Low Muscle and High Fat Percentages Are Associated with Low Natural Killer Cell Activity: A Cross-Sectional Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Study Population
2.2. Correlations between IFN-γ Levels and Other Variables, Including Muscle and Fat Percentages
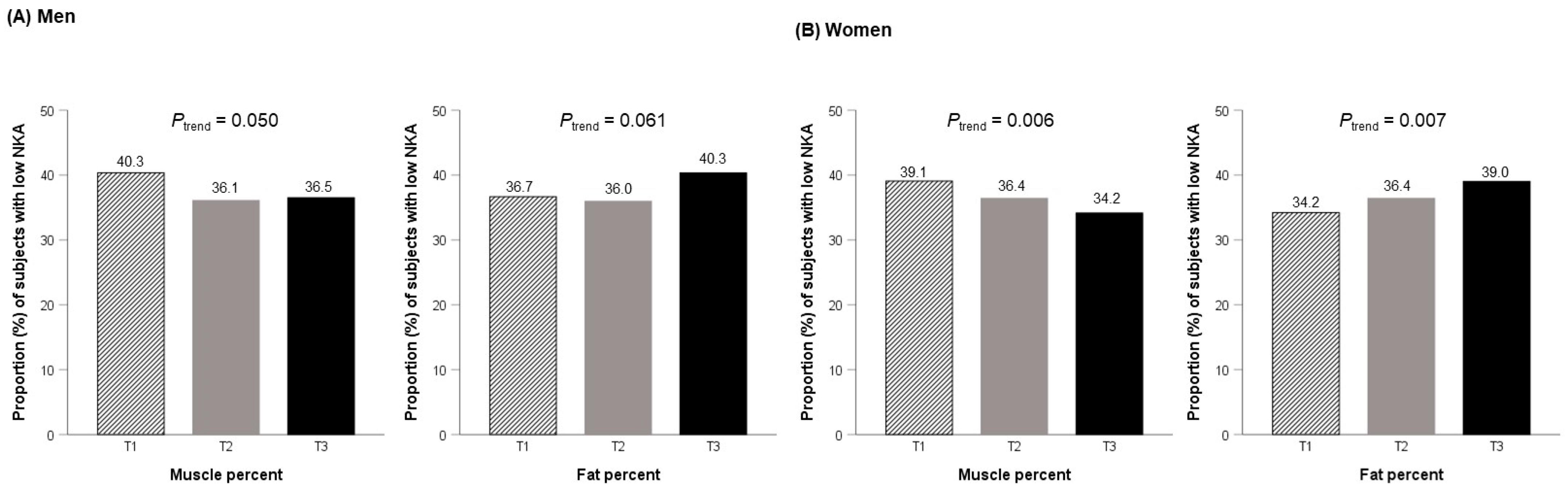
2.3. Relationship between Tertiles of Muscle and Fat Percentages and Low NKA
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Assessment of Body Composition
4.3. IFN-γ Measurement for NKA
4.4. Data Collection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the iarc working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honce, R.; Schultz-Cherry, S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Pugliese, G.; Laudisio, D.; Castellucci, B.; Barrea, L.; Savastano, S.; Colao, A. The impact of obesity on immune response to infection: Plausible mechanisms and outcomes. Obes. Rev. 2021, 22, e13216. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Bumbacea, R.S.; Pappa, A.K.; Papadopoulos, A.N.; Bumbacea, D. Obesity and infection: What have we learned from the COVID-19 pandemic. Front. Nutr. 2022, 9, 931313. [Google Scholar] [CrossRef]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G.; Faggioni, R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000, 68, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001, 1, 41–49. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. Nk cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Minami, S.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef]
- Ogata, K.; Yokose, N.; Tamura, H.; An, E.; Nakamura, K.; Dan, K.; Nomura, T. Natural killer cells in the late decades of human life. Clin. Immunol. Immunopathol. 1997, 84, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of nk cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.; Vuletić, A.; Mirjačić Martinović, K.; Colović, N.; Čolović, M.; Jurišić, V. Decreased cd161 activating and increased cd158a inhibitory receptor expression on nk cells underlies impaired nk cell cytotoxicity in patients with multiple myeloma. J. Clin. Pathol. 2016, 69, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.; Jurisić, V.; Banićevic, B.; Spuzić, I. The difference in nk-cell activity between patients with non-hodgkin’s lymphomas and hodgkin’s disease. Br. J. Haematol. 1999, 104, 144–151. [Google Scholar] [CrossRef]
- Bähr, I.; Goritz, V.; Doberstein, H.; Hiller, G.G.; Rosenstock, P.; Jahn, J.; Pörtner, O.; Berreis, T.; Mueller, T.; Spielmann, J.; et al. Diet-induced obesity is associated with an impaired nk cell function and an increased colon cancer incidence. J. Nutr. Metab. 2017, 2017, 4297025. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Kim, J.K.; Han, S.H.; Lim, J.S.; Kim, K.I.; Cho, D.H.; Lee, M.S.; Lee, J.H.; Yoon, D.Y.; Yoon, S.R.; et al. Adiponectin is a negative regulator of nk cell cytotoxicity. J. Immunol. 2006, 176, 5958–5964. [Google Scholar] [CrossRef] [Green Version]
- Huebner, L.; Engeli, S.; Wrann, C.D.; Goudeva, L.; Laue, T.; Kielstein, H. Human nk cell subset functions are differentially affected by adipokines. PLoS ONE 2013, 8, e75703. [Google Scholar] [CrossRef] [Green Version]
- Bähr, I.; Jahn, J.; Zipprich, A.; Pahlow, I.; Spielmann, J.; Kielstein, H. Impaired natural killer cell subset phenotypes in human obesity. Immunol. Res. 2018, 66, 234–244. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, D.; Cawood, T.J.; O’Farrelly, C.; Lynch, L. Natural killer cells in obesity: Impaired function and increased susceptibility to the effects of cigarette smoke. PLoS ONE 2010, 5, e8660. [Google Scholar] [CrossRef] [Green Version]
- Laue, T.; Wrann, C.D.; Hoffmann-Castendiek, B.; Pietsch, D.; Hübner, L.; Kielstein, H. Altered nk cell function in obese healthy humans. BMC Obes. 2015, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Besson, L.; Charrier, E.; Marçais, A.; Disse, E.; Bienvenu, J.; Walzer, T.; Dumontet, C. Alteration of natural killer cell phenotype and function in obese individuals. Clin. Immunol. 2017, 177, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Keilen, J.; Gar, C.; Rottenkolber, M.; Fueessl, L.U.; Joseph, A.T.; Draenert, R.; Seissler, J.; Lechner, A. No association of natural killer cell number and function in peripheral blood with overweight/obesity and metabolic syndrome in a cohort of young women. Physiol. Rep. 2022, 10, e15148. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.M.; Marguti, I.; Peron, J.P.; Halpern, A.; Rizzo, L.V. Bariatric surgery reverses natural killer (nk) cell activity and nk-related cytokine synthesis impairment induced by morbid obesity. Obes. Surg. 2011, 21, 112–118. [Google Scholar] [CrossRef]
- Jahn, J.; Spielau, M.; Brandsch, C.; Stangl, G.I.; Delank, K.S.; Bähr, I.; Berreis, T.; Wrann, C.D.; Kielstein, H. Decreased nk cell functions in obesity can be reactivated by fat mass reduction. Obesity 2015, 23, 2233–2241. [Google Scholar] [CrossRef] [Green Version]
- De Barra, C.; Khalil, M.; Mat, A.; O’Donnell, C.; Shaamile, F.; Brennan, K.; O’Shea, D.; Hogan, A.E. Glucagon-like peptide-1 therapy in people with obesity restores natural killer cell metabolism and effector function. Obesity 2023, 31, 1787–1797. [Google Scholar] [CrossRef]
- Valiathan, R.; Lewis, J.E.; Melillo, A.B.; Leonard, S.; Ali, K.H.; Asthana, D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand. J. Immunol. 2012, 75, 455–462. [Google Scholar] [CrossRef]
- Lee, S.B.; Cha, J.; Kim, I.K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of nk cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Jensen, M.D.; Thomas, R.J.; Squires, R.W.; Allison, T.G.; Korinek, J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur. Heart J. 2007, 28, 2087–2093. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Braun, W.; Geisler, C.; Norman, K.; Müller, M.J. Body composition and cardiometabolic health: The need for novel concepts. Eur. J. Clin. Nutr. 2018, 72, 638–644. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Diagnosis of obesity based on body composition-associated health risks-time for a change in paradigm. Obes. Rev. 2021, 22 (Suppl. S2), e13190. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Nieman, D.C.; Austin, M.D.; Jin, F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010, 104, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, L.M.; Mavinkurve, M.; Carolan, E.; Kinlen, D.; O’Brien, E.C.; Little, M.A.; Finlay, D.K.; Cody, D.; Hogan, A.E.; O’Shea, D. Nk cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight 2017, 2, e94939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.J.; Braun, W.; Pourhassan, M.; Geisler, C.; Bosy-Westphal, A. Application of standards and models in body composition analysis. Proc. Nutr. Soc. 2016, 75, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bähr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-associated alterations of natural killer cells and immunosurveillance of cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Afzali, A.M.; Müntefering, T.; Wiendl, H.; Meuth, S.G.; Ruck, T. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun. Rev. 2018, 17, 518–529. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and cd8 t cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar]
- Barra, N.G.; Fan, I.Y.; Gillen, J.B.; Chew, M.; Marcinko, K.; Steinberg, G.R.; Gibala, M.J.; Ashkar, A.A. High intensity interval training increases natural killer cell number and function in obese breast cancer-challenged mice and obese women. J. Cancer Prev. 2017, 22, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Miller, A.R.; Henson, D.A.; Warren, B.J.; Gusewitch, G.; Johnson, R.L.; Davis, J.M.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Effects of high- vs moderate-intensity exercise on natural killer cell activity. Med. Sci. Sports Exerc. 1993, 25, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; Morley, J.E.; Bloom, E.T.; Benton, D.; Solomon, G.F.; Makinodan, T. The effect of exercise on natural killer cell activity in young and old subjects. J. Gerontol. 1989, 44, M37–M45. [Google Scholar] [CrossRef]
- Crist, D.M.; Mackinnon, L.T.; Thompson, R.F.; Atterbom, H.A.; Egan, P.A. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology 1989, 35, 66–71. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Flynn, M.G.; Phillips, M.D.; Stewart, L.K.; Timmerman, K.L. Chronic resistance exercise training improves natural killer cell activity in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1315–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, J.; Hebert, J.R.; Reddy, M.M. Dietary fat and natural-killer-cell activity. Am. J. Clin. Nutr. 1989, 50, 861–867. [Google Scholar] [CrossRef]
- Wing, E.J.; Stanko, R.T.; Winkelstein, A.; Adibi, S.A. Fasting-enhanced immune effector mechanisms in obese subjects. Am. J. Med. 1983, 75, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Griffin, C.; Abrishami, S.; Eter, L.; Lanzetta, N.; Hak, L.; Clemente, J.; Agarwal, D.; Lerner, A.; Westerhoff, M.; et al. Sex hormones regulate metainflammation in diet-induced obesity in mice. J. Biol. Chem. 2021, 297, 101229. [Google Scholar] [CrossRef]
- Ter Horst, R.; van den Munckhof, I.C.L.; Schraa, K.; Aguirre-Gamboa, R.; Jaeger, M.; Smeekens, S.P.; Brand, T.; Lemmers, H.; Dijkstra, H.; Galesloot, T.E.; et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1787–1800. [Google Scholar] [CrossRef]
- Aglago, K.E.; Menchawy, I.E.; Kari, K.E.; Hamdouchi, A.E.; Barkat, A.; Bengueddour, R.; Haloui, N.E.; Mokhtar, N.; Aguenaou, H. Development and validation of bioelectrical impedance analysis equations for predicting total body water and fat-free mass in north-african adults. Eur. J. Clin. Nutr. 2013, 67, 1081–1086. [Google Scholar] [CrossRef]
- Kim, H.; Kim, C.H.; Kim, D.W.; Park, M.; Park, H.S.; Min, S.S.; Han, S.H.; Yee, J.Y.; Chung, S.; Kim, C. External cross-validation of bioelectrical impedance analysis for the assessment of body composition in korean adults. Nutr. Res. Pract. 2011, 5, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, K.C.; Shim, D.H.; Yang, C.M.; Lee, S.B.; Kim, S.M.; Shin, T.Y.; Kim, K.H.; Yoon, H.G.; Rha, K.H.; Lee, J.M.; et al. Reduction of the cd16(-)cd56bright nk cell subset precedes nk cell dysfunction in prostate cancer. PLoS ONE 2013, 8, e78049. [Google Scholar] [CrossRef] [Green Version]
- Nederby, L.; Jakobsen, A.; Hokland, M.; Hansen, T.F. Quantification of nk cell activity using whole blood: Methodological aspects of a new test. J. Immunol. Methods 2018, 458, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Haam, J.H.; Cho, S.H.; Kim, Y.S. Cross-sectional and time-dependent analyses on inflammatory markers following natural killer cell activity. Diagnostics 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for ifn-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The r package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, K.; Lee, S.B.; Kang, S.; Park, J.S.; Ahn, C.W.; Nam, J.S. Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. J. Diabetes Investig. 2019, 10, 1223–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solana, R.; Mariani, E. Nk and nk/t cells in human senescence. Vaccine 2000, 18, 1613–1620. [Google Scholar] [CrossRef]

| Men | Women | |||||
|---|---|---|---|---|---|---|
| Normal NKA | Low NKA | p | Normal NKA | Low NKA | p | |
| Number, n | 2296 | 1386 | 2777 | 1599 | ||
| Age, years | 48.5 ± 11.5 | 50.2 ± 11.5 | <0.001 | 47.1 ± 11.8 | 47.1 ± 11.6 | 0.960 |
| Height, cm | 174.2 ± 5.9 | 173.3 ± 5.9 | <0.001 | 161.7 ± 5.7 | 161.5 ± 5.7 | 0.267 |
| Weight, kg | 76.5 ± 10.8 | 75.5 ± 11.8 | 0.007 | 56.7 ± 8.4 | 57.0 ± 9.6 | 0.351 |
| BMI, kg/m2 | 25.2 ± 3.2 | 25.1 ± 3.5 | 0.288 | 21.7 ± 3.2 | 21.9 ± 3.6 | 0.206 |
| Muscle mass, kg | 55.0 ± 6.3 | 53.9 ± 6.6 | <0.001 | 37.3 ± 4.1 | 37.0 ± 4.2 | 0.025 |
| Muscle percent, % | 72.3 ± 5.4 | 71.9 ± 5.7 | 0.026 | 66.4 ± 6.3 | 65.8 ± 6.5 | 0.001 |
| Fat mass, kg | 18.3 ± 6.5 | 18.5 ± 7.1 | 0.517 | 17.1 ± 6.1 | 17.7 ± 6.9 | 0.007 |
| Fat percent, % | 23.5 ± 5.7 | 24.0 ± 5.9 | 0.027 | 29.5 ± 6.6 | 30.2 ± 6.9 | 0.002 |
| WBC, cells/μL | 5.47 ± 1.38 | 5.99 ± 1.75 | <0.001 | 4.99 ± 1.29 | 5.60 ± 1.63 | <0.001 |
| CRP, mg/dL | 0.16 ± 0.32 | 0.21 ± 0.50 | 0.003 | 0.14 ± 0.28 | 0.21 ± 0.68 | 0.001 |
| Glucose, mg/dL | 94.7 ± 20.0 | 95.4 ± 21.0 | 0.329 | 85.6 ± 13.5 | 85.2 ± 15.1 | 0.404 |
| Insulin, µIU/mL | 7.5 ± 6.2 | 7.5 ± 5.4 | 0.973 | 5.3 ± 3.8 | 5.6 ± 4.3 | 0.091 |
| HOMA-IR | 1.84 ± 1.99 | 1.84 ± 1.70 | 0.967 | 1.18 ± 1.02 | 1.25 ± 1.12 | 0.109 |
| IFN-γ, pg/mL | 1665.4 ± 885.8 | 228.5 ± 140.2 | <0.001 | 1692.9 ± 894.6 | 216.8 ± 142.6 | <0.001 |
| Hypertension, n (%) | 500 (21.8) | 295 (21.3) | 0.424 | 233 (8.4) | 137 (8.6) | 0.545 |
| Diabetes, n (%) | 190 (8.3) | 129 (9.3) | 0.253 | 70 (2.5) | 47 (2.9) | 0.412 |
| Dyslipidemia, n (%) | 400 (17.4) | 250 (18.0) | 0.410 | 315 (11.3) | 160 (10.0) | 0.226 |
| Men | Women | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age, years | −0.068 | <0.001 | −0.020 | 0.178 |
| Height, cm | 0.050 | 0.002 | 0.025 | 0.102 |
| Weight, kg | 0.033 | 0.047 | −0.019 | 0.208 |
| BMI, kg/m2 | 0.014 | 0.409 | −0.028 | 0.067 |
| Muscle mass, kg | 0.079 | <0.001 | 0.032 | 0.033 |
| Muscle percent, % | 0.049 | 0.003 | 0.053 | <0.001 |
| Fat mass, kg | −0.026 | 0.116 | −0.048 | 0.001 |
| Fat percent, % | −0.050 | 0.002 | −0.053 | <0.001 |
| WBC count, cells/μL | −0.169 | <0.001 | −0.225 | <0.001 |
| CRP, mg/dL | −0.037 | 0.047 | −0.059 | 0.001 |
| Glucose, mg/dL | −0.024 | 0.139 | 0.009 | 0.555 |
| Insulin, µIU/mL | −0.048 | 0.018 | −0.046 | 0.013 |
| HOMA-IR | −0.045 | 0.024 | −0.039 | 0.034 |
| Muscle Percent | T1 | T2 | T3 | |||
| OR (95% CI) | p | OR (95% CI) | p | Overall p | ||
| Men | ||||||
| Unadjusted | 1 (Ref.) | 0.84 (0.71–0.98) | 0.030 | 0.85 (0.72–1.00) | 0.051 | 0.056 |
| Model 1 | 1 (Ref.) | 0.76 (0.63–0.91) | 0.002 | 0.74 (0.61–0.92) | 0.005 | 0.005 |
| Model 2 | 1 (Ref.) | 0.69 (0.55–0.86) | 0.001 | 0.74 (0.57–0.95) | 0.019 | 0.005 |
| Women | ||||||
| Unadjusted | 1 (Ref.) | 0.89 (0.77–1.04) | 0.136 | 0.81 (0.70–0.94) | 0.006 | 0.022 |
| Model 1 | 1 (Ref.) | 0.86 (0.72–1.02) | 0.090 | 0.76 (0.62–0.93) | 0.009 | 0.033 |
| Model 2 | 1 (Ref.) | 0.93 (0.74–1.15) | 0.491 | 0.76 (0.59–0.99) | 0.042 | 0.087 |
| Fat Percent | T1 | T2 | T3 | |||
| OR (95% CI) | p | OR (95% CI) | p | Overall p | ||
| Men | ||||||
| Unadjusted | 1 (Ref.) | 0.97 (0.82–1.14) | 0.715 | 1.17 (0.99–1.37) | 0.063 | 0.056 |
| Model 1 | 1 (Ref.) | 1.00 (0.84–1.19) | 0.989 | 1.32 (1.08–1.63) | 0.008 | 0.005 |
| Model 2 | 1 (Ref.) | 0.92 (0.74–1.14) | 0.439 | 1.31 (1.01–1.69) | 0.043 | 0.009 |
| Women | ||||||
| Unadjusted | 1 (Ref.) | 1.10 (0.95–1.28) | 0.215 | 1.23 (1.06–1.43) | 0.007 | 0.026 |
| Model 1 | 1 (Ref.) | 1.13 (0.96–1.32) | 0.141 | 1.31 (1.07–1.61) | 0.010 | 0.038 |
| Model 2 | 1 (Ref.) | 1.21 (0.99–1.48) | 0.061 | 1.29 (1.00–1.68) | 0.054 | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, A.-R.; Suh, E.; Oh, H.; Cho, B.H.; Gil, M.; Lee, Y.-K. Low Muscle and High Fat Percentages Are Associated with Low Natural Killer Cell Activity: A Cross-Sectional Study. Int. J. Mol. Sci. 2023, 24, 12505. https://doi.org/10.3390/ijms241512505
Cho A-R, Suh E, Oh H, Cho BH, Gil M, Lee Y-K. Low Muscle and High Fat Percentages Are Associated with Low Natural Killer Cell Activity: A Cross-Sectional Study. International Journal of Molecular Sciences. 2023; 24(15):12505. https://doi.org/10.3390/ijms241512505
Chicago/Turabian StyleCho, A-Ra, Eunkyung Suh, Hyoju Oh, Baek Hwan Cho, Minchan Gil, and Yun-Kyong Lee. 2023. "Low Muscle and High Fat Percentages Are Associated with Low Natural Killer Cell Activity: A Cross-Sectional Study" International Journal of Molecular Sciences 24, no. 15: 12505. https://doi.org/10.3390/ijms241512505
APA StyleCho, A.-R., Suh, E., Oh, H., Cho, B. H., Gil, M., & Lee, Y.-K. (2023). Low Muscle and High Fat Percentages Are Associated with Low Natural Killer Cell Activity: A Cross-Sectional Study. International Journal of Molecular Sciences, 24(15), 12505. https://doi.org/10.3390/ijms241512505






