Depletion of SNORA33 Abolishes ψ of 28S-U4966 and Affects the Ribosome Translational Apparatus
Abstract
:1. Introduction
2. Results
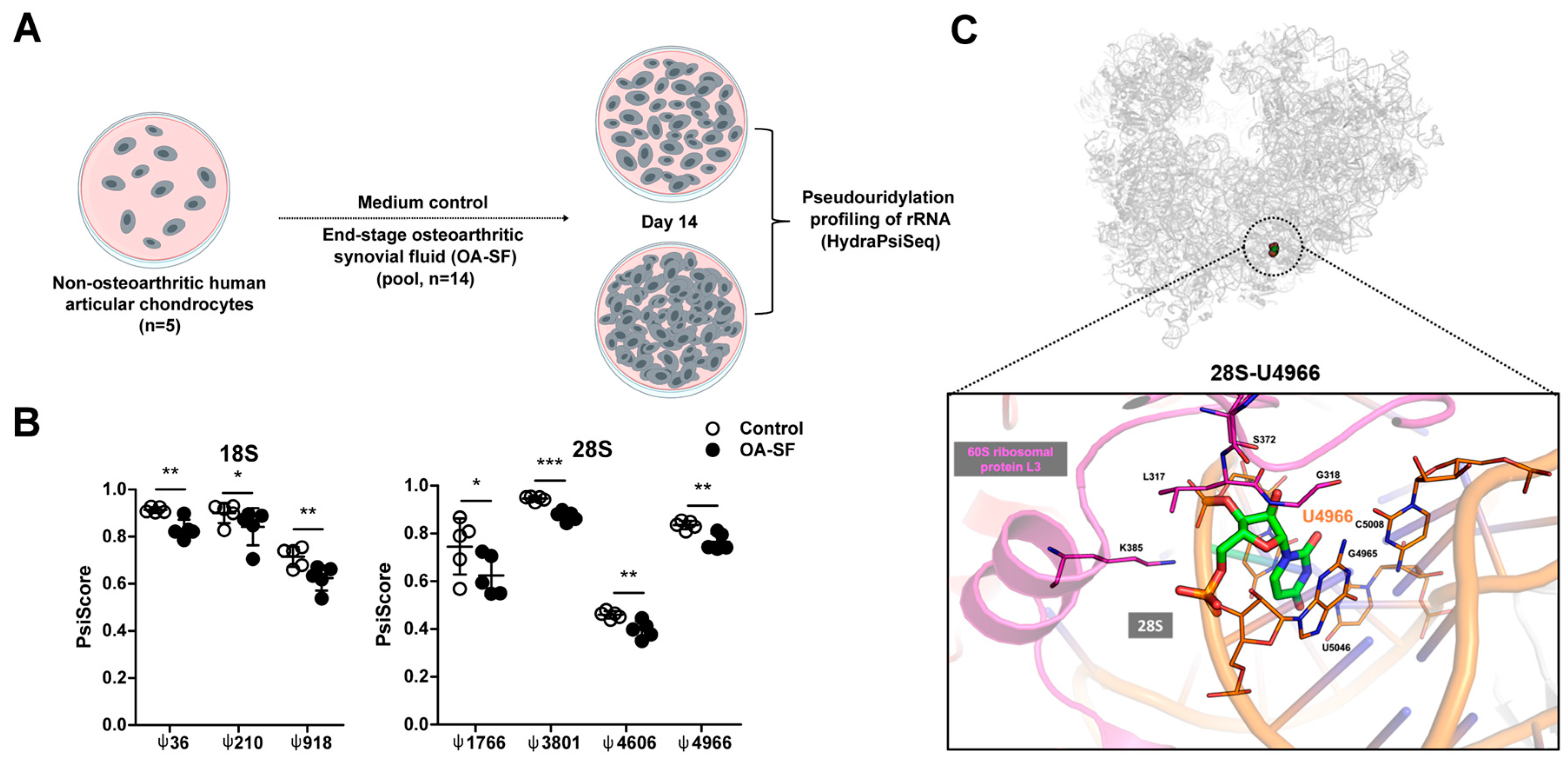
2.1. A Chronic Disease Microenvironment Provokes Site-Specific Changes in rRNA ψ Profiles
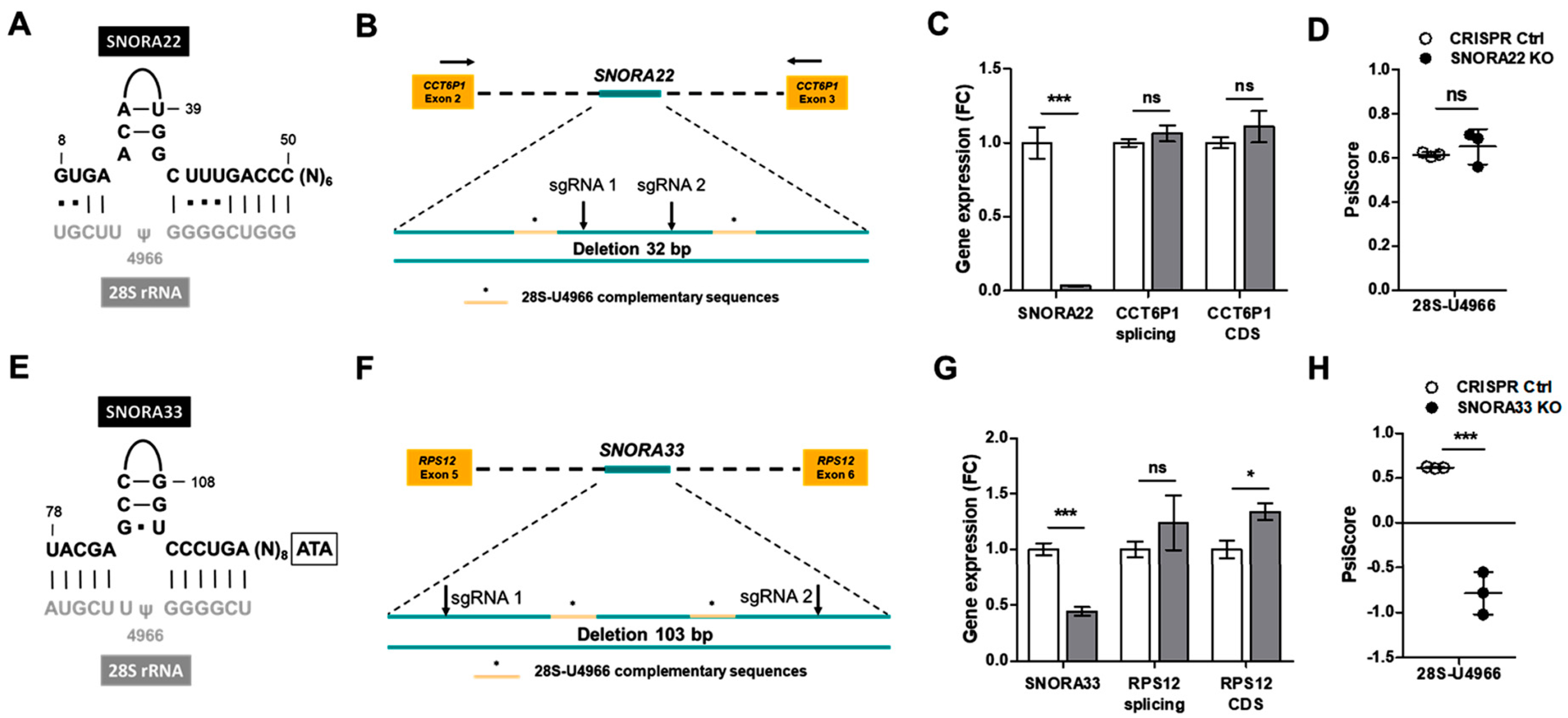
2.2. Depletion of SNORA33 but Not SNORA22 Reduces 28S-ψ4966 Levels
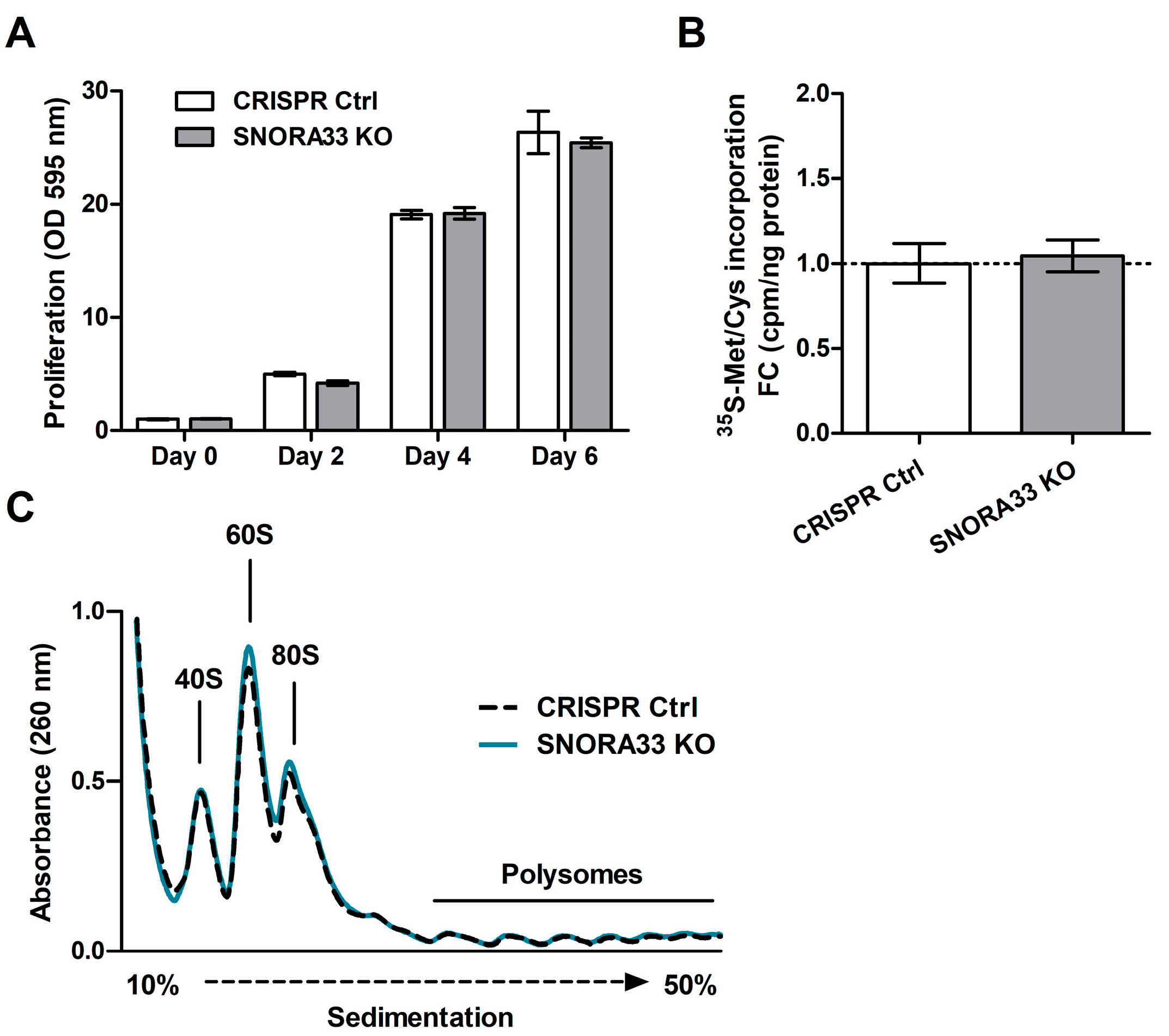
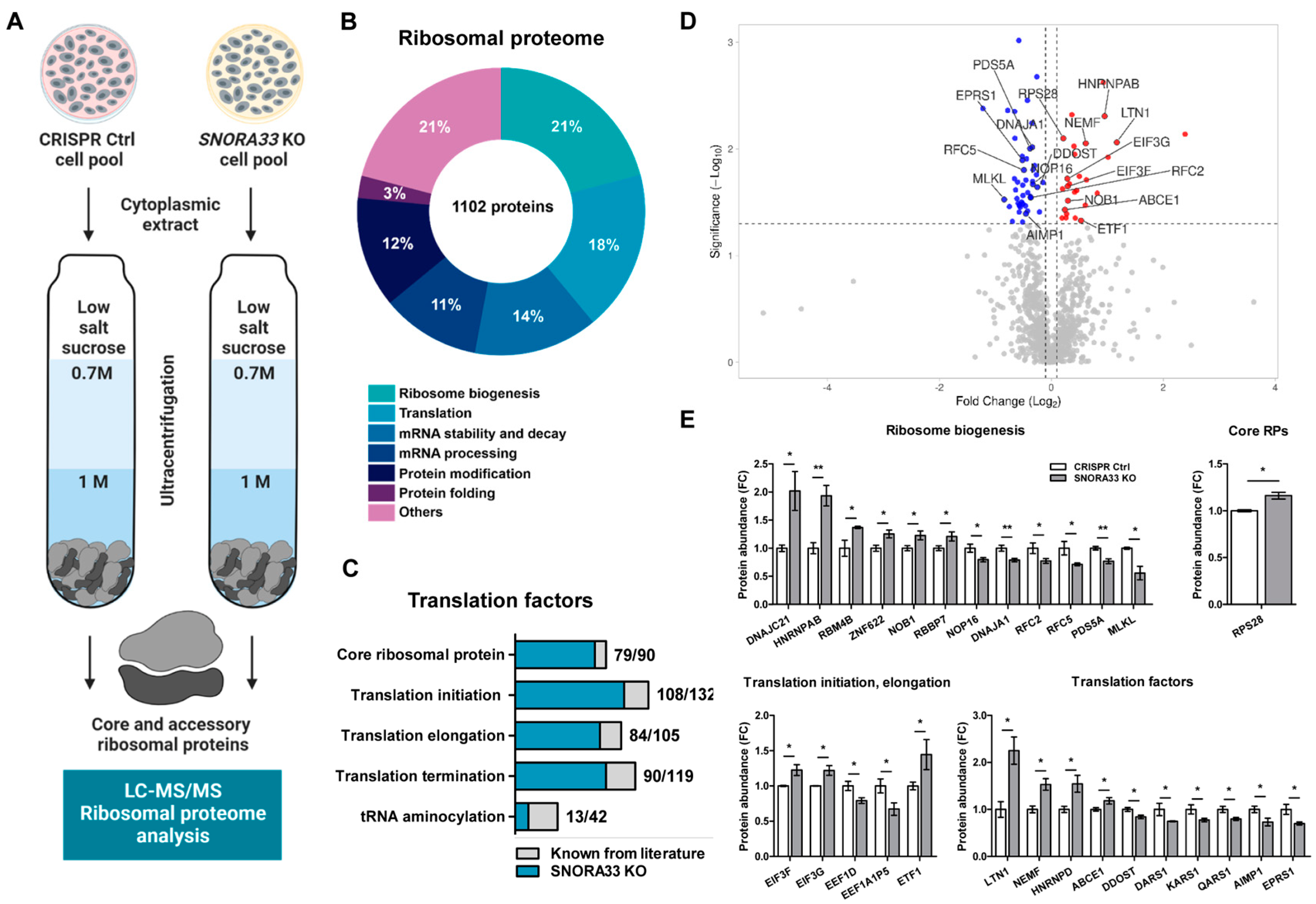
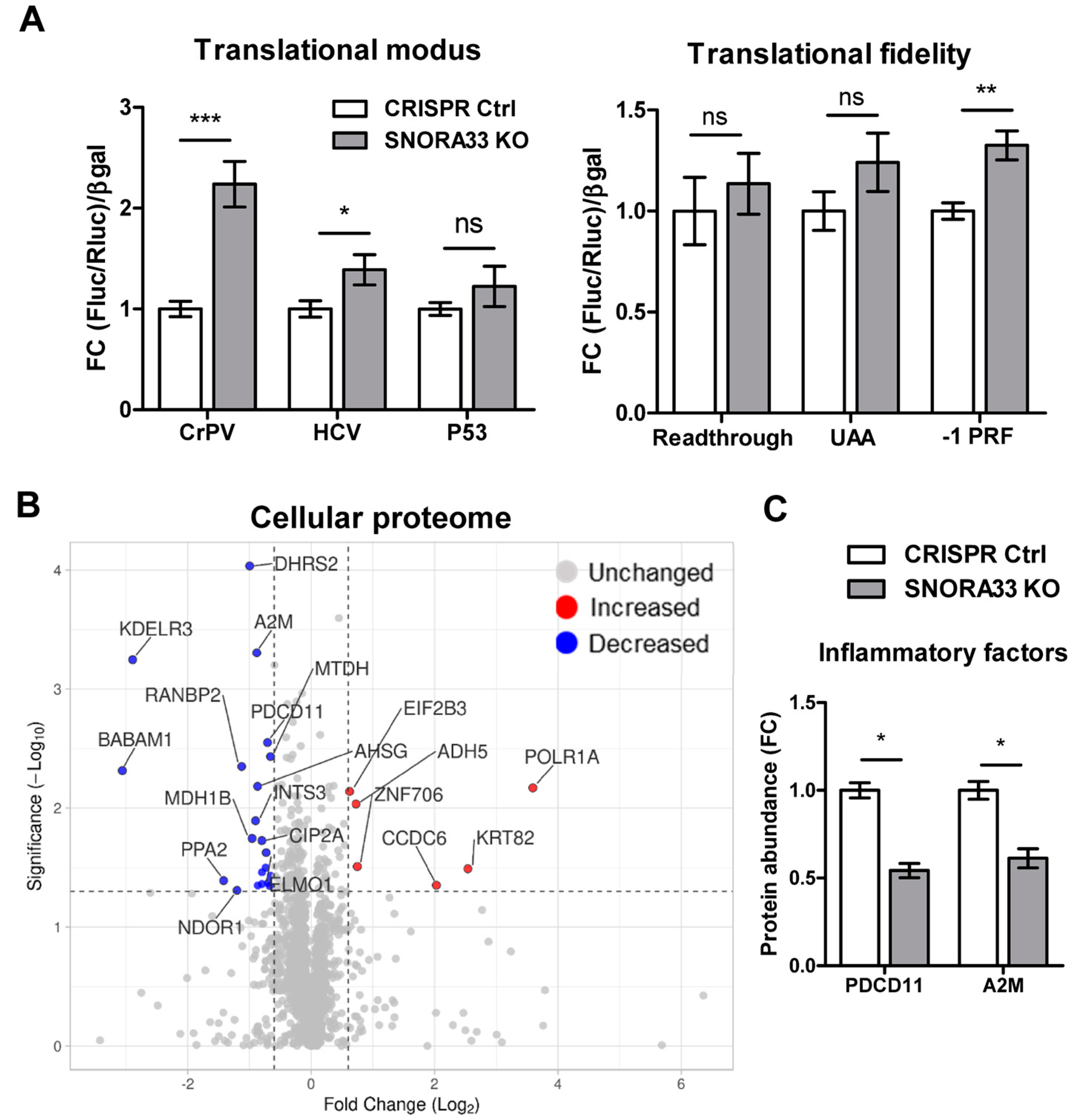
2.3. SNORA33 Depletion Affects Ribosome Composition, Function, and the Cellular Proteome
3. Discussion
4. Materials and Methods
4.1. Human Articular Chondrocytes Isolation and Culture
4.2. Synovial Fluid Collection
4.3. RNA Isolation and HydraPsiSeq
4.4. Visualization of the Human Ribosome and PTM Mapping
4.5. Lentiviral CRISPR/Cas9 Targeting snoRNAs
4.6. DNA Isolation and Surveyor Assay
4.7. RT-qPCR
4.8. Proliferation Assay
4.9. Total Protein Synthesis
4.10. Polysome Profiling
4.11. Translational Modus and Fidelity Assays
4.12. Label-Free Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis of Cellular and Ribosomal Proteome
4.13. Statistical Testing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-O-me | 2′-O-methylation |
| A-A | antibiotic–antimycotic |
| ANOVA | analysis of variance |
| ANS | anisomycin |
| AP1G1 | adaptor related protein complex 1 subunit gamma 1 |
| BCA | bicinchoninic acid |
| CHX | cycloheximide |
| CPM | counts per minute |
| DE | differentially expressed |
| DLR | dual-luciferase |
| DMEM/F-12 | Dulbecco’s modified eagle medium/nutrient mixture F-12 |
| dTTP | deoxythymidine triphosphate |
| ELISA | enzyme-linked immunoassay |
| EMT | emetine |
| FA | fucidic acid |
| FBL | fibrillarin |
| FC | fold change |
| FCS | fetal calf serum |
| gDNA | genomic DNA |
| GFP | green fluorescent protein |
| sgRNA | single guide RNA |
| HAC | human articular chondrocyte |
| KCl | potassium chloride |
| KO | knockout |
| M | male |
| MgCl2 | magnesium chloride |
| NaCl | sodium chloride |
| NPM1 | nucleophosmin |
| nm | nanometer |
| OA | osteoarthritis |
| OA-SF | osteoarthritic synovial fluid |
| PBS | phosphate-buffered saline |
| RLU | relative light unit |
| RIN | RNA integrity number |
| RIPA | radioimmunoprecipitation assay |
| rRNA | ribosomal RNA |
| RT-qPCR | quantitative reverse transcription polymerase chain reaction |
| snoRNA | small nucleolar RNA |
References
- Khatter, H.; Myasnikov, A.G.; Natchiar, S.K.; Klaholz, B.P. Structure of the human 80S ribosome. Nature 2015, 520, 640–645. [Google Scholar] [CrossRef]
- Taoka, M.; Nobe, Y.; Yamaki, Y.; Sato, K.; Ishikawa, H.; Izumikawa, K.; Yamauchi, Y.; Hirota, K.; Nakayama, H.; Takahashi, N.; et al. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res. 2018, 46, 9289–9298. [Google Scholar] [CrossRef]
- Marchand, V.; Pichot, F.; Neybecker, P.; Ayadi, L.; Bourguignon-Igel, V.; Wacheul, L.; Lafontaine, D.L.J.; Pinzano, A.; Helm, M.; Motorin, Y. HydraPsiSeq: A method for systematic and quantitative mapping of pseudouridines in RNA. Nucleic Acids Res. 2020, 48, e110. [Google Scholar] [CrossRef]
- Motorin, Y.; Quinternet, M.; Rhalloussi, W.; Marchand, V. Constitutive and variable 2′-O-methylation (Nm) in human ribosomal RNA. RNA Biol. 2021, 18 (Suppl. 1), 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Malla, S.; Lyons, S.M. snoRNPs: Functions in Ribosome Biogenesis. Biomolecules 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T. Small nucleolar RNAs: An abundant group of noncoding RNAs with diverse cellular functions. Cell 2002, 109, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganot, P.; Bortolin, M.L.; Kiss, T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 1997, 89, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Decatur, W.A.; Fournier, M.J. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002, 27, 344–351. [Google Scholar] [CrossRef]
- Gay, D.M.; Lund, A.H.; Jansson, M.D. Translational control through ribosome heterogeneity and functional specialization. Trends Biochem. Sci. 2022, 47, 66–81. [Google Scholar] [CrossRef]
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Jaafar, M.; Paraqindes, H.; Gabut, M.; Diaz, J.J.; Marcel, V.; Durand, S. 2’O-Ribose Methylation of Ribosomal RNAs: Natural Diversity in Living Organisms, Biological Processes, and Diseases. Cells 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.; Bellodi, C.; Landry, D.M.; Niederer, R.O.; Meskauskas, A.; Musalgaonkar, S.; Kopmar, N.; Krasnykh, O.; Dean, A.M.; Thompson, S.R.; et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell 2011, 44, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Penzo, M.; Montanaro, L. Turning Uridines around: Role of rRNA Pseudouridylation in Ribosome Biogenesis and Ribosomal Function. Biomolecules 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchi, L.; Pacilli, A.; Sethi, R.; Penzo, M.; Schneider, R.J.; Trere, D.; Brigotti, M.; Montanaro, L. Dyskerin depletion increases VEGF mRNA internal ribosome entry site-mediated translation. Nucleic Acids Res. 2013, 41, 8308–8318. [Google Scholar] [CrossRef] [Green Version]
- Penzo, M.; Rocchi, L.; Brugiere, S.; Carnicelli, D.; Onofrillo, C.; Coute, Y.; Brigotti, M.; Montanaro, L. Human ribosomes from cells with reduced dyskerin levels are intrinsically altered in translation. FASEB J. 2015, 29, 3472–3482. [Google Scholar] [CrossRef] [Green Version]
- Jansson, M.D.; Hafner, S.J.; Altinel, K.; Tehler, D.; Krogh, N.; Jakobsen, E.; Andersen, J.V.; Andersen, K.L.; Schoof, E.M.; Menard, P.; et al. Regulation of translation by site-specific ribosomal RNA methylation. Nat. Struct. Mol. Biol. 2021, 28, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Heiss, N.S.; Knight, S.W.; Vulliamy, T.J.; Klauck, S.M.; Wiemann, S.; Mason, P.J.; Poustka, A.; Dokal, I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998, 19, 32–38. [Google Scholar] [CrossRef]
- Knight, S.W.; Heiss, N.S.; Vulliamy, T.J.; Greschner, S.; Stavrides, G.; Pai, G.S.; Lestringant, G.; Varma, N.; Mason, P.J.; Dokal, I.; et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am. J. Hum. Genet. 1999, 65, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, S.A.; Alter, B.P. Dyskeratosis congenita. Hematol. Oncol. Clin. N. Am. 2009, 23, 215–231. [Google Scholar] [CrossRef]
- Ejby, M.; Sorensen, M.A.; Pedersen, S. Pseudouridylation of helix 69 of 23S rRNA is necessary for an effective translation termination. Proc. Natl. Acad. Sci. USA 2007, 104, 19410–19415. [Google Scholar] [CrossRef]
- Gutgsell, N.S.; Deutscher, M.P.; Ofengand, J. The pseudouridine synthase RluD is required for normal ribosome assembly and function in Escherichia coli. RNA 2005, 11, 1141–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudin-Baillieu, A.; Fabret, C.; Liang, X.H.; Piekna-Przybylska, D.; Fournier, M.J.; Rousset, J.P. Nucleotide modifications in three functionally important regions of the Saccharomyces cerevisiae ribosome affect translation accuracy. Nucleic Acids Res. 2009, 37, 7665–7677. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.H.; Liu, Q.; Fournier, M.J. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol. Cell 2007, 28, 965–977. [Google Scholar] [CrossRef]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Nam, S.W.; Park, W.S.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Absence of DKC1 exon 3 mutation in common human cancers. Acta Oncol. 2006, 45, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wen, J.; Huang, Z.; Chen, X.P.; Zhang, B.X.; Chu, L. Small Nucleolar RNAs: Insight Into Their Function in Cancer. Front. Oncol. 2019, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Contreras, A.; Holm, M.; Uechi, T.; Forester, C.M.; Pang, X.; Jackson, C.; Calvert, M.E.; Chen, B.; Quigley, D.A.; et al. A single H/ACA small nucleolar RNA mediates tumor suppression downstream of oncogenic RAS. Elife 2019, 8, e48847. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, L.; Nirujogi, R.S.; Ahmad, S.; Bhattacharjee, M.; Manda, S.S.; Renuse, S.; Kelkar, D.S.; Subbannayya, Y.; Raju, R.; Goel, R.; et al. Proteomic analysis of human osteoarthritis synovial fluid. Clin. Proteom. 2014, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Carlson, A.K.; Rawle, R.A.; Adams, E.; Greenwood, M.C.; Bothner, B.; June, R.K. Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem. Biophys. Res. Commun. 2018, 499, 182–188. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Ingelmark, B.E. The nutritive supply and nutritional value of synovial fluid. Acta Orthop. Scand. 1950, 20, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef] [Green Version]
- van den Akker, G.G.H.; Caron, M.M.J.; Peffers, M.J.; Welting, T.J.M. Ribosome dysfunction in osteoarthritis. Curr. Opin. Rheumatol. 2022, 34, 61–67. [Google Scholar] [CrossRef]
- Chabronova, A.; van den Akker, G.G.H.; Housmans, B.A.C.; Caron, M.M.J.; Cremers, A.; Surtel, D.A.M.; Wichapong, K.; Peffers, M.M.J.; van Rhijn, L.W.; Marchand, V.; et al. Ribosomal RNA-based epitranscriptomic regulation of chondrocyte translation and proteome in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Housmans, B.A.C.; Neefjes, M.; Surtel, D.A.M.; Vitik, M.; Cremers, A.; van Rhijn, L.W.; van der Kraan, P.M.; van den Akker, G.G.H.; Welting, T.J.M. Synovial fluid from end-stage osteoarthritis induces proliferation and fibrosis of articular chondrocytes via MAPK and RhoGTPase signaling. Osteoarthr. Cartil. 2022, 30, 862–874. [Google Scholar] [CrossRef]
- Tonna, E.A.; Singh, I.J. An autoradiographic investigation of 3H-uridine utilization by aging mouse cartilage cells. Exp. Gerontol. 1976, 11, 231–234. [Google Scholar] [CrossRef]
- Kiss, A.M.; Jady, B.E.; Bertrand, E.; Kiss, T. Human box H/ACA pseudouridylation guide RNA machinery. Mol. Cell. Biol. 2004, 24, 5797–5807. [Google Scholar] [CrossRef] [Green Version]
- Belin, S.; Hacot, S.; Daudignon, L.; Therizols, G.; Pourpe, S.; Mertani, H.C.; Rosa-Calatrava, M.; Diaz, J.J. Purification of ribosomes from human cell lines. Curr. Protoc. Cell Biol. 2010, 49, 3–40. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Seither, P.; Coy, J.F.; Pouska, A.; Grummt, I. Molecular cloning and characterization of the cDNA encoding the largest subunit of mouse RNA polymerase I. Mol. Gen. Genet. 1997, 255, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Housmans, B.A.C.; van den Akker, G.G.H.; Neefjes, M.; Timur, U.T.; Cremers, A.; Peffers, M.J.; Caron, M.M.J.; van Rhijn, L.W.; Emans, P.J.; Boymans, T.A.E.J.; et al. Direct comparison of non-osteoarthritic and osteoarthritic synovial fluid-induced intracellular chondrocyte signaling and phenotype changes. Osteoarthr. Cartil. 2023, 31, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Pisarev, A.V.; Kolupaeva, V.G.; Yusupov, M.M.; Hellen, C.U.; Pestova, T.V. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J. 2008, 27, 1609–1621. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Kavdia, K.; Pagala, V.; Palmer, L.; Finkelstein, D.; Fan, Y.; Peng, J.; Demontis, F. An age-downregulated ribosomal RpS28 protein variant regulates the muscle proteome. G3 (Bethesda) 2021, 11, jkab165. [Google Scholar] [CrossRef]
- Gripp, K.W.; Curry, C.; Olney, A.H.; Sandoval, C.; Fisher, J.; Chong, J.X.; Genomics, U.W.C.f.M.; Pilchman, L.; Sahraoui, R.; Stabley, D.L.; et al. Diamond-Blackfan anemia with mandibulofacial dystostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. Am. J. Med. Genet. A 2014, 164A, 2240–2249. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Godet, A.C.; David, F.; Hantelys, F.; Tatin, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.C. IRES Trans-Acting Factors, Key Actors of the Stress Response. Int. J. Mol. Sci. 2019, 20, 924. [Google Scholar] [CrossRef] [Green Version]
- Bellodi, C.; Krasnykh, O.; Haynes, N.; Theodoropoulou, M.; Peng, G.; Montanaro, L.; Ruggero, D. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 2010, 70, 6026–6035. [Google Scholar] [CrossRef] [Green Version]
- Ruggero, D.; Grisendi, S.; Piazza, F.; Rego, E.; Mari, F.; Rao, P.H.; Cordon-Cardo, C.; Pandolfi, P.P. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science 2003, 299, 259–262. [Google Scholar] [CrossRef]
- Yoon, A.; Peng, G.; Brandenburger, Y.; Zollo, O.; Xu, W.; Rego, E.; Ruggero, D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 2006, 312, 902–906. [Google Scholar] [CrossRef]
- Paek, K.Y.; Kim, C.S.; Park, S.M.; Kim, J.H.; Jang, S.K. RNA-binding protein hnRNP D modulates internal ribosome entry site-dependent translation of hepatitis C virus RNA. J. Virol. 2008, 82, 12082–12093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Zhuang, J.; Peltz, S.; Dougherty, J. Identification of a cellular factor that modulates HIV-1 programmed ribosomal frameshifting. J. Biol. Chem. 2010, 285, 19776–19784. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.F.; Crowe-McAuliffe, C.; Graves, R.; Cardno, T.S.; McKinney, C.; Poole, E.S.; Tate, W.P. The highly conserved codon following the slippery sequence supports -1 frameshift efficiency at the HIV-1 frameshift site. PLoS ONE 2015, 10, e0122176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef]
- Sweet, T.; Khalili, K.; Sawaya, B.E.; Amini, S. Identification of a novel protein from glial cells based on its ability to interact with NF-kappaB subunits. J. Cell. Biochem. 2003, 90, 884–891. [Google Scholar] [CrossRef]
- Yang, R.; Zhan, M.; Guo, M.; Yuan, H.; Wang, Y.; Zhang, Y.; Zhang, W.; Chen, S.; de The, H.; Chen, Z.; et al. Yolk sac-derived Pdcd11-positive cells modulate zebrafish microglia differentiation through the NF-kappaB-Tgfbeta1 pathway. Cell Death Differ. 2021, 28, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, X.; Zhou, J.; Zhang, J.; Li, K.; Chen, Q.; Terek, R.; Fleming, B.C.; Goldring, M.B.; Ehrlich, M.G.; et al. Identification of alpha2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol. 2014, 66, 1843–1853. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wei, X.; Browning, S.; Scuderi, G.; Hanna, L.S.; Wei, L. Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res. Ther. 2017, 19, 175. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Cao, C.; Zhao, T.; Guo, H.; Fleming, B.C.; Owens, B.; Beveridge, J.; McAllister, S.; Wei, L. A2M inhibits inflammatory mediators of chondrocytes by blocking IL-1beta/NF-kappaB pathway. J. Orthop. Res. 2022, 41, 241–248. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natchiar, S.K.; Myasnikov, A.G.; Kratzat, H.; Hazemann, I.; Klaholz, B.P. Visualization of chemical modifications in the human 80S ribosome structure. Nature 2017, 551, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef] [Green Version]
- Pauli, C.; Liu, Y.; Rohde, C.; Cui, C.; Fijalkowska, D.; Gerloff, D.; Walter, C.; Krijgsveld, J.; Dugas, M.; Edemir, B.; et al. Site-specific methylation of 18S ribosomal RNA by SNORD42A is required for acute myeloid leukemia cell proliferation. Blood 2020, 135, 2059–2070. [Google Scholar] [CrossRef]
- Di Ceglie, I.; van den Akker, G.G.; Ascone, G.; Ten Harkel, B.; Hacker, H.; van de Loo, F.A.; Koenders, M.I.; van der Kraan, P.M.; de Vries, T.J.; Vogl, T.; et al. Genetic modification of ER-Hoxb8 osteoclast precursors using CRISPR/Cas9 as a novel way to allow studies on osteoclast biology. J. Leukoc. Biol. 2017, 101, 957–966. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelson, T.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G.; et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.C.; Martindale, J.L.; Gorospe, M. Polysome Fractionation to Analyze mRNA Distribution Profiles. Bio. Protoc. 2017, 7, e2126. [Google Scholar] [CrossRef] [Green Version]
- van den Akker, G.G.H.; Zacchini, F.; Housmans, B.A.C.; van der Vloet, L.; Caron, M.M.J.; Montanaro, L.; Welting, T.J.M. Current Practice in Bicistronic IRES Reporter Use: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 5193. [Google Scholar] [CrossRef]
- Ripmeester, E.G.J.; Caron, M.M.J.; van den Akker, G.G.H.; Surtel, D.A.M.; Cremers, A.; Balaskas, P.; Dyer, P.; Housmans, B.A.C.; Chabronova, A.; Smagul, A.; et al. Impaired chondrocyte U3 snoRNA expression in osteoarthritis impacts the chondrocyte protein translation apparatus. Sci. Rep. 2020, 10, 13426. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.; Screen, H.R.; Clegg, P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabronova, A.; van den Akker, G.; Housmans, B.A.C.; Caron, M.M.J.; Cremers, A.; Surtel, D.A.M.; Peffers, M.J.; van Rhijn, L.W.; Marchand, V.; Motorin, Y.; et al. Depletion of SNORA33 Abolishes ψ of 28S-U4966 and Affects the Ribosome Translational Apparatus. Int. J. Mol. Sci. 2023, 24, 12578. https://doi.org/10.3390/ijms241612578
Chabronova A, van den Akker G, Housmans BAC, Caron MMJ, Cremers A, Surtel DAM, Peffers MJ, van Rhijn LW, Marchand V, Motorin Y, et al. Depletion of SNORA33 Abolishes ψ of 28S-U4966 and Affects the Ribosome Translational Apparatus. International Journal of Molecular Sciences. 2023; 24(16):12578. https://doi.org/10.3390/ijms241612578
Chicago/Turabian StyleChabronova, Alzbeta, Guus van den Akker, Bas A. C. Housmans, Marjolein M. J. Caron, Andy Cremers, Don A. M. Surtel, Mandy J. Peffers, Lodewijk W. van Rhijn, Virginie Marchand, Yuri Motorin, and et al. 2023. "Depletion of SNORA33 Abolishes ψ of 28S-U4966 and Affects the Ribosome Translational Apparatus" International Journal of Molecular Sciences 24, no. 16: 12578. https://doi.org/10.3390/ijms241612578
APA StyleChabronova, A., van den Akker, G., Housmans, B. A. C., Caron, M. M. J., Cremers, A., Surtel, D. A. M., Peffers, M. J., van Rhijn, L. W., Marchand, V., Motorin, Y., & Welting, T. J. M. (2023). Depletion of SNORA33 Abolishes ψ of 28S-U4966 and Affects the Ribosome Translational Apparatus. International Journal of Molecular Sciences, 24(16), 12578. https://doi.org/10.3390/ijms241612578








