Production of Tissue-Engineered Skin Substitutes for Clinical Applications: Elimination of Serum
Abstract
:1. Introduction
2. Results and Discussion
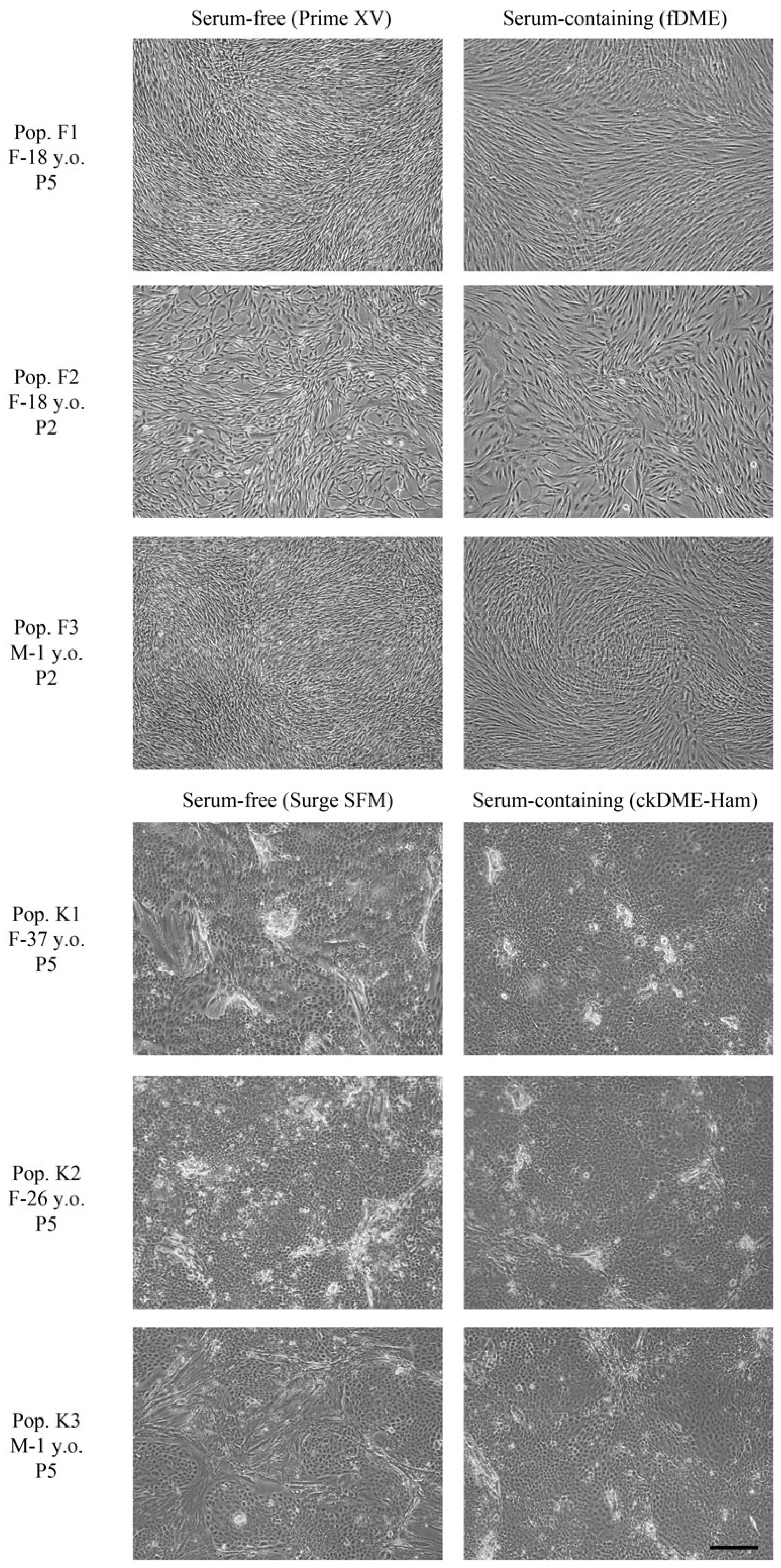
2.1. Fibroblasts and Epithelial Cell Culture in Serum-Free Media
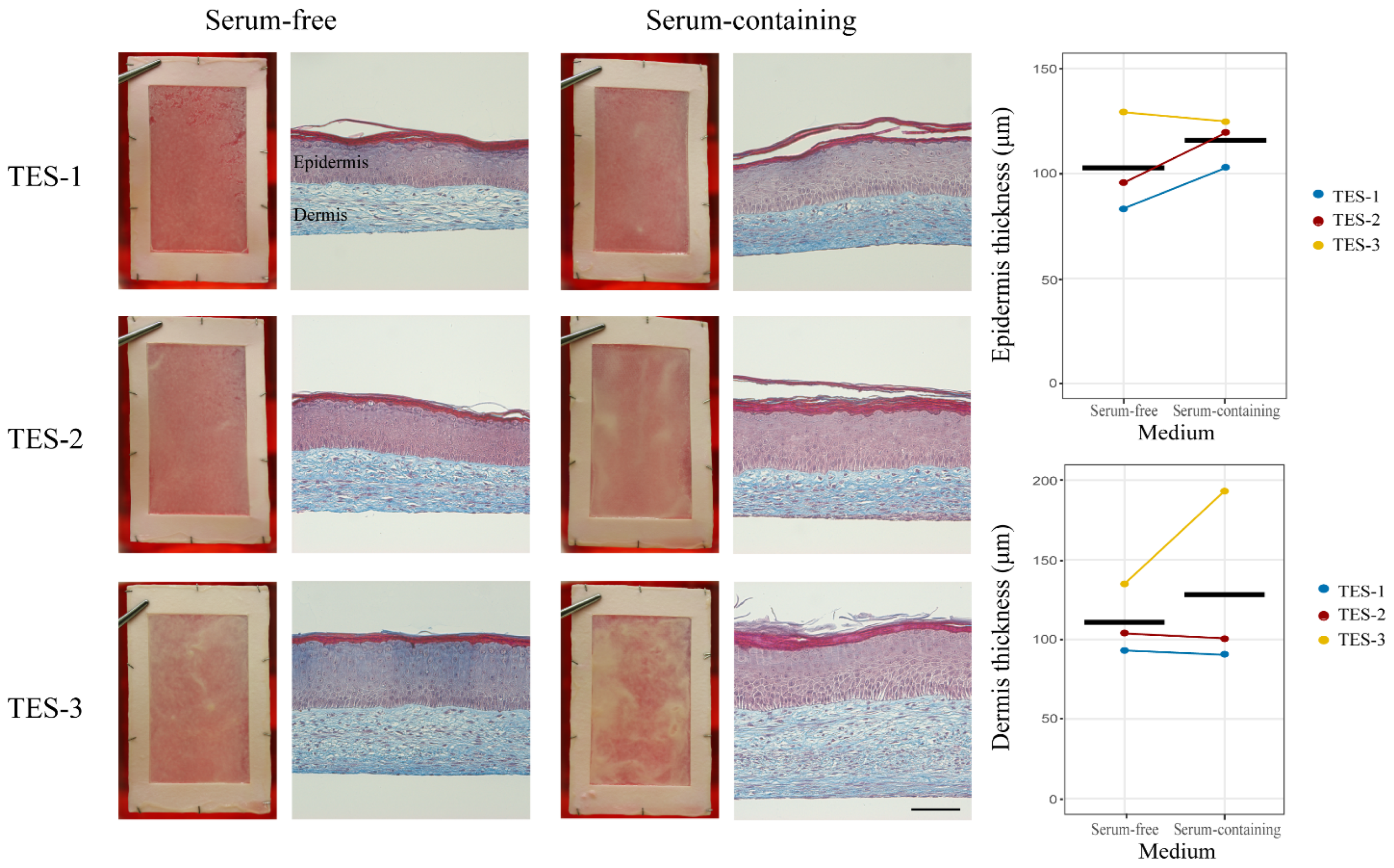
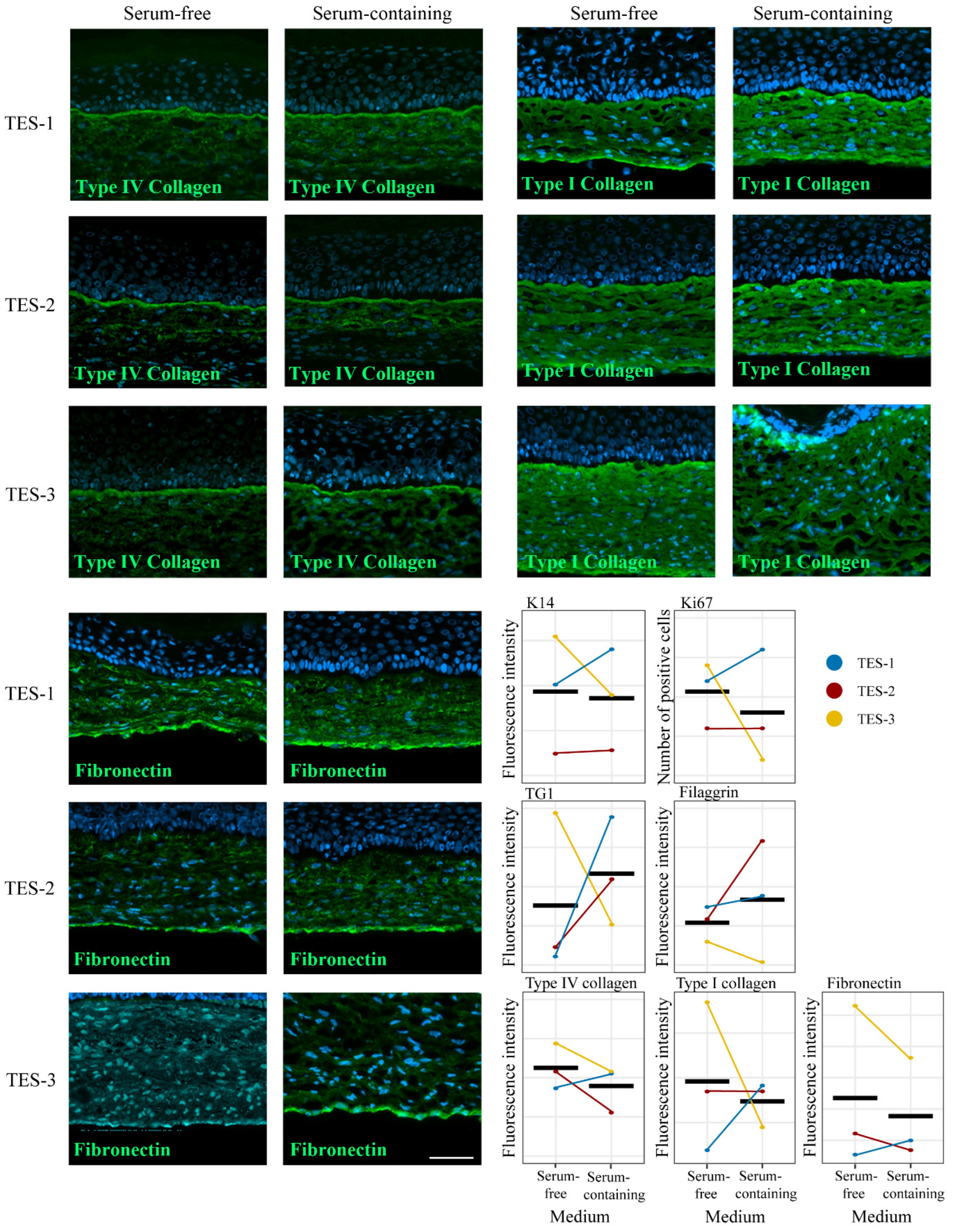
2.2. General Aspects of the Skin Substitutes
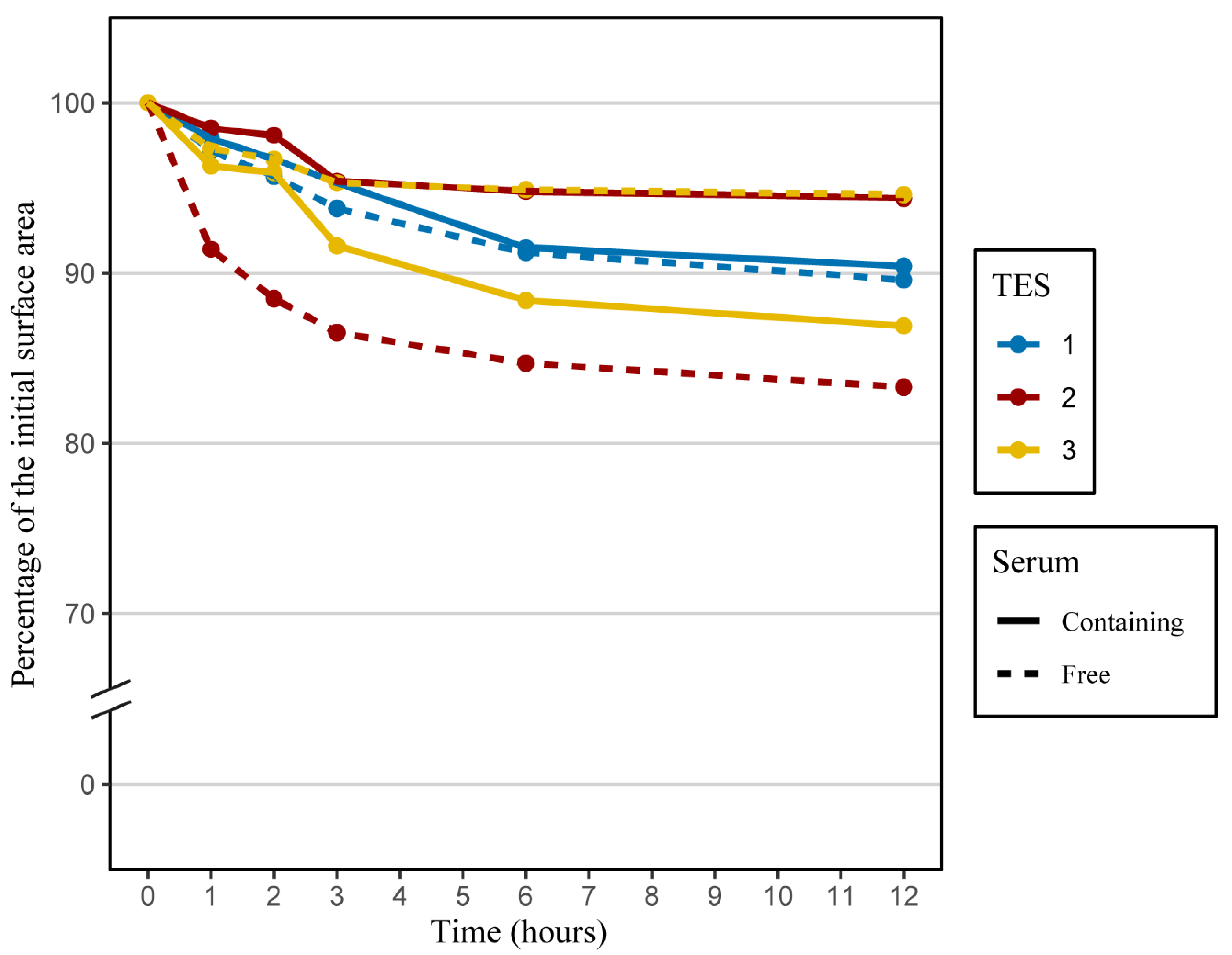
2.3. Contraction Assessment
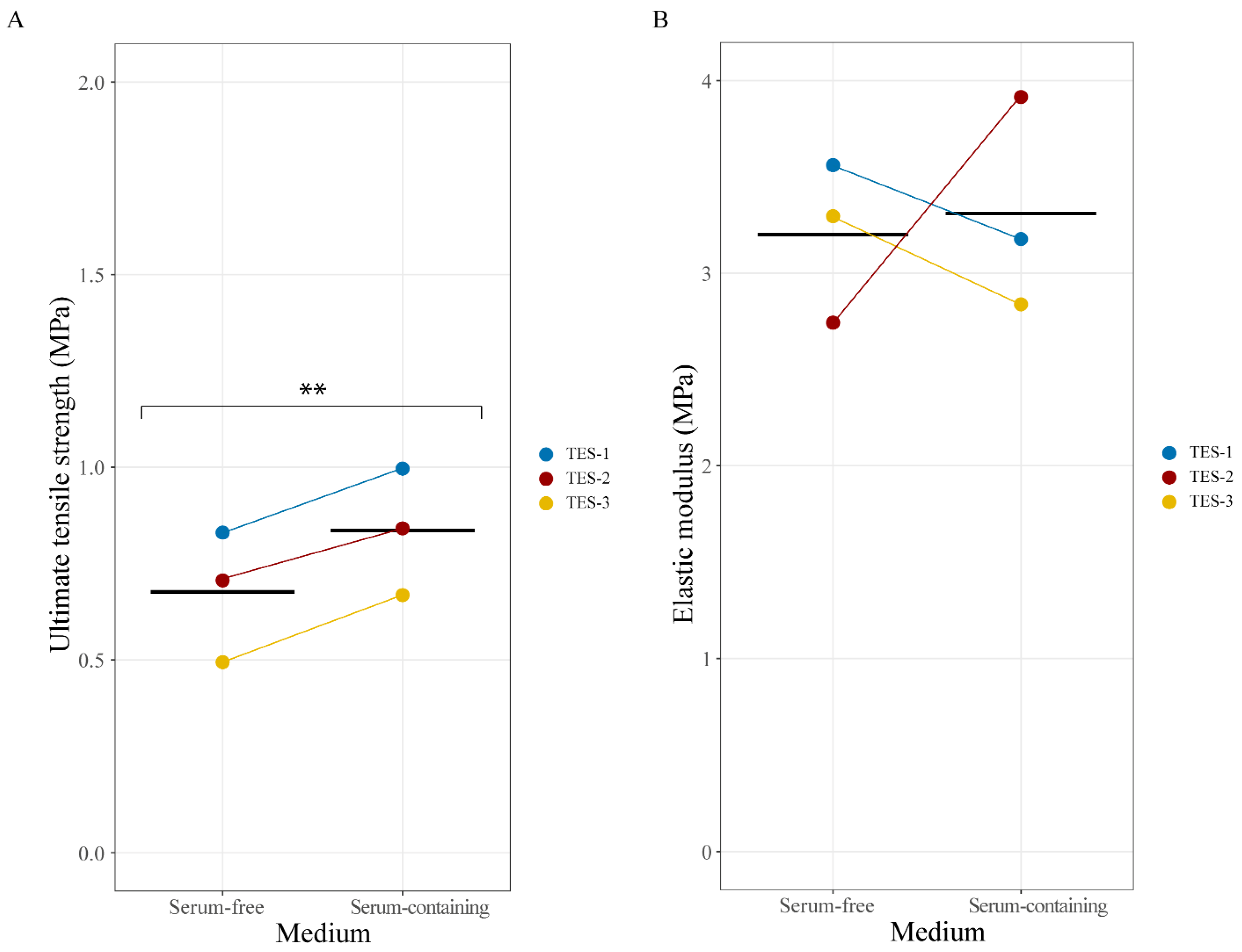
2.4. Mechanical Properties of Skin Substitutes
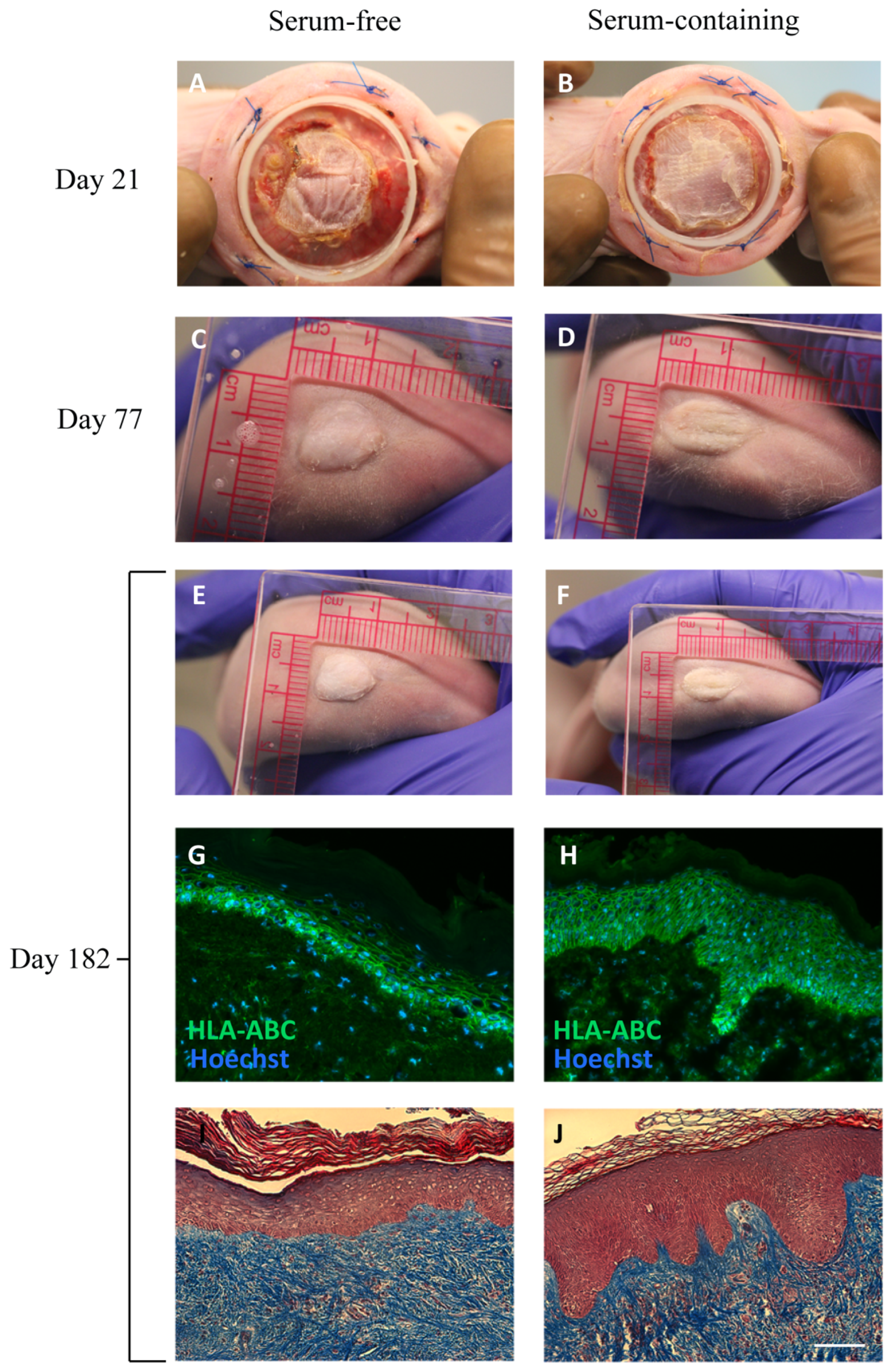
2.5. In Vivo Assessment
3. Materials and Methods
3.1. Ethical Considerations
3.2. Cell Populations
3.3. Cell Culture
3.4. Skin Substitute Production
3.5. Histological and Immunofluorescence Analyses
3.6. Contraction Tests
3.7. Tensile Strength Tests
3.8. Skin Substitute Grafting on Nude Mice
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyce, S.T.; Kagan, R.J.; Meyer, N.A.; Yakuboff, K.P.; Warden, G.D. The 1999 Clinical Research Award Cultured Skin Substitutes Combined with Integra Artificial Skin to Replace Native Skin Autograft and Allograft for the Closure of Excised Full–Thickness Burns. J. Burn Care Rehabil. 1999, 20, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous Bilayered Self-Assembled Skin Substitutes (SASSs) as Permanent Grafts: A Case Series of 14 Severely Burned Patients Indicating Clinical Effectiveness. eCM 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Llames, S.; García, E.; García, V.; del Río, M.; Larcher, F.; Jorcano, J.L.; López, E.; Holguín, P.; Miralles, F.; Otero, J.; et al. Clinical Results of an Autologous Engineered Skin. Cell Tissue Bank. 2006, 7, 47–53. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Formation of a Keratinizing Epithelium in Culture by a Cloned Cell Line Derived from a Teratoma. Cell 1975, 6, 317–330. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Epidermal Growth Factor and the Multiplication of Cultured Human Epidermal Keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Cortez Ghio, S.; Larouche, D.; Doucet, E.J.; Germain, L. The Role of Cultured Autologous Bilayered Skin Substitutes as Epithelial Stem Cell Niches after Grafting: A Systematic Review of Clinical Studies. Burn. Open 2021, 5, 56–66. [Google Scholar] [CrossRef]
- Jochems, C.E.A.; van der Valk, J.B.F.; Stafleu, F.R.; Baumans, V. The Use of Fetal Bovine Serum: Ethical or Scientific Problem? Altern. Lab. Anim. 2002, 30, 219–227. [Google Scholar] [CrossRef]
- Baker, M. Reproducibility: Respect Your Cells! Nature 2016, 537, 433–435. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.; Mellor, D.; Brands, R.; Fischer, R.; Gruber, F.; Gstraunthaler, G.; Hellebrekers, L.; Hyllner, J.; Jonker, F.H.; Prieto, P.; et al. The Humane Collection of Fetal Bovine Serum and Possibilities for Serum-Free Cell and Tissue Culture. Toxicol. Vitr. 2004, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.; Sato, G. Methods for Growth of Cultured Cells in Serum-Free Medium. Anal. Biochem. 1980, 102, 255–270. [Google Scholar] [CrossRef]
- McGillicuddy, N.; Floris, P.; Albrecht, S.; Bones, J. Examining the Sources of Variability in Cell Culture Media Used for Biopharmaceutical Production. Biotechnol. Lett. 2018, 40, 5–21. [Google Scholar] [CrossRef]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A Plea to Reduce or Replace Fetal Bovine Serum in Cell Culture Media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, J.; Brunner, D.; De Smet, K.; Fex Svenningsen, Å.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of Chemically Defined Cell Culture Media—Replacing Fetal Bovine Serum in Mammalian In Vitro Methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Jayme, D.W.; Epstein, D.A.; Conrad, D.R. Fetal Bovine Serum Alternatives. Nature 1988, 334, 547–548. [Google Scholar] [CrossRef]
- Hawkes, P.W. Fetal Bovine Serum: Geographic Origin and Regulatory Relevance of Viral Contamination. Bioresour. Bioprocess. 2015, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.A.; Manktelow, A.; Johnson, M.; Deserres, S.; Herzog, S.; Peterson, H.D. Antibody Response to Xenogeneic Proteins in Burned Patients Receiving Cultured Keratinocyte Grafts. J. Trauma Inj. Infect. Crit. Care 1988, 28, 1054–1059. [Google Scholar] [CrossRef]
- Cortez Ghio, S.; Barbier, M.A.; Doucet, E.J.; Debbah, I.; Safoine, M.; Le-Bel, G.; Cartier, A.; Jolibois, E.; Morissette, A.; Larouche, D.; et al. A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 1821. [Google Scholar] [CrossRef]
- Safoine, M.; Côté, A.; Leloup, R.; Hayward, C.J.; Plourde Campagna, M.-A.; Ruel, J.; Fradette, J. Engineering naturally-derived human connective tissues for clinical applications using a serum-free production system. Biomed. Mater. 2022, 17, 055011. [Google Scholar] [CrossRef] [PubMed]
- Heathman, T.R.J.; Glyn, V.A.M.; Picken, A.; Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Kara, B.; Hewitt, C.J. Expansion, Harvest and Cryopreservation of Human Mesenchymal Stem Cells in a Serum-Free Microcarrier Process: Expansion, Harvest and Preservation of HMSCs. Biotechnol. Bioeng. 2015, 112, 1696–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.N.F.B.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem. Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Paquet, C.; Fradette, J.; Carrier, P.; Auger, F.A.; Germain, L. Regeneration of Skin and Cornea by Tissue Engineering. In Stem Cells in Regenerative Medicine; Audet, J., Stanford, W.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 482, pp. 233–256. [Google Scholar] [CrossRef]
- Lamb, R.; Ambler, C.A. Keratinocytes Propagated in Serum-Free, Feeder-Free Culture Conditions Fail to Form Stratified Epidermis in a Reconstituted Skin Model. PLoS ONE 2013, 8, e52494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium Regulation of Growth and Differentiation of Mouse Epidermal Cells in Culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.; Mattey, D.; Garrod, D. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J. Cell. Biol. 1984, 99, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. Available online: Imagej.org (accessed on 2 August 2023).
- Tuan, T.-L.; Grinnell, F. Fibronectin and Fibrinolysis Are Not Required for Fibrin Gel Contraction by Human Skin Fibroblasts. J. Cell. Physiol. 1989, 140, 577–583. [Google Scholar] [CrossRef]
- Gillery, P.; Maquart, F.-X.; Borel, J.-P. Fibronectin Dependence of the Contraction of Collagen Lattices by Human Skin Fibroblasts. Exp. Cell Res. 1986, 167, 29–37. [Google Scholar] [CrossRef]
- Gauvin, R.; Larouche, D.; Marcoux, H.; Guignard, R.; Auger, F.A.; Germain, L. Minimal Contraction for Tissue-Engineered Skin Substitutes When Matured at the Air-Liquid Interface: Epidermis Influences Engineered Skin Structural Stability. J. Tissue Eng. Regen. Med. 2012, 7, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, R.; Parenteau-Bareil, R.; Larouche, D.; Marcoux, H.; Bisson, F.; Bonnet, A.; Auger, F.A.; Bolduc, S.; Germain, L. Dynamic Mechanical Stimulations Induce Anisotropy and Improve the Tensile Properties of Engineered Tissues Produced without Exogenous Scaffolding. Acta Biomater. 2011, 7, 3294–3301. [Google Scholar] [CrossRef]
- Beaudoin Cloutier, C.; Guignard, R.; Bernard, G.; Gauvin, R.; Larouche, D.; Lavoie, A.; Lacroix, D.; Moulin, V.J.; Germain, L.; Auger, F.A. Production of a Bilayered Self-Assembled Skin Substitute Using a Tissue-Engineered Acellular Dermal Matrix. Tissue Eng. Part C Methods 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Bisson, F.; Rochefort, É.; Lavoie, A.; Larouche, D.; Zaniolo, K.; Simard-Bisson, C.; Damour, O.; Auger, F.; Guérin, S.; Germain, L. Irradiated Human Dermal Fibroblasts Are as Efficient as Mouse Fibroblasts as a Feeder Layer to Improve Human Epidermal Cell Culture Lifespan. Int. J. Mol. Sci. 2013, 14, 4684–4704. [Google Scholar] [CrossRef] [Green Version]
- Cartier, A.; Barbier, M.A.; Larouche, D.; Morissette, A.; Bussières, A.; Montalin, L.; Beaudoin Cloutier, C.; Germain, L. Tie-Over Bolster Pressure Dressing Improves Outcomes of Skin Substitutes Xenografts on Athymic Mice. Int. J. Mol. Sci. 2022, 23, 5507. [Google Scholar] [CrossRef]
- Goyer, B.; Larouche, D.; Kim, D.H.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.A.; Germain, L. Immune Tolerance of Tissue-Engineered Skin Produced with Allogeneic or Xenogeneic Fibroblasts and Syngeneic Keratinocytes Grafted on Mice. Acta Biomater. 2019, 90, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package. Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2017; Version 3. [Google Scholar]

| TES | Keratinocytes | Donor Age (Years) | Fibroblasts | Donor Age (Years) |
|---|---|---|---|---|
| TES-1 | K1 | D1 (37) | F1 | D5 (18) |
| TES-2 | K2 | D2 (26) | F2 | D6 (18) |
| TES-3 | K3 | D3 (1) | F3 | D3 (1) |
| TES-4 | K4 | D4 (56) | F4 | D6 (18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doucet, E.J.; Cortez Ghio, S.; Barbier, M.A.; Savard, É.; Magne, B.; Safoine, M.; Larouche, D.; Fradette, J.; Germain, L. Production of Tissue-Engineered Skin Substitutes for Clinical Applications: Elimination of Serum. Int. J. Mol. Sci. 2023, 24, 12537. https://doi.org/10.3390/ijms241612537
Doucet EJ, Cortez Ghio S, Barbier MA, Savard É, Magne B, Safoine M, Larouche D, Fradette J, Germain L. Production of Tissue-Engineered Skin Substitutes for Clinical Applications: Elimination of Serum. International Journal of Molecular Sciences. 2023; 24(16):12537. https://doi.org/10.3390/ijms241612537
Chicago/Turabian StyleDoucet, Emilie J., Sergio Cortez Ghio, Martin A. Barbier, Étienne Savard, Brice Magne, Meryem Safoine, Danielle Larouche, Julie Fradette, and Lucie Germain. 2023. "Production of Tissue-Engineered Skin Substitutes for Clinical Applications: Elimination of Serum" International Journal of Molecular Sciences 24, no. 16: 12537. https://doi.org/10.3390/ijms241612537
APA StyleDoucet, E. J., Cortez Ghio, S., Barbier, M. A., Savard, É., Magne, B., Safoine, M., Larouche, D., Fradette, J., & Germain, L. (2023). Production of Tissue-Engineered Skin Substitutes for Clinical Applications: Elimination of Serum. International Journal of Molecular Sciences, 24(16), 12537. https://doi.org/10.3390/ijms241612537







