Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations
- Umbilical cord samples represent a safe, non-invasive method to obtain valuable information regarding intrauterine conditions [77]. However, the type of delivery may affect the gas analytic values: fetal oxygenation of neonates born by vaginal delivery may, in fact, be altered by the engagement of the fetus through the birth canal or by the reduction in blood flow during uterine contractions [78], as well as by cesarean section, which can affect fetal well-being due to the cardiovascular effects of anesthesia, maternal position, and maternal ventilation [79,80]. Although the impact of delivery type on the gas test result is well known [13], in this study we did not find a different incidence of vaginal or cesarean delivery at different gestational ages, and therefore the blood samples were analyzed as a whole without further stratification.
- In this study, only umbilical cord blood samples from preterm infants were analyzed. The merging of data obtained from preterm and term newborns would have provided a better interpretation of the fluctuations in fetal oxygenation. However, the pooling of such data would not have been methodologically suitable, as umbilical cord blood samples in preterm neonates were collected within 60 sec after birth (“early cord clamping”), while samples in term neonates were collected approximately 60 sec after birth (“delayed cord clamping”) [13].
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eastman, N.J. Mount Everest in utero. Am. J. Obstet. Gynecol. 1954, 67, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef]
- Soothill, P.W.; Nicolaides, K.H.; Rodeck, C.H.; Campbell, S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986, 1, 168–175. [Google Scholar] [CrossRef]
- Weiner, C.P.; Sipes, S.L.; Wenstrom, K. The effect of fetal age upon normal fetal laboratory values and venous pressure. Obstet. Gynecol. 1992, 79, 713–718. [Google Scholar]
- Pardi, G.; Cetin, I.; Marconi, A.M.; Lanfranchi, A.; Bozzetti, P.; Ferrazzi, E.; Buscaglia, M.; Battaglia, F.C. Diagnostic value of blood sampling in fetuses with growth retardation. N. Engl. J. Med. 1993, 328, 692–696. [Google Scholar] [CrossRef]
- Nava, S.; Bocconi, L.; Zuliani, G.; Kustermann, A.; Nicolini, U. Aspects of fetal physiology from 18 to 37 weeks’ gestation as assessed by blood sampling. Obstet. Gynecol. 1996, 87, 975–980. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Santos, V.; Shih, A.Y. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef]
- Aguilar, E.; Dorrell, M.I.; Friedlander, D.; Jacobson, R.A.; Johnson, A.; Marchetti, V.; Moreno, S.K.; Ritter, M.R.; Friedlander, M. Chapter 6. Ocular models of angiogenesis. Methods Enzymol. 2008, 444, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.W.; Strube, Y.N. Retinopathy of Prematurity. In Pediatric Ophthalmology and Strabismus; Wright, K.W., Strube, Y.N., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 957–992. [Google Scholar]
- Filippi, L.; Scaramuzzo, R.T.; Pascarella, F.; Pini, A.; Morganti, R.; Cammalleri, M.; Bagnoli, P.; Ciantelli, M. Fetal oxygenation in the last weeks of pregnancy evaluated through the umbilical cord blood gas analysis. Front. Pediatr. 2023, 11, 1140021. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.S.; de Vrijer, B.; Brown, H.K.; Stitt, L.; Choo, S.; Regnault, T.R.H. Gestational age impacts birth to placental weight ratio and umbilical cord oxygen values with implications for the fetal oxygen margin of safety. Early Hum. Dev. 2022, 164, 105511. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Placental Gas Exchange and the Oxygen Supply to the Fetus. Compr. Physiol. 2015, 5, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Fathollahipour, S.; Patil, P.S.; Leipzig, N.D. Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells Tissues Organs 2018, 205, 350–371. [Google Scholar] [CrossRef]
- Houghton, F.D. Hypoxia and reproductive health: Hypoxic regulation of preimplantation embryos: Lessons from human embryonic stem cells. Reproduction 2021, 161, F41–F51. [Google Scholar] [CrossRef]
- Cowden Dahl, K.D.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol. Cell. Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef] [Green Version]
- Pringle, K.G.; Kind, K.L.; Sferruzzi-Perri, A.N.; Thompson, J.G.; Roberts, C.T. Beyond oxygen: Complex regulation and activity of hypoxia inducible factors in pregnancy. Hum. Reprod. Update 2010, 16, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of human placental development by oxygen tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef]
- Aplin, J.D. Hypoxia and human placental development. J. Clin. Investig. 2000, 105, 559–560. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Landers, K.; Mortimer, R.H.; Richard, K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell. 2009, 17, 755–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 1993, 99, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yedwab, G.A.; Paz, G.; Homonnai, T.Z.; David, M.P.; Kraicer, P.F. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of woman and uterus of rats during the cycle. Fertil. Steril. 1976, 27, 304–309. [Google Scholar] [CrossRef]
- Ottosen, L.D.; Hindkaer, J.; Husth, M.; Petersen, D.E.; Kirk, J.; Ingerslev, H.J. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reprod. Biomed. Online 2006, 13, 380–385. [Google Scholar] [CrossRef]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The Impact of Hypoxia in Early Pregnancy on Placental Cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O.; Houghton, F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010, 139, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, H.; Harris, L.J.; Zhang, P.; McIlhenny, S.; Srinivas, V.; Tulenko, T.; DiMuzio, P.J. The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 2011, 165, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Leese, H.J. Metabolic control during preimplantation mammalian development. Hum. Reprod. Update 1995, 1, 63–72. [Google Scholar] [CrossRef]
- Thompson, J.G.; Partridge, R.J.; Houghton, F.D.; Cox, C.I.; Leese, H.J. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J. Reprod. Fertil. 1996, 106, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Prather, R.S. A role for the Warburg effect in preimplantation embryo development: Metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 2012, 79, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.N.; Huang, X.B.; Muyayalo, K.P.; Mor, G.; Liao, A.H. Lactic Acid: A Novel Signaling Molecule in Early Pregnancy? Front. Immunol. 2020, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Adelman, D.M.; Gertsenstein, M.; Nagy, A.; Simon, M.C.; Maltepe, E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000, 14, 3191–3203. [Google Scholar] [CrossRef]
- Robins, J.C.; Heizer, A.; Hardiman, A.; Hubert, M.; Handwerger, S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta 2007, 28, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Wakeland, A.K.; Parast, M.M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 2018, 236, R43–R56. [Google Scholar] [CrossRef] [Green Version]
- Meschia, G.; Cotter, J.R.; Breathnach, C.S.; Barron, D.H. The hemoglobin, oxygen, carbon dioxide and hydrogen ion concentrations in the umbilical bloods of sheep and goats as sampled via indwelling plastic catheters. Q. J. Exp. Physiol. Cogn. Med. Sci. 1965, 50, 185–195. [Google Scholar] [CrossRef]
- Comline, R.S.; Silver, M. Daily changes in foetal and maternal blood of conscious pregnant ewes, with catheters in umbilical and uterine vessels. J. Physiol. 1970, 209, 567–586. [Google Scholar] [CrossRef]
- Dawes, G.S.; Fox, H.E.; Leduc, B.M.; Liggins, G.C.; Richards, R.T. Respiratory movements and rapid eye movement sleep in the foetal lamb. J. Physiol. 1972, 220, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Clewlow, F.; Dawes, G.S.; Johnston, B.M.; Walker, D.W. Changes in breathing, electrocortical and muscle activity in unanaesthetized fetal lambs with age. J. Physiol. 1983, 341, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Taylor, P.M.; White, K.L.; Forhead, A.J. Ontogenic and nutritionally induced changes in fetal metabolism in the horse. J. Physiol. 2000, 528, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Forhead, A.J.; Fowden, A.L. Development of cardiovascular function in the horse fetus. J. Physiol. 2005, 565, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Yamaleyeva, L.M.; Sun, Y.; Bledsoe, T.; Hoke, A.; Gurley, S.B.; Brosnihan, K.B. Photoacoustic imaging for in vivo quantification of placental oxygenation in mice. FASEB J. 2017, 31, 5520–5529. [Google Scholar] [CrossRef] [Green Version]
- Basak, K.; Luís Deán-Ben, X.; Gottschalk, S.; Reiss, M.; Razansky, D. Non-invasive determination of murine placental and foetal functional parameters with multispectral optoacoustic tomography. Light Sci. Appl. 2019, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Saini, B.; Morrison, J.; Seed, M. Gas Exchange across the Placenta. In Respiratory Disease in Pregnancy; Lapinsky, S., Plante, L., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 34–56. [Google Scholar]
- Schneider, H. Oxygenation of the placental-fetal unit in humans. Respir. Physiol. Neurobiol. 2011, 178, 51–58. [Google Scholar] [CrossRef]
- Templeton, A.; Kelman, G.R. Maternal blood-gases, (PAo2-Pao2), physiological shunt and VD/VT in normal pregnancy. Br. J. Anaesth. 1976, 48, 1001–1004. [Google Scholar] [CrossRef]
- Assali, N.S.; Rauramo, L.; Peltonen, T. Measurement of uterine blood flow and uterine metabolism. VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am. J. Obstet. Gynecol. 1960, 79, 86–98. [Google Scholar] [CrossRef]
- Varma, S.; Cohen, H.J. Co-transactivation of the 3’ erythropoietin hypoxia inducible enhancer by the HIF-1 protein. Blood Cells Mol. Dis. 1997, 23, 169–176. [Google Scholar] [CrossRef]
- Jopling, J.; Henry, E.; Wiedmeier, S.E.; Christensen, R.D. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: Data from a multihospital health care system. Pediatrics 2009, 123, e333–e337. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, V.G.; Xu, J.; Orkin, S.H. Advances in the understanding of haemoglobin switching. Br. J. Haematol. 2010, 149, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.N.M.; Campbell, A.; Devito, L.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell. Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mattia, M.; Mauro, A.; Citeroni, M.R.; Dufrusine, B.; Peserico, A.; Russo, V.; Berardinelli, P.; Dainese, E.; Cimini, A.; Barboni, B. Insight into Hypoxia Stemness Control. Cells 2021, 10, 2161. [Google Scholar] [CrossRef]
- Semenza, G.L. Dynamic regulation of stem cell specification and maintenance by hypoxia-inducible factors. Mol. Aspects Med. 2016, 47–48, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.J.; Csete, M.; Groves, A.K.; Melega, W.; Wold, B.; Anderson, D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000, 20, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Studer, L.; Csete, M.; Lee, S.H.; Kabbani, N.; Walikonis, J.; Wold, B.; McKay, R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 2000, 20, 7377–7383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Porat, R.M.; Alon, T.; Keshet, E.; Stone, J. Tissue oxygen levels control astrocyte movement and differentiation in developing retina. Brain Res. Dev. Brain Res. 1999, 118, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Ishizu, A.; Kurihara, T.; Okuno, Y.; Ozawa, Y.; Kishi, K.; Goda, N.; Tsubota, K.; Okano, H.; Suda, T.; Kubota, Y. The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev. Biol. 2012, 363, 106–114. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Zeng, Y.; Li, Q.; Zou, T.; Chen, S.; Wu, Q.; Fu, C.; Xu, H.; Yin, Z.Q. Intermittent high oxygen influences the formation of neural retinal tissue from human embryonic stem cells. Sci. Rep. 2016, 6, 29944. [Google Scholar] [CrossRef] [Green Version]
- Heinis, M.; Simon, M.T.; Ilc, K.; Mazure, N.M.; Pouysségur, J.; Scharfmann, R.; Duvillié, B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010, 59, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, M.A.; Sinitsyna, N.N.; Qin, Q.; Rice, R.H. Oxygen-dependent differentiation of human keratinocytes. J. Investig. Dermatol. 2007, 127, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wenum, M.; Adam, A.A.A.; van der Mark, V.A.; Chang, J.C.; Wildenberg, M.E.; Hendriks, E.J.; Jongejan, A.; Moerland, P.D.; van Gulik, T.M.; Oude Elferink, R.P.; et al. Oxygen drives hepatocyte differentiation and phenotype stability in liver cell lines. J. Cell Commun. Signal. 2018, 12, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Berthelemy, N.; Kerdjoudj, H.; Schaaf, P.; Prin-Mathieu, C.; Lacolley, P.; Stoltz, J.F.; Voegel, J.C.; Menu, P. O2 level controls hematopoietic circulating progenitor cells differentiation into endothelial or smooth muscle cells. PLoS ONE 2009, 4, e5514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.D.; Ryan, S.L.; Ingram, D.A.; Seedorf, G.J.; Abman, S.H.; Balasubramaniam, V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am. J. Respir. Crit. Care Med. 2009, 180, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Wisgrill, L.; Schüller, S.; Bammer, M.; Berger, A.; Pollak, A.; Radke, T.F.; Kögler, G.; Spittler, A.; Helmer, H.; Husslein, P.; et al. Hematopoietic stem cells in neonates: Any differences between very preterm and term neonates? PLoS ONE 2014, 9, e106717. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.J.; Mead, L.E.; Prater, D.; Bessler, W.K.; Foster, D.; Case, J.; Goebel, W.S.; Yoder, M.C.; Haneline, L.S.; Ingram, D.A. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr. Res. 2008, 64, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Borghesi, A.; Massa, M.; Campanelli, R.; Bollani, L.; Tzialla, C.; Figar, T.A.; Ferrari, G.; Bonetti, E.; Chiesa, G.; de Silvestri, A.; et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2009, 180, 540–546. [Google Scholar] [CrossRef]
- Cammalleri, M.; Amato, R.; Dal Monte, M.; Filippi, L.; Bagnoli, P. The β3 adrenoceptor in proliferative retinopathies: “Cinderella” steps out of its family shadow. Pharmacol. Res. 2023, 190, 106713. [Google Scholar] [CrossRef]
- Puebla, M.; Tapia, P.J.; Espinoza, H. Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis. Int. J. Mol. Sci. 2022, 23, 2646. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 2014, 41, 47–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, B.L.; Mychaliska, G.B. Milestones for clinical translation of the artificial placenta. Semin. Fetal Neonatal Med. 2022, 27, 101408. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Dal Monte, M. β3-Adrenoceptor, a novel player in the round-trip from neonatal diseases to cancer: Suggestive clues from embryo. Med. Res. Rev. 2022, 42, 1179–1201. [Google Scholar] [CrossRef]
- Armstrong, L.; Stenson, B.J. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F430–F434. [Google Scholar] [CrossRef]
- Brotanek, V.; Hendricks, C.H.; Yoshida, T. Changes in uterine blood flow during uterine contractions. Am. J. Obstet. Gynecol. 1969, 103, 1108–1116. [Google Scholar] [CrossRef]
- Szymanowski, P.; Szepieniec, W.K.; Zarawski, M.; Gruszecki, P.; Szweda, H.; Jóźwik, M. The impact of birth anesthesia on the parameters of oxygenation and acid-base balance in umbilical cord blood. J. Matern. Fetal Neonatal Med. 2020, 33, 3445–3452. [Google Scholar] [CrossRef]
- Raghuraman, N.; Temming, L.A.; Doering, M.M.; Stoll, C.R.; Palanisamy, A.; Stout, M.J.; Colditz, G.A.; Cahill, A.G.; Tuuli, M.G. Maternal Oxygen Supplementation Compared with Room Air for Intrauterine Resuscitation: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021, 175, 368–376. [Google Scholar] [CrossRef]
- Ayres-de-Campos, D.; Arulkumaran, S.; FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Physiology of fetal oxygenation and the main goals of intrapartum fetal monitoring. Int. J. Gynaecol. Obstet. 2015, 131, 5–8. [Google Scholar] [CrossRef] [Green Version]
- D’Alton, M.E.; Hankins, G.D.V.; Berkowitz, R.L.; Bienstock, J.; Ghidini, A.; Goldsmith, J.; Higgins, R.; Moore, T.R.; Natale, R.; Nelson, K.B.; et al. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet. Gynecol. 2014, 123, 896–901. [Google Scholar] [CrossRef]
- Westgate, J.; Garibaldi, J.M.; Greene, K.R. Umbilical cord blood gas analysis at delivery: A time for quality data. Br. J. Obstet. Gynaecol. 1994, 101, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.G. Clinical application of the Henderson-Hasselbalch equation. Appl. Ther. 1965, 7, 730–736. [Google Scholar] [PubMed]
- Siggaard-Andersen, O.; Engel, K. A new acid-base nomogram. An improved method for the calculation of the relevant blood acid-base data. Scand. J. Clin. Lab. Investig. 1960, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
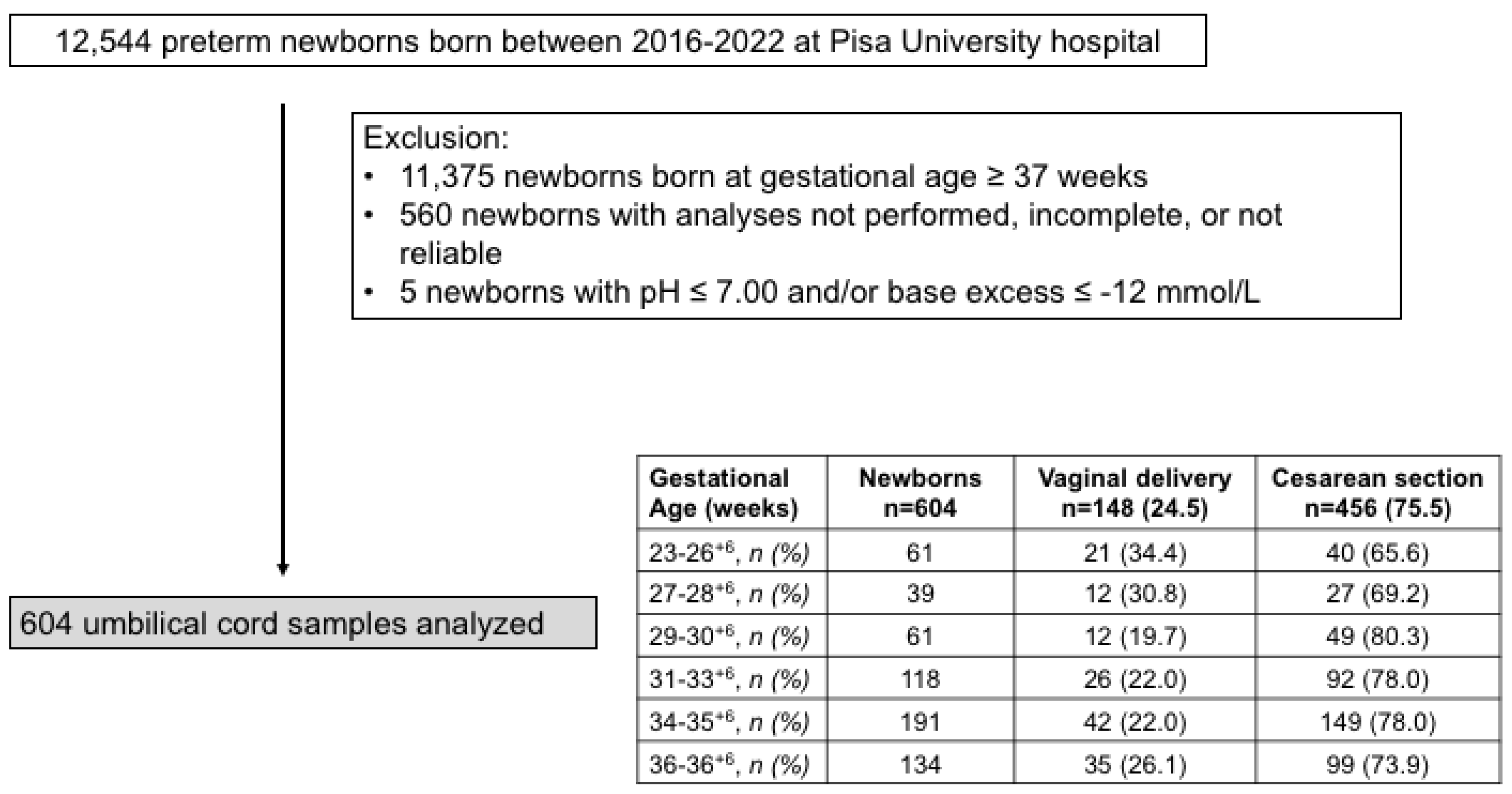

| All Preterm Newborns n = 604 | Vaginal Delivery n = 148 | Cesarean Section n = 456 | p Value | |
|---|---|---|---|---|
| GA, days, mean (SD) | 230 (24) | 228 (28) | 231 (23) | 0.127 |
| Birth weight, g, mean (SD) | 1855 (687) | 1926 (728) | 1832 (672) | 0.155 |
| Male, n (%) | 310 (51.2) | 80 (54.0) | 230 (50.4) | 0.474 |
| Apgar Score at 5 min, mean (SD) | 7.6 (1.2) | 7.6 (1.4) | 7.7 (1.1) | 0.781 |
| Umbilical venous cord sampling | ||||
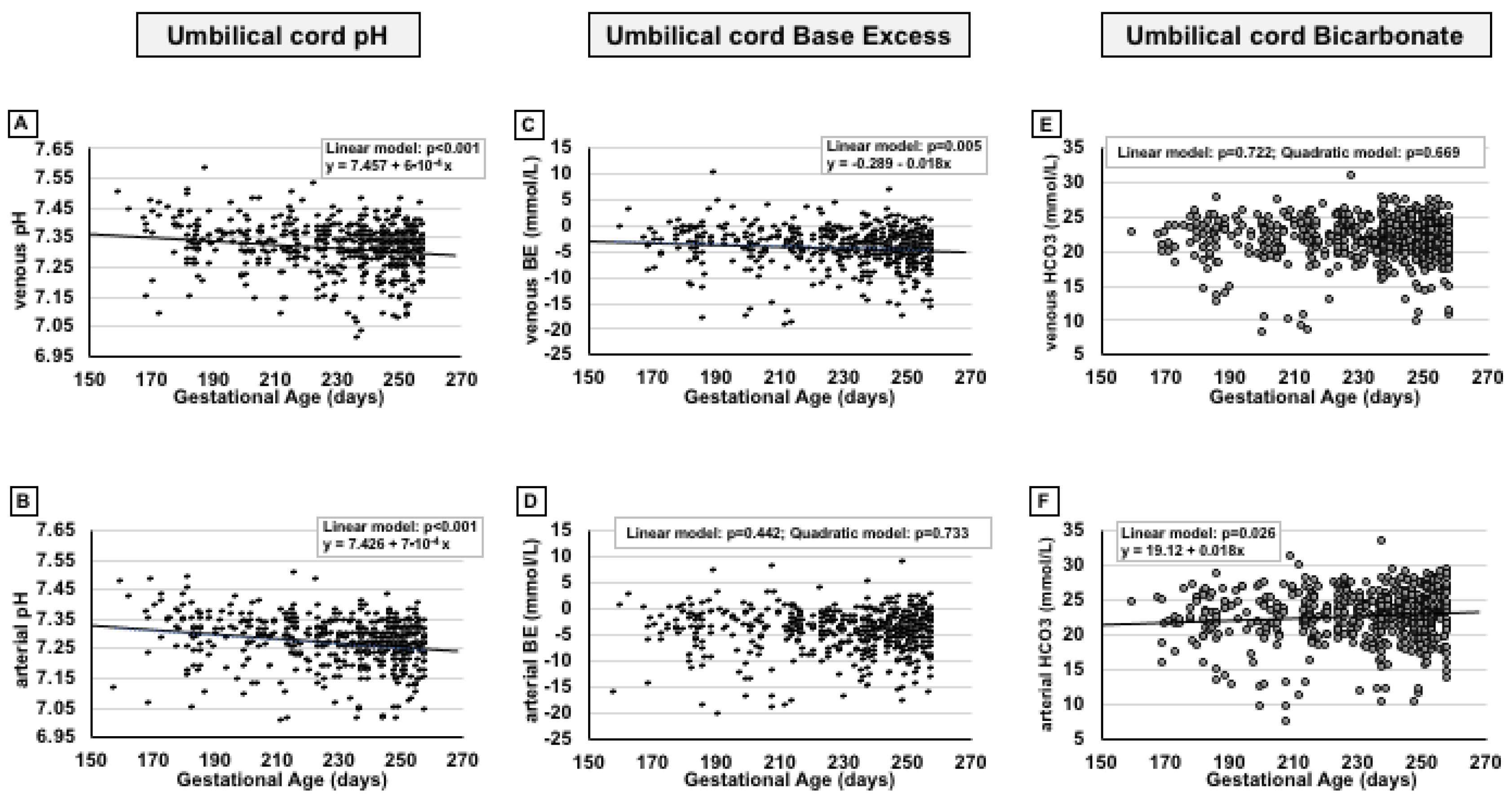
| pH, mean (SD) | 7.315 (0.08) | 7.334 (0.08) | 7.309 (0.08) | <0.001 |
| pCO2, mmHg, mean (SD) | 43.2 (9.4) | 39.9 (9.5) | 44.3 (9.2) | <0.001 |
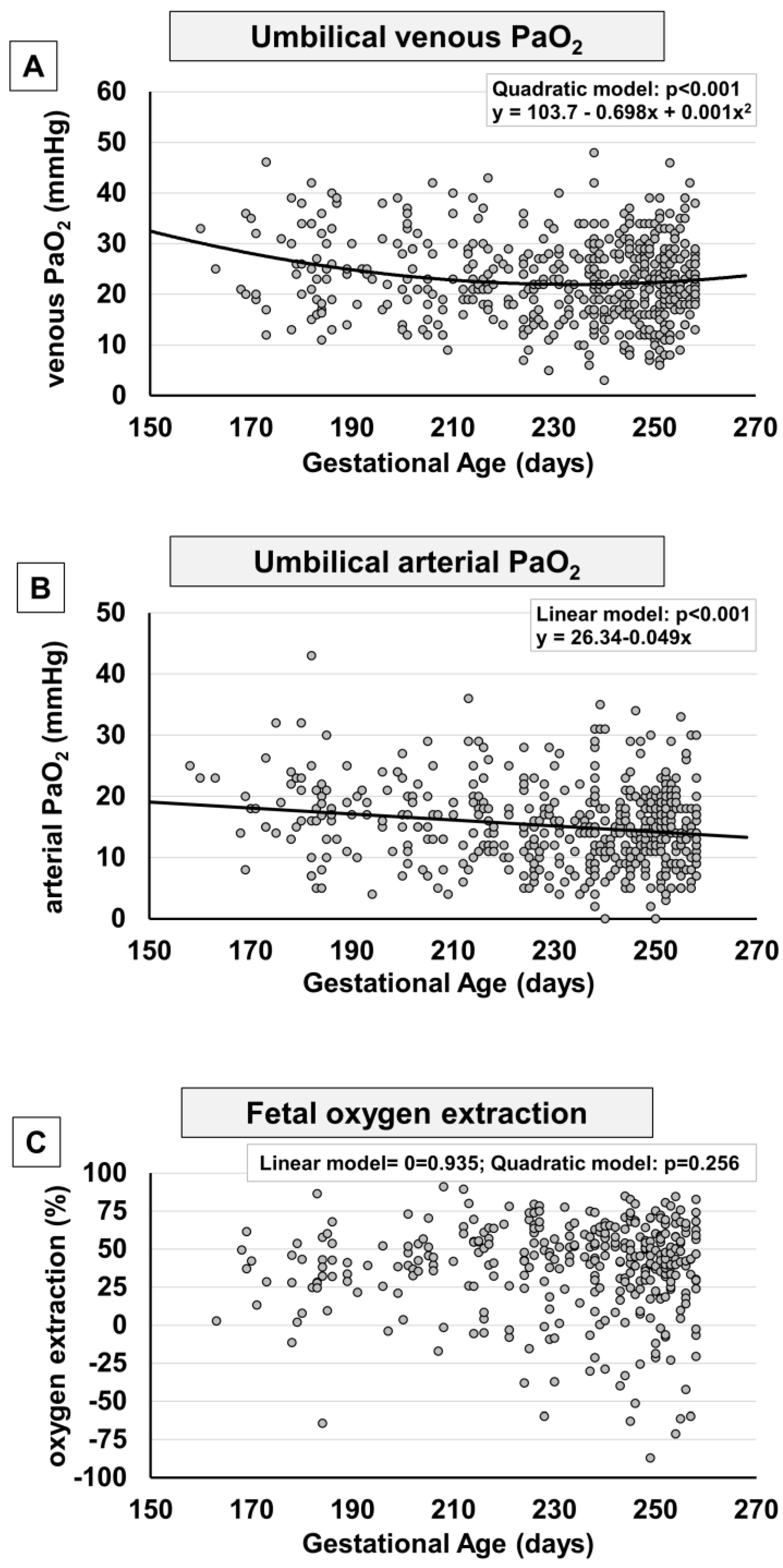
| pO2, mmHg, mean (SD) | 22.8 (7.9) | 25.3 (8.3) | 22.0 (7.6) | <0.001 |
| Bicarbonate, mmol/L, mean (SD) | 21.7 (3.1) | 20.9 (2.7) | 22.0 (3.1) | <0.001 |
| BE(B), mmol/L, mean (SD) | −4.3 (3.6) | −4.6 (3.5) | −4.3 (3.6) | 0.250 |
| Lactate, mmol/L, mean (SD) | 2.8 (1.4) | 3.3 (1.4) | 2.7 (1.3) | <0.001 |
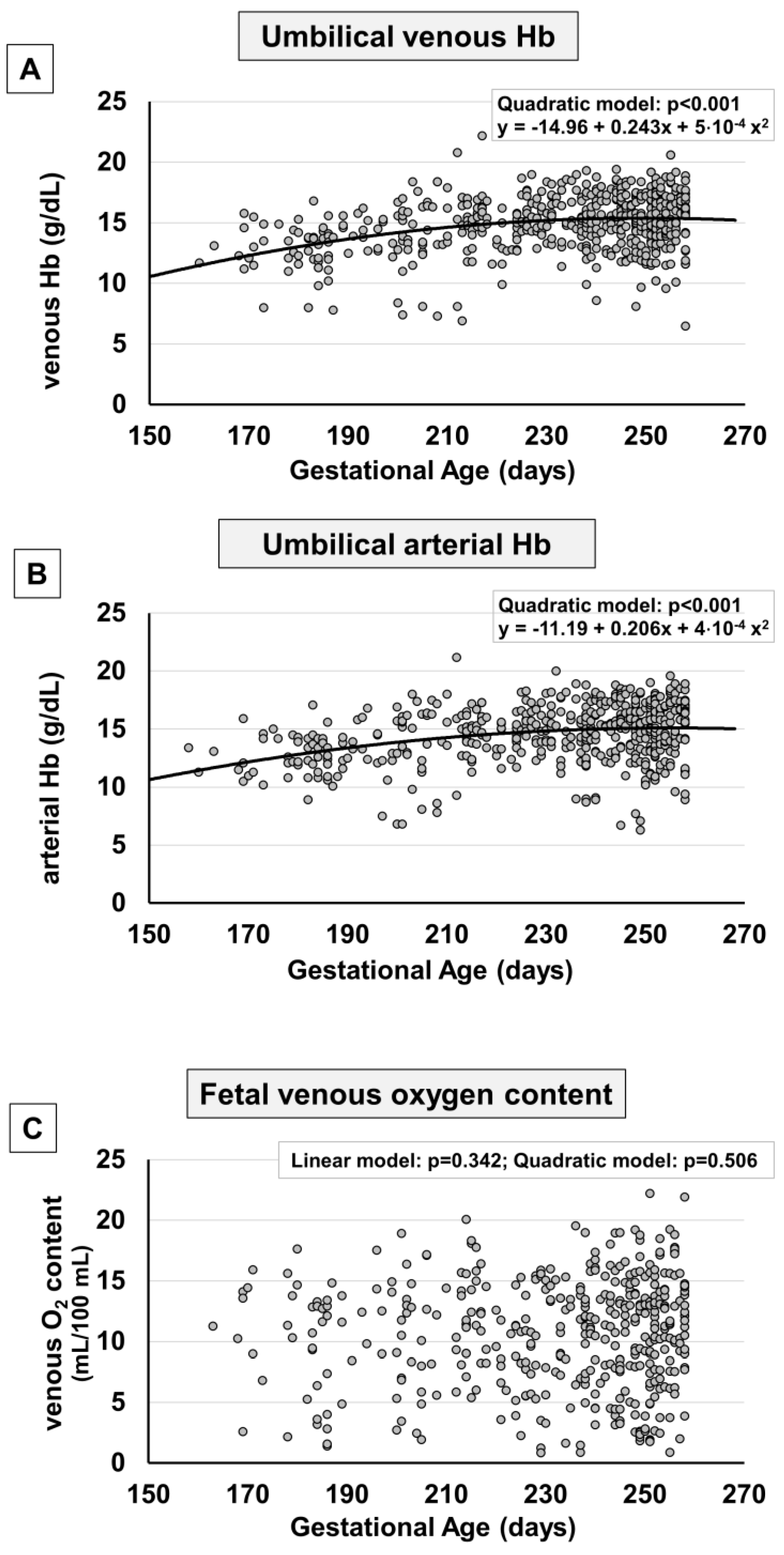
| Hemoglobin, g/dL, mean (SD) | 14.96 (2.3) | 15.5 (1.9) | 14.8 (2.4) | 0.001 |
| SaO2, %, mean (SD) | 52.9 (22.1) | 57.4 (21.4) | 51.3 (22.2) | 0.011 |
| Umbilical arterial cord sampling | ||||
| pH, mean (SD) | 7.267 (0.08) | 7.274 (0.09) | 7.278 (0.08) | 0.327 |
| pCO2, mmHg, mean (SD) | 50.7 (11.4) | 49.7 (11.8) | 51.0 (11.3) | 0.243 |
| pO2, mmHg, mean (SD) | 15.2 (6.9) | 17.6 (6.7) | 14.4 (6.8) | <0.001 |
| Bicarbonate, mmol/L, mean (SD) | 22.7 (3.8) | 22.3 (3.2) | 22.8 (3.9) | 0.227 |
| BE(B), mmol/L, mean (SD) | −4.6 (4.1) | −4.8 (3.7) | −4.5 (4.3) | 0.414 |
| Lactate, mmol/L, mean (SD) | 3.1 (1.5) | 3.7 (1.6) | 2.9 (1.5) | <0.001 |
| Hemoglobin, g/dL, mean (SD) | 14.96 (2.3) | 15.3 (2.1) | 14.4 (2.5) | <0.001 |
| SaO2, %, mean (SD) | 33.0 (19.2) | 40.4 (20.0) | 30.0 (18.2) | <0.001 |
| Veno-arterial O2 difference, mmHg, mean (SD) | 7.7 (6.4) | 7.5 (7.1) | 7.8 (6.2) | 0.665 |
| Fetal oxygen extraction, %, mean (SD) | 35.7 (35.8) | 27.3 (32.0) | 38.6 (36.8) | 0.007 |
| GA = gestational age; pO2 = partial pressure of oxygen; pCO2 = partial pressure of carbon dioxide; BE = base excess. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, L.; Pascarella, F.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Morganti, R.; Innocenti, F.; Castagnini, N.; Melosi, A.; Scaramuzzo, R.T. Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis. Int. J. Mol. Sci. 2023, 24, 12487. https://doi.org/10.3390/ijms241512487
Filippi L, Pascarella F, Pini A, Cammalleri M, Bagnoli P, Morganti R, Innocenti F, Castagnini N, Melosi A, Scaramuzzo RT. Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis. International Journal of Molecular Sciences. 2023; 24(15):12487. https://doi.org/10.3390/ijms241512487
Chicago/Turabian StyleFilippi, Luca, Francesca Pascarella, Alessandro Pini, Maurizio Cammalleri, Paola Bagnoli, Riccardo Morganti, Francesca Innocenti, Nicola Castagnini, Alice Melosi, and Rosa Teresa Scaramuzzo. 2023. "Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis" International Journal of Molecular Sciences 24, no. 15: 12487. https://doi.org/10.3390/ijms241512487
APA StyleFilippi, L., Pascarella, F., Pini, A., Cammalleri, M., Bagnoli, P., Morganti, R., Innocenti, F., Castagnini, N., Melosi, A., & Scaramuzzo, R. T. (2023). Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis. International Journal of Molecular Sciences, 24(15), 12487. https://doi.org/10.3390/ijms241512487








