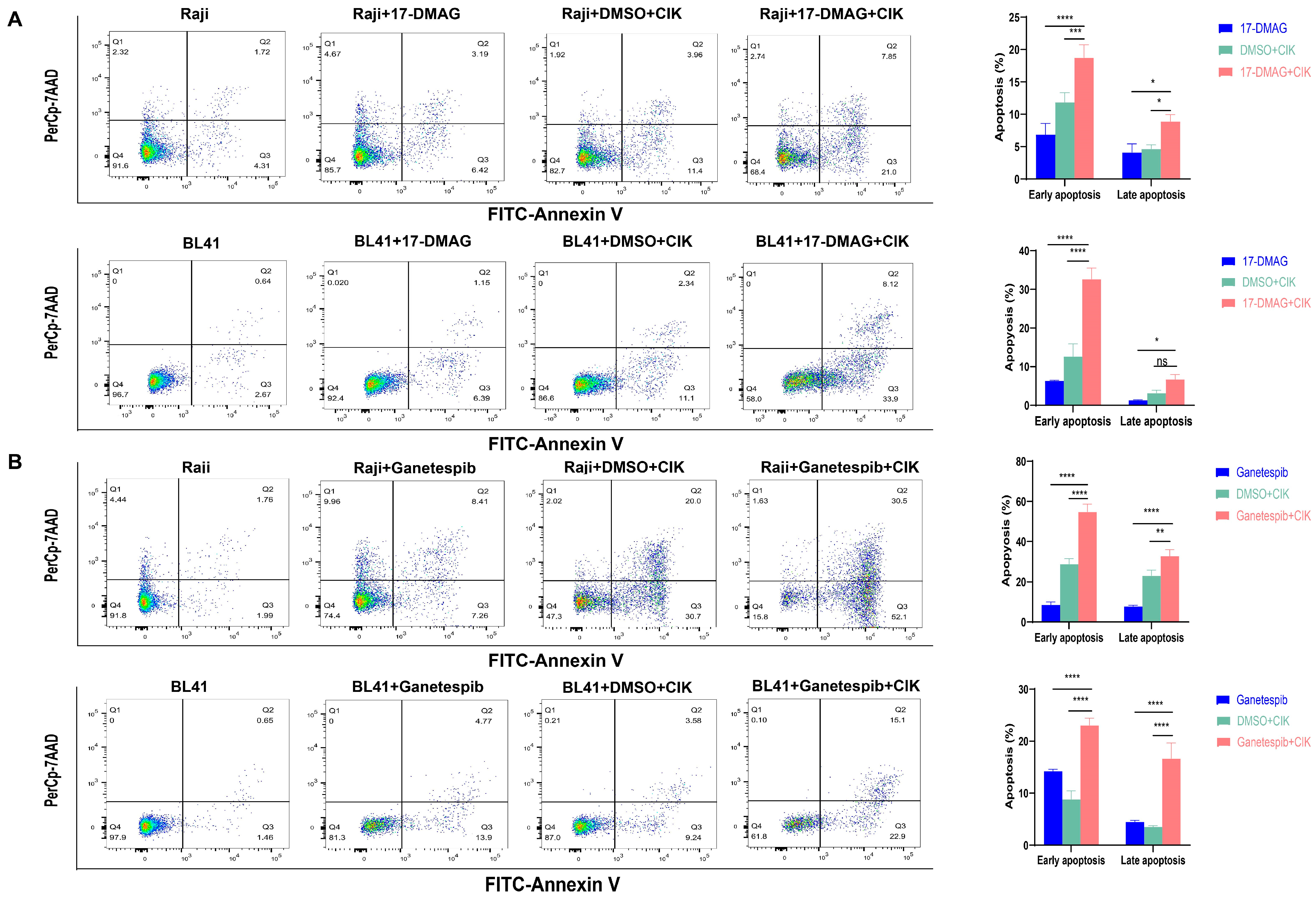
2.3. CIK Cells Combined with HSP90 Inhibitors Primarily Induced Apoptosis in BL
2.4. Increased Expression of Fas May Lead to the Induction of Caspase 3/7-Dependent Apoptosis in BL Cells via HSP90 Inhibitors
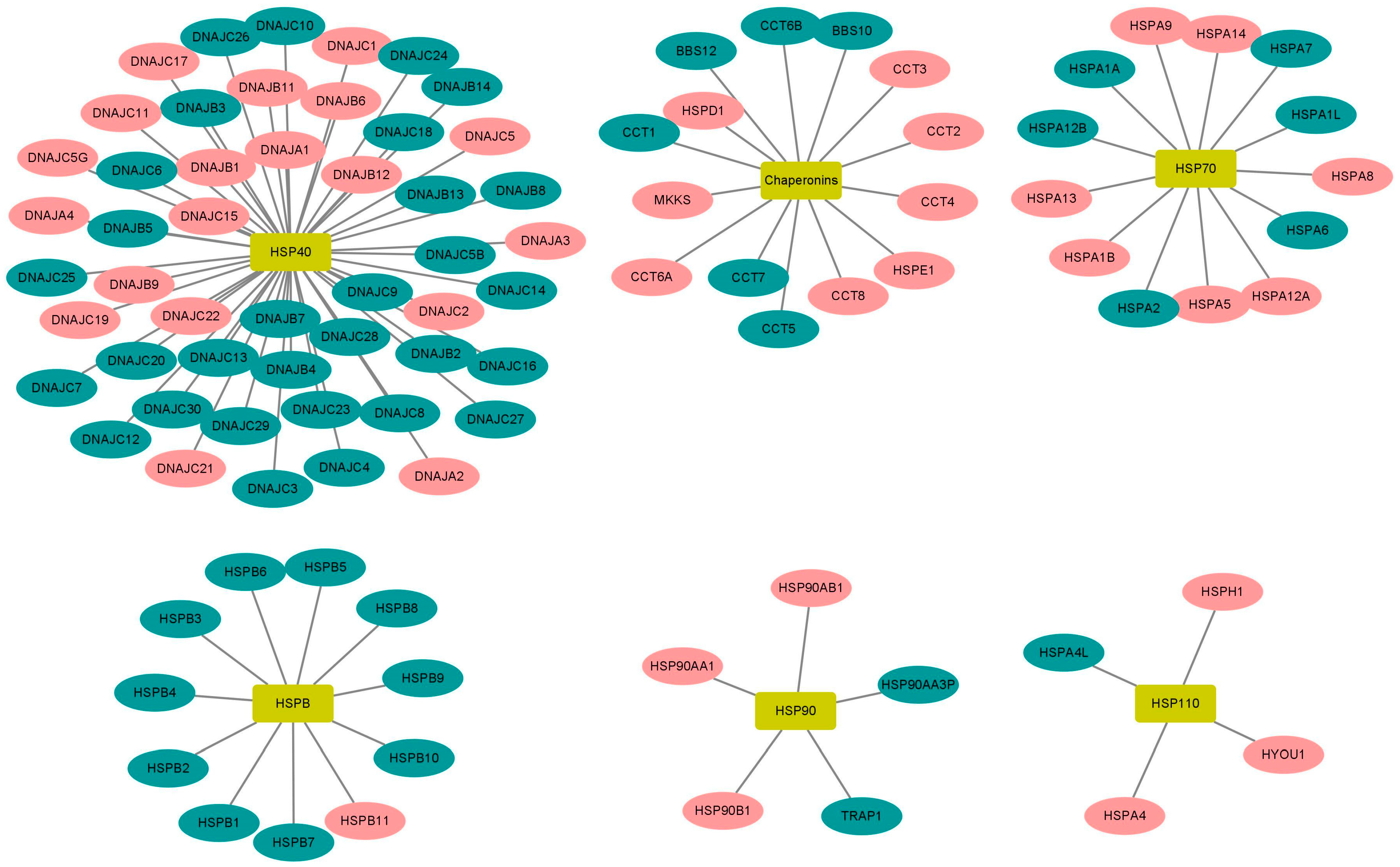
2.5. High Expression of HSP90 Genes Is Significantly Associated with Reduced Overall Survival in BL Patients
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Reagents
4.3. Generation of Cytokine-Induced Killer (CIK) Cells
4.4. CCK8 Assay
4.5. Phenotype Expression of CIK Cells
4.6. Cytotoxicity of CIK Cells
4.7. MICA/B Expression
4.8. The Apoptosis of Burkitt’s Lymphoma Cells
4.9. Caspase 3/7 Activity
4.10. Fas Expression
4.11. Correlation of HSP Genes with BL Patients
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, H.; Luo, K.; Sharma, A.; Sun, X. Prognostic gene expression signature revealed the involvement of mutational pathways in cancer genome. J. Cancer 2020, 11, 4510–4520. [Google Scholar] [CrossRef]
- Sharma, A.; Liu, H.; Herwig-Carl, M.C.; Chand Dakal, T.; Schmidt-Wolf, I.G.H. Epigenetic Regulatory Enzymes: Mutation Prevalence and Coexistence in Cancers. Cancer Investig. 2021, 39, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Dhabhai, B.; Sharma, A.; Maciaczyk, J.; Dakal, T.C. X-Linked Tumor Suppressor Genes Act as Presumed Contributors in the Sex Chromosome-Autosome Crosstalk in Cancers. Cancer Investig. 2022, 40, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Ochiana, S.O.; Dunphy, M.P.; Gerecitano, J.F.; Corben, A.D.; Peter, R.I.; Janjigian, Y.Y.; Gomes-DaGama, E.M.; Koren, J.; Modi, S.; et al. Heat shock protein 90 inhibitors in the treatment of cancer: Current status and future directions. Expert Opin. Investig. Drugs 2014, 23, 611–628. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, T.; Schwartz, S.J.; Sun, D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: Mechanisms, clinical perspective and more potential. Drug Resist. Updat. 2009, 12, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Amatya, E.; Blagg, B.S.J. Recent advances toward the development of Hsp90 C-terminal inhibitors. Bioorg. Med. Chem. Lett. 2023, 80, 129111. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Barrott, J.J.; Haystead, T.A.J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, G.; Khandelwal, A.; Blagg, B.S.J. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-N.; Luo, Y. HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review). Oncol. Rep. 2023, 49, 6. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [Green Version]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.; Linka, R.M.; Reinke, H. HSP90 affects the stability of BMAL1 and circadian gene expression. J. Biol. Rhythms. 2014, 29, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Coskunpinar, E.; Akkaya, N.; Yildiz, P.; Oltulu, Y.M.; Aynaci, E.; Isbir, T.; Yaylim, I. The significance of HSP90AA1, HSP90AB1 and HSP90B1 gene polymorphisms in a Turkish population with non-small cell lung cancer. Anticancer Res. 2014, 34, 753–757. [Google Scholar] [PubMed]
- Liu, K.; Kang, M.; Li, J.; Qin, W.; Wang, R. Prognostic value of the mRNA expression of members of the HSP90 family in non-small cell lung cancer. Exp. Ther. Med. 2019, 17, 2657–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wengert, L.A.; Backe, S.J.; Bourboulia, D.; Mollapour, M.; Woodford, M.R. TRAP1 Chaperones the Metabolic Switch in Cancer. Biomolecules 2022, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, J.R.; Rassidakis, G.Z.; Lin, P.; Atwell, C.; Georgakis, G.V.; Younes, A.; Jones, D.; Medeiros, L.J. Expression of heat-shock protein-90 in non-Hodgkin’s lymphomas. Mod. Pathol. 2005, 18, 1343–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulino-Roth, L.; van Besien, H.J.; Dalton, T.; Totonchy, J.E.; Rodina, A.; Taldone, T.; Bolaender, A.; Erdjument-Bromage, H.; Sadek, J.; Chadburn, A.; et al. Inhibition of Hsp90 Suppresses PI3K/AKT/mTOR Signaling and Has Antitumor Activity in Burkitt Lymphoma. Mol. Cancer Ther. 2017, 16, 1779–1790. [Google Scholar] [CrossRef] [Green Version]
- Walter, R.; Pan, K.-T.; Doebele, C.; Comoglio, F.; Tomska, K.; Bohnenberger, H.; Young, R.M.; Jacobs, L.; Keller, U.; Bönig, H.; et al. HSP90 promotes Burkitt lymphoma cell survival by maintaining tonic B-cell receptor signaling. Blood 2017, 129, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.J.; Zheng, W.; Lee, H.; Young, D.; Lodh, A.; Chadli, A.; van Riggelen, J. Targeting the MYC Oncogene in Burkitt Lymphoma through HSP90 Inhibition. Cancers 2018, 10, 448. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cao, Y.; Zhang, Q.; Liu, W.; Zhou, X.; Ming, X.; Meng, F.; Zhang, Y.; Li, C.; Huang, L.; et al. Chimeric Antigen Receptor-Modified T Cell Immunotherapy for Relapsed and Refractory Adult Burkitt Lymphoma. Front. Immunol. 2022, 13, 879983. [Google Scholar] [CrossRef] [PubMed]
- Zayac, A.S.; Olszewski, A.J. Burkitt lymphoma: Bridging the gap between advances in molecular biology and therapy. Leuk Lymphoma 2020, 61, 1784–1796. [Google Scholar] [CrossRef]
- Sharma, A.; Schmidt-Wolf, I.G.H. 30 years of CIK cell therapy: Recapitulating the key breakthroughs and future perspective. J. Exp. Clin. Cancer Res. 2021, 40, 388. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Lefterova, P.; Mehta, B.A.; Fernandez, L.P.; Huhn, D.; Blume, K.G.; Weissman, I.L.; Negrin, R.S. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp. Hematol. 1993, 21, 1673–1679. [Google Scholar]
- Schmidt-Wolf, I.G.; Lefterova, P.; Johnston, V.; Huhn, D.; Blume, K.G.; Negrin, R.S. Propagation of large numbers of T cells with natural killer cell markers. Br. J. Haematol. 1994, 87, 453–458. [Google Scholar] [CrossRef]
- Pievani, A.; Belussi, C.; Klein, C.; Rambaldi, A.; Golay, J.; Introna, M. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood 2011, 117, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Wang, S.M.; Hui, K.M. Comparative gene expression profiling of cytokine-induced killer cells in response to acute myloid leukemic and acute lymphoblastic leukemic stimulators using oligonucleotide arrays. Exp. Hematol. 2005, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, I.G.; Finke, S.; Trojaneck, B.; Denkena, A.; Lefterova, P.; Schwella, N.; Heuft, H.G.; Prange, G.; Korte, M.; Takeya, M.; et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br. J. Cancer 1999, 81, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sharma, A.; Weiher, H.; Schmid, M.; Kristiansen, G.; Schmidt-Wolf, I.G.H. Clinical Studies on Cytokine-Induced Killer Cells: Lessons from Lymphoma Trials. Cancers 2021, 13, 6007. [Google Scholar] [CrossRef]
- Wei, S.; Yin, D.; Yu, S.; Lin, X.; Savani, M.R.; Du, K.; Ku, Y.; Wu, D.; Li, S.; Liu, H.; et al. Antitumor Activity of a Mitochondrial-Targeted HSP90 Inhibitor in Gliomas. Clin. Cancer Res. 2022, 28, 2180–2195. [Google Scholar] [CrossRef]
- Lang, J.E.; Forero-Torres, A.; Yee, D.; Yau, C.; Wolf, D.; Park, J.; Parker, B.A.; Chien, A.J.; Wallace, A.M.; Murthy, R.; et al. Safety and efficacy of HSP90 inhibitor ganetespib for neoadjuvant treatment of stage II/III breast cancer. NPJ Breast Cancer 2022, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Zhang, B.; Li, L.; Su, Z.; Pu, W.; Zhao, C.; Wei, L.; Lian, P.; Lu, R.; Wang, R.; et al. Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer. Front. Pharmacol. 2021, 12, 724192. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.A.; Kumar, V.; Tailor, D.; Garcia-Marques, F.J.; Hsu, E.-C.; Liu, S.; Bermudez, A.; Kanchustambham, V.; Shankar, V.; Inde, Z.; et al. SU086, an inhibitor of HSP90, impairs glycolysis and represents a treatment strategy for advanced prostate cancer. Cell Rep. Med. 2022, 3, 100502. [Google Scholar] [CrossRef] [PubMed]
- Kryeziu, K.; Bruun, J.; Guren, T.K.; Sveen, A.; Lothe, R.A. Combination therapies with HSP90 inhibitors against colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 240–247. [Google Scholar] [CrossRef]
- Lancet, J.E.; Gojo, I.; Burton, M.; Quinn, M.; Tighe, S.M.; Kersey, K.; Zhong, Z.; Albitar, M.X.; Bhalla, K.; Hannah, A.L.; et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia 2010, 24, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Zhang, L.L.; Wu, W.; Guo, H.; Li, Y.; Sukhanova, M.; Venkataraman, G.; Huang, S.; Zhang, H.; Alikhan, M.; et al. Activation of MYC, a bona fide client of HSP90, contributes to intrinsic ibrutinib resistance in mantle cell lymphoma. Blood Adv. 2018, 2, 2039–2051. [Google Scholar] [CrossRef]
- Diamanti, P.; Cox, C.V.; Moppett, J.P.; Blair, A. Dual targeting of Hsp90 in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2016, 180, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Ikebe, E.; Shimosaki, S.; Hasegawa, H.; Iha, H.; Tsukamoto, Y.; Wang, Y.; Sasaki, D.; Imaizumi, Y.; Miyazaki, Y.; Yanagihara, K.; et al. TAS-116 (pimitespib), a heat shock protein 90 inhibitor, shows efficacy in preclinical models of adult T-cell leukemia. Cancer Sci. 2022, 113, 684–696. [Google Scholar] [CrossRef]
- Chen, T.L.; Harrington, B.; Truxall, J.; Wasmuth, R.; Prouty, A.; Sloan, S.; Lehman, A.M.; Sampath, D.; Orlemans, E.; Baiocchi, R.A.; et al. Preclinical evaluation of the Hsp90 inhibitor SNX-5422 in ibrutinib resistant CLL. J. Hematol Oncol. 2021, 14, 36. [Google Scholar] [CrossRef]
- Kawazoe, A.; Itahashi, K.; Yamamoto, N.; Kotani, D.; Kuboki, Y.; Taniguchi, H.; Harano, K.; Naito, Y.; Suzuki, M.; Fukutani, M.; et al. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clin. Cancer Res. 2021, 27, 6709–6715. [Google Scholar] [CrossRef]
- Gutierrez, M.; Guo, R.; Giaccone, G.; Liu, S.V.; Hao, Z.; Hilton, C.; Hinson, J.M.; Kris, M.G.; Orlemans, E.O.; Drilon, A. Phase 1 multicenter study of the HSP90 inhibitor SNX-5422 plus carboplatin and paclitaxel in patients with lung cancers. Lung Cancer 2021, 162, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.; Hussain, S.; Saad, F.; Garcia, J.; Picus, J.; Ferraldeschi, R.; Crespo, M.; Flohr, P.; Riisnaes, R.; Lin, C.; et al. Pharmacodynamic and Clinical Results from a Phase I/II Study of the HSP90 Inhibitor Onalespib in Combination with Abiraterone Acetate in Prostate Cancer. Clin. Cancer Res. 2019, 25, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- McCaig, A.M.; Cosimo, E.; Leach, M.T.; Michie, A.M. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br. J. Haematol. 2011, 153, 199–211. [Google Scholar] [CrossRef]
- Cavenagh, J.; Oakervee, H.; Baetiong-Caguioa, P.; Davies, F.; Gharibo, M.; Rabin, N.; Kurman, M.; Novak, B.; Shiraishi, N.; Nakashima, D.; et al. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br. J. Cancer 2017, 117, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oki, Y.; Younes, A.; Knickerbocker, J.; Samaniego, F.; Nastoupil, L.; Hagemeister, F.; Romaguera, J.; Fowler, N.; Kwak, L.; Westin, J. Experience with HSP90 inhibitor AUY922 in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica 2015, 100, e272–e274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalla Pietà, A.; Cappuzzello, E.; Palmerini, P.; Ventura, A.; Visentin, A.; Astori, G.; Chieregato, K.; Mozzo, V.; Perbellini, O.; Tisi, M.C.; et al. Innovative therapeutic strategy for B-cell malignancies that combines obinutuzumab and cytokine-induced killer cells. J. Immunother Cancer 2021, 9, e002475. [Google Scholar] [CrossRef]
- Stephan, D.; Weiher, H.; Schmidt-Wolf, I.G.H. CIK Cells and HDAC Inhibitors in Multiple Myeloma. Int. J. Mol. Sci. 2017, 18, 945. [Google Scholar] [CrossRef] [Green Version]
- Linn, Y.C.; Lau, S.K.J.; Liu, B.H.; Ng, L.H.; Yong, H.X.; Hui, K.M. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology 2009, 126, 423–435. [Google Scholar] [CrossRef]
- Wu, X.; Sharma, A.; Oldenburg, J.; Weiher, H.; Essler, M.; Skowasch, D.; Schmidt-Wolf, I.G.H. NKG2D Engagement Alone Is Sufficient to Activate Cytokine-Induced Killer Cells While 2B4 Only Provides Limited Coactivation. Front. Immunol. 2021, 12, 731767. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z. High HSPA8 expression predicts adverse outcomes of acute myeloid leukemia. BMC Cancer 2021, 21, 475. [Google Scholar] [CrossRef]
- Wang, S.-F.; Huang, K.-H.; Tseng, W.-C.; Lo, J.-F.; Li, A.F.-Y.; Fang, W.-L.; Chen, C.-F.; Yeh, T.-S.; Chang, Y.-L.; Chou, Y.-C.; et al. DNAJA3/Tid1 Is Required for Mitochondrial DNA Maintenance and Regulates Migration and Invasion of Human Gastric Cancer Cells. Cancers 2020, 12, 3463. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhu, X.; Liang, J.; Wei, M.; Huang, S.; Pan, X. Upregulation of HSPA1A/HSPA1B/HSPA7 and Downregulation of HSPA9 Were Related to Poor Survival in Colon Cancer. Front. Oncol. 2021, 11, 749673. [Google Scholar] [CrossRef]
- Guo, H.; Deng, Q.; Wu, C.; Hu, L.; Wei, S.; Xu, P.; Kuang, D.; Liu, L.; Hu, Z.; Miao, X.; et al. Variations in HSPA1B at 6p21.3 are associated with lung cancer risk and prognosis in Chinese populations. Cancer Res. 2011, 71, 7576–7586. [Google Scholar] [CrossRef] [Green Version]
- Losmanova, T.; Zens, P.; Scherz, A.; Schmid, R.A.; Tschan, M.P.; Berezowska, S. Chaperone-Mediated Autophagy Markers LAMP2A and HSPA8 in Advanced Non-Small Cell Lung Cancer after Neoadjuvant Therapy. Cells 2021, 10, 2731. [Google Scholar] [CrossRef]
- Yang, C.; Shao, Y.; Wang, X.; Wang, J.; Wang, P.; Huang, C.; Wang, W.; Wang, J. The Effect of the Histone Chaperones HSPA8 and DEK on Tumor Immunity in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 2653. [Google Scholar] [CrossRef]
- Winkler, R.; Mägdefrau, A.-S.; Piskor, E.-M.; Kleemann, M.; Beyer, M.; Linke, K.; Hansen, L.; Schaffer, A.-M.; Hoffmann, M.E.; Poepsel, S.; et al. Targeting the MYC interaction network in B-cell lymphoma via histone deacetylase 6 inhibition. Oncogene 2022, 41, 4560–4572. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, Y.-M.; Hong, S. DNAJB9 suppresses the metastasis of triple-negative breast cancer by promoting FBXO45-mediated degradation of ZEB1. Cell Death Dis. 2021, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-W.; Chao, M.-W.; Tu, H.-J.; Chen, L.-C.; Hsu, K.-C.; Liou, J.-P.; Yang, C.-R.; Yen, S.-C.; HuangFu, W.-C.; Pan, S.-L. A novel dual HDAC and HSP90 inhibitor, MPT0G449, downregulates oncogenic pathways in human acute leukemia in vitro and in vivo. Oncogenesis 2021, 10, 39. [Google Scholar] [CrossRef]
- Li, Y.; Sharma, A.; Wu, X.; Weiher, H.; Skowasch, D.; Essler, M.; Schmidt-Wolf, I.G.H. A Combination of Cytokine-Induced Killer Cells With PD-1 Blockade and ALK Inhibitor Showed Substantial Intrinsic Variability Across Non-Small Cell Lung Cancer Cell Lines. Front. Oncol. 2022, 12, 713476. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, F.; Wang, Y.; Sharma, A.; Yang, Y.; Liu, H.; Essler, M.; Jaehde, U.; Schmidt-Wolf, I.G.H. Cytokine-Induced Killer Cells in Combination with Heat Shock Protein 90 Inhibitors Functioning via the Fas/FasL Axis Provides Rationale for a Potential Clinical Benefit in Burkitt’s lymphoma. Int. J. Mol. Sci. 2023, 24, 12476. https://doi.org/10.3390/ijms241512476
Ge F, Wang Y, Sharma A, Yang Y, Liu H, Essler M, Jaehde U, Schmidt-Wolf IGH. Cytokine-Induced Killer Cells in Combination with Heat Shock Protein 90 Inhibitors Functioning via the Fas/FasL Axis Provides Rationale for a Potential Clinical Benefit in Burkitt’s lymphoma. International Journal of Molecular Sciences. 2023; 24(15):12476. https://doi.org/10.3390/ijms241512476
Chicago/Turabian StyleGe, Fangfang, Yulu Wang, Amit Sharma, Yu Yang, Hongde Liu, Markus Essler, Ulrich Jaehde, and Ingo G. H. Schmidt-Wolf. 2023. "Cytokine-Induced Killer Cells in Combination with Heat Shock Protein 90 Inhibitors Functioning via the Fas/FasL Axis Provides Rationale for a Potential Clinical Benefit in Burkitt’s lymphoma" International Journal of Molecular Sciences 24, no. 15: 12476. https://doi.org/10.3390/ijms241512476






