Reducing 3D Hydrogel Stiffness, Addition of Oestradiol in a Physiological Concentration and Increasing FSH Concentration Improve In Vitro Growth of Murine Preantral Follicles
Abstract
:1. Introduction
2. Results
2.1. Effect of Oestradiol and Follicle Re-Embedding Halfway through the Culture
2.2. Addition of Oestradiol in a Physiological Concentration Promotes Follicle Growth
2.3. Reduction of Alginate Concentration during Culture Can Promote Follicle Growth In Vitro
2.4. Concentration of Anti-Müllerian Hormone (AMH) in the Condition Medium
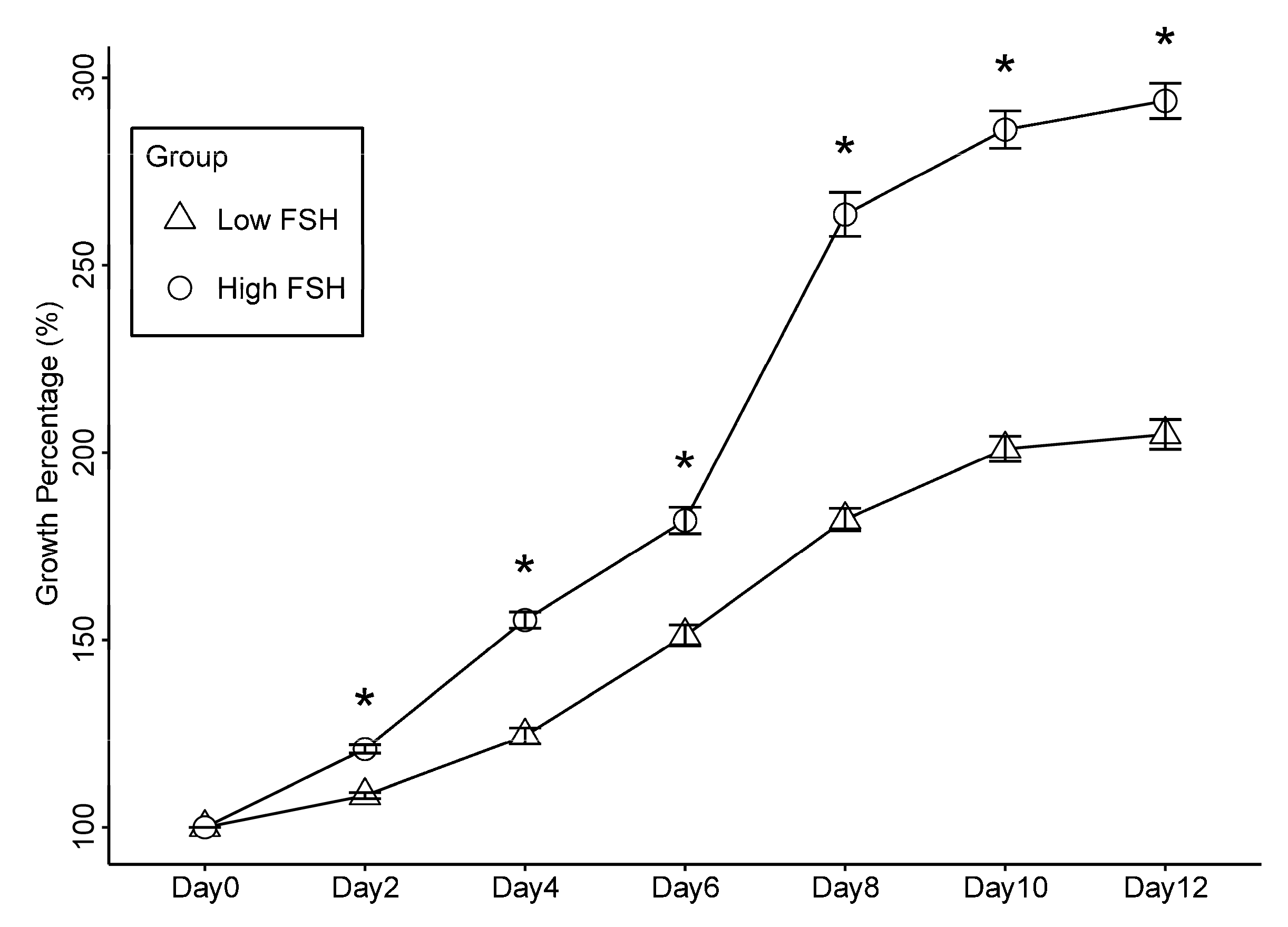
2.5. Increased Level of FSH Promotes the Growth of Secondary Follicles in Culture
3. Discussion
4. Materials and Methods
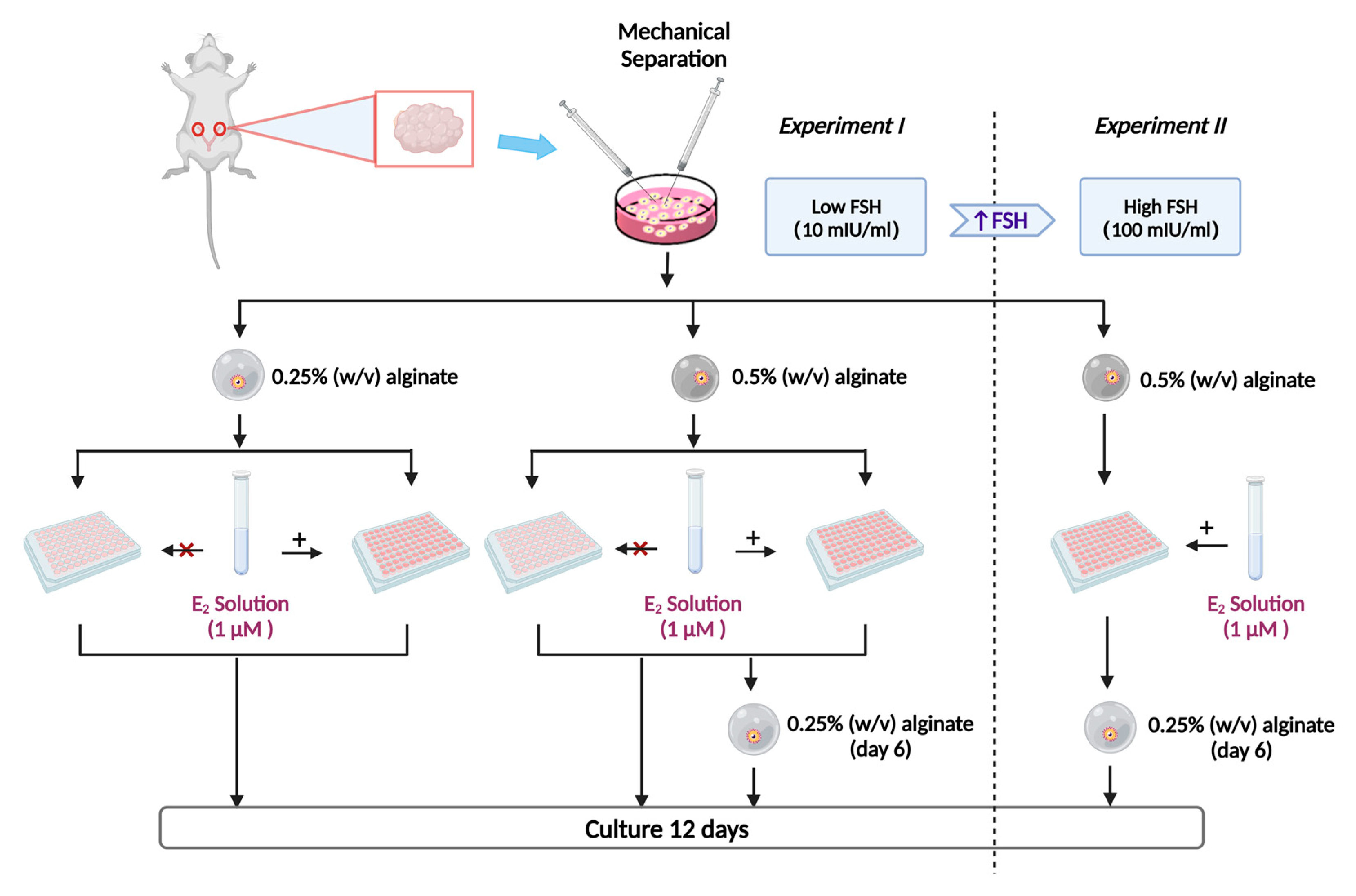
4.1. Experiment Design
4.2. Animals
4.3. Follicle Isolation, Encapsulation, Re-Encapsulation and Culture
4.4. Alginate Hydrogel Preparation
4.5. Follicle Measurement
4.6. AMH Hormone Assays
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, I.R.; Lima, I.M.T.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Figueiredo, J.R. Three-dimensional systems for in vitro follicular culture: Overview of alginate-based matrices. Reprod. Fertil. Dev. 2014, 26, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Green, L.J.; Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 2016, 86, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Woodruff, T.K.; Shea, L.D. Bioengineering and the ovarian follicle. Cancer Treat. Res. 2007, 138, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Pais, A.S.; Reis, S.; Laranjo, M.; Caramelo, F.; Silva, F.; Botelho, M.F.; Almeida-Santos, T. The challenge of ovarian tissue culture: 2D versus 3D culture. J. Ovarian Res. 2021, 14, 147. [Google Scholar] [CrossRef]
- Khunmanee, S.; Park, H. Three-Dimensional Culture for In Vitro Folliculogenesis in the Aspect of Methods and Materials. Tissue Eng. Part B Rev. 2022, 28, 1242–1257. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Brito, I.R.; Silva, C.M.G.; Duarte, A.B.G.; Lima, I.M.T.; Rodrigues, G.Q.; Rossetto, R.; Sales, A.D.; Lobo, C.H.; Bernuci, M.P.; Rosa-E-Silva, A.C.J.S.; et al. Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol. Reprod. Dev. 2014, 81, 636–645. [Google Scholar] [CrossRef]
- Xu, M.; West, E.; Shea, L.D.; Woodruff, T.K. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol. Reprod. 2006, 75, 916–923. [Google Scholar] [CrossRef] [Green Version]
- West, E.R.; Xu, M.; Woodruff, T.K.; Shea, L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007, 28, 4439–4448. [Google Scholar] [CrossRef] [Green Version]
- Vanacker, J.; Amorim, C.A. Alginate: A Versatile Biomaterial to Encapsulate Isolated Ovarian Follicles. Ann. Biomed. Eng. 2017, 45, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Shea, L.D. A new hypothesis regarding ovarian follicle development: Ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Genet. 2011, 28, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendell, J.J.; Dorrington, J. Estradiol-17 beta stimulates DNA synthesis in rat granulosa cells: Action mediated by transforming growth factor-beta. Endocrinology 1991, 128, 2663–2665. [Google Scholar] [CrossRef]
- Palter, S.F.; Tavares, A.B.; Hourvitz, A.; Veldhuis, J.D.; Adashi, E.Y. Are estrogens of import to primate/human ovarian folliculogenesis? Endocr. Rev. 2001, 22, 389–424. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.N.; Greenwald, G.S. Synergistic effects of steroids with FSH on folliculogenesis, steroidogenesis and FSH- and hCG-receptors in hypophysectomized mice. J. Reprod. Fertil. 1993, 99, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couse, J.F.; Yates, M.M.; Deroo, B.J.; Korach, K.S. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005, 146, 3247–3262. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Vaitukaitis, J.L.; Ross, G.T. Estrogen and follicle stimulation hormone interactions on follicle growth in rats. Endocrinology 1972, 90, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Robker, R.L.; Richards, J.S. Hormone-induced proliferation and differentiation of granulosa cells: A coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol. Endocrinol. 1998, 12, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Spears, N.; Murray, A.A.; Allison, V.; Boland, N.I.; Gosden, R.G. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J. Reprod. Fertil. 1998, 113, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatum, M.; Gyo, Y.; Diana, P.; Laufer, N.; Simon, A. Is estradiol mandatory for an adequate follicular and embryo development? A mouse model using aromatase inhibitor (anastrozole). J. Assist. Reprod. Genet. 2006, 23, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Tarumi, W.; Itoh, M.T.; Suzuki, N. Effects of 5α-dihydrotestosterone and 17β-estradiol on the mouse ovarian follicle development and oocyte maturation. PLoS ONE 2014, 9, e99423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.A.K. ERβ Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef]
- Khristi, V.; Chakravarthi, V.P.; Singh, P.; Ghosh, S.; Pramanik, A.; Ratri, A.; Borosha, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Mol. Cell. Endocrinol. 2018, 474, 214–226. [Google Scholar] [CrossRef]
- Richards, J.S. Maturation of ovarian follicles: Actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 1980, 60, 51–89. [Google Scholar] [CrossRef]
- Gore-Langton, R.E.; Daniel, S.A. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol. Reprod. 1990, 43, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, S.L.; Richards, J.S. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology 1991, 129, 1452–1462. [Google Scholar] [CrossRef]
- Knecht, M.; Darbon, J.M.; Ranta, T.; Baukal, A.J.; Catt, K.J. Estrogens enhance the adenosine 3′,5′-monophosphate-mediated induction of follicle-stimulating hormone and luteinizing hormone receptors in rat granulosa cells. Endocrinology 1984, 115, 41–49. [Google Scholar] [CrossRef]
- Rajabi, Z.; Yazdekhasti, H.; Noori Mugahi, S.M.H.; Abbasi, M.; Kazemnejad, S.; Shirazi, A.; Majidi, M.; Zarnani, A.-H. Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod. Biol. 2018, 18, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef]
- Tagler, D.; Makanji, Y.; Anderson, N.R.; Woodruff, T.K.; Shea, L.D. Supplemented αMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol. Bioeng. 2013, 110, 3258–3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrados, C.S.; Cadenas, J.; Zheng, M.; Lund, S.; Larsen, E.C.; Tanvig, M.H.; Greve, V.H.; Blanche, P.; Andersen, C.Y.; Kristensen, S.G. Human platelet lysate improves the growth and survival of cultured human pre-antral follicles. Reprod. Biomed. Online, 2023; in press. [Google Scholar] [CrossRef]
- Spearow, J.L.; Barkley, M. Genetic control of hormone-induced ovulation rate in mice. Biol. Reprod. 1999, 61, 851–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, K.; Fenwick, M.; Mora, J.; Laird, M.; Thomson, K.; Franks, S. Onset and Heterogeneity of Responsiveness to FSH in Mouse Preantral Follicles in Culture. Endocrinology 2017, 158, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Nayudu, P.L.; Vitt, U.A.; Barrios De Tomasi, J.; Pancharatna, K.; Ulloa-Aguirre, A. Intact follicle culture: What it can tell us about the roles of FSH glycoforms during follicle development. Reprod. Biomed. Online 2002, 5, 240–253. [Google Scholar] [CrossRef]
- Vitt, U.A.; Kloosterboer, H.J.; Rose, U.M.; Mulders, J.W.; Kiesel, P.S.; Bete, S.; Nayudu, P.L. Isoforms of human recombinant follicle-stimulating hormone: Comparison of effects on murine follicle development in vitro. Biol. Reprod. 1998, 59, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Garavelas, A.; Mallis, P.; Michalopoulos, E.; Nikitos, E. Clinical Benefit of Autologous Platelet-Rich Plasma Infusion in Ovarian Function Rejuvenation: Evidence from a Before-After Prospective Pilot Study. Medicines 2023, 10, 19. [Google Scholar] [CrossRef]
- Yin, H.; Kristensen, S.G.; Jiang, H.; Rasmussen, A.; Andersen, C.Y. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum. Reprod. 2016, 31, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.A.; de Jong, F.H.; Laven, J.S.E.; Themmen, A.P.N. Anti-Müllerian hormone: A new marker for ovarian function. Reproduction 2006, 131, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 1 October 2022).
- Zeileis, A.; Köll, S.; Graham, N. Various Versatile Variances: An Object-Oriented Implementation of Clustered Covariances in R. J. Stat. Soft. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Zeileis, A. Econometric Computing with HC and HAC Covariance Matrix Estimators. J. Stat. Soft. 2004, 11, 1–17. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319242774. [Google Scholar]
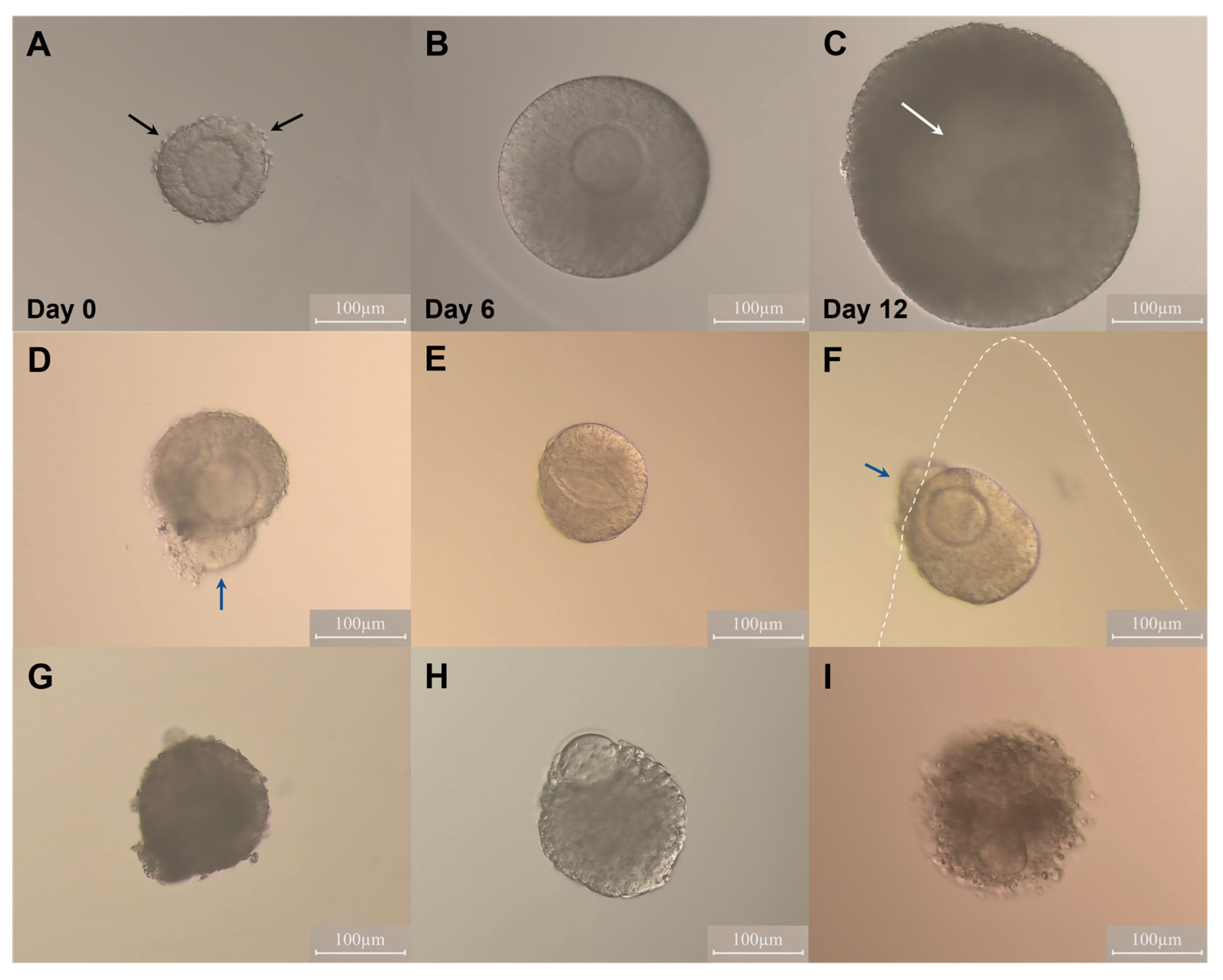
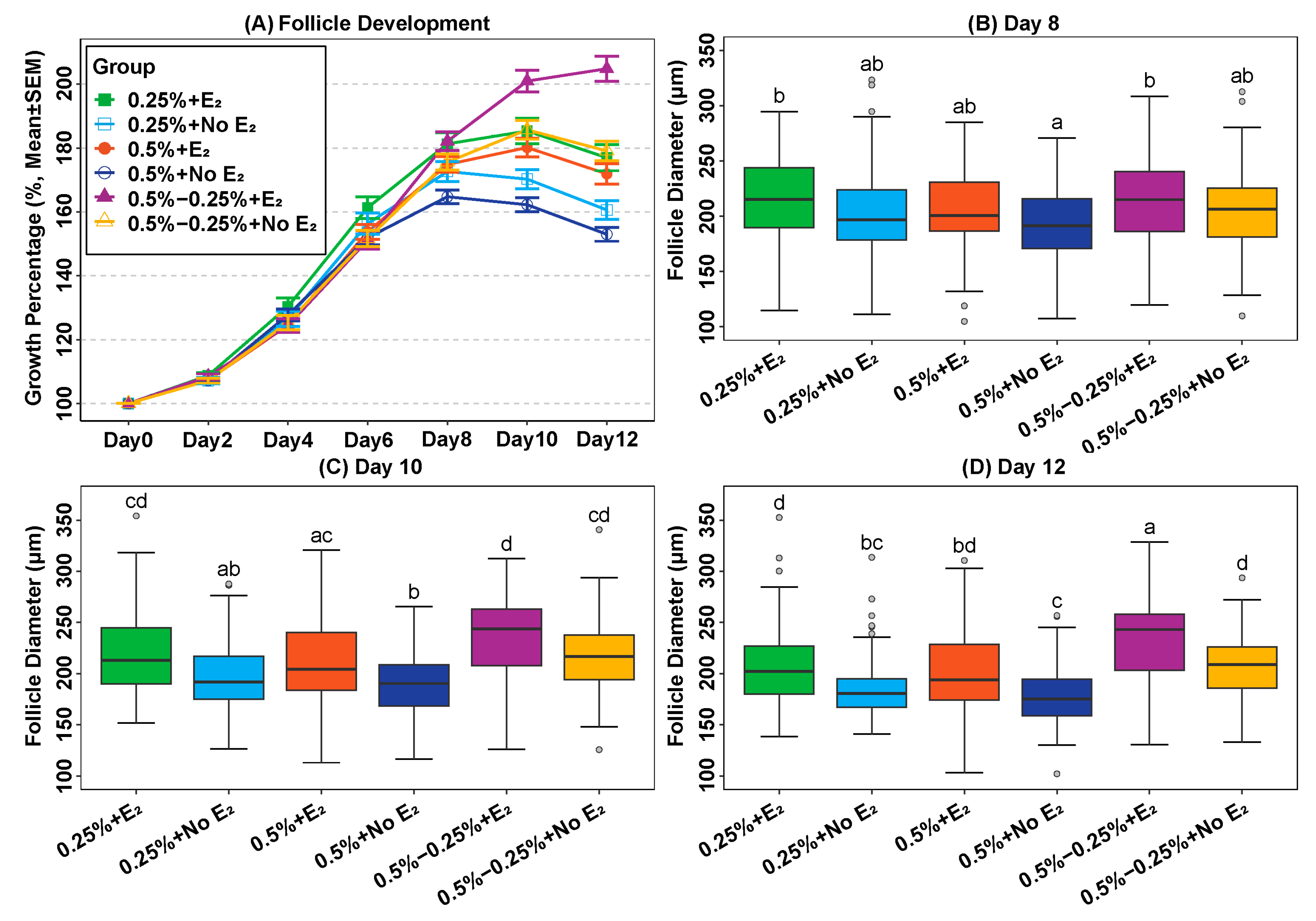
| Groups | N | Follicle Diameter (μm, mean (SD)) | Survival (%) | Antrum Formation (%) | |||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 8 | Day 10 | Day 12 | ||||
| 0.25% alginate + E2 | 85 | 118 (9) | 214 b (39) | 218 c,d (40) | 208 d (40) | 84% a | 13% a,b,c |
| 0.25% alginate + no E2 | 88 | 117 (8) | 202 a,b (39) | 198 a,b (33) | 187 b,c (32) | 78% a | 1% d |
| 0.5% alginate + E2 | 123 | 117 (8) | 205 a,b (34) | 210 a,c (38) | 202 b,d (40) | 72% a,b | 4% c,d |
| 0.5% alginate + no E2 | 125 | 118 (8) | 193 a (30) | 190 b (29) | 180 c (29) | 71% a,b | 0% d |
| 0.5–0.25% alginate + E2 | 103 | 117 (8) | 213 b (41) | 232 d (41) | 233 a (42) | 56% b | 20% b |
| 0.5–0.25% alginate + no E2 | 109 | 117 (8) | 206 a,b (36) | 215 c,d (36) | 206 d (34) | 54% b | 3% a,c,d |
| Comparison Groups | Est. Diff (µm) | 95% CI | p-Value | |
|---|---|---|---|---|
| Day 0 | 0.5–0.25% vs. 0.25% | −0.6 | (−2.6; 1.4) | 0.75 |
| 0.50% vs. 0.25% | 0 | (−2.0; 2.0) | 1.00 | |
| 0.50% vs. 0.5–0.25% | 0.6 | (−1.2; 2.4) | 0.69 | |
| 0.5–0.25% vs. 0.25% | −0.7 | (−4.4; 2.9) | 0.89 | |
| Day 2 | 0.50% vs. 0.25% | 0 | (−4.2; 2.4) | 0.8 |
| 0.50% vs. 0.5–0.25% | −0.2 | (−3.2; 2.9) | 0.99 | |
| 0.5–0.25% vs. 0.25% | −4.8 | (−13.1; 3.6) | 0.37 | |
| Day 4 | 0.50% vs. 0.25% | −2.8 | (−10.5; 4.8) | 0.66 |
| 0.50% vs. 0.5–0.25% | 2 | (−5.1; 9.0) | 0.79 | |
| 0.5–0.25% vs. 0.25% | −9.5 | (−19.8; 0.7) | 0.07 | |
| Day 6 | 0.50% vs. 0.25% | −7.4 | (−16.9; 2.2) | 0.17 |
| 0.50% vs. 0.5–0.25% | 2.2 | (−6.3; 10.6) | 0.82 | |
| 0.5–0.25% vs. 0.25% | 1.5 | (−8.1; 11.0) | 0.93 | |
| Day 8 | 0.50% vs. 0.25% | −8.8 | (−17.3; −0.3) | 0.04 * |
| 0.50% vs. 0.5–0.25% | −10.3 | (−18.3; −2.2) | 0.01 * | |
| 0.5–0.25% vs. 0.25% | 15.8 | (5.9; 25.8) | <0.001 * | |
| Day 10 | 0.50% vs. 0.25% | −7.7 | (−16.4; 0.9) | 0.09 |
| 0.50% vs. 0.5–0.25% | −23.6 | (−32.6; −14.5) | <0.001 * | |
| 0.5–0.25% vs. 0.25% | 22 | (11.1; 33.0) | <0.001 * | |
| Day 12 | 0.50% vs. 0.25% | −6.6 | (−16.1; 2.9) | 0.23 |
| 0.50% vs. 0.5–0.25% | −28.6 | (−39.0; −18.3) | <0.001 * | |
| Day 0 | E2 vs. without E2 | 0.2 | (−1.1; 1.5) | 0.76 |
| Day 2 | 1.3 | (−0.9; 3.5) | 0.26 | |
| Day 4 | −0.6 | (−5.7; 4.5) | 0.81 | |
| Day 6 | 2.4 | (−3.8; 8.6) | 0.45 | |
| Day 8 | 10 | (4.2; 15.8) | <0.001 * | |
| Day 10 | 19.1 | (13.0; 25.3) | <0.001 * | |
| Day 12 | 22.9 | (16.1; 29.8) | <0.001 * |
| AMH Concentration (ng/mL) | 0.25% + E2 | 0.25% + No E2 | 0.5% + E2 | 0.5% + No E2 | 0.5−0.25%+ E2 | 0.5–0.25% + No E2 |
|---|---|---|---|---|---|---|
| (N = 14) | (N = 16) | (N = 10) | (N = 15) | (N = 9) | (N = 3) | |
| Day 2 + Day 4 | ||||||
| Mean (SD) | 3.1 (0.3) | 0.9 (0.5) | 0.6 (0.5) | 1.2 (1.1) | 3.0 (3.2) | 1.7 (1.3) |
| Median (Min, Max) | 3.3 (2.8, 3.4) | 0.9 (0.1, 1.6) | 0.5 (0.1, 1.3) | 1.1 (0.1, 2.3) | 1.8 (0.7, 9.5) | 1.7 (0.8, 2.6) |
| Invalid tests | 11 (78.6%) | 10 (62.5%) | 6 (60.0%) | 12 (80.0%) | 1 (11.1%) | 1 (33.3%) |
| Day 6 + Day 8 | ||||||
| Mean (SD) | 3.5 (4.0) | 1.2 (1.7) | 3.6 (2.9) | 1.7 (1.1) | 4.3 (2.7) | 5.9 (1.9) |
| Median (Min, Max) | 2.3 (0.1, 12) | 0.50 (0.0, 4.2) | 2.9 (1.5, 11) | 1.3 (0.3, 3.9) | 3.8 (0.4, 8.8) | 6.9 (3.8, 7.1) |
| Invalid tests | 6 (42.9%) | 11 (68.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Day 10 + Day 12 | ||||||
| Mean (SD) | 2.7 (1.9) | 1.5 (0.6) | 1.1 (0.5) | 1.0 (0.6) | 1.5 (1.5) | 2.0 (1.3) |
| Median (Min, Max) | 2.6 (0.1, 5.5) | 1.4 (0.5, 2.5) | 1.1 (0.6, 1.7) | 0.9 (0.1, 2.5) | 1.0 (0.5, 4.4) | 2.0 (1.1, 3.0) |
| Invalid tests | 0 (0%) | 0 (0%) | 6 (60.0%) | 2 (13.3%) | 3 (33.3%) | 1 (33.3%) |
| Comparison Groups | Est. Diff | 95% CI | p-Value | |
|---|---|---|---|---|
| Day 2 + Day 4 | 0.50% vs. 0.25% | −0.9 | (−2.3; 0.4) | 0.23 |
| 0.5–0.25% vs. 0.25% | 0.2 | (−1.1; 1.6) | 0.92 | |
| 0.5–0.25% vs. 0.50% | 1.1 | (−0.2; 2.5) | 0.11 | |
| 0.50% vs. 0.25% | 0.3 | (−0.5; 1.1) | 0.65 | |
| Day 6 + Day 8 | 0.5–0.25% vs. 0.25% | 0.8 | (−0.1; 1.7) | 0.11 |
| 0.5–0.25% vs. 0.50% | 0.5 | (−0.3; 1.3) | 0.31 | |
| 0.50% vs. 0.25% | −0.6 | (−1.1; −0.1) | 0.016 * | |
| Day 10 + Day 12 | 0.5–0.25% vs. 0.25% | −0.4 | (−1.3; 0.5) | 0.53 |
| 0.5–0.25% vs. 0.50% | 0.2 | (−0.7; 1.1) | 0.82 | |
| Day 2 + Day 4 | NO E2 vs. E2 | −0.6 | (−1.5; 0.4) | 0.24 |
| Day 6 + Day 8 | −0.5 | (−1.1; 0.01) | 0.054 | |
| Day 10 + Day 12 | −0.3 | (−0.7; 0.1) | 0.18 |
| Groups | N | Survival (%) | Follicle Diameter (μm, Mean (SD)) | Antrum Formation (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | ||||
| Low FSH group (10 mIU/mL) | 103 | 56% | 117 (8) | 127 a (15) | 146 a (32) | 178 a (41) | 213 a (41) | 232 a (41) | 233 a (42) | 20% a |
| High FSH group (100 mIU/mL) | 33 | 67% | 118 (6) | 142 b (12) | 183 b (21) | 215 b (31) | 311 b (48) | 337 b (40) | 343 b (34) | 82% b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Cadenas, J.; Pors, S.E.; Esa, T.; Kristensen, S.G.; Mamsen, L.S.; Adrados, C.S.; Andersen, C.Y. Reducing 3D Hydrogel Stiffness, Addition of Oestradiol in a Physiological Concentration and Increasing FSH Concentration Improve In Vitro Growth of Murine Preantral Follicles. Int. J. Mol. Sci. 2023, 24, 12499. https://doi.org/10.3390/ijms241512499
Zheng M, Cadenas J, Pors SE, Esa T, Kristensen SG, Mamsen LS, Adrados CS, Andersen CY. Reducing 3D Hydrogel Stiffness, Addition of Oestradiol in a Physiological Concentration and Increasing FSH Concentration Improve In Vitro Growth of Murine Preantral Follicles. International Journal of Molecular Sciences. 2023; 24(15):12499. https://doi.org/10.3390/ijms241512499
Chicago/Turabian StyleZheng, Mengxue, Jesús Cadenas, Susanne Elisabeth Pors, Tasnim Esa, Stine Gry Kristensen, Linn Salto Mamsen, Cristina Subiran Adrados, and Claus Yding Andersen. 2023. "Reducing 3D Hydrogel Stiffness, Addition of Oestradiol in a Physiological Concentration and Increasing FSH Concentration Improve In Vitro Growth of Murine Preantral Follicles" International Journal of Molecular Sciences 24, no. 15: 12499. https://doi.org/10.3390/ijms241512499





