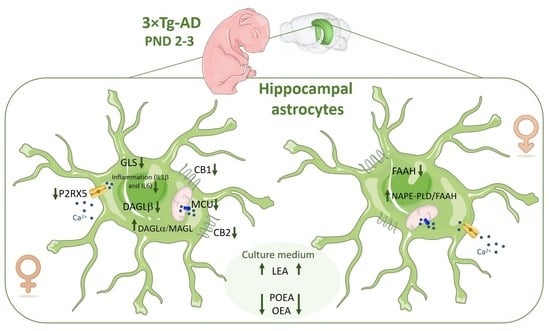
Sex-Dependent Altered Expression of Cannabinoid Signaling in Hippocampal Astrocytes of the Triple Transgenic Mouse Model of Alzheimer’s Disease: Implications for Controlling Astroglial Activity
Abstract
:1. Introduction
2. Results
2.1. mRNA Expression of Inflammation Markers
2.2. mRNA Expression of Cannabinoid-Related Receptors
2.3. Cannabinoid Enzymes mRNA Expression
2.4. Cannabinoid Enzymes Protein Expression
2.5. Culture Medium Endocannabinoids Levels
2.6. mRNA Expression of Ca2+ Signaling-Related Genes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Model
4.3. Primary Cultures of Hippocampal Astrocytes
4.4. RNA Isolation and Real-Time Quantitative PCR Analysis
4.5. Western Blot Analysis
4.6. Endocannabinoid Quantification
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [PubMed] [Green Version]
- Heneka, M.T.; O’Banion, M.K. Inflammatory Processes in Alzheimer’s Disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Evidence of Oxidative Stress in Alzheimer’s Disease Brain and Antioxidant Therapy: Lights and Shadows. Ann. N. Y. Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [PubMed]
- McGeer, P.L.; McGeer, E.G.; Yasojima, K. Alzheimer Disease and Neuroinflammation. J. Neural Transm. Suppl. 2000, 59, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35, 419–432. [Google Scholar] [PubMed] [Green Version]
- Beggiato, S.; Cassano, T.; Ferraro, L.; Tomasini, M.C. Astrocytic Palmitoylethanolamide Pre-Exposure Exerts Neuroprotective Effects in Astrocyte-Neuron Co-Cultures from a Triple Transgenic Mouse Model of Alzheimer’s Disease. Life Sci. 2020, 257, 118037. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E.; Wharton, S.B.; Simpson, J.E.; Heath, P.R.; Sharp, P.; Berwick, J. Review: Neuropathology and Behavioural Features of Transgenic Murine Models of Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2017, 43, 553–570. [Google Scholar] [PubMed] [Green Version]
- Edison, P.; Donat, C.K.; Sastre, M. In Vivo Imaging of Glial Activation in Alzheimer’s Disease. Front. Neurol. 2018, 9, 625. [Google Scholar] [PubMed] [Green Version]
- Saito, T.; Saido, T.C. Neuroinflammation in Mouse Models of Alzheimer’s Disease. Clin. Exp. Neuroimmunol. 2018, 9, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaney, A.; Williams, S.R.; Boutin, H. In Vivo Molecular Imaging of Neuroinflammation in Alzheimer’s Disease. J. Neurochem. 2019, 149, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid Signaling in Astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Wadhwa, P.; Jadhav, H. Reactive Astrogliosis: Role in Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets 2015, 14, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An Integral Player in the Pathogenesis of Alzheimer’s Disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar]
- Fernández-Moncada, I.; Marsicano, G. Astroglial CB1 Receptors, Energy Metabolism, and Gliotransmission: An Integrated Signaling System? Essays Biochem. 2023, 67, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose Metabolism Links Astroglial Mitochondria to Cannabinoid Effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef]
- Dörnyei, G.; Vass, Z.; Juhász, C.B.; Nádasy, G.L.; Hunyady, L.; Szekeres, M. Role of the Endocannabinoid System in Metabolic Control Processes and in the Pathogenesis of Metabolic Syndrome: An Update. Biomedicines 2023, 11, 306. [Google Scholar]
- Abd-Nikfarjam, B.; Dolati-Somarin, A.; Baradaran Rahimi, V.; Askari, V.R. Cannabinoids in Neuroinflammatory Disorders: Focusing on Multiple Sclerosis, Parkinsons, and Alzheimers Diseases. BioFactors 2023, 49, 560–583. [Google Scholar]
- Centonze, D.; Finazzi-Agrò, A.; Bernardi, G.; Maccarrone, M. The Endocannabinoid System in Targeting Inflammatory Neurodegenerative Diseases. Trends Pharmacol. Sci. 2007, 28, 180–187. [Google Scholar]
- Pennant, N.M.; Hinton, C.V. The Evolution of Cannabinoid Receptors in Cancer. WIREs Mech. Dis. 2023, 15, e1602. [Google Scholar]
- Aguirre-Rueda, D.; Guerra-Ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Valles, S.L. WIN 55,212-2, Agonist of Cannabinoid Receptors, Prevents Amyloid Β1-42 Effects on Astrocytes in Primary Culture. PLoS ONE 2015, 10, e0122843. [Google Scholar] [CrossRef]
- Klein, T.W. Cannabinoid-Based Drugs as Anti-Inflammatory Therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar]
- Berry, A.J.; Zubko, O.; Reeves, S.J.; Howard, R.J. Endocannabinoid System Alterations in Alzheimer’s Disease: A Systematic Review of Human Studies. Brain Res. 2020, 1749, 147135. [Google Scholar] [CrossRef]
- Di Marzo, V. “Endocannabinoids” and Other Fatty Acid Derivatives with Cannabimimetic Properties: Biochemistry and Possible Physiopathological Relevance. Biochim. Biophys. Acta-Lipids Lipid Metab. 1998, 1392, 153–175. [Google Scholar]
- Marcu, J.P.; Schechter, J.B. Molecular Pharmacology of CB1 and CB2 Cannabinoid Receptors. In Neuropathology of Drug Addictions and Substance Misuse Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [PubMed]
- Shu-Jung Hu, S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. In Handbook of Experimental Pharmacology; Springer: Cham, Swizerland, 2015; Volume 231, pp. 59–93. [Google Scholar]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [PubMed]
- Bernal-Chico, A.; Tepavcevic, V.; Manterola, A.; Utrilla, C.; Matute, C.; Mato, S. Endocannabinoid Signaling in Brain Diseases: Emerging Relevance of Glial Cells. Glia 2023, 71, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, M.A.; Romli, M.H.; Abas, R.; Vidyadaran, S.; Hidayat Baharuldin, M.T.; Nasaruddin, M.L.; Thirupathirao, V.; Sura, S.; Warsito, K.; Mohd Nor, N.H.; et al. Regulatory Role of the Endocannabinoid System on Glial Cells toward Cognitive Function in Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Animal Studies. Front. Pharmacol. 2023, 14, 1053680. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Kong, V.; Devenyi, G.A.; Gallino, D.; Ayranci, G.; Germann, J.; Rollins, C.; Chakravarty, M.M. Early-in-Life Neuroanatomical and Behavioural Trajectories in a Triple Transgenic Model of Alzheimer’s Disease. Brain Struct. Funct. 2018, 223, 3365–3382. [Google Scholar] [CrossRef]
- Liu, Y.; Bilen, M.; McNicoll, M.M.; Harris, R.A.; Fong, B.C.; Iqbal, M.A.; Paul, S.; Mayne, J.; Walker, K.; Wang, J.; et al. Early Postnatal Defects in Neurogenesis in the 3xTg Mouse Model of Alzheimer’s Disease. Cell Death Dis. 2023, 14, 138. [Google Scholar] [CrossRef]
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in Physiological Aging and Alzheimer’s Disease. Neuroscience 2016, 323, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Rodríguez, J.J.; Parpura, V. Astroglia Dynamics in Ageing and Alzheimer’s Disease. Curr. Opin. Pharmacol. 2016, 26, 74–79. [Google Scholar]
- Medina-Vera, D.; Rosell-Valle, C.; López-Gambero, A.J.; Navarro, J.A.; Zambrana-Infantes, E.N.; Rivera, P.; Santín, L.J.; Suarez, J.; de Fonseca, F.R. Imbalance of Endocannabinoid/Lysophosphatidylinositol Receptors Marks the Severity of Alzheimer’s Disease in a Preclinical Model: A Therapeutic Opportunity. Biology 2020, 9, 377. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.I.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef]
- Frost, G.R.; Li, Y.M. The Role of Astrocytes in Amyloid Production and Alzheimer’s Disease. Open Biol. 2017, 7, 170228. [Google Scholar] [PubMed] [Green Version]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nuñez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic Mouse Models of Alzheimer’s Disease: An Integrative Analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [PubMed]
- Gomez-Arboledas, A.; Davila, J.C.; Sanchez-Mejias, E.; Navarro, V.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Sanchez-Mico, M.V.; Trujillo-Estrada, L.; Fernandez-Valenzuela, J.J.; Vizuete, M.; et al. Phagocytic Clearance of Presynaptic Dystrophies by Reactive Astrocytes in Alzheimer’s Disease. Glia 2018, 66, 637–653. [Google Scholar] [CrossRef] [Green Version]
- Spanos, F.; Liddelow, S.A. An Overview of Astrocyte Responses in Genetically Induced Alzheimer’s Disease Mouse Models. Cells 2020, 9, 2415. [Google Scholar]
- Fu, H.; Hussaini, S.A.; Wegmann, S.; Profaci, C.; Daniels, J.D.; Herman, M.; Emrani, S.; Figueroa, H.Y.; Hyman, B.T.; Davies, P.; et al. 3D Visualization of the Temporal and Spatial Spread of Tau Pathology Reveals Extensive Sites of Tau Accumulation Associated with Neuronal Loss and Recognition Memory Deficit in Aged Tau Transgenic Mice. PLoS ONE 2016, 11, e0159463. [Google Scholar] [CrossRef] [Green Version]
- Nilson, A.N.; English, K.C.; Gerson, J.E.; Barton Whittle, T.; Nicolas Crain, C.; Xue, J.; Sengupta, U.; Castillo-Carranza, D.L.; Zhang, W.; Gupta, P.; et al. Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2017, 55, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.W.; Grant, P.A.; Rissman, E.F. Sex Differences in Histone Modifications in the Neonatal Mouse Brain. Epigenetics 2009, 4, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, L.; Obst, S.; Labusek, N.; Abberger, H.; Köster, C.; Klein-Hitpass, L.; Schumann, S.; Kleinschnitz, C.; Hermann, D.M.; Felderhoff-Müser, U.; et al. Regulatory T Cells Contribute to Sexual Dimorphism in Neonatal Hypoxic-Ischemic Brain Injury. Stroke 2022, 53, 381–390. [Google Scholar] [CrossRef]
- Jash, S.; Sharma, S. Pathogenic Infections during Pregnancy and the Consequences for Fetal Brain Development. Pathogens 2022, 11, 193. [Google Scholar]
- Zhang, M.; Zhou, Y.; Jiang, Y.; Lu, Z.; Xiao, X.; Ning, J.; Sun, H.; Zhang, X.; Luo, H.; Can, D.; et al. Profiling of Sexually Dimorphic Genes in Neural Cells to Identify Eif2s3y, Whose Overexpression Causes Autism-Like Behaviors in Male Mice. Front. Cell Dev. Biol. 2021, 9, 669798. [Google Scholar] [CrossRef]
- Bedse, G.; Romano, A.; Cianci, S.; Lavecchia, A.M.; Lorenzo, P.; Elphick, M.R.; Laferla, F.M.; Vendemiale, G.; Grillo, C.; Altieri, F.; et al. Altered Expression of the CB1 Cannabinoid Receptor in the Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 40, 701–712. [Google Scholar] [CrossRef]
- Kalifa, S.; Polston, E.K.; Allard, J.S.; Manaye, K.F. Distribution Patterns of Cannabinoid CB1 Receptors in the Hippocampus of APPswe/PS1ΔE9 Double Transgenic Mice. Brain Res. 2011, 1376, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30. [Google Scholar]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2017, 15, 508–518. [Google Scholar] [CrossRef]
- Cuellar-Santoyo, A.O.; Ruiz-Rodríguez, V.M.; Mares-Barbosa, T.B.; Patrón-Soberano, A.; Howe, A.G.; Portales-Pérez, D.P.; Miquelajáuregui Graf, A.; Estrada-Sánchez, A.M. Revealing the Contribution of Astrocytes to Glutamatergic Neuronal Transmission. Front. Cell. Neurosci. 2023, 16, 1037641. [Google Scholar] [PubMed]
- Yang, J.T.; Wang, Z.J.; Cai, H.Y.; Yuan, L.; Hu, M.M.; Wu, M.N.; Qi, J.S. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/Tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar] [CrossRef]
- Venance, L.; Piomelli, D.; Glowinski, J.; Glaume, C. Inhibition by Anandamide of Gap Junctions and Intercellular Calcium Signalling in Striatal Astrocytes. Nature 1995, 376, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Fuente-Martín, E.; Garciá-Cáceres, C.; Argente-Arizón, P.; Diáz, F.; Granado, M.; Freire-Regatillo, A.; Castro-González, D.; Ceballos, M.L.; Frago, L.M.; Dickson, S.L.; et al. Ghrelin Regulates Glucose and Glutamate Transporters in Hypothalamic Astrocytes. Sci. Rep. 2016, 6, 23673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, P.; Guerra-Cantera, S.; Vargas, A.; Díaz, F.; García-Úbeda, R.; Tovar, R.; Ramírez-López, M.T.; Argente, J.; de Fonseca, F.R.; Suárez, J.; et al. Maternal Hypercaloric Diet Affects Factors Involved in Lipid Metabolism and the Endogenous Cannabinoid Systems in the Hypothalamus of Adult Offspring: Sex-Specific Response of Astrocytes to Palmitic Acid and Anandamide. Nutr. Neurosci. 2020, 25, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Decara, J.; Rivera, P.; Arrabal, S.; Vargas, A.; Serrano, A.; Pavón, F.J.; Dieguez, C.; Nogueiras, R.; Rodríguez de Fonseca, F.; Suárez, J. Cooperative Role of the Glucagon-like Peptide-1 Receptor and Β3-Adrenergic-Mediated Signalling on Fat Mass Reduction through the Downregulation of PKA/AKT/AMPK Signalling in the Adipose Tissue and Muscle of Rats. Acta Physiol. 2018, 222, e13008. [Google Scholar] [CrossRef]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; De La Torre, R. Analysis of ECs and Related Compounds in Plasma: Artifactual Isomerization and Ex Vivo Enzymatic Generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Symbol | Assay ID | GenBank Accession Number | Amplicon Length (bp) |
|---|---|---|---|
| Actb | Mm02619580 | NM_007393.5 | 143 |
| Gfap | Mm01253033 | NM_001131020.1 | 75 |
| Vim (Vimentin) | Mm01333430 | NM_011701.4 | 62 |
| Tnfa | Mm00443258 | NM_001278601.1 | 81 |
| Il1b | Mm00434228 | NM_008361.3 | 90 |
| Gls | Mm01257297 | NM_001081081.2 | 114 |
| Gls2 | Mm01164862 | NM_001033264.3 | 118 |
| Cnr1 (CB1) | Mm01212171 | NM_007726.3 | 66 |
| Cnr2 (CB2) | Mm02620087 | NM_009924.4 | 171 |
| Gpr55 | Mm02621622 | NM_001033290.2 | 102 |
| Ppara | Mm00440939 | NM_001113418.1 | 74 |
| Trpv1 | Mm01246300 | NM_001001445.2 | 56 |
| Trpa1 | Mm01227437 | NM_177781.4 | 61 |
| Dagla | Mm00813830 | NM_198114.2 | 69 |
| Daglb | Mm00523381 | NM_144915.3 | 72 |
| Nape-pld | Mm00724596 | NM_178728.5 | 85 |
| Mgll | Mm00449274 | NM_001166249.1 | 78 |
| Faah | Mm00515684 | NM_010173.4 | 62 |
| P2rx5 | Mm00473677 | NM_033321.3 | 104 |
| Mcu | Mm01168773 | NM_001033259.4 | 71 |
| Nsmf | Mm00480341 | NM_001039386.1 | 70 |
| Itpr1 | Mm00439907 | NM_010585.5 | 58 |
| Il6 | Mm00446190 | NM_031168.1 | 78 |
| Ptgs2 | Mm00478374 | NM_011198.3 | 80 |
| Antigen | Manufacturing | Dilution |
|---|---|---|
| βactin | Sigma (St. Louis, MO, USA) #2535L, Mouse monoclonal antibody. | 1:2000 |
| DAGLa | bioNova Científica (Madrid, España) (#orb156533). Rabbit polyclonal antibody | 1:100 |
| DAGLb | Biorbyt (Cambridge, United Kingdom)(orb182976). Rabbit polyclonal antibody | 1:200 |
| NAPE-PLD | Abcam (Cambridge, United Kingdom). Rabbit polyclonal antibody. Ab95397 | 1:200 |
| MAGL | Abcam (Cambridge, United Kingdom). Rabbit polyclonal antibody. (Ab24701) | 1:200 |
| FAAH | Cayman (Ann Arbor, MI, USA). Rabbit polyclonal antibody. Cat. No: 101600 | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Sánchez, B.; Tovar, R.; Ben Rabaa, M.; Sánchez-Salido, L.; Vargas, A.; Suárez, J.; Rodríguez de Fonseca, F.; Rivera, P. Sex-Dependent Altered Expression of Cannabinoid Signaling in Hippocampal Astrocytes of the Triple Transgenic Mouse Model of Alzheimer’s Disease: Implications for Controlling Astroglial Activity. Int. J. Mol. Sci. 2023, 24, 12598. https://doi.org/10.3390/ijms241612598
Pacheco-Sánchez B, Tovar R, Ben Rabaa M, Sánchez-Salido L, Vargas A, Suárez J, Rodríguez de Fonseca F, Rivera P. Sex-Dependent Altered Expression of Cannabinoid Signaling in Hippocampal Astrocytes of the Triple Transgenic Mouse Model of Alzheimer’s Disease: Implications for Controlling Astroglial Activity. International Journal of Molecular Sciences. 2023; 24(16):12598. https://doi.org/10.3390/ijms241612598
Chicago/Turabian StylePacheco-Sánchez, Beatriz, Rubén Tovar, Meriem Ben Rabaa, Lourdes Sánchez-Salido, Antonio Vargas, Juan Suárez, Fernando Rodríguez de Fonseca, and Patricia Rivera. 2023. "Sex-Dependent Altered Expression of Cannabinoid Signaling in Hippocampal Astrocytes of the Triple Transgenic Mouse Model of Alzheimer’s Disease: Implications for Controlling Astroglial Activity" International Journal of Molecular Sciences 24, no. 16: 12598. https://doi.org/10.3390/ijms241612598