Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet?
Abstract
:1. Introduction
1.1. Gases as Signaling Molecules
1.2. Gasotransmitter ID
- Being gaseous at temperature and pressure compatible with life;
- Dimensions and physicochemical features (low molar mass, between 28 and 34 g/mol; low solubility in water, between 0.004 and 3.980 g/L) allowing permeability through cellular outer and inner membranes (no receptor required for action);
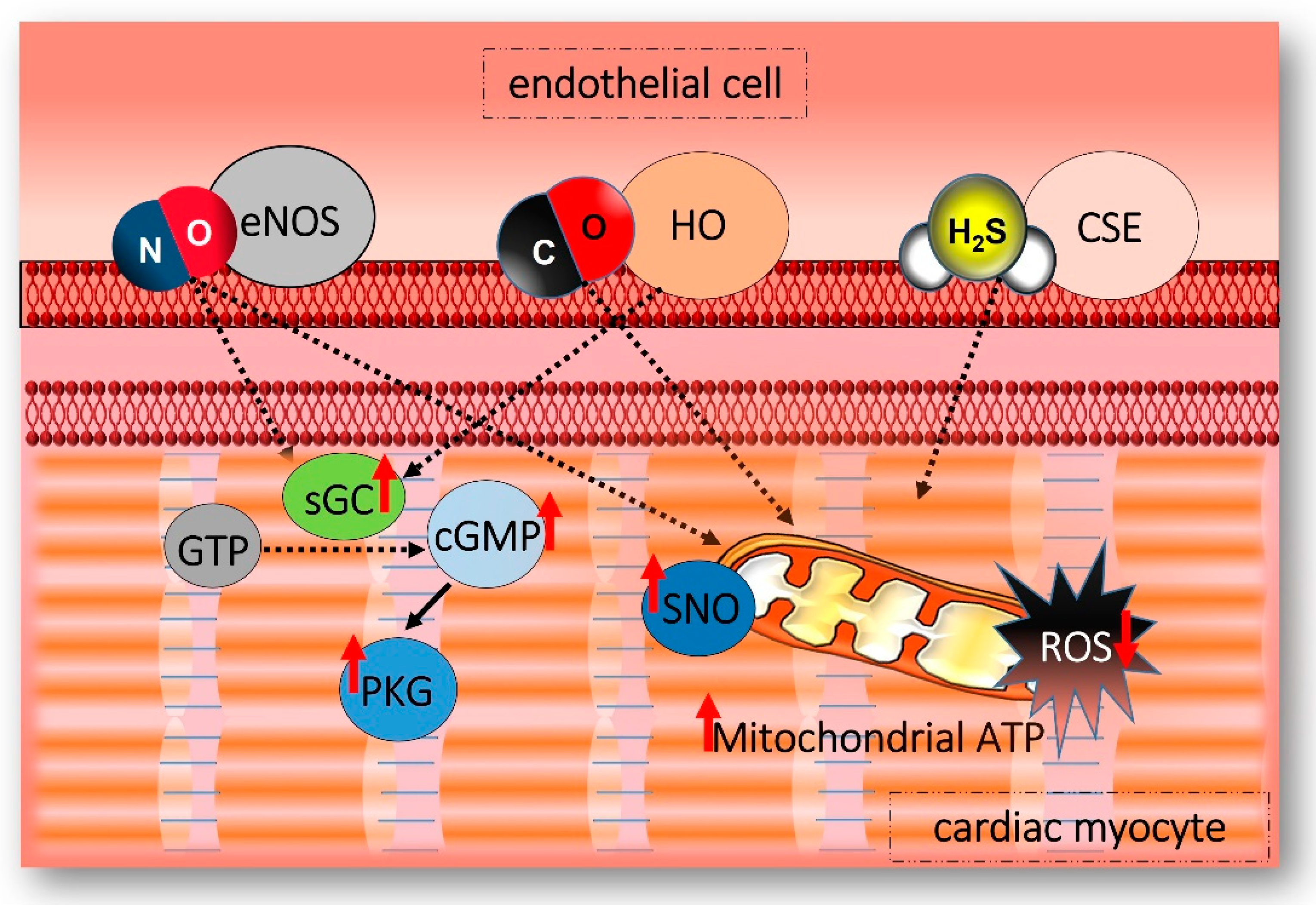
- Regulated endogenous production by constitutively expressed enzymes (Figure 1);
- Basal levels exert fundamental and well-defined physiological functions;
- Toxic effects at high concentrations;
- Targeting of different organs/systems;
- Interaction with one or more member(s) of the family;
- Exclusion criteria: being a respiratory gas.
2. Hydrogen Sulfide
2.1. H2S Biology in the Heart and Vessels
- Between 50 and 100 ppm: mild conjunctivitis and airway irritation after an hour. May cause loss of appetite;
- Between 100 and 150 ppm: loss of smell (“olfactory fatigue”);
- Between 150 and 300 ppm: severe conjunctivitis and airway irritation after one hour. If the exposure is prolonged, it may cause pulmonary edema;
- Between 500 and 700 ppm: collapse within 5 min, eye damage within 30 min, and death after 30–60 min;
- Between 700 and 1000 ppm: rapid unconsciousness, immediate collapse (“knockdown”) in one or two breaths, and death within a few minutes;
2.2. H2S and Ischemia/Reperfusion Injury
2.3. H2S and Mitochondria
2.4. H2S and Clinical Applications
3. Nitric Oxide (NO)
3.1. NO Biology in the Heart and Vessels
3.2. NO in Myocardial Ischemia/Reperfusion Injury
- (a)
- The lack of direct measures of myocardial NO concentration and/or NOS expression;
- (b)
- The lack of attention to non-enzymatic NO production as a potential source of NO;
- (c)
- The lack of consideration for plasma/blood components influencing NO delivery and metabolism.
3.3. NO as a Mediator of Cardioprotection
3.4. NO, Cardioprotection, and Mitochondria
3.5. NO and Clinical Applications
4. Carbon Monoxide (CO)
4.1. CO Biology in the Heart and Vessels
4.2. CO in Myocardial Ischemia-Reperfusion Injury and Cardioprotection
4.3. CO, Cardioprotection, and Mitochondria
4.4. CO and Clinical Applications
5. Gasotransmitters Interplay within the Cardiovascular System
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef] [PubMed]
- General Modes of Intercellular Signaling. Goodman’s Medical Cell Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Wang, R. Two’s company, three’s a crowd: Can H 2 S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancardi, D.; Ottolenghi, S.; Attanasio, U.; Tocchetti, C.G.; Paroni, R.; Pagliaro, P.; Samaja, M. Janus, or the Inevitable Battle Between Too Much and Too Little Oxygen. Antioxid. Redox Signal. 2022, 37, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Rumbeiha, W.; Whitley, E.; Anantharam, P.; Kim, D.-S.; Kanthasamy, A. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: Challenges for translational neuroprotective research. Ann. N. Y. Acad. Sci. 2016, 1378, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [Green Version]
- Mancardi, D.; Penna, C.; Merlino, A.; Del Soldato, P.; Wink, D.A.; Pagliaro, P. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. Biochim. Biophys. Acta 2009, 1787, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Li, S.; Ping, N.-N.; Cao, L.; Mi, Y.-N.; Cao, Y.-X. H2S induces vasoconstriction of rat cerebral arteries via cAMP/adenylyl cyclase pathway. Toxicol. Appl. Pharmacol. 2015, 289, 389–396. [Google Scholar] [CrossRef]
- Kubo, S.; Kajiwara, M.; Kawabata, A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology 2007, 15, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cheang, W.S.; Wong, W.T.; Shen, B.; Lau, C.W.; Tian, X.Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y.; Huang, Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul. Pharmacol. 2010, 53, 94–98. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; De La Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Tang, G.; Cao, K.; Wu, L.; Wang, R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid. Redox Signal. 2010, 12, 1167–1178. [Google Scholar] [CrossRef]
- Hedegaard, E.R.; Gouliaev, A.; Winther, A.K.; Arcanjo, D.D.R.; Aalling, M.; Renaltan, N.S.; Wood, M.E.; Whiteman, M.; Skovgaard, N.; Simonsen, U. Involvement of Potassium Channels and Calcium-Independent Mechanisms in Hydrogen Sulfide-Induced Relaxation of Rat Mesenteric Small Arteries. J. Pharmacol. Exp. Ther. 2016, 356, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Gonzalez Bosc, L.V.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef] [Green Version]
- Jackson-Weaver, O.; Paredes, D.; Gonzalez Bosc, L.; Walker, B.; Kanagy, N. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ. Res. 2011, 108, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Avanzato, D.; Moccia, F.; Mancardi, D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium 2013, 53, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pupo, E.; Fiorio Pla, A.; Avanzato, D.; Moccia, F.; Avelino Cruz, J.-E.; Tanzi, F.; Merlino, A.; Mancardi, D.; Munaron, L. Hydrogen sulfide promotes calcium signals and migration in tumor-derived endothelial cells. Free Radic. Biol. Med. 2011, 51, 1765–1773. [Google Scholar] [CrossRef]
- Moccia, F.; Bertoni, G.; Pla, A.F.; Dragoni, S.; Pupo, E.; Merlino, A.; Mancardi, D.; Munaron, L.; Tanzi, F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr. Pharm. Biotechnol. 2011, 12, 1416–1426. [Google Scholar] [CrossRef]
- Altaany, Z.; Moccia, F.; Munaron, L.; Mancardi, D.; Wang, R. Hydrogen sulfide and endothelial dysfunction: Relationship with nitric oxide. Curr. Med. Chem. 2014, 21, 3646–3661. [Google Scholar] [CrossRef] [Green Version]
- Mancardi, D.; Florio Pla, A.; Moccia, F.; Tanzi, F.; Munaron, L. Old and New Gasotransmitters in the Cardiovascular System: Focus on the Role of Nitric Oxide and Hydrogen Sulfide in Endothelial Cells and Cardiomyocytes. Curr. Pharm. Biotechnol. 2011, 12, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, D.; Merlino, A.; Porrera, S.; Wang, R.; Munaron, L.; Mancardi, D. Role of calcium channels in the protective effect of hydrogen sulfide in rat cardiomyoblasts. Cell. Physiol. Biochem. 2014, 33, 1205–1214. [Google Scholar] [CrossRef]
- Luan, H.F.; Zhao, Z.B.; Zhao, Q.H.; Zhu, P.; Xiu, M.Y.; Ji, Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz. J. Med. Biol. Res. 2012, 45, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Osipov, R.M.; Robich, M.P.; Feng, J.; Liu, Y.; Clements, R.T.; Glazer, H.P.; Sodha, N.R.; Szabo, C.; Bianchi, C.; Sellke, F.W. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: Comparison of different administration regimens and characterization of the cellular mechanisms of protection. J. Cardiovasc. Pharmacol. 2009, 54, 287–297. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The cardioprotective effects of hydrogen sulfide in heart diseases: From molecular mechanisms to therapeutic potential. Oxid. Med. Cell. Longev. 2015, 2015, 925167. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.Z.; Zhong, J.W.; Ho, P.; Yoke, Y.L.; Yi, C.Z.; Shan, H.H.; Chee, S.T.; Whiteman, M.; Lu, J.; Moore, P.K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 2007, 102, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-Nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, J.L.; Burger, N.; Gawel, J.M.; Mulvey, J.F.; Norman, A.A.I.; Nishimura, T.; Tsujihata, Y.; Logan, A.; Sauchanka, O.; Caldwell, S.T.; et al. Rapid and selective generation of H2S within mitochondria protects against cardiac ischemia-reperfusion injury. Redox Biol. 2022, 55, 102429. [Google Scholar] [CrossRef] [PubMed]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Szabõ, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of cystathionine γ-Lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [Green Version]
- Pascovici, D.; Wu, J.X.; McKay, M.J.; Joseph, C.; Noor, Z.; Kamath, K.; Wu, Y.; Ranganathan, S.; Gupta, V.; Mirzaei, M. Clinically Relevant Post-Translational Modification Analyses-Maturing Workflows and Bioinformatics Tools. Int. J. Mol. Sci. 2018, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H2S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef]
- Domán, A.; Dóka, É.; Garai, D.; Bogdándi, V.; Balla, G.; Balla, J.; Nagy, P. Interactions of reactive sulfur species with metalloproteins. Redox Biol. 2023, 60, 102617. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Vitvitsky, V.; Bostelaar, T.; Bak, D.W.; Lee, H.J.; Sakamoto, N.; Fearon, E.; Lyssiotis, C.A.; Weerapana, E.; Banerjee, R. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 2019, 294, 12077–12090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, S.F.; Moochhala, S.M.; MacAry, P.A.; Bhatia, M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLoS ONE 2011, 6, e24535. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.A.; Shen, X.; Shah, S.H.; Pardue, S.; Glawe, J.D.; Zhang, W.W.; Reddy, P.; Akkus, N.I.; Varma, J.; Kevil, C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013, 2, e000387. [Google Scholar] [CrossRef] [Green Version]
- Coletta, C.; Szabo, C. Potential Role of Hydrogen Sulfide in the Pathogenesis of Vascular Dysfunction in Septic Shock. Curr. Vasc. Pharmacol. 2013, 11, 208–221. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef]
- Sun, L.; Sun, S.; Li, Y.F.; Pan, W.; Xie, Y.; Wang, S.S.; Zhang, Z.W. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chin. Med. J. 2014, 127, 893–899. [Google Scholar] [CrossRef]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Li, H.; Tang, C.; Geng, B.; Qi, Y.; Liu, X. Hydrogen sulfide attenuates the pathogenesis of pulmonary fibrosis induced by bleomycin in rats. Can. J. Physiol. Pharmacol. 2009, 87, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. ChemBioChem 2021, 22, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Kumar, R.; Libiad, M.; Maebius, A.; Landry, A.P.; Banerjee, R. The mitochondrial NADH pool is involved in hydrogen sulfide signaling and stimulation of aerobic glycolysis. J. Biol. Chem. 2021, 296, 100736. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Tiranti, V.; Zeviani, M. Altered sulfide (H2S) metabolism in ethylmalonic encephalopathy. Cold Spring Harb. Perspect. Biol. 2013, 5, a011437. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [Green Version]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.K.; Vitvitsky, V.; Carballal, S.; Seravalli, J.; Banerjee, R. Thioredoxin regulates human mercaptopyruvate sulfurtransferase at physiologically-relevant concentrations. J. Biol. Chem. 2020, 295, 6299–6311. [Google Scholar] [CrossRef] [Green Version]
- Fräsdorf, B.; Radon, C.; Leimkühler, S. Characterization and interaction studies of two isoforms of the dual localized 3-mercaptopyruvate sulfurtransferase TUM1 from humans. J. Biol. Chem. 2014, 289, 34543–34556. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Sawada, N. The Mercaptopyruvate Pathway in Cysteine Catabolism: A Physiologic Role and Related Disease of the Multifunctional 3-Mercaptopyruvate Sulfurtransferase. Curr. Med. Chem. 2006, 13, 1219–1230. [Google Scholar] [CrossRef]
- Nagahara, N.; Katayama, A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine-sulfenate in the maintenance of redox homeostasis. J. Biol. Chem. 2005, 280, 34569–34576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völkel, S.; Grieshaber, M.K. Mitochondrial sulfide oxidation in Arenicola marina Evidence for alternative electron pathways. Eur. J. Biochem. 1996, 235, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Coletta, C.; Erdélyi, K.; Papapetropoulos, A.; Szabo, C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013, 27, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide-Biol. Chem. 2014, 41, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.L.; Wu, H.C.; Li, Z.L.; Geng, B.; Tang, C.S. Changes of the new gaseous transmitter H2S in patients with coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao 2005, 25, 951–954. [Google Scholar]
- Hashimoto, A.; Nishikawa, T.; Konno, R.; Niwa, A.; Yasumura, Y.; Oka, T.; Takahashi, K. Free d-serine, d-aspartate and d-alanine in central nervous system and serum in mutant mice lacking d-amino acid oxidase. Neurosci. Lett. 1993, 152, 33–36. [Google Scholar] [CrossRef]
- Huang, J.; Niknahad, H.; Khan, S.; O’Brien, P.J. Hepatocyte-catalysed detoxification of cyanide by L- and D-cysteine. Biochem. Pharmacol. 1998, 55, 1983–1990. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from D-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Roth, M.B.; Nystul, T. Buying time in suspended animation. Sci. Am. 2005, 292, 48–55. [Google Scholar] [CrossRef]
- Lindell, S.L.; Klahn, S.L.; Piazza, T.M.; Mangino, M.J.; Torrealba, J.R.; Southard, J.H.; Carey, H.V. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G473–G480. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Bhushan, S.; King, A.; Prabhu, S.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.; Gojon, G.; Wang, R.; et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G.; Gojon, G.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol. Sci. 2007, 28, 501–505. [Google Scholar] [CrossRef]
- Glanville, J.R.W.; Jalali, P.; Flint, J.D.; Patel, A.A.; Maini, A.A.; Wallace, J.L.; Hosin, A.A.; Gilroy, D.W. Potent anti-inflammatory effects of an H2S-releasing naproxen (ATB-346) in a human model of inflammation. FASEB J. 2021, 35, e21913. [Google Scholar] [CrossRef]
- Wallace, J.L.; Nagy, P.; Feener, T.D.; Allain, T.; Ditrói, T.; Vaughan, D.J.; Muscara, M.N.; de Nucci, G.; Buret, A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2020, 177, 769–777. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Shimizu, S.; Izumi, H. Hydrotherapy can modulate peripheral leukocytes: An approach to alternative medicine. In Proceedings of the Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Prandelli, C.; Parola, C.; Buizza, L.; Delbarba, A.; Marziano, M.; Salvi, V.; Zacchi, V.; Memo, M.; Sozzani, S.; Calza, S.; et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 633–646. [Google Scholar] [CrossRef]
- Contoli, M.; Gnesini, G.; Forini, G.; Marku, B.; Pauletti, A.; Padovani, A.; Casolari, P.; Taurino, L.; Ferraro, A.; Chicca, M.; et al. Reducing agents decrease the oxidative burst and improve clinical outcomes in COPD patients: A randomised controlled trial on the effects of sulphurous thermal water inhalation. Sci. World J. 2013, 2013, 927835. [Google Scholar] [CrossRef] [Green Version]
- Stuehr, D.J.; Santolini, J.; Wang, Z.Q.; Wei, C.C.; Adak, S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004, 279, 36167–36170. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.D.; Rezende, B.A.; Cortes, S.F.; Lemos, V.S. Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front. Physiol. 2016, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Fisslthaler, B.; Loot, A.E.; Mohamed, A.; Busse, R.; Fleming, I. Inhibition of Endothelial Nitric Oxide Synthase activity by Proline-Rich Tyrosine Kinase 2 in response to fluid shear stress and insulin. Circ. Res. 2008, 102, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Kumar, S.; Yu, Y.; Aggarwal, S.; Gross, C.; Wang, Y.; Chakraborty, T.; Verin, A.D.; Catravas, J.D.; Lucas, R.; et al. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+ -toxins. PLoS ONE 2014, 9, e99823. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Xiao, H.; Rizzo, A.N.; Zhang, W.; Mai, Y.; Ye, M. Endothelial nitric oxide synthase dimerization is regulated by heat shock protein 90 rather than by phosphorylation. PLoS ONE 2014, 9, e105479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, G.A.M.; Zhang, J.; Goodwin, D.; Stewart, S.; Xu, L.; Knapton, A.; González, C.; Bancos, S.; Zhang, L.; Lawton, M.P.; et al. The Role of eNOS Phosphorylation in Causing Drug-induced Vascular Injury. Toxicol. Pathol. 2014, 42, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.R.; Chen, B.R.; Hsieh, C.C.; Lin, W.C.; Wu, K.K.; Hwu, Y.; Chen, P.F. The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495and Ser1177. Biosci. Rep. 2014, 34, 443–455. [Google Scholar] [CrossRef]
- Gopalakrishna, D.; Pennington, S.; Karaa, A.; Clemens, M.G. ET-1 Stimulates Superoxide Production by eNOS Following Exposure of Vascular Endothelial Cells to Endotoxin. Shock 2016, 46, 60–66. [Google Scholar] [CrossRef]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol.-Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [Green Version]
- Zweier, J.L.; Samouilov, A.; Kuppusamy, P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta-Bioenerg. 1999, 1411, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R. Cardioprotective Function of Inducible Nitric Oxide Synthase and Role of Nitric Oxide in Myocardial Ischemia and Preconditioning: An Overview of a Decade of Research. J. Mol. Cell. Cardiol. 2001, 33, 1897–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.P.; Bolli, R. The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell. Cardiol. 2006, 40, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rastaldo, R.; Pagliaro, P.; Cappello, S.; Penna, C.; Mancardi, D.; Westerhof, N.; Losano, G. Nitric oxide and cardiac function. Life Sci. 2007, 81, 779–793. [Google Scholar] [CrossRef]
- Dunkerly-Eyring, B.; Kass, D.A. Myocardial Phosphodiesterases and Their Role in cGMP Regulation. J. Cardiovasc. Pharmacol. 2020, 75, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Shiva, S.; Gladwin, M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide-Biol. Chem. 2009, 21, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Kelm, M.; Heusch, G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Finkel, M.S.; Oddis, C.V.; Jacob, T.D.; Watkins, S.C.; Hattler, B.G.; Simmons, R.L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992, 257, 387–389. [Google Scholar] [CrossRef]
- Patel, V.C.; Yellon, D.M.; Singh, K.J.; Neild, G.H.; Woolfson, R.G. Inhibition of nitric oxide limits infarct size in the in situ rabbit heart. Biochem. Biophys. Res. Commun. 1993, 194, 234–238. [Google Scholar] [CrossRef]
- Mori, E.; Haramaki, N.; Ikeda, H.; Imaizumi, T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc. Res. 1998, 40, 113–123. [Google Scholar] [CrossRef]
- Wildhirt, S.M.; Weismueller, S.; Schulze, C.; Conrad, N.; Kornberg, A.; Reichart, B. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: Evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc. Res. 1999, 43, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Lu, X.; Jones, D.L.; Shen, J.; Arnold, J.M.O. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation 2001, 104, 700–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, H.K.; Xu, Y.J.; Zhang, M.; Liu, P.P.; Kirshenbaum, L.A.; Dhalla, N.S. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp. Clin. Cardiol. 2005, 10, 213–222. [Google Scholar]
- Hu, A.; Jiao, X.; Gao, E.; Koch, W.J.; Sharifi-Azad, S.; Grunwald, Z.; Ma, X.L.; Sun, J.Z. Chronic β-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J. Pharmacol. Exp. Ther. 2006, 318, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Yao, Q.; Wang, W.; Yao, H.; Chao, J. iNOS induces vascular endothelial cell migration and apoptosis via autophagy in ischemia/reperfusion injury. Cell. Physiol. Biochem. 2016, 38, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, S.; Ghasemi, A.; Asgari, A.; Nezami-Asl, A. Role of inducible nitric oxide synthase in myocardial ischemia-reperfusion injury in sleep-deprived rats. Sleep Breath. 2018, 22, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Tang, X.L.; Knowlton, A.A.; Park, S.W.; Qiu, Y.; Bolli, R. Late preconditioning against myocardial stunning: An endogenous protective mechanism that confers resistance to postischemic dysfunction 24 h after brief ischemia in conscious pigs. J. Clin. Investig. 1995, 95, 388–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshida, S.; Yamashita, N.; Otsu, K.; Hori, M. The importance of manganese superoxide dismutase in delayed preconditioning: Involvement of reactive oxygen species and cytokines. Cardiovasc. Res. 2002, 55, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Mancardi, D.; Pagliaro, P.; Ridnour, L.A.; Tocchetti, C.G.; Miranda, K.; Juhaszova, M.; Sollott, S.J.; Wink, D.A.; Paolocci, N. HNO Protects the Myocardium against Reperfusion Injury, Inhibiting the mPTP Opening via PKCε Activation. Antioxidants 2022, 11, 382. [Google Scholar] [CrossRef]
- Penna, C.; Mancardi, D.; Rastaldo, R.; Losano, G.; Pagliaro, P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc. Res. 2007, 75, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, J.; Zhu, H.; Wu, X.; Zhou, L.; Song, Y.; Zhu, S.; Hao, M.; Liu, C.; Fan, Y.; et al. Ischemic postconditioning protects the heart against ischemia–reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria. Cell Death Dis. 2016, 7, e2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvert, J.W.; Condit, M.E.; Aragón, J.P.; Nicholson, C.K.; Moody, B.F.; Hood, R.L.; Sindler, A.L.; Gundewar, S.; Seals, D.R.; Barouch, L.A.; et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β3-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ. Res. 2011, 108, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Kleindienst, A.; Bolea, G.; Meyer, G.; Gayrard, S.; Geny, B.; Obert, P.; Cazorla, O.; Tanguy, S.; Reboul, C. Exercise-induced cardioprotection: A role for eNOS uncoupling and NO metabolites. Basic Res. Cardiol. 2013, 108, 389. [Google Scholar] [CrossRef]
- Ren, J.; Yang, L.; Tian, W.; Zhu, M.; Liu, J.; Lu, P.; Li, J.; Yang, L.; Qi, Z. Nitric oxide synthase inhibition abolishes exercise-mediated protection against isoproterenol-induced cardiac hypertrophy in female mice. Cardiology 2015, 130, 175–184. [Google Scholar] [CrossRef]
- Aragón, J.P.; Condit, M.E.; Bhushan, S.; Predmore, B.L.; Patel, S.S.; Grinsfelder, D.B.; Gundewar, S.; Jha, S.; Calvert, J.W.; Barouch, L.A.; et al. Beta 3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J. Am. Coll. Cardiol. 2011, 58, 2683–2691. [Google Scholar] [CrossRef] [Green Version]
- Comità, S.; Femmino, S.; Thairi, C.; Alloatti, G.; Boengler, K.; Pagliaro, P.; Penna, C. Regulation of STAT3 and its role in cardioprotection by conditioning: Focus on non-genomic roles targeting mitochondrial function. Basic Res. Cardiol. 2021, 116, 56. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Hyodo, F.; Pappan, L.K.; Abu-Asab, M.; Tsokos, M.; Krishna, M.C.; Frazier, W.A.; Roberts, D.D. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2582–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Rogers, N.M.; Csányi, G.; Rodriguez, A.I.; Ross, M.A.; St. Croix, C.; Knupp, H.; Novelli, E.M.; Thomson, A.W.; Pagano, P.J.; et al. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal Ischemia reperfusion injury. J. Am. Soc. Nephrol. 2014, 25, 1171–1186. [Google Scholar] [CrossRef] [Green Version]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002, 33, 1451–1464. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Schulz, R.; Girao, H.; Kwak, B.R.; De Stefani, D.; Rizzuto, R.; Bernardi, P.; Di Lisa, F. Mitochondrial ion channels as targets for cardioprotection. J. Cell. Mol. Med. 2020, 24, 7102–7114. [Google Scholar] [CrossRef]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, V.; Schildknecht, S. Sensing hypoxia by mitochondria: A unifying hypothesis involving S-Nitrosation. Antioxid. Redox Signal. 2014, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Hendgen-Cotta, U.B.; Rassaf, T. Nitrite-nitric oxide signaling and cardioprotection. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 982. [Google Scholar]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cochemé, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methner, C.; Chouchani, E.T.; Buonincontri, G.; Pell, V.R.; Sawiak, S.J.; Murphy, M.P.; Krieg, T. Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts. Eur. J. Heart Fail. 2014, 16, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, P.; Gattullo, D.; Penna, C. Nitroglycerine and sodium trioxodinitrate: From the discovery to the preconditioning effect. J. Cardiovasc. Med. 2013, 14, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Ignarro, L.J.; Lippton, H.; Edwards, J.C.; Baricos, W.H.; Hyman, A.L.; Kadowitz, P.J.; Gruetter, C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: Evidence for the involvement of S-Nitrosothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981, 218, 739–749. [Google Scholar]
- Kleschyov, A.L.; Oelze, M.; Daiber, A.; Huang, Y.; Mollnau, H.; Schulz, E.; Sydow, K.; Fichtlscherer, B.; Mülsch, A.; Münzel, T. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ. Res. 2003, 93, e104–e112. [Google Scholar] [CrossRef]
- Pasero, D.; Rana, N.K.; Bonato, R.; Ribezzo, M.; Ivaldi, F.; Ricci, D.; Grosso Marra, W.; Checco, L.; Lupo, M.; Boffini, M.; et al. Inhaled nitric oxide versus sodium nitroprusside for preoperative evaluation of pulmonary hypertension in heart transplant candidates. In Proceedings of the Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Siddiqi, N.; Bruce, M.; Neil, C.J.; Jagpal, B.; Maclennon, G.; Cotton, S.C.; Papadopoulo, S.A.; Bunce, N.; Lim, P.; Schwarz, K.; et al. Protocol: Does sodium nitrite administration reduce ischaemia-reperfusion injury in patients presenting with acute ST segment elevation myocardial infarction? Nitrites in acute myocardial infarction (NIAMI). J. Transl. Med. 2013, 11, 116–118. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P. Differential biological effects of products of nitric oxide (NO) synthase: It is not enough to say NO. Life Sci. 2003, 73, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P.; Mancardi, D.; Rastaldo, R.; Penna, C.; Gattullo, D.; Miranda, K.M.; Feelisch, M.; Wink, D.A.; Kass, D.A.; Paolocci, N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003, 34, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Miranda, K.M.; Katori, T.; Mancardi, D.; Thomas, D.D.; Ridnour, L.; Espey, M.G.; Feelisch, M.; Colton, C.A.; Fukuto, J.M.; et al. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: A novel redox paradigm. Am. J. Physiol.-Hear. Circ. Physiol. 2003, 285, H2264–H2276. [Google Scholar] [CrossRef] [Green Version]
- Tocchetti, C.G.; Stanley, B.A.; Murray, C.I.; Sivakumaran, V.; Donzelli, S.; Mancardi, D.; Pagliaro, P.; Gao, W.D.; Van Eyk, J.; Kass, D.A.; et al. Playing with cardiac redox switches: The HNO way to modulate cardiac function. Antioxid. Redox Signal. 2011, 14, 1687–1698. [Google Scholar] [CrossRef] [Green Version]
- Penna, C.; Angotti, C.; Pagliaro, P. Protein S-nitrosylation in preconditioning and postconditioning. Exp. Biol. Med. 2014, 239, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Rogers, N.M.; Sharifi-Sanjani, M.; Yao, M.; Ghimire, K.; Bienes-Martinez, R.; Mutchler, S.M.; Knupp, H.E.; Baust, J.; Novelli, E.M.; Ross, M.; et al. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc. Res. 2017, 113, 15–29. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Romeo, M.J.; Maxhimer, J.B.; Smedley, J.; Frazier, W.A.; Roberts, D.D. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: Implications for human disease. Ann. Surg. 2008, 247, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, Y.; Wang, H.; Sun, L.; Fu, Y.; Yang, Y.G. Elimination of donor CD47 protects against vascularized allograft rejection in mice. Xenotransplantation 2019, 26, e12459. [Google Scholar] [CrossRef]
- George, I.; Xydas, S.; Topkara, V.K.; Ferdinando, C.; Barnwell, E.C.; Gableman, L.; Sladen, R.N.; Naka, Y.; Oz, M.C. Clinical Indication for Use and Outcomes After Inhaled Nitric Oxide Therapy. Ann. Thorac. Surg. 2006, 82, 2161–2169. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donald, J.A.; Cameron, M.S. Carbon monoxide. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1087–1090. [Google Scholar]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme oxygenase-1: A metabolic nike. Antioxid. Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.D.; Wang, X.Y.; Nisar, M.F.; Lin, M.; Zhong, J.L. Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 5416728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterbein, L.E.; Foresti, R.; Motterlini, R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act between Danger Signaling and Pro-Survival. Circ. Res. 2016, 118, 1940–1959. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.H.W.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Chu, L.M.; Shaefi, S.; Byrne, J.D.; Alves de Souza, R.W.; Otterbein, L.E. Carbon monoxide and a change of heart. Redox Biol. 2021, 48, 102183. [Google Scholar] [CrossRef]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef]
- Leffler, C.W.; Parfenova, H.; Jaggar, J.H. Carbon monoxide as an endogenous vascular modulator. Am. J. Physiol.-Hear. Circ. Physiol. 2011, 301, H1–H11. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-H.; Choi, S. Therapeutic Aspects of Carbon Monoxide in Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2381. [Google Scholar] [CrossRef] [Green Version]
- Foresti, R.; Goatly, H.; Green, C.J.; Motterlini, R. Role of heme oxygenase-1 in hypoxia-reoxygenation: Requirement of substrate heme to promote cardioprotection. Am. J. Physiol.-Hear. Circ. Physiol. 2001, 281, H1976–H1984. [Google Scholar] [CrossRef] [PubMed]
- Lancel, S.; Montaigne, D.; Marechal, X.; Marciniak, C.; Hassoun, S.M.; Decoster, B.; Ballot, C.; Blazejewski, C.; Corseaux, D.; Lescure, B.; et al. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS ONE 2012, 7, e41836. [Google Scholar] [CrossRef] [Green Version]
- Bilska-Wilkosz, A.; Górny, M.; Iciek, M. Biological and Pharmacological Properties of Carbon Monoxide: A General Overview. Oxygen 2022, 2, 130–151. [Google Scholar] [CrossRef]
- Stein, A.B.; Guo, Y.; Tan, W.; Wu, W.J.; Zhu, X.; Li, Q.; Luo, C.; Dawn, B.; Johnson, T.R.; Motterlini, R.; et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J. Mol. Cell. Cardiol. 2005, 38, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Knauert, M.; Vangala, S.; Haslip, M.; Lee, P.J. Therapeutic applications of carbon monoxide. Oxid. Med. Cell. Longev. 2013, 2013, 360815. [Google Scholar] [CrossRef]
- Ryter, S.W. Therapeutic potential of heme oxygenase-1 and carbon monoxide in acute organ injury, critical illness, and inflammatory disorders. Antioxidants 2020, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Otterbein, L.E.; Alam, J.; Flavell, R.A.; Davis, R.J.; Choi, A.M.K.; Lee, P.J. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J. Biol. Chem. 2003, 278, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.S.; Moon, J.S.; Xu, J.F.; Ifedigbo, E.; Ryter, S.W.; Choi, A.M.K.; Nakahira, K. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L1058–L1067. [Google Scholar] [CrossRef] [Green Version]
- Bannenberg, G.L.; Vieira, H.L.A. Therapeutic applications of the gaseous mediators carbon monoxide and hydrogen sulfide. Expert Opin. Ther. Pat. 2009, 19, 663–682. [Google Scholar] [CrossRef]
- Lavitrano, M.; Smolenski, R.T.; Musumeci, A.; Maccherini, M.; Slominska, E.; Florio, E.; Bracco, A.; Mancini, A.; Stassi, G.; Patti, M.; et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004, 18, 1093–1095. [Google Scholar] [CrossRef]
- Suliman, H.B.; Carraway, M.S.; Ali, A.S.; Reynolds, C.M.; Welty-Wolf, K.E.; Piantadosi, C.A. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J. Clin. Investig. 2007, 117, 3730–3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancel, S.; Hassoun, S.M.; Favory, R.; Decoster, B.; Motterlini, R.; Neviere, R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 2009, 329, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo-Pereira, C.; Dias-Pedroso, D.; Soares, N.L.; Vieira, H.L.A. CO-mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 2020, 32, 101470. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Cytoprotective and Anti-Inflammatory Actions of Carbon Monoxide in Organ Injury and Sepsis Models. In Sepsis: New Insights, New Therapies; John Wiley & Sons, Ltd.: Chichester, UK, 2008; Volume 280. [Google Scholar]
- Ryter, S.W.; Choi, A.M.K. Carbon monoxide in exhaled breath testing and therapeutics. J. Breath Res. 2013, 7, 017111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juin, S.K.; Ouseph, R.; Gondim, D.D.; Jala, V.R.; Sen, U. Diabetic Nephropathy and Gaseous Modulators. Antioxidants 2023, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Junho, C.V.C.; Carneiro-Ramos, M.S.; Seabra, A.B. H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol. Res. 2020, 161, 105121. [Google Scholar] [CrossRef]
- Minamishima, S.; Bougaki, M.; Sips, P.Y.; Yu, J.D.; Minamishima, Y.A.; Elrod, J.W.; Lefer, D.J.; Bloch, K.D.; Ichinose, F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 2009, 120, 888–896. [Google Scholar] [CrossRef] [Green Version]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.-X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Xiong, Y.; Zhu, D.; Mao, Y.; Zhu, Y.Z. Novel H2S-NO hybrid molecule (ZYZ-803) promoted synergistic effects against heart failure. Redox Biol. 2018, 15, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Bir, S.C.; Yuan, S.; Shen, X.; Pardue, S.; Wang, R.; Kevil, C.G. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc. Res. 2015, 107, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef]
- Wang, R. Shared signaling pathways among gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef]
- Bianco, C.L.; Fukuto, J.M. Examining the reaction of NO and H2S and the possible cross-talk between the two signaling pathways. Proc. Natl. Acad. Sci. USA 2015, 112, 10573–10574. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Yuan, S.; Kevil, C.G. Gasotransmitter heterocellular signaling. Antioxid. Redox Signal. 2017, 26, 936–960. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanović-Burmazović, I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Y.T.; Zhang, Y.Q.; Hsieh, C.W.; Wung, B.S. Carbon monoxide releasing molecule induces endothelial nitric oxide synthase activation through a calcium and phosphatidylinositol 3-kinase/Akt mechanism. Vascul. Pharmacol. 2016, 87, 209–218. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, Y.-M. Regulation of Endothelial and Vascular Functions by Carbon Monoxide via Crosstalk with Nitric Oxide. Front. Cardiovasc. Med. 2021, 8, 649630. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.G.F.; Nunes, J.; Tomé, C.S.; Zuhra, K.; Costa, J.M.F.; Antunes, A.M.M.; Giuffrè, A.; Vicente, J.B. Human cystathionine γ-lyase is inhibited by s-nitrosation: A new crosstalk mechanism between no and H2S. Antioxidants 2021, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Subramaniam, S. Gasotransmitter-Induced Therapeutic Angiogenesis: A Biomaterial Prospective. ACS Omega 2022, 7, 45849–45866. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yan, X.H.; Chen, S.J. H(2)S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng Li Xue Bao 2008, 60, 221–227. [Google Scholar] [PubMed]
- Ananthakrishnan, R.; Li, Q.; O’Shea, K.M.; Quadri, N.; Wang, L.; Abuchowski, A.; Schmidt, A.M.; Ramasamy, R. Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif. Cells Nanomed. Biotechnol. 2013, 41, 428–436. [Google Scholar] [CrossRef]
- Shao, J.; Miao, C.; Geng, Z.; Gu, M.; Wu, Y.; Li, Q. Effect of eNOS on Ischemic Postconditioning-Induced Autophagy against Ischemia/Reperfusion Injury in Mice. Biomed Res. Int. 2019, 2019, 5201014. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, X.; Zhang, Q.; Li, X.; Li, S.; Ma, J.; Zhu, W.; Liu, X.; Wei, M.; Tu, W.; et al. Hydrogen sulfide restores sevoflurane postconditioning mediated cardioprotection in diabetic rats: Role of SIRT1/Nrf2 signaling-modulated mitochondrial dysfunction and oxidative stress. J. Cell. Physiol. 2021, 236, 5052–5068. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Keusch, G.; Boengler, K.; Schulz, R. Cardioprotection: Nitric oxide, protein kinases, and mitochondria. Circulation 2008, 118, 1915–1919. [Google Scholar]
- Pagliaro, P.; Moro, F.; Tullio, F.; Perrelli, M.G.; Penna, C. Cardioprotective pathways during reperfusion: Focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 2011, 14, 833–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]


| Study Title | Condition | Intervention | Outcome(s) | Location(s) | Trial ID |
|---|---|---|---|---|---|
| Clinical trials with H2S donors | |||||
| A Double-Blind, Controlled Study to Compare the Gastrointestinal Safety of a 14-Day Oral Dosing Regimen of ATB-346 to Sodium Naproxen in Healthy Subjects | Gastric ulcer | ATB-346 (H2S-NSAID) vs. naproxen sodium (NSAID) | Gastroduodenal ulcers ≥ 3 mm diameter (Time frame: after 14 days of oral dosing) Gastroduodenal ulcers ≥ 5 mm diameter; gastroduodenal erosions; dyspepsia; hematocrit; thromboxane B2 levels (Time frame: after 14 days of oral dosing) | Topstone Clinical Research Toronto, Ontario, Canada, M9C 4Z5 | NCT03291418 |
| Groningen Intervention Study for the Preservation of Cardiac Function With Sodium Thiosulfate After ST-segment Elevation Myocardial Infarction * | Myocardial infarction HF | sodium thiosulfate vs. placebo | Myocardial infarct size measured with late gadolinium enhancement cardiac MRI (Time frame: 4 months after randomization) LV ejection fraction as assessed with cardiac MRI; NT-proBNP level (ng/L) (Time frame: 4 months after randomization) All-cause mortality; combined major CV-AEs; incidence of stroke; incidence of stent thrombosis; incidence of ICD implantation; hospitalization for HF or chest pain (Time frame: 4 months after randomization and after 2-year follow-up) Enzymatic infarct size as assessed with peak CK-MB (U/L) (Time frame: 0–3 days after randomization) Health related quality of life: EuroQol EQ-5D-5L; general affective status (Time frame: 0–5 days after randomization and at 4 months follow up) CK (U/L); troponin T (ng/mL) (Time frame: 0–3 days after randomization) NT-proBNP (ng/L) (Time frame: 0–5 days after randomization) | Treant Scheper Hospital Emmen, Drenthe, Netherlands, 7824 AA University Medical Centre Groningen Groningen, Netherlands, 9700RB University Medical Center Utrecht Utrecht, Netherlands, 3584 CX | NCT02899364 |
| A Dose Escalation Study to Assess the Safety and Ability of SG1002 to Overcome Circulating Deficits in Hydrogen Sulfide Found in Heart Failure | HF | SG1002 (α-sulfur/sodium sulfate) vs. placebo | Number of subjects with AEs (Time frame: following 7 days of treatment at each dose) Changes in peak H2S levels in HF subjects following SG1002 administration (Time frame: 24 h) Potential clinical benefits of SG1002 administration by analyzing BNP levels (Time frame: 7 days at each dose) | Alfred Health Melbourne, Victoria, Australia, 3004 Nucleus Network Melbourne, Victoria, Australia, 3004 | NCT01989208 |
| Patients Peripheral Vascular Effects of Sulfhydryl-containing Antihypertensive Pharmacotherapy on Microvascular Function and Vessel Remodeling in Hypertensive Humans * | Hypertension | Captopril (ACEi+SH) vs. enalapril (ACEi) vs. hydrochlorothiazide (diuretic) | Laser Doppler blood flow (Time frame: 16 weeks) SBP; DBP (Time frame: 16 Weeks) | Pennsylvania State University University Park, Pennsylvania, United States, 16802 | NCT03179163 |
| Abbreviations: HF = heart failure; MRI = magnetic resonance imaging; LV = left centricular; NTproBNP = N-terminal pro type B natriuretic peptide; CV-AEs = cardiovascular adverse events; ICD = implantable cardioverter-defibrillator; CK-MB = creatin kinase-muscle brain; CK = creatin kinase; ACEi = ACE inhibitor; SBP = systolic blood pressure; DBP = diastolic blood pressure; AEs: adverse events; BNP = B type natriuretic peptide * = ongoing studies | |||||
| Clinical trials with NO donors/NO potentiators | |||||
| Effects of Nitric Oxide on Vascular Responsiveness and on Endothelial Cells During Hemolysis in Patients With Pre-operative Endothelial Dysfunction Undergoing Prolonged Cardiopulmonary Bypass * | Endothelial dysfunction Intravascular hemolysis | iNO vs. placebo | RHI (Time frame: perioperatively—before anesthesia induction—and at 24 h after CPB during ICU admission) eNOS enzymatic activity (Time frame: perioperatively—before anesthesia induction—and at 24 h after CPB during ICU admission) PVR; SVR (Time frame: every 6 h after surgery for 24 h after CPB start) | Massachusetts General Hospital Boston, Massachusetts, United States, 02114 Boston Medical Center Boston, Massachusetts, United States, 02118 | NCT03748082 |
| Effects of Prolonged Delivery of Nitric Oxide Gas on Plasma Reduction-Oxidation Reactions in Cardiac Surgical Patients * | Endothelial dysfunction | iNO vs. placebo | Changes in the concentration and electric potential of GSH/GSSG and Cys/CysSS couples in the plasma after cardiac surgery (Time frame: before cardiac surgery and during the first 48 h aftersurgery) Changes in concentration of plasma and RBCs of NO metabolites after cardiac surgery (Time frame: before cardiac surgery and after the first 48 h after surgery) | Massachusetts General Hospital Boston, Massachusetts, United States, 02114 Southampton General Hospital Southampton, Hampshire, United Kingdom, SO16 6YD | NCT04022161 |
| Improving Outcomes in Cardiac Arrest With Inhaled Nitric Oxide * | Cardiac arrest | iNO | Rate of return of spontaneous circulation; change in cerebral oxygenation (Time frame: 1 day) Neurologic outcomes at hospital discharge; short term survival (Time frame: up to 24 weeks) | Stony Brook University S. Setauket, New York, United States, 11720 | NCT04134078 |
| Nebivolol and the Endothelin (ET)-1 System | Prehypertension Hypertension | nebivolol (β-blocker with NO-potentiating properties) vs. metroprolol (β-blocker) vs. placebo | SBP; DBP (Time frame: before and after intervention) Percent change in FBF response to BQ-123 (100 Nmol/Min) (Time Frame: 0–60 min before and after the intervention) Percent change in FBF response to BQ-123 (100 Nmol/Min) + BQ-788 (50 Nmol/Min) (Time frame: 0–120 min before and after the intervention) FBF response to ACh or sodium nitroprusside; FBF response to ACh w/ or w/o BQ-123+BQ-788 (Time frame: before and after the intervention) | UC-Boulder Clinical and Translational Research Center Boulder, Colorado, United States, 80309 | NCT01395329 |
| Acute Effects of Inhaled Sodium Nitrite on Cardiovascular Hemodynamics in Heart Failure With Preserved Ejection Fraction | HF | inhaled sodium nitrite vs. placebo | Change in pulmonary capillary wedge pressure (mmHg) during exercise (Time frame: baseline, after study drug dosing, approximately 4 min after starting exercise) | Mayo Clinic Rochester, Minnesota, United States, 55905 | NCT02262078 |
| A Randomized, Double-blinded, Placebo-controlled, Phase IIa Dose-ranging Study to Assess the Safety, Pharmacokinetics, and Tolerability of Multiple Doses of Sodium Nitrite in Patients With Peripheral Arterial Disease (PAD)—SONIC | Peripheral arterial disease | 80 mg sodium nitrite vs. 40 mg sodium nitrite vs. placebo | Reporting of AEs during 11 week treatment period (Time frame: 11 weeks) Assessment of changes in brachial artery FMD 10 weeks after baseline (Time frame: 10 weeks) Assessment of changes in walking distance (Timeframe: 10 weeks) Assessment of improvement in quality of life using the WIQ and SF-36 Questionnaire (Time frame: 10 weeks) | University of Colorado Denver Health Sciences Center Aurora, Colorado, United States, 80045 Emory University Atlanta, Georgia, United States, 30322 University of Iowa Iowa City, Iowa, United States, 52242 University of Cincinnati Cincinnati, Ohio, United States, 45267 Cleveland Clinic Cleveland, Ohio, United States, 44106 Ohio State University Columbus, Ohio, United States, 43210 University of Pennsylvania Philadelphia, Pennsylvania, United States, 19104 Vanderbilt Heart and Vascular Institute Nashville, Tennessee, United States, 37232 Medical College of Wisconsin Milwaukee, Wisconsin, United States, 53226 | NCT01401517 |
| Efficacy of Oral Sodium Nitrite for Improving Physiological Functions in Older Adults | Aging | sodium nitrite vs. placebo | Vascular function (Time frame: 3 months) Motor function (Time frame: 3 months) Systemic oxidative stress and inflammation (Time frame: 3 months) Number of participants with additional measures of motor ability (Time frame: 3 months) Endothelial cell oxidative stress and inflammation (Time frame: 3 months) Plasma metabolites (Time frame: 3 months) | Clinical Translational Center Boulder, Colorado, United States, 80309 | NCT02393742 |
| The Effects of Inorganic Nitrate on Cardiac Muscle: Physiology, Pharmacology and Therapeutic Potential in Patients Suffering From Angina | Chronic stable angina | sodium nitrate vs. placebo | Time to 1 mm ST depression (Time frame: 12 weeks) Onset of chest pain (Time frame: 12 weeks) Change in TDI systolic peak velocity (Time frame: 12 weeks) Angina frequency (Time frame: 12 weeks) Nitrate/nitrite in plasma (Time frame: 12 weeks) Metabolic, inflammatory, and angiogenic plasma markers (Time frame: 12 weeks) | Cardiovascular Research Facility, University of Aberdeen Aberdeen, United Kingdom, AB24 3FX | NCT02078921 |
| Abbreviations: AEs = adverse events; iNO = inhaled NO; RHI = reactive hyperaemia index; CPB = cardiopulmonary bypass; ICU = intensive care unit; RBCs = red blood cells; SBP = systolic blood pressure; DBP = diastolic blood pressure; FMD = flow-mediated dilation; FBF = forearm blood flow; ACh = acetyl choline; WIQ = Walking Impairment Questionnaire; TDI = tissue doppler imaging * = ongoing studies | |||||
| Clinical trials with CO donors | |||||
| Modification of Chronic Inflammation by Inhaled Carbon Monoxide in Patients With Stable COPD | COPD | iCO vs. placebo | Percentage of neutrophils in induced sputum (Time frame: 17 h after the last inhalation) Methacholine provocation threshold; exhaled CO/NO; FEV1, FVC, RAW, sGAW; inflammatory parameters in sputum and blood; 8-isoprostane in exhaled breath (Time frame: 17 h after the last inhalation) | University Medical Center Groningen, Department of Pulmonary Diseases Groningen, Netherlands, 9700RB | NCT00122694 |
| Abbreviations: iCO = inhaled CO; FEV1 = forced exhaled volume in 1 s; FVC = forced vital capacity; RAW = airway resistance; sGAW = specific airway conductance | |||||
| NOS Isoform | Cellular Localization | Modulation | |||
|---|---|---|---|---|---|
| eNOS | Plasma membrane (linked to caveolin) | Enzymatic Regulation (phosphorylation) | |||
| Enzyme | P-site | Stimuli | Effect | ||
| Akt | S615 | Shear stress; VEGF; statins; BK; 8-Br-cAMP | ↑ activity | ||
| PKA; Pim1 | S633 | ? | |||
| Akt1; AMPK; PKA; CaMKII | S1177 | Estrogens; VEGF; IGF-1; insulin; BK; shear stress; 8-Br-cAMP; statins; leptin; adiponectin; sphingosine 1-P | |||
| PKC | T495 (constitutively phosphorylated) | - | ↓ activity | ||
| PYK2 | Y657 | ? | ↑ activity | ||
| Non-enzymatic regulation | |||||
| Trigger | Mechanism | Effect | |||
| ↑ iCa2+ | ↑ CaM binding | ↑ activity | |||
| Allosteric regulation | |||||
| Molecule | Mechanism | Effect | |||
| HSP90 | Dimer stabilization | ↑ activity | |||
| iNOS | Cytoplasm | Transcriptional regulation | |||
| Trigger | Mechanism | Stimuli | Effect | ||
| LPS | NF-κB | Inflammation; infections; ischemia; mechanical overload | ↑ expression | ||
| IL-1β | |||||
| INF-γ | JAK-STAT | ||||
| nNOS | Sarcoplasmic reticulum (linked to ryanodine receptor) | Enzymatic regulation (phosphorylation) | |||
| Enzyme | P-site | Effect | |||
| CaMKI | S741 | ↓ activity | |||
| CaMKII | S852 | ||||
| ? | S1212 | ↑ activity | |||
| Non-enzymatic regulation | |||||
| Trigger | Mechanism | Effect | |||
| ↑ iCa2+ | ↑ CaM binding | ↑ activity | |||
| Allosteric regulation | |||||
| Molecule | Mechanism | Effect | |||
| PIN | Dimer destabilization | ↓ activity | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigo, E.; Comità, S.; Pagliaro, P.; Penna, C.; Mancardi, D. Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet? Int. J. Mol. Sci. 2023, 24, 12480. https://doi.org/10.3390/ijms241512480
Arrigo E, Comità S, Pagliaro P, Penna C, Mancardi D. Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet? International Journal of Molecular Sciences. 2023; 24(15):12480. https://doi.org/10.3390/ijms241512480
Chicago/Turabian StyleArrigo, Elisa, Stefano Comità, Pasquale Pagliaro, Claudia Penna, and Daniele Mancardi. 2023. "Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet?" International Journal of Molecular Sciences 24, no. 15: 12480. https://doi.org/10.3390/ijms241512480
APA StyleArrigo, E., Comità, S., Pagliaro, P., Penna, C., & Mancardi, D. (2023). Clinical Applications for Gasotransmitters in the Cardiovascular System: Are We There Yet? International Journal of Molecular Sciences, 24(15), 12480. https://doi.org/10.3390/ijms241512480








