Insights into Hepatocellular Carcinoma in Patients with Thalassemia: From Pathophysiology to Novel Therapies
Abstract
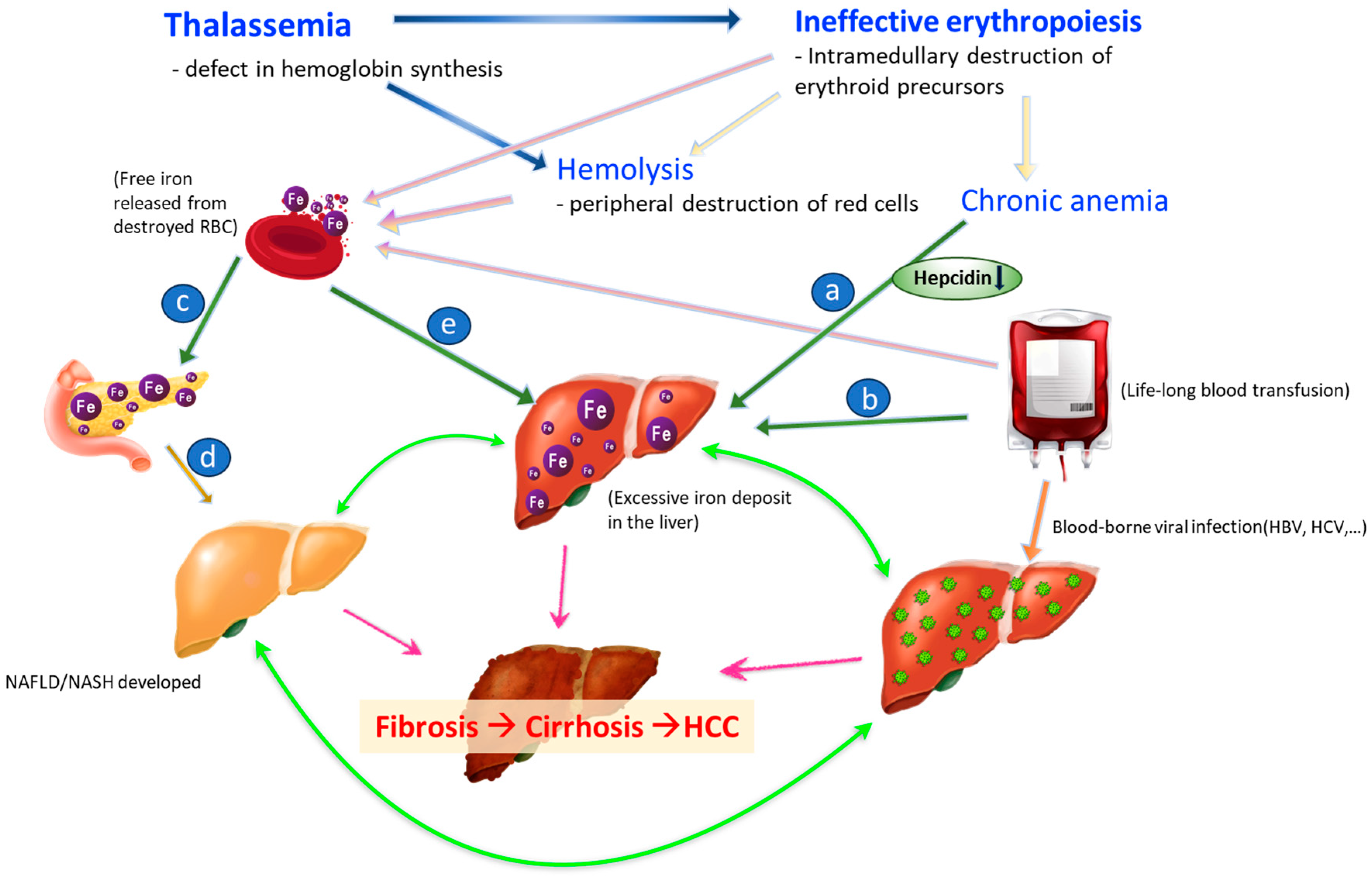
:1. Introduction
2. Risk Factors for HCC
2.1. Risk Factors for HCC in the General Population Focusing on Hepatitis Virus and Fatty Liver Disease
2.2. Risk Factors for HCC in the Thalassemia Group Focusing on Hepatitis Virus and Fatty Liver Disease
2.3. Risk Factors for HCC in the Thalassemia Group Focusing on Iron Overload
3. Pathogenesis of Viral Infection and NAFLD with HCC
3.1. HBV/HCV-Associated HCC
3.2. NAFLD/NASH-Associated HCC
4. Pathogenesis of Iron Overload and HCC
4.1. Iron and Inflammation
4.2. Iron and Reactive Oxygen Species (ROS)
4.3. Hepcidin and Ferriportin
5. Management
5.1. Management of Iron Overload
5.2. Management of HCC
6. Prognosis
7. Surveillance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinberg, M.H.; Forget, B.G.; Higgs, D.R.; Weatherall, D.J. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood J. Am. Soc. Hematol. 2010, 115, 4331–4336. [Google Scholar]
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar]
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of β-thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [PubMed]
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [PubMed]
- Piel, F.B.; Weatherall, D.J. The α-thalassemias. N. Engl. J. Med. 2014, 371, 1908–1916. [Google Scholar]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Program Book 2017, 2017, 265–271. [Google Scholar]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar]
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86. [Google Scholar]
- Mancuso, A.; Sciarrino, E.; Concetta Renda, M.; Maggio, A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin 2006, 30, 119–124. [Google Scholar]
- Moukhadder, H.M.; Halawi, R.; Cappellini, M.D.; Taher, A.T. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: A comprehensive review. Cancer 2017, 123, 751–758. [Google Scholar] [PubMed]
- Chung, W.-S.; Lin, C.-L.; Lin, C.-L.; Kao, C.-H. Thalassaemia and risk of cancer: A population-based cohort study. J. Epidemiol. Community Health 2015, 69, 1066–1070. [Google Scholar] [PubMed]
- Taher, A.; Musallam, K.; Cappellini, M.D. Guidelines for the Management of Non-Transfusion Dependent Thalassaemia (NTDT); TIF Publications: Nicosia, Cyprus, 2017. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Zhang, C.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [PubMed]
- Sangiovanni, A.; Del Ninno, E.; Fasani, P.; De Fazio, C.; Ronchi, G.; Romeo, R.; Morabito, A.; De Franchis, R.; Colombo, M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004, 126, 1005–1014. [Google Scholar]
- Jepsen, P.; Ott, P.; Andersen, P.K.; Sørensen, H.T.; Vilstrup, H. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: A Danish nationwide cohort study. Ann. Intern. Med. 2012, 156, 841–847. [Google Scholar]
- Lin, C.-W.; Lin, C.-C.; Mo, L.-R.; Chang, C.-Y.; Perng, D.-S.; Hsu, C.-C.; Lo, G.-H.; Chen, Y.-S.; Yen, Y.-C.; Hu, J.-T.; et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J. Hepatol. 2013, 58, 730–735. [Google Scholar]
- Sinn, D.H.; Kang, D.; Guallar, E.; Chang, Y.; Ryu, S.; Zhao, D.; Hong, Y.S.; Cho, J.; Gwak, G.-Y. Alcohol intake and mortality in patients with chronic viral hepatitis: A nationwide cohort study. Am. J. Gastroenterol. 2021, 116, 329–335. [Google Scholar]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [PubMed]
- Borgna-Pignatti, C.; Garani, M.C.; Forni, G.L.; Cappellini, M.D.; Cassinerio, E.; Fidone, C.; Spadola, V.; Maggio, A.; Restivo Pantalone, G.; Piga, A.; et al. Hepatocellular carcinoma in thalassaemia: An update of the Italian Registry. Br. J. Haematol. 2014, 167, 121–126. [Google Scholar] [PubMed]
- Wonke, B.; Hoffbrand, A.; Brown, D.; Dusheiko, G. Antibody to hepatitis C virus in multiply transfused patients with thalassaemia major. J. Clin. Pathol. 1990, 43, 638–640. [Google Scholar] [PubMed] [Green Version]
- He, L.-N.; Chen, W.; Yang, Y.; Xie, Y.-J.; Xiong, Z.-Y.; Chen, D.-Y.; Lu, D.; Liu, N.-Q.; Yang, Y.-H.; Sun, X.-F. Elevated prevalence of abnormal glucose metabolism and other endocrine disorders in patients with-thalassemia major: A meta-analysis. BioMed Res. Int. 2019, 2019, 6573497. [Google Scholar]
- Memaj, P.; Jornayvaz, F.R. Non-alcoholic fatty liver disease in type 1 diabetes: Prevalence and pathophysiology. Front. Endocrinol. 2022, 13, 1031633. [Google Scholar]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and anemia: A tight relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar]
- Frey, P.A.; Reed, G.H. The Ubiquity of Iron; ACS Publications: Washington, DC, USA, 2012. [Google Scholar]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar]
- Grosse, S.D.; Gurrin, L.C.; Bertalli, N.A.; Allen, K.J. Clinical penetrance in hereditary hemochromatosis: Estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet. Med. 2018, 20, 383–389. [Google Scholar]
- Atkins, J.L.; Pilling, L.C.; Masoli, J.A.; Kuo, C.-L.; Shearman, J.D.; Adams, P.C.; Melzer, D. Association of hemochromatosis HFE p. C282Y homozygosity with hepatic malignancy. JAMA 2020, 324, 2048–2057. [Google Scholar]
- Turlin, B.; Juguet, F.; Moirand, R.; Le Quilleuc, D.; Loréal, O.; Campion, J.-P.; Launois, B.; Ramée, M.-p.; Brissot, P.; Deugnier, Y. Increased liver iron stores in patients with hepatocellular carcinoma developed on a noncirrhotic liver. Hepatology 1995, 22, 446–450. [Google Scholar] [PubMed]
- Zanella, S.; Garani, M.C.; Borgna-Pignatti, C. Malignancies and thalassemia: A review of the literature. Ann. N. Y. Acad. Sci. 2016, 1368, 140–148. [Google Scholar] [PubMed]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 8, 435–450. [Google Scholar]
- Simas, C.; Larson, H.J. Overcoming vaccine hesitancy in low-income and middle-income regions. Nat. Rev. Dis. Primers 2021, 7, 41. [Google Scholar]
- Xie, Y. Hepatitis B virus-associated hepatocellular carcinoma. In Infectious Agents Associated Cancers: Epidemiology and Molecular Biology; Springer: Singapore, 2017; pp. 11–21. [Google Scholar]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic mechanisms involved in HCV-induced hepatocellular carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [PubMed]
- Benhammou, J.N.; Lin, J.; Hussain, S.K.; El-Kabany, M. Emerging risk factors for nonalcoholic fatty liver disease associated hepatocellular carcinoma. Hepatoma Res. 2020, 6, 35. [Google Scholar]
- Said, A.; Ghufran, A. Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World J. Clin. Oncol. 2017, 8, 429. [Google Scholar]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar]
- Tian, Z.; Xu, C.; Yang, P.; Lin, Z.; Wu, W.; Zhang, W.; Ding, J.; Ding, R.; Zhang, X.; Dou, K. Molecular pathogenesis: Connections between viral hepatitis-induced and non-alcoholic steatohepatitis-induced hepatocellular carcinoma. Front. Immunol. 2022, 13, 984728. [Google Scholar]
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.-J.; Kim, W.K.; Bae, K.-H.; Oh, K.-J. Metabolic spectrum of liver failure in type 2 diabetes and obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495. [Google Scholar]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar]
- Garrido, A.; Djouder, N. Cirrhosis: A questioned risk factor for hepatocellular carcinoma. Trends Cancer 2021, 7, 29–36. [Google Scholar] [PubMed]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB–regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [PubMed] [Green Version]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [PubMed]
- Hoshida, Y.; Villanueva, A.; Sangiovanni, A.; Sole, M.; Hur, C.; Andersson, K.L.; Chung, R.T.; Gould, J.; Kojima, K.; Gupta, S.; et al. Prognostic gene expression signature for patients with hepatitis C–related early-stage cirrhosis. Gastroenterology 2013, 144, 1024–1030. [Google Scholar] [PubMed] [Green Version]
- Budhu, A.; Forgues, M.; Ye, Q.-H.; Jia, H.-L.; He, P.; Zanetti, K.A.; Kammula, U.S.; Chen, Y.; Qin, L.-X.; Tang, Z.-Y.; et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006, 10, 99–111. [Google Scholar]
- Moeini, A.; Torrecilla, S.; Tovar, V.; Montironi, C.; Andreu-Oller, C.; Peix, J.; Higuera, M.; Pfister, D.; Ramadori, P.; Pinyol, R.; et al. An immune gene expression signature associated with development of human hepatocellular carcinoma identifies mice that respond to chemopreventive agents. Gastroenterology 2019, 157, 1383–1397.e11. [Google Scholar] [PubMed] [Green Version]
- Adams, P.C. Is there a threshold of hepatic iron concentration that leads to cirrhosis in C282Y hemochromatosis? Am. J. Gastroenterol. 2001, 96, 567–569. [Google Scholar]
- Angelucci, E.; Muretto, P.; Nicolucci, A.; Baronciani, D.; Erer, B.; Gaziev, J.; Ripalti, M.; Sodani, P.; Tomassoni, S.; Visani, G.; et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood J. Am. Soc. Hematol. 2002, 100, 17–21. [Google Scholar]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [PubMed]
- Moloney, J.N.; Cotter, T.G. Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 50–64. [Google Scholar]
- Choi, J.-E.; Kim, J.-H.; Song, N.-Y.; Suh, J.; Kim, D.-H.; Kim, S.-J.; Na, H.-K.; Nadas, J.; Dong, Z.; Cha, Y.-N.; et al. 15-Deoxy-Δ12, 14-prostaglandin J2 stabilizes hypoxia inducible factor-1α through induction of heme oxygenase-1 and direct modification ofprolyl-4-hydroxylase 2. Free Radic. Res. 2016, 50, 1140–1152. [Google Scholar] [PubMed]
- Cao, L.; Liu, J.; Zhang, L.; Xiao, X.; Li, W. Curcumin inhibits H2O2-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol. Rep. 2016, 36, 2245–2251. [Google Scholar]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Cho, Y.; Lee, G.; You, D.; Yoo, Y.; Kim, Y. A direct role for hepatitis B virus X protein in inducing mitochondrial membrane permeabilization. J. Viral Hepat. 2018, 25, 412–420. [Google Scholar]
- Lambrecht, R.W.; Sterling, R.K.; Naishadham, D.; Stoddard, A.M.; Rogers, T.; Morishima, C.; Morgan, T.R.; Bonkovsky, H.L.; Group, H.-C.T. Iron levels in hepatocytes and portal tract cells predict progression and outcomes of patients with advanced chronic hepatitis C. Gastroenterology 2011, 140, 1490–1500.e3. [Google Scholar]
- Krause, A.; Neitz, S.; Mägert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [Green Version]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kijima, H.; Sawada, T.; Tomosugi, N.; Kubota, K. Expression of hepcidin mRNA is uniformly suppressed in hepatocellular carcinoma. BMC Cancer 2008, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.G.K.; Kumarasinghe, M.P.; Wang, S.M.; Ooi, L.L.P.; Aw, S.E.; Hui, K.M. Modulation of iron-regulatory genes in human hepatocellular carcinoma and its physiological consequences. Exp. Biol. Med. 2009, 234, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Chang, J.G.; Hwang, Y.H.; Yeh, K.T.; Chen, Y.L.; Yu, H.S. Expression of hepcidin and other iron-regulatory genes in human hepatocellular carcinoma and its clinical implications. J. Cancer Res. Clin. Oncol. 2009, 135, 1413–1420. [Google Scholar] [CrossRef]
- Kessler, S.M.; Barghash, A.; Laggai, S.; Helms, V.; Kiemer, A.K. Hepatic hepcidin expression is decreased in cirrhosis and HCC. J. Hepatol. 2015, 62, 977–979. [Google Scholar] [CrossRef] [Green Version]
- Han, C.Y.; Koo, J.H.; Kim, S.H.; Gardenghi, S.; Rivella, S.; Strnad, P.; Hwang, S.J.; Kim, S.G. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat. Commun. 2016, 7, 13817. [Google Scholar] [CrossRef] [Green Version]
- Joachim, J.H.; Mehta, K.J. Hepcidin in hepatocellular carcinoma. Br. J. Cancer 2022, 127, 185–192. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Guo, W.; Yuan, L.; Zhang, D.; Xu, Y.; Nemeth, E.; Ganz, T.; Liu, S. Disordered hepcidin-ferroportin signaling promotes breast cancer growth. Cell. Signal. 2014, 26, 2539–2550. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, S.; Chen, Y.; Zhang, D.; Yuan, L.; Cong, H.; Liu, S. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim. Biophys. Sin. 2015, 47, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Rah, B.; Farhat, N.M.; Hamad, M.; Muhammad, J.S. JAK/STAT signaling and cellular iron metabolism in hepatocellular carcinoma: Therapeutic implications. Clin. Exp. Med. 2023. [Google Scholar] [CrossRef]
- Finianos, A.; Matar, C.F.; Taher, A. Hepatocellular carcinoma in β-thalassemia patients: Review of the literature with molecular insight into liver carcinogenesis. Int. J. Mol. Sci. 2018, 19, 4070. [Google Scholar] [PubMed] [Green Version]
- Hatairaktham, S.; Masaratana, P.; Hantaweepant, C.; Srisawat, C.; Sirivatanauksorn, V.; Siritanaratkul, N.; Panichkul, N.; Kalpravidh, R.W. Curcuminoids supplementation ameliorates iron overload, oxidative stress, hypercoagulability, and inflammation in non-transfusion-dependent β-thalassemia/Hb E patients. Ann. Hematol. 2021, 100, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Tamaddoni, A.; Qujeq, D.; Nasseri, E.; Zayeri, F.; Zand, H.; Gholami, M.; Mir, S.M. An investigation of the effects of curcumin on iron overload, hepcidin level, and liver function in β-thalassemia major patients: A double-blind randomized controlled clinical trial. Phytother. Res. 2018, 32, 1828–1835. [Google Scholar]
- Al-Momen, H.; Hussein, H.K.; Al-Attar, Z.; Hussein, M.J. Green tea influence on iron overload in thalassemia intermedia patients: A randomized controlled trial. FResearch 2020, 9, 1136. [Google Scholar]
- Saeidnia, M.; Fazeli, P.; Erfani, M.; Nowrouzi-Sohrabi, P.; Tamaddon, G.; Karimi, M. The Effect of Curcumin on Iron Overload in Patients with Beta-Thalassemia Intermedia. Clin. Lab. 2022, 68, 210629. [Google Scholar]
- Farmakis, D.; Porter, J.; Taher, A.; Cappellini, M.D.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. HemaSphere 2022, 6, e732. [Google Scholar]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar]
- Llovet, J.; Kelley, R.; Villanueva, A. Hepatocellular carcinoma. Nat Rev Dis Primers. PubMed Artic. 2021, 7, 7. [Google Scholar]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.W.; Lee, I.C.; Chi, C.T.; Lee, R.C.; Liu, C.A.; Chiu, N.C.; Hwang, H.E.; Chao, Y.; Hou, M.C.; Huang, Y.H. Redefining Tumor Burden in Patients with Intermediate-Stage Hepatocellular Carcinoma: The Seven-Eleven Criteria. Liver Cancer 2021, 10, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Borgna-Pignatti, C.; Vergine, G.; Lombardo, T.; Cappellini, M.D.; Cianciulli, P.; Maggio, A.; Renda, D.; Lai, M.E.; Mandas, A.; Forni, G.; et al. Hepatocellular carcinoma in the thalassaemia syndromes. Br. J. Haematol. 2004, 124, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Kountouras, D.; Malagari, K.; Tampaki, M.; Theochari, M.; Koskinas, J. Characteristics and prognosis of hepatocellular carcinoma in multi-transfused patients with β-thalassemia. Experience of a single tertiary center. Mediterr. J. Hematol. 2020, 12, e2020013. [Google Scholar] [CrossRef]
- Filippiadis, D.; Velonakis, G.; Charalampopoulos, G.; Masala, S.; Kelekis, A.; Kelekis, N.J.C.; Radiology, I. Percutaneous Microwave Ablation for the Management of Hepatocellular Carcinoma in Transfusion-Dependent Beta-Thalassemia Patients. CardioVascular Interv. Radiol. 2022, 45, 709–711. [Google Scholar] [CrossRef]
- Ricchi, P.; Costantini, S.; Spasiano, A.; Cinque, P.; Esposito, S.; Filosa, A. Hepatocellular carcinoma in patients with thalassemia in the post-DAA era: Not a disappearing entity. Ann. Hematol. 2021, 100, 1907–1910. [Google Scholar]
- Maakaron, J.E.; Cappellini, M.D.; Graziadei, G.; Ayache, J.B.; Taher, A.T. Hepatocellular carcinoma in hepatitis-negative patients with thalassemia intermedia: A closer look at the role of siderosis. Ann. Hepatol. 2013, 12, 142–146. [Google Scholar]
- Restivo Pantalone, G.; Renda, D.; Valenza, F.; D’Amato, F.; Vitrano, A.; Cassarà, F.; Rigano, P.; Salvo, V.D.; Giangreco, A.; Bevacqua, E.; et al. Hepatocellular carcinoma in patients with thalassaemia syndromes: Clinical characteristics and outcome in a long term single centre experience. Br. J. Haematol. 2010, 150, 245–247. [Google Scholar]
- Mancuso, A.; Perricone, G. Time to define a new strategy for management of hepatocellular carcinoma in thalassaemia? Br. J. Haematol. 2015, 168, 304–305. [Google Scholar] [CrossRef]
- Mangia, A.; Bellini, D.; Cillo, U.; Laghi, A.; Pelle, G.; Valori, V.M.; Caturelli, E. Hepatocellular carcinoma in adult thalassemia patients: An expert opinion based on current evidence. BMC Gastroenterol. 2020, 20, 251. [Google Scholar]
- Girardi, D.M.; Sousa, L.P.; Miranda, T.A.; Haum, F.N.; Pereira, G.C.; Pereira, A.A. Systemic Therapy for Advanced Hepatocellular Carcinoma: Current Stand and Perspectives. Cancers 2023, 15, 1680. [Google Scholar] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular carcinoma: An overview of the changing landscape of treatment options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [PubMed]
- Chan, S.L.; Wong, N.; Lam, W.J.; Kuang, M. Personalized treatment for hepatocellular carcinoma: Current status and Future perspectives. J. Gastroenterol. Hepatol. 2022, 37, 1197–1206. [Google Scholar] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- O’Rourke, J.M.; Sagar, V.M.; Shah, T.; Shetty, S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J. Gastroenterol. 2018, 24, 4436–4447. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar]
- Goltyaev, M.V.; Varlamova, E.G. The Role of Selenium Nanoparticles in the Treatment of Liver Pathologies of Various Natures. Int. J. Mol. Sci. 2023, 24, 10547. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Z. Survival improvement and prognosis for hepatocellular carcinoma: Analysis of the SEER database. BMC Cancer 2021, 21, 1157. [Google Scholar]
- Taher, A.T.; Cappellini, M.D. How I manage medical complications of β-thalassemia in adults. Blood J. Am. Soc. Hematol. 2018, 132, 1781–1791. [Google Scholar]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Alansary, N.; Kattamis, A.; Skafida, M.; Galati, M.C.; Christou, S.; Campisi, S.; Messina, G.; et al. A concise review on the frequency, major risk factors and surveillance of hepatocellular carcinoma (HCC) in β-thalassemias: Past, present and future perspectives and the ICET-A experience. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020006. [Google Scholar] [PubMed]
- Sheu, J.-C.; Sung, J.-L.; Chen, D.-S.; Yang, P.-M.; Lai, M.-Y.; Lee, C.-S.; Hsu, H.-C.; Chuang, C.-N.; Yang, P.-C.; Wang, T.-H.; et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985, 89, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar]
- Johnson, P.J.; Pirrie, S.J.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The Detection of Hepatocellular Carcinoma Using a Prospectively Developed and Validated Model Based on Serological BiomarkersDetection of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Schlosser, S.; Tümen, D.; Volz, B.; Neumeyer, K.; Egler, N.; Kunst, C.; Tews, H.C.; Schmid, S.; Kandulski, A.; Müller, M.; et al. HCC biomarkers–state of the old and outlook to future promising biomarkers and their potential in everyday clinical practice. Front. Oncol. 2022, 12, 1016952. [Google Scholar]
- Shahini, E.; Pasculli, G.; Solimando, A.G.; Tiribelli, C.; Cozzolongo, R.; Giannelli, G. Updating the Clinical Application of Blood Biomarkers and Their Algorithms in the Diagnosis and Surveillance of Hepatocellular Carcinoma: A Critical Review. Int. J. Mol. Sci. 2023, 24, 4286. [Google Scholar] [PubMed]
- Foda, Z.H.; Annapragada, A.V.; Boyapati, K.; Bruhm, D.C.; Vulpescu, N.A.; Medina, J.E.; Mathios, D.; Cristiano, S.; Niknafs, N.; Luu, H.T.; et al. Detecting liver cancer using cell-free DNA fragmentomes. Cancer Discov. 2023, 13, 616–631. [Google Scholar] [CrossRef]
- Tran, N.H.; Kisiel, J.; Roberts, L.R. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep. 2021, 3, 100304. [Google Scholar]
- Zhang, X.; Wang, Z.; Tang, W.; Wang, X.; Liu, R.; Bao, H.; Chen, X.; Wei, Y.; Wu, S.; Bao, H.; et al. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology 2022, 76, 317–329. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-C.; Hsu, W.-Y.; Lee, P.-Y.; Hsu, S.-H.; Chiou, S.-S. Insights into Hepatocellular Carcinoma in Patients with Thalassemia: From Pathophysiology to Novel Therapies. Int. J. Mol. Sci. 2023, 24, 12654. https://doi.org/10.3390/ijms241612654
Lin P-C, Hsu W-Y, Lee P-Y, Hsu S-H, Chiou S-S. Insights into Hepatocellular Carcinoma in Patients with Thalassemia: From Pathophysiology to Novel Therapies. International Journal of Molecular Sciences. 2023; 24(16):12654. https://doi.org/10.3390/ijms241612654
Chicago/Turabian StyleLin, Pei-Chin, Wan-Yi Hsu, Po-Yi Lee, Shih-Hsien Hsu, and Shyh-Shin Chiou. 2023. "Insights into Hepatocellular Carcinoma in Patients with Thalassemia: From Pathophysiology to Novel Therapies" International Journal of Molecular Sciences 24, no. 16: 12654. https://doi.org/10.3390/ijms241612654




