Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD
Abstract
1. Introduction
1.1. Free Fatty Acids (FFAs) Role
1.2. Nuclear Receptors
1.2.1. Peroxisome Proliferator-Activated Receptors (PPARs)
1.2.2. Liver X Receptors (LXRs)
1.2.3. Farnesoid X Receptors (FXRs)
1.3. Gut–Liver Axis in NAFLD
1.4. Genetic Component of NAFLD
1.5. De Novo Lipogenesis
2. Possible Therapeutic Approaches
2.1. Incretins: GLP-1 Agonists
| Drug | Mechanism | Clinical Trial Status | Administration Route/Posology | Dosage | Main Liver-Related Outcomes | Clinical Trial Ref | Published Results |
|---|---|---|---|---|---|---|---|
| Liraglutide | GLP-1 agonist | 5 completed; 1 active, not recruiting; 1 recruiting; 1 unknown. | Subcutaneous, 1/day | 0.6–1.8 mg/day | ↓ Liver volume ↓ Liver fat content ↓ Hepatic de novo lipogenesis ↓ Serum ALT, AST ↓ TG, LDL ↑ Serum HDL | NCT02147925 NCT03068065 NCT01399645 NCT03233178 NCT01237119 NCT05041673 NCT05779644 NCT02654665 | [136] [137] [138] [139] [140] [120] [141] |
| Exenatide | GLP-1 agonist | 4 completed; 1 terminated. | Subcutaneous, 2/day or 1/week (extended release) | 5–10 μg twice a day | ↓ Liver volume ↓↓ Liver fat content ↓ Serum ALT, AST, GGT ↓ Serum LDL | NCT02303730 NCT01006889 NCT01208649 NCT00650546 NCT00529204 | [142] |
| Semaglutide | GLP-1 agonist | 5 completed; 2 active, not recruiting; 9 recruiting; 3 not yet recruiting; 1 suspended. | Subcutaneous, 1/week, 1/day OR Oral, 1/day | 0.24–2.4 mg weekly; 0.05–0.4 mg daily; 3–14 mg daily (oral tablets). | ↓ Liver volume ↓ Liver fat content ↓ Serum ALT, AST, GGT ↓ TG ↓ Fibrosis | NCT03987074 NCT02970942 NCT03357380 NCT03987451 NCT04944992 NCT04216589 NCT05654051 NCT05813249 NCT03884075 NCT03919929 NCT04639414 NCT04822181 NCT05766709 NCT04971785 NCT05016882 NCT05779644 NCT05067621 NCT05195944 NCT05877547 NCT05751720 NCT05424003 | [122] [123] [143] [144] [125] [145] [123] [146] |
| Dulaglutide | GLP-1 agonist | 1 completed; 1 not yet recruiting. | Subcutaneous, 1/week | 0.75–1.5 mg weekly | ↓ Liver fat content ↓ GGT | NCT03590626 NCT03648554 | [147] |
| Cotadutide | Dual GLP-1 and glucacon receptor agonist | 2 completed; 1 recruiting; 1 not yet recruiting; 1 terminated. | Subcutaneous, 1/day | 300 μg daily | ↓ Liver fat content ↓ Serum ALT, AST, GGT ↓↓ Serum cholesterol, TG ↓ Pro-C3 ↓ FIB-4, NFS | NCT03235050 NCT04019561 NCT05364931 NCT05294458 NCT05668936 | [131] |
| Tirzepatide | Dual GIP and GLP-1 receptor agonist | 1 active, not recruiting; 1 not yet recruiting. | Subcutaneous, 1/week | 0.25–15 mg weekly | ↓ Body weight | NCT04166773 NCT05751720 | [148] [134] |
2.2. THR-β Agonists
2.3. PPAR Agonists
2.3.1. PPARα
2.3.2. PPARγ
2.4. FXR Agonists
2.5. Natural Compounds
2.5.1. Natural Compounds in Trials for NAFL/NASH
Vitamin E
Silymarin
Resveratrol
Curcumin
Caffeine
| Compound | Identifier Trial | Condition | Drug | Dosage | Status | Citations |
|---|---|---|---|---|---|---|
| Vitamin E | NCT02690792 | NAFLD | Vitamin E | 200 IU capsule, two capsules each morning and each evening every 4 months | Completed | - |
| NCT01792115 | NAFLD | Vitamin E | 200–400–800 IU/day for 24 weeks + optional extension for up to 120 weeks | Completed | [274] | |
| NCT02962297 | NASH | Vitamin E | Softgel 100 mg, oral adiministration for 96 weeks. | Completed | [272] | |
| NCT04198805 (PUVENAFLD) | NAFLD | Vitamin E and DHA-EE | Vitamin E 1000 mg capsules/DHA EE (1.89 g) alone or in combination once daily for 6 months | Completed | Results posted | |
| NCT01147523 | NAFLD | Vitamin E and spironolactone | Spironolactone, tablets, 25 mg and vitamin E capsules 400 mg daily, for 52 weeks | Completed | [273] | |
| NCT01934777 | NAFLD (4–16 years) | Docosahexaenoic acid plus vitamin E plus choline | DHA 250 mg plus vitamin E (39 UI) plus choline 201 mg per day for 6 months | Completed | - | |
| NCT00655018 (VITENAFLD) | NAFLD (3–20 years) | Vitamin E, vitamin C | a-tocopherol 600 IU/d plus ascorbic acid 500 mg/d | Completed | [273] | |
| Resveratrol | NCT01464801 (LIRMOI3) | NAFLD | Resveratrol | Resveratrol 500 mg tablet, three times daily for 6 months | Completed | - |
| NCT02030977 | NASH | Resveratrol | Resveratrol 500 mg/d, one capsule per day for 12 weeks | Completed | [4] | |
| NCT01446276 | NAFLD | Resveratrol | 500 mg, three times daily for 6 months | Completed | [279] | |
| NCT02216552 | NAFLD/IR | ResVida (13–18 years) | Resveratrol 75 mg twice daily for 30 days | Completed | - | |
| Silibilin | NCT05497765 | NAFLD | Silibilin | Silibilin extract, four tablets with warm water twice a day | Recruiting | - |
| NCT04640324 | NAFLD | Silybin–phospholipids, vitamin D, vitamin E | 303 mg of silybin-phospholipid complex, 10 ug of vitamin D, and 15 mg of vitamin E, twice a day for 6 months | Completed | [284] | |
| Ginger | NCT02289235 (GinLivDM) | NAFLD/ T2DM | Ginger | Ginger capsule 1000 mg twice daily for 3 months | Completed | - |
| Berberine | NCT04049396 | NAFLD | Berberine | 6.25 g/day berberine for 6 weeks | Completed | - |
| NCT05523024 | NAFLD | Berberine | 1500 mg of berberine extract per day in three doses. | Recruiting | - | |
| NCT03198572 | NASH | Berberine | Tablets 0.5 g, 30 min before each meal, for 48 weeks | Unknown | - | |
| Silymarin | NCT03749070 (The SILIVER Trial) | NAFLD | Silymarin | 700 mg of silymarin, 8 mg Vitamin E and 50 mg phosphatidilcholine, daily for 12 weeks | Recruiting | [277] |
| NCT02006498 | NASH | Silymarin | 700 mg, administered three times daily for 48 weeks | Completed | [275] | |
| NCT00680407 | NASH/HCV | Legalon | 420 or 700 mg dose of silymarin (five pills, three times daily) for 48–50 weeks | Completed | [276] | |
| NCT05051527 | NAFLD | Legalon | 140 mg of silymarin for 6 months | Recruiting | - | |
| Caffeine | NCT02929901 | NAFDL/ T2DM | Caffeine | caffeine (200 mg)/chlorogenic acid (200 mg) or combination of the two, one capsule/day for 6 months | Completed | [282] |
| Curcumin | NCT02908152 | T2DM/NAFLD | Curcumin capsules 1.5 g/d | 1.5 g/die | Completed | [280] |
| NCT03864783 | NAFLD/IR | Phospholipid curcumin capsules | One tablet (200 mg curcumin) twice daily for 3–4 days | Completed | [281] | |
| NCT04315350 | NAFLD/IR | Curcumin tablets | One tablet (100 mg curcumin) twice daily + prednisolon 50 mg every morning | Recruiting | - | |
| NCT02369536 | NAFLD | Fish oil 70%, DHA, silymarine, curcumin, D-a-tocopherol, choline bitartrate, phosphatidilcoline in sunflower oil | Two soft gelatin capsules of 800 mg per day | Completed | Results posted | |
| Mastiha | NCT03135873 (MAST4HEALTH) | NAFLD | Mastiha | 2.1 g/day for 6 months | Completed | [285] |
2.5.2. Challenges in the Use of Natural Compounds
2.6. Obstacles and Challenges in Clinical Trials
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How Does Hepatic Lipid Accumulation Lead to Lipotoxicity in Non-Alcoholic Fatty Liver Disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Burchill, M.A.; Finlon, J.M.; Goldberg, A.R.; Gillen, A.E.; Dahms, P.A.; McMahan, R.H.; Tye, A.; Winter, A.B.; Reisz, J.A.; Bohrnsen, E.; et al. Oxidized Low-Density Lipoprotein Drives Dysfunction of the Liver Lymphatic System. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 573–595. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of Fatty Acids Stored in Liver and Secreted via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Recent Insights into Hepatic Lipid Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Viswanathan, S.; Adami, E.; Singh, B.K.; Chothani, S.P.; Ng, B.; Lim, W.W.; Zhou, J.; Tripathi, M.; Ko, N.S.J.; et al. Hepatocyte-Specific IL11 Cis-Signaling Drives Lipotoxicity and Underlies the Transition from NAFLD to NASH. Nat. Commun. 2021, 12, 66. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Hamid, O.; Eltelbany, A.; Mohammed, A.; Alsabbagh Alchirazi, K.; Trakroo, S.; Asaad, I. The Epidemiology of Non-Alcoholic Steatohepatitis (NASH) in the United States between 2010–2020: A Population-Based Study. Ann. Hepatol. 2022, 27, 100727. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL). EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Canbay, L.; Gerken, G. Lipid Metabolism in the Liver. Z. Gastroenterol. 2007, 45, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M. Intestinal Lipid Absorption and Lipoprotein Formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding Lipotoxicity in NAFLD Pathogenesis: Is CD36 a Key Driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.F.P.; Bonen, A.; Glatz, J.F.C. Fatty Acid Transport across the Cell Membrane: Regulation by Fatty Acid Transporters. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 149–154. [Google Scholar] [CrossRef]
- Garbacz, W.G.; Lu, P.; Miller, T.M.; Poloyac, S.M.; Eyre, N.S.; Mayrhofer, G.; Xu, M.; Ren, S.; Xie, W. Hepatic Overexpression of CD36 Improves Glycogen Homeostasis and Attenuates High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance. Mol. Cell Biol. 2016, 36, 2715–2727. [Google Scholar] [CrossRef]
- Bradbury, M.W. Lipid Metabolism and Liver Inflammation. I. Hepatic Fatty Acid Uptake: Possible Role in Steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 194–198. [Google Scholar] [CrossRef]
- Enooku, K.; Tsutsumi, T.; Kondo, M.; Fujiwara, N.; Sasako, T.; Shibahara, J.; Kado, A.; Okushin, K.; Fujinaga, H.; Nakagomi, R.; et al. Hepatic FATP5 Expression Is Associated with Histological Progression and Loss of Hepatic Fat in NAFLD Patients. J. Gastroenterol. 2020, 55, 227–243. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Morgantini, C.; Lewis, G.F. New Insights into the Regulation of Chylomicron Production. Annu. Rev. Nutr. 2015, 35, 265–294. [Google Scholar] [CrossRef]
- Grundy, S.M. Absorption and Metabolism of Dietary Cholesterol. Annu. Rev. Nutr. 1983, 3, 71–96. [Google Scholar] [CrossRef]
- Mocciaro, G.; Gastaldelli, A. Obesity-Related Insulin Resistance: The Central Role of Adipose Tissue Dysfunction. In From Obesity to Diabetes; Eckel, J., Clément, K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 145–164. ISBN 978-3-030-99995-7. [Google Scholar]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin Resistance in Non-Diabetic Patients with Non-Alcoholic Fatty Liver Disease: Sites and Mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Kalavalapalli, S.; Leiva, E.G.; Lomonaco, R.; Chi, X.; Shrestha, S.; Dillard, R.; Budd, J.; Romero, J.P.; Li, C.; Bril, F.; et al. Adipose Tissue Insulin Resistance Predicts the Severity of Liver Fibrosis in Patients with Type 2 Diabetes and NAFLD. J. Clin. Endocrinol. Metab. 2023, 108, 1192–1201. [Google Scholar] [CrossRef]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of Adipose Tissue Insulin Resistance on Metabolic Parameters and Liver Histology in Obese Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef]
- Guerra, S.; Mocciaro, G.; Gastaldelli, A. Adipose Tissue Insulin Resistance and Lipidome Alterations as the Characterizing Factors of Non-Alcoholic Steatohepatitis. Eur. J. Clin. Investig. 2022, 52, e13695. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Høy, C.E. The Digestion of Dietary Triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic Lipid Partitioning and Liver Damage in Nonalcoholic Fatty Liver Disease: Role of Stearoyl-Coa Desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef]
- Falcon, A.; Doege, H.; Fluitt, A.; Tsang, B.; Watson, N.; Kay, M.A.; Stahl, A. FATP2 Is a Hepatic Fatty Acid Transporter and Peroxisomal Very Long-Chain Acyl-CoA Synthetase. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E384–E393. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid β-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal 2022, 36, 1081–1100. [Google Scholar] [CrossRef]
- Chen, C.L.; Lin, Y.C. Autophagy Dysregulation in Metabolic Associated Fatty Liver Disease: A New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 10055. [Google Scholar] [CrossRef]
- Heebøll, S.; Poulsen, M.K.; Ornstrup, M.J.; Kjær, T.N.; Pedersen, S.B.; Nielsen, S.; Grønbæk, H.; Handberg, A. Circulating SCD36 Levels in Patients with Non-Alcoholic Fatty Liver Disease and Controls. Int. J. Obes. 2017, 41, 262–267. [Google Scholar] [CrossRef]
- Zhang, P.; Ge, Z.; Wang, H.; Feng, W.; Sun, X.; Chu, X.; Jiang, C.; Wang, Y.; Zhu, D.; Bi, Y. Prolactin Improves Hepatic Steatosis via CD36 Pathway. J. Hepatol. 2018, 68, 1247–1255. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Kashirskikh, D.A.; Sukhorukov, V.N.; Kalmykov, V.; Omelchenko, A.V.; Orekhov, A.N. Cholesterol Transport Dysfunction and Its Involvement in Atherogenesis. Int. J. Mol. Sci. 2022, 2022, 1332. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Ronca, A.; D’erasmo, L.; Manfredini, M.; Baratta, F.; Pastori, D.; Di Martino, M.; Ceci, F.; Angelico, F.; Del Ben, M.; et al. Hdl-Mediated Cholesterol Efflux and Plasma Loading Capacities Are Altered in Subjects with Metabolically-but Not Genetically Driven Non-Alcoholic Fatty Liver Disease (Nafld). Biomedicines 2020, 8, 625. [Google Scholar] [CrossRef]
- Wellington, C.L.; Walker, E.K.Y.; Suarez, A.; Kwok, A.; Bissada, N.; Singaraja, R.; Yang, Y.-Z.; Zhang, L.-H.; James, E.; Wilson, J.E.; et al. ABCA1 MRNA and Protein Distribution Patterns Predict Multiple Different Roles and Levels of Regulation. Lab. Investig. 2002, 82, 273–283. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Wang, Y.; An, W. Suppression of ABCA1 by Unsaturated Fatty Acids Leads to Lipid Accumulation in HepG2 Cells. Biochimie 2010, 92, 958–963. [Google Scholar] [CrossRef]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S.; et al. Hepatic Fatty Acid Transporter Cd36 Is a Common Target of LXR, PXR, and PPARγ in Promoting Steatosis. Gastroenterology 2008, 134, 556–567.e1. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic Role of the Transmembrane Glycoprotein CD36 (SR-B2) in Cellular Fatty Acid Uptake and Utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef]
- Nassir, F.; Adewole, O.L.; Brunt, E.M.; Abumrad, N.A. CD36 Deletion Reduces VLDL Secretion, Modulates Liver Prostaglandins, and Exacerbates Hepatic Steatosis in Ob/Ob Mice. J. Lipid Res. 2013, 54, 2988–2997. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, H.; Liao, M.; Zheng, E.; Luo, X.; Xiao, A.; Li, Y.; Chen, L.; Wei, L.; Zhao, L.; et al. CD36 Promotes de Novo Lipogenesis in Hepatocytes through INSIG2-Dependent SREBP1 Processing. Mol. Metab. 2022, 57, 101428. [Google Scholar] [CrossRef]
- Yeon, J.E.; Choi, K.M.; Baik, S.H.; Kim, K.O.; Lim, H.J.; Park, K.H.; Kim, J.Y.; Park, J.J.; Kim, J.S.; Bak, Y.T.; et al. Reduced Expression of Peroxisome Proliferator-Activated Receptor-α May Have an Important Role in the Development of Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2004, 19, 799–804. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Bulgakov, A. Lipid Metabolism Disorders in the Comorbid Course of Nonalcoholic Fatty Liver Disease and Chronic Obstructive Pulmonary Disease. Cells 2021, 10, 2978. [Google Scholar] [CrossRef]
- Wu, H.M.; Ni, X.X.; Xu, Q.Y.; Wang, Q.; Li, X.Y.; Hua, J. Regulation of Lipid-Induced Macrophage Polarization through Modulating Peroxisome Proliferator-Activated Receptor-Gamma Activity Affects Hepatic Lipid Metabolism via a Toll-like Receptor 4/NF-ΚB Signaling Pathway. J. Gastroenterol. Hepatol. 2020, 35, 1998–2008. [Google Scholar] [CrossRef]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear Receptors and Nonalcoholic Fatty Liver Disease. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2016, 1859, 1083–1099. [Google Scholar] [CrossRef]
- Lee, S.M.; Muratalla, J.; Karimi, S.; Diaz-Ruiz, A.; Frutos, M.D.; Guzman, G.; Ramos-Molina, B.; Cordoba-Chacon, J. Hepatocyte PPARγ Contributes to the Progression of Non-Alcoholic Steatohepatitis in Male and Female Obese Mice. Cell. Mol. Life Sci. 2023, 80, 39. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, F.; Zhang, Y.; Zhang, X.; Chen, J.; Jiang, Y. Pparγ Attenuates Hepatic Inflammation and Oxidative Stress of Non-Alcoholic Steatohepatitis via Modulating the Mir-21-5p/Sfrp5 Pathway. Mol. Med. Rep. 2021, 24, 823. [Google Scholar] [CrossRef]
- Ni, X.X.; Ji, P.X.; Chen, Y.X.; Li, X.Y.; Sheng, L.; Lian, M.; Guo, C.J.; Hua, J. Regulation of the Macrophage-Hepatic Stellate Cell Interaction by Targeting Macrophage Peroxisome Proliferator-Activated Receptor Gamma to Prevent Non-Alcoholic Steatohepatitis Progression in Mice. Liver Int. 2022, 42, 2696–2712. [Google Scholar] [CrossRef]
- Choi, Y.; Song, M.-J.; Jung, W.-J.; Jeong, H.; Park, S.; Yang, B.; Lee, E.-C.; Joo, J.-S.; Choi, D.; Koo, S.-H.; et al. Liver-Specific Deletion of Mouse CTCF Leads to Hepatic Steatosis via Augmented PPARγ Signaling. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1761–1787. [Google Scholar] [CrossRef]
- Lee, S.M.; Muratalla, J.; Diaz-Ruiz, A.; Remon-Ruiz, P.; McCann, M.; Liew, C.W.; Kineman, R.D.; Cordoba-Chacon, J. Rosiglitazone Requires Hepatocyte PPARγ Expression to Promote Steatosis in Male Mice with Diet-Induced Obesity. Endocrinology 2021, 162, bqab175. [Google Scholar] [CrossRef]
- Hajri, T.; Zaiou, M.; Fungwe, T.V.; Ouguerram, K.; Besong, S. Epigenetic Regulation of Peroxisome Proliferator-Activated Receptor Gamma Mediates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease. Cells 2021, 10, 1355. [Google Scholar] [CrossRef]
- Lee, S.M.; Pusec, C.M.; Norris, G.H.; De Jesus, A.; Diaz-Ruiz, A.; Muratalla, J.; Sarmento-Cabral, A.; Guzman, G.; Layden, B.T.; Cordoba-Chacon, J. Hepatocyte-Specific Loss of PPARγ Protects Mice From NASH and Increases the Therapeutic Effects of Rosiglitazone in the Liver. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1291–1311. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-Regulation of PPAR-γ MRNA Expression in the Liver of Obese Patients: An Additional Reinforcing Lipogenic Mechanism to SREBP-1c Induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Ghoneim, R.H.; Sock, E.T.N.; Lavoie, J.M.; Piquette-Miller, M. Effect of a High-Fat Diet on the Hepatic Expression of Nuclear Receptors and Their Target Genes: Relevance to Drug Disposition. Br. J. Nutr. 2015, 113, 507–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lima-Cabello, E.; García-Mediavilla, M.V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lozano-Rodríguez, T.; Fernández-Bermejo, M.; Olcoz, J.L.; González-Gallego, J.; García-Monzón, C.; Sánchez-Campos, S. Enhanced Expression of Pro-Inflammatory Mediators and Liver X-Receptor-Regulated Lipogenic Genes in Non-Alcoholic Fatty Liver Disease and Hepatitis C. Clin. Sci. 2010, 120, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Li, H. Role of Farnesoid X Receptor in Hepatic Steatosis in Nonalcoholic Fatty Liver Disease. Biomed. Pharmacother. 2020, 121, 109609. [Google Scholar] [CrossRef]
- Hubbert, M.L.; Zhang, Y.; Lee, F.Y.; Edwards, P.A. Regulation of Hepatic Insig-2 by the Farnesoid X Receptor. Mol. Endocrinol. 2007, 21, 1359–1369. [Google Scholar] [CrossRef][Green Version]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X Receptor Is Essential for Normal Glucose Homeostasis. J. Clin. Investig. 2006, 116, 1102–1109. [Google Scholar] [CrossRef]
- Kunne, C.; Acco, A.; Duijst, S.; de Waart, D.R.; Paulusma, C.C.; Gaemers, I.; Oude Elferink, R.P.J. FXR-Dependent Reduction of Hepatic Steatosis in a Bile Salt Deficient Mouse Model. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 739–746. [Google Scholar] [CrossRef]
- Aguilar-Olivos, N.E.; Carrillo-Córdova, D.; Oria-Hernández, J.; Sánchez-Valle, V.; Ponciano-Rodríguez, G.; Ramírez-Jaramillo, M.; Chablé-Montero, F.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. The Nuclear Receptor FXR, but Not LXR, up-Regulates Bile Acid Transporter Expression in Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2015, 14, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Mosca, A.; Della Corte, C.; Veraldi, S.; De Vito, R.; De Stefanis, C.; D’Oria, V.; Jahnel, J.; Zohrer, E.; et al. Hepatic Farnesoid X Receptor Protein Level and Circulating Fibroblast Growth Factor 19 Concentration in Children with NAFLD. Liver Int. 2018, 38, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-Driven Gut Vascular Barrier Disruption Is a Prerequisite for Non-Alcoholic Steatohepatitis Development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, K.; Agarwal, S.; Sharma, S.; Goswami, P.; Singh, N.; Sachdev, V.; Poudel, S.; Das, P.; Yadav, R.; Kumar, D.; et al. Demonstration of Gut-Barrier Dysfunction in Early Stages of Non-Alcoholic Fatty Liver Disease: A Proof-Of-Concept Study. J. Clin. Exp. Hepatol. 2022, 12, 1102–1113. [Google Scholar] [CrossRef]
- Zhuang, Y.P.; Zhang, Y.T.; Zhang, R.X.; Zhong, H.J.; He, X.X. The Gut-Liver Axis in Nonalcoholic Fatty Liver Disease: Association of Intestinal Permeability with Disease Severity and Treatment Outcomes. Int. J. Clin. Pract. 2022, 2022, 4797453. [Google Scholar] [CrossRef] [PubMed]
- Delik, A.; Dinçer, S.; Ülger, Y.; Akkız, H.; Karaoğullarından, Ü. Metagenomic Identification of Gut Microbiota Distribution on the Colonic Mucosal Biopsy Samples in Patients with Non-Alcoholic Fatty Liver Disease. Gene 2022, 833, 146587. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wei, W.; Tang, L.; Tian, Y.; Zhu, Y.; Luo, Y.; Liu, S. CONSORT-Characteristics and Metabolic Phenotype of Gut Microbiota in NAFLD Patients. Medicine 2022, 101, E29347. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, J.; Xu, X.; Xu, W.; Tong, B.; Wang, S.; Ji, R.; Tan, Y.; Zhu, Y. Characteristics of Gut Microbiota in Patients with Metabolic Associated Fatty Liver Disease. Sci. Rep. 2023, 13, 9988. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, C.; Taminiau, B.; García-García, A.; Cueto, A.; Robles-Díaz, M.; Ortega-Alonso, A.; Martín-Reyes, F.; Daube, G.; Sanabria-Cabrera, J.; Jimenez-Perez, M.; et al. Microbiota Diversity in Nonalcoholic Fatty Liver Disease and in Drug-Induced Liver Injury. Pharmacol. Res. 2022, 182, 106348. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular Phenomics and Metagenomics of Hepatic Steatosis in Non-Diabetic Obese Women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta-omics-based Approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.; Li, Z.; Galuppo, B.; Graf, J.; Santoro, N. Insights from Shotgun Metagenomics into Bacterial Species and Metabolic Pathways Associated with NAFLD in Obese Youth. Hepatol. Commun. 2022, 6, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, L.; Li, Q.; Gu, M.; Qu, Q.; Yang, X.; Yi, Q.; Gu, K.; Kuang, L.; Hao, M.; et al. Characterization of Microbiome and Metabolite Analyses in Patients with Metabolic Associated Fatty Liver Disease and Type II Diabetes Mellitus. BMC Microbiol. 2022, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, P.; Arendt, B.M.; Schwenger, K.J.P.; Sivaraj, S.; Bhat, M.; Comelli, E.M.; Lou, W.; Allard, J.P. Relationship Between Hepatic Gene Expression, Intestinal Microbiota, and Inferred Functional Metagenomic Analysis in NAFLD. Clin. Transl. Gastroenterol. 2022, 13, e00466. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-Producing Bacteria in Gut Microbiome of Human NAFLD as a Putative Link to Systemic T-Cell Activation and Advanced Disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Barrow, F.; Khan, S.; Fredrickson, G.; Wang, H.; Dietsche, K.; Parthiban, P.; Robert, S.; Kaiser, T.; Winer, S.; Herman, A.; et al. Microbiota-Driven Activation of Intrahepatic B Cells Aggravates NASH Through Innate and Adaptive Signaling. Hepatology 2021, 74, 2021. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined Effects of Host Genetics and Diet on Human Gut Microbiota and Incident Disease in a Single Population Cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Mazzini, F.N.; Cook, F.; Gounarides, J.; Marciano, S.; Haddad, L.; Tamaroff, A.J.; Casciato, P.; Narvaez, A.; Mascardi, M.F.; Anders, M.; et al. Plasma and Stool Metabolomics to Identify Microbiota Derived-Biomarkers of Metabolic Dysfunction-Associated Fatty Liver Disease: Effect of PNPLA3 Genotype. Metabolomics 2021, 17, 58. [Google Scholar] [CrossRef]
- De Faria Ghetti, F.; Oliveira, D.G.; de Oliveira, J.M.; de Castro Ferreira, L.E.V.V.; Cesar, D.E.; Moreira, A.P.B. Effects of Dietary Intervention on Gut Microbiota and Metabolic-Nutritional Profile of Outpatients with Non-Alcoholic Steatohepatitis: A Randomized Clinical Trial. J. Gastrointest. Liver Dis. 2019, 28, 279–287. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A Randomized Controlled Trial for Response of Microbiome Network to Exercise and Diet Intervention in Patients with Nonalcoholic Fatty Liver Disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.M.; Disciglio, V.; Franco, I.; Sorino, P.; Bonfiglio, C.; Bianco, A.; Campanella, A.; Lippolis, T.; Pesole, P.L.; Polignano, M.; et al. A Low Glycemic Index Mediterranean Diet Combined with Aerobic Physical Activity Rearranges the Gut Microbiota Signature in NAFLD Patients. Nutrients 2022, 14, 1773. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Luukkonen, P.; Sädevirta, S.; Yki-Järvinen, H.; Salonen, A. Impact of Short-Term Overfeeding of Saturated or Unsaturated Fat or Sugars on the Gut Microbiota in Relation to Liver Fat in Obese and Overweight Adults. Clin. Nutr. 2021, 40, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, K.; Lang, S.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.J.G.T.; Martin, A.; Nowag, A.; Kretzschmar, A.; Scholz, C.J.; Roderburg, C.; et al. Dietary Omega-6/Omega-3 Ratio Is Not Associated with Gut Microbiota Composition and Disease Severity in Patients with Nonalcoholic Fatty Liver Disease. Nutr. Res. 2022, 107, 12–25. [Google Scholar] [CrossRef]
- Basuray, S.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. The PNPLA3 Variant Associated with Fatty Liver Disease (I148M) Accumulates on Lipid Droplets by Evading Ubiquitylation. Hepatology 2017, 66, 1111–1124. [Google Scholar] [CrossRef]
- Chandrasekharan, K.; Alazawi, W. Genetics of Non-Alcoholic Fatty Liver and Cardiovascular Disease: Implications for Therapy? Front. Pharmacol. 2019, 10, 1413. [Google Scholar] [CrossRef]
- Bruschi, F.V.; Claudel, T.; Tardelli, M.; Caligiuri, A.; Stulnig, T.M.; Marra, F.; Trauner, M. The PNPLA3 I148M Variant Modulates the Fibrogenic Phenotype of Human Hepatic Stellate Cells. Hepatology 2017, 65, 1875–1890. [Google Scholar] [CrossRef]
- Teo, K.; Abeysekera, K.W.M.; Adams, L.; Aigner, E.; Anstee, Q.M.; Banales, J.M.; Banerjee, R.; Basu, P.; Berg, T.; Bhatnagar, P.; et al. Rs641738C>T near MBOAT7 Is Associated with Liver Fat, ALT and Fibrosis in NAFLD: A Meta-Analysis. J. Hepatol. 2021, 74, 20–30. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.; Guo, Y.; Zhu, T.; Garcia-Barrio, M.T.; Jiang, Z.; Willer, C.J.; Zhang, J.; Eugene Chen, Y. Hepatic Transmembrane 6 Superfamily Member 2 Regulates Cholesterol Metabolism in Mice. Gastroenterology 2016, 150, 1208–1218. [Google Scholar] [CrossRef]
- Smagris, E.; Gilyard, S.; BasuRay, S.; Cohen, J.C.; Hobbs, H.H. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2016, 291, 10659–10676. [Google Scholar] [CrossRef] [PubMed]
- Newberry, E.P.; Hall, Z.; Xie, Y.; Molitor, E.A.; Bayguinov, P.O.; Strout, G.W.; Fitzpatrick, J.A.J.; Brunt, E.M.; Griffin, J.L.; Davidson, N.O. Liver-Specific Deletion of Mouse Tm6sf2 Promotes Steatosis, Fibrosis, and Hepatocellular Cancer. Hepatology 2021, 74, 2021. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant Rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [PubMed]
- Thangapandi, V.R.; Knittelfelder, O.; Brosch, M.; Patsenker, E.; Vvedenskaya, O.; Buch, S.; Hinz, S.; Hendricks, A.; Nati, M.; Herrmann, A.; et al. Loss of Hepatic Mboat7 Leads to Liver Fibrosis. Gut 2021, 70, 940–950. [Google Scholar] [CrossRef]
- Gellert-Kristensen, H.; Richardson, T.G.; Smith, G.D.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Stender, S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020, 72, 845–856. [Google Scholar] [CrossRef]
- Su, H.; Haque, M.; Becker, S.; Edlund, K.; Duda, J.; Wang, Q.; Reißing, J.; Marschall, H.U.; Candels, L.S.; Mohamed, M.; et al. Long-Term Hypercaloric Diet Exacerbates Metabolic Liver Disease in PNPLA3 I148M Animals. Liver Int. 2023, 43, 1699–1713. [Google Scholar] [CrossRef]
- Lang, S.; Martin, A.; Zhang, X.; Farowski, F.; Wisplinghoff, H.; Maria, J.G.T.; Vehreschild, M.; Krawczyk, M.; Nowag, A.; Kretzschmar, A.; et al. Combined Analysis of Gut Microbiota, Diet and PNPLA3 Polymorphism in Biopsy-Proven Non-Alcoholic Fatty Liver Disease. Liver Int. 2021, 41, 1576–1591. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut Microbiome Analysis as a Tool towards Targeted Non-Invasive Biomarkers for Early Hepatocellular Carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Pirola, C.J.; Salatino, A.; Quintanilla, M.F.; Castaño, G.O.; Garaycoechea, M.; Sookoian, S. The Influence of Host Genetics on Liver Microbiome Composition in Patients with NAFLD. eBioMedicine 2022, 76, 103858. [Google Scholar] [CrossRef]
- Sanders, F.W.B.; Griffin, J.L. De Novo Lipogenesis in the Liver in Health and Disease: More than Just a Shunting Yard for Glucose. Biol. Rev. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and Sugar: A Major Mediator of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Iizuka, K. The Transcription Factor Carbohydrate-Response Element-Binding Protein (ChREBP): A Possible Link between Metabolic Disease and Cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 474–485. [Google Scholar] [CrossRef]
- Hall, A.M.; Finck, B.N. ChREBP Refines the Hepatic Response to Fructose to Protect the Liver from Injury. J. Clin. Investig. 2017, 127, 2533–2535. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos–Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De Novo Lipogenesis Is a Distinct Characteristic of Individuals with Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De Novo Lipogenesis in Health and Disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Caballero, F.; Fernández, A.; De Lacy, A.M.; Fernández-Checa, J.C.; Caballería, J.; García-Ruiz, C. Enhanced Free Cholesterol, SREBP-2 and StAR Expression in Human NASH. J. Hepatol. 2009, 50, 789–796. [Google Scholar] [CrossRef]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A.; et al. Sterol Regulatory Element-Binding Protein-1 (SREBP-1) Is Required to Regulate Glycogen Synthesis and Gluconeogenic Gene Expression in Mouse Liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef]
- Hunt, J.E.; Holst, J.J.; Jeppesen, P.B.; Kissow, H. Glp-1 and Intestinal Diseases. Biomedicines 2021, 9, 383. [Google Scholar] [CrossRef]
- Al-Dwairi, A.; Alqudah, T.E.; Al-Shboul, O.; Alqudah, M.; Mustafa, A.G.; Alfaqih, M.A. Glucagon-like Peptide-1 Exerts Anti-Inflammatory Effects on Mouse Colon Smooth Muscle Cells through the Cyclic Adenosine Monophosphate/ Nuclear Factor-ΚB Pathway in Vitro. J. Inflamm. Res. 2018, 11, 95–109. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.A.; et al. GLP-1R Agonists Modulate Enteric Immune Responses through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J. V Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.L.; Softic, S.; Mouzaki, M. Evolving Role for Pharmacotherapy in NAFLD/NASH. Clin. Transl. Sci. 2021, 14, 11–19. [Google Scholar] [CrossRef]
- Wong, C.; Lee, M.H.; Yaow, C.Y.L.; Chin, Y.H.; Goh, X.L.; Ng, C.H.; Lim, A.Y.L.; Muthiah, M.D.; Khoo, C.M. Glucagon-Like Peptide-1 Receptor Agonists for Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: A Meta-Analysis. Front. Endocrinol. 2021, 12, 609110. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Metabolites Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef]
- Ji, J.; Feng, M.; Huang, Y.; Niu, X. Liraglutide Inhibits Receptor for Advanced Glycation End Products (RAGE)/Reduced Form of Nicotinamide-Adenine Dinucleotide Phosphate (NAPDH) Signaling to Ameliorate Non-Alcoholic Fatty Liver Disease (NAFLD) in Vivo and Vitro. Bioengineered 2022, 13, 5091–5102. [Google Scholar] [CrossRef]
- Khalifa, O.; AL-Akl, N.S.; Errafii, K.; Arredouani, A. Exendin-4 Alleviates Steatosis in an in Vitro Cell Model by Lowering FABP1 and FOXA1 Expression via the Wnt/-Catenin Signaling Pathway. Sci. Rep. 2022, 12, 2226. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Li, Y.; Lei, R.; Lei, H.; Xiong, Q.; Xie, F.; Yao, C.; Feng, P. Side Effect Profile of Pharmacologic Therapies for Liver Fibrosis in Nonalcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1–14. [Google Scholar] [CrossRef]
- Alkhouri, N.; Herring, R.; Kabler, H.; Kayali, Z.; Hassanein, T.; Kohli, A.; Huss, R.S.; Zhu, Y.; Billin, A.N.; Damgaard, L.H.; et al. Safety and Efficacy of Combination Therapy with Semaglutide, Cilofexor and Firsocostat in Patients with Non-Alcoholic Steatohepatitis: A Randomised, Open-Label Phase II Trial. J. Hepatol. 2022, 77, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Armstrong, M.J.; Funuyet-Salas, J.; Mangla, K.K.; Ladelund, S.; Sejling, A.; Shrestha, I.; Sanyal, A.J. Improved Health-related Quality of Life with Semaglutide in People with Non-alcoholic Steatohepatitis: A Randomised Trial. Aliment. Pharmacol. Ther. 2023, 58, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Abdelmalek, M.F.; Armstrong, M.J.; Jara, M.; Kjær, M.S.; Krarup, N.; Lawitz, E.; Ratziu, V.; Sanyal, A.J.; Schattenberg, J.M.; et al. Semaglutide 2·4 Mg Once Weekly in Patients with Non-Alcoholic Steatohepatitis-Related Cirrhosis: A Randomised, Placebo-Controlled Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Inia, J.A.; Stokman, G.; Morrison, M.C.; Worms, N.; Verschuren, L.; Caspers, M.P.M.; Menke, A.L.; Petitjean, L.; Chen, L.; Petitjean, M.; et al. Semaglutide Has Beneficial Effects on Non-Alcoholic Steatohepatitis in Ldlr-/-.Leiden Mice. Int. J. Mol. Sci. 2023, 24, 8494. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, L.; Zhang, W.; Kong, T.; Zhou, R.; He, Y.; Deng, C.; Yang, L.; Kong, J.; Chen, Y.; et al. Comparative Efficacy of 5 Sodium-Glucose Cotransporter Protein-2 (SGLT-2) Inhibitor and 4 Glucagon-like Peptide-1 (GLP-1) Receptor Agonist Drugs in Non-Alcoholic Fatty Liver Disease: A GRADE-Assessed Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2023, 14, 1102792. [Google Scholar] [CrossRef]
- Malik, A.; Amjad, W.; Inayat, F.; Nadeem, M.; Weissman, S.; Imran Malik, M.; Ahmad Jajja, A.; Khan, A.; Tabibian, J.H. The Effects of Liraglutide on Liver Enzymes and Metabolic Factors in Patients with Nonalcoholic Steatohepatitis: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol. Rev. 2022, 18, 100–109. [Google Scholar] [CrossRef]
- Tsankof, A.; Neokosmidis, G.; Koureta, E.; Veneti, S.; Cholongitas, E.; Tziomalos, K. Which Is the Optimal Antiobesity Agent for Patients with Nonalcoholic Fatty Liver Disease? Front. Endocrinol. 2022, 13, 984041. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Gozar, M.; Da, B.L.; Bernstein, D.; Satapathy, S.K. Pharmacotherapeutic Efficacy on Noninvasive Fibrosis Progression in Nonalcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 102–111. [Google Scholar] [CrossRef]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults with Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442. [Google Scholar] [CrossRef]
- Marcondes-de-Castro, I.A.; Oliveira, T.F.; Spezani, R.; Marinho, T.S.; Cardoso, L.E.M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Cotadutide Effect in Liver and Adipose Tissue in Obese Mice. J. Mol. Endocrinol. 2023, 70, e220168. [Google Scholar] [CrossRef] [PubMed]
- Nestor, J.J.; Parkes, D.; Feigh, M.; Suschak, J.J.; Harris, M.S. Effects of ALT-801, a GLP-1 and Glucagon Receptor Dual Agonist, in a Translational Mouse Model of Non-Alcoholic Steatohepatitis. Sci. Rep. 2022, 12, 6666. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jin, K.; Yue, M.; Chen, X.; Chen, J. Research Progress on the GIP/GLP-1 Receptor Coagonist Tirzepatide, a Rising Star in Type 2 Diabetes. J. Diabetes Res. 2023, 2023, 5891532. [Google Scholar] [CrossRef]
- Perakakis, N.; Stefanakis, K.; Feigh, M.; Veidal, S.S.; Mantzoros, C.S. Elafibranor and Liraglutide Improve Differentially Liver Health and Metabolism in a Mouse Model of Non-Alcoholic Steatohepatitis. Liver Int. 2021, 41, 1853–1866. [Google Scholar] [CrossRef]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef]
- Feng, W.; Gao, C.; Bi, Y.; Wu, M.; Li, P.; Shen, S.; Chen, W.; Yin, T.; Zhu, D. Randomized Trial Comparing the Effects of Gliclazide, Liraglutide, and Metformin on Diabetes with Non-Alcoholic Fatty Liver Disease. J. Diabetes 2017, 9, 800–809. [Google Scholar] [CrossRef]
- Khoo, J.; Hsiang, J.C.; Taneja, R.; Koo, S.H.; Soon, G.H.; Kam, C.J.; Law, N.M.; Ang, T.L. Randomized Trial Comparing Effects of Weight Loss by Liraglutide with Lifestyle Modification in Non-Alcoholic Fatty Liver Disease. Liver Int. 2019, 39, 941–949. [Google Scholar] [CrossRef]
- Tang, A.; Rabasa-Lhoret, R.; Castel, H.; Wartelle-Bladou, C.; Gilbert, G.; Massicotte-Tisluck, K.; Chartrand, G.; Olivié, D.; Julien, A.S.; De Guise, J.; et al. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients with Type 2 Diabetes: A Randomized Trial. Diabetes Care 2015, 38, 1339–1346. [Google Scholar] [CrossRef]
- Bajaj, H.S.; Brown, R.E.; Bhullar, L.; Sohi, N.; Kalra, S.; Aronson, R. SGLT2 Inhibitors and Incretin Agents: Associations with Alanine Aminotransferase Activity in Type 2 Diabetes. Diabetes Metab. 2018, 44, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like Peptide 1 Decreases Lipotoxicity in Non-Alcoholic Steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.W.; Wang, D.; Duan, B.H.; Kuang, H.Y. Exenatide Attenuates Non-Alcoholic Steatohepatitis by Inhibiting the Pyroptosis Signaling Pathway. Front. Endocrinol. 2021, 12, 663039. [Google Scholar] [CrossRef]
- Andersen, A.; Knop, F.K.; Vilsbøll, T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs 2021, 81, 1003–1030. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Sundby Palle, M.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised Clinical Trial: Semaglutide versus Placebo Reduced Liver Steatosis but Not Liver Stiffness in Subjects with Non-Alcoholic Fatty Liver Disease Assessed by Magnetic Resonance Imaging. Aliment. Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Calanna, S.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.; Sejling, A.-S.; Newsome, P.N. Semaglutide for the Treatment of Non-Alcoholic Steatohepatitis: Trial Design and Comparison of Non-Invasive Biomarkers. Contemp. Clin. Trials 2020, 97, 106174. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.A.; Allen, A.M. Hepatic Steatosis Provides the Terroir That Promotes the Development of Cardiovascular Risk Factors and Disease. Hepatology 2023, 77, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of Dulaglutide on Liver Fat in Patients with Type 2 Diabetes and NAFLD: Randomised Controlled Trial (D-LIFT Trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef]
- Naseralallah, L.; Aboujabal, B. Profile of Tirzepatide in the Management of Type 2 Diabetes Mellitus: Design, Development, and Place in Therapy. Expert. Opin. Pharmacother. 2023, 24, 407–418. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Delitala, A.P.; Delitala, G.; Sioni, P.; Fanciulli, G. Thyroid Hormone Analogs for the Treatment of Dyslipidemia: Past, Present, and Future. Curr. Med. Res. Opin. 2017, 33, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.A.; Leow, M.K.S.; et al. Low-Dose Levothyroxine Reduces Intrahepatic Lipid Content in Patients with Type 2 Diabetes Mellitus and NAFLD. J. Clin. Endocrinol. Metab. 2018, 103, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Hatziagelaki, E.; Paschou, S.A.; Schön, M.; Psaltopoulou, T.; Roden, M. NAFLD and Thyroid Function: Pathophysiological and Therapeutic Considerations. Trends Endocrinol. Metab. 2022, 33, 755–768. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yu, X.; Qi, X. Thyroid Function and Risk of Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects. Ann. Hepatol. 2018, 17, 779–788. [Google Scholar] [CrossRef]
- Van den Berg, E.H.; van Tienhoven-Wind, L.J.N.; Amini, M.; Schreuder, T.C.M.A.; Faber, K.N.; Blokzijl, H.; Dullaart, R.P.F. Higher Free Triiodothyronine Is Associated with Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects: The Lifelines Cohort Study. Metabolism 2017, 67, 62–71. [Google Scholar] [CrossRef]
- Martínez-Escudé, A.; Pera, G.; Costa-Garrido, A.; Rodríguez, L.; Arteaga, I.; Expósito-Martínez, C.; Torán-Monserrat, P.; Caballería, L. TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population. J. Clin. Med. 2021, 10, 2907. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of Non-Alcoholic Fatty Liver Disease with Thyroid Function: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kube, I.; Jastrow, H.; Führer, D.; Zwanziger, D. Thyroid Hormone Deficiency Modifies Hepatic Lipid Droplet Morphology and Molecular Properties in Lithogenic-Diet Supplemented Mice. Exp. Clin. Endocrinol. Diabetes 2021, 129, 926–930. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Campi, I.; Maggioni, M.; Perbellini, R.; Giammona, E.; Stucchi, R.; Borghi, M.; Degasperi, E.; De Silvestri, A.; Persani, L.; et al. The Relationship between Liver Histology and Thyroid Function Tests in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). PLoS ONE 2021, 16, e0249614. [Google Scholar] [CrossRef]
- Manka, P.; Bechmann, L.; Best, J.; Sydor, S.; Claridge, L.C.; Coombes, J.D.; Canbay, A.; Moeller, L.; Gerken, G.; Wedemeyer, H.; et al. Low Free Triiodothyronine Is Associated with Advanced Fibrosis in Patients at High Risk for Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2019, 64, 2351–2358. [Google Scholar] [CrossRef]
- Vatner, D.F.; Weismann, D.; Beddow, S.A.; Kumashiro, N.; Erion, D.M.; Liao, X.H.; Grover, G.J.; Webb, P.; Phillips, K.J.; Weiss, R.E.; et al. Thyroid Hormone Receptor-β Agonists Prevent Hepatic Steatosis in Fat-Fed Rats but Impair Insulin Sensitivity via Discrete Pathways. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E89–E100. [Google Scholar] [CrossRef]
- Sjouke, B.; Langslet, G.; Ceska, R.; Nicholls, S.J.; Nissen, S.E.; Öhlander, M.; Ladenson, P.W.; Olsson, A.G.; Hovingh, G.K.; Kastelein, J.J.P. Eprotirome in Patients with Familial Hypercholesterolaemia (the AKKA Trial): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Diabetes Endocrinol. 2014, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Neutel, J.; Mohseni, R.; Bernard, D.; Severance, R.; Dao, M.; Saini, S.; Margaritescu, C.; Homer, K.; Tran, B.; et al. LBP-20-VK2809, a Novel Liver-Directed Thyroid Receptor Beta Agonist, Significantly Reduces Liver Fat with Both Low and High Doses in Patients with Non-Alcoholic Fatty Liver Disease: A Phase 2 Randomized, Placebo-Controlled Trial. J. Hepatol. 2019, 70, e150–e151. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the Treatment of Non-Alcoholic Steatohepatitis: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Kannt, A.; Wohlfart, P.; Madsen, A.N.; Veidal, S.S.; Feigh, M.; Schmoll, D. Activation of Thyroid Hormone Receptor-β Improved Disease Activity and Metabolism Independent of Body Weight in a Mouse Model of Non-Alcoholic Steatohepatitis and Fibrosis. Br. J. Pharmacol. 2021, 178, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Geng, L.; Tanaka, N.; Ye, B. Resmetirom Ameliorates NASH-Model Mice by Suppressing STAT3 and NF-ΚB Signaling Pathways in an RGS5-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 5843. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; Mccarty, K.; Frias, J.P.; Taub, R.; Alkhouri, N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients with NASH. Hepatol. Commun. 2021, 5, 2021. [Google Scholar] [CrossRef]
- Hu, L.; Gu, Y.; Liang, J.; Ning, M.; Yang, J.; Zhang, Y.; Qu, H.; Yang, Y.; Leng, Y.; Zhou, B. Discovery of Highly Potent and Selective Thyroid Hormone Receptor β Agonists for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2023, 66, 3284–3300. [Google Scholar] [CrossRef]
- Kirschberg, T.; Jones, C.; Xu, Y.; Wang, Y.; Fenaux, M.; Klucher, K. TERN-501 a potent and selective agonist of thyroid hormone receptor beta, strongly reduces histological features and biomarkers of non-alcoholic steatohepatitis associated pathology in rodent models. In Proceedings of the Digital International Liver Congress, Online, 27–29 August 2020. [Google Scholar]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Small-Molecule, Liver-Directed, β-Selective THR Agonist for the Treatment of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Eur. Endocrinol. 2023, 19, 60. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. Tg68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of Nafld. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef]
- Larsen, A.T.; Gydesen, S.; Sonne, N.; Karsdal, M.A.; Henriksen, K. The Dual Amylin and Calcitonin Receptor Agonist KBP-089 and the GLP-1 Receptor Agonist Liraglutide Act Complimentarily on Body Weight Reduction and Metabolic Profile. BMC Endocr. Disord. 2021, 21, 10. [Google Scholar] [CrossRef]
- Mathiesen, D.S.; Lund, A.; Vilsbøll, T.; Knop, F.K.; Bagger, J.I. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front. Endocrinol. 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Cuzzocrea, S. The role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (ppar-α) in the regulation of inflammation in macrophages. Shock 2009, 32, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Shiri-Sverdlov, R.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; Noel, B.; Buffat, L.; Staels, B.; Maeda, N.; van Bilsen, M.; Hofker, M.H. Early Diet-Induced Non-Alcoholic Steatohepatitis in APOE2 Knock-in Mice and Its Prevention by Fibrates. J. Hepatol. 2006, 44, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Wouters, K.; van Bilsen, M.; van Gorp, P.J.; Bieghs, V.; Lütjohann, D.; Kerksiek, A.; Staels, B.; Hofker, M.H.; Shiri-Sverdlov, R. Intrahepatic Cholesterol Influences Progression, Inhibition and Reversal of Non-Alcoholic Steatohepatitis in Hyperlipidemic Mice. FEBS Lett. 2010, 584, 1001–1005. [Google Scholar] [CrossRef]
- Jiang, S.; Uddin, M.J.; Yu, X.; Piao, L.; Dorotea, D.; Oh, G.T.; Ha, H. Peroxisomal Fitness: A Potential Protective Mechanism of Fenofibrate against High Fat Diet-Induced NonAlcoholic Fatty Liver Disease in Mice. Diabetes Metab. J. 2022, 46, 829–842. [Google Scholar] [CrossRef]
- Fernández-Miranda, C.; Pérez-Carreras, M.; Colina, F.; López-Alonso, G.; Vargas, C.; Solís-Herruzo, J.A. A Pilot Trial of Fenofibrate for the Treatment of Non-Alcoholic Fatty Liver Disease. Dig. Liver Dis. 2008, 40, 200–205. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Jafari, S.; Sajedi, B.; Gohari, S.; Akbarieh, S.; Heydari, A.H.; Jameshoorani, M. Comparison of Fenofibrate and Pioglitazone Effects on Patients with Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1385–1388. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Kiezel-Tsugunova, M.; Kiss, O.; Uddin, M.; Sundén, M.; Ryaboshapkina, M.; Lind, L.; Oscarsson, J.; Nicolaou, A. Omega-3 Carboxylic Acids and Fenofibrate Differentially Alter Plasma Lipid Mediators in Patients with Non-Alcoholic Fatty Liver Disease. FASEB J. 2021, 35, e21976. [Google Scholar] [CrossRef]
- Oscarsson, J.; Önnerhag, K.; Risérus, U.; Sundén, M.; Johansson, L.; Jansson, P.-A.; Moris, L.; Nilsson, P.M.; Eriksson, J.W.; Lind, L. Effects of Free Omega-3 Carboxylic Acids and Fenofibrate on Liver Fat Content in Patients with Hypertriglyceridemia and Non-Alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Lipidol. 2018, 12, 1390–1403.e4. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, L.; Chen, C.; Wang, Y.; Zhang, Q.; Qi, R. Fenofibrate Nanoliposome: Preparation and Its Inhibitory Effects on Nonalcoholic Fatty Liver Disease in Mice. Nanomedicine 2016, 12, 2449–2458. [Google Scholar] [CrossRef]
- Du, K.; Huang, X.; Peng, A.; Yang, Q.; Chen, D.; Zhang, J.; Qi, R. Engineered Fenofibrate as Oxidation-Sensitive Nanoparticles with ROS Scavenging and PPARα-Activating Bioactivity to Ameliorate Nonalcoholic Fatty Liver Disease. Mol. Pharm. 2023, 20, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.-C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Katsuyama, H.; Hakoshima, M. Effects of a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator, Pemafibrate, on Metabolic Parameters: A Retrospective Longitudinal Study. Biomedicines 2022, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Tahara, T.; Miura, K.; Kawarai Lefor, A.; Yamamoto, H. Pemafibrate Therapy for Non-Alcoholic Fatty Liver Disease Is More Effective in Lean Patients than Obese Patients. Clin. Exp. Hepatol. 2022, 8, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef]
- Jain, M.R.; Giri, S.R.; Bhoi, B.; Trivedi, C.; Rath, A.; Rathod, R.; Ranvir, R.; Kadam, S.; Patel, H.; Swain, P.; et al. Dual PPARα/γ Agonist Saroglitazar Improves Liver Histopathology and Biochemistry in Experimental NASH Models. Liver Int. 2018, 38, 1084–1094. [Google Scholar] [CrossRef]
- Akbari, R.; Behdarvand, T.; Afarin, R.; Yaghooti, H.; Jalali, M.T.; Mohammadtaghvaei, N. Saroglitazar Improved Hepatic Steatosis and Fibrosis by Modulating Inflammatory Cytokines and Adiponectin in an Animal Model of Non-Alcoholic Steatohepatitis. BMC Pharmacol. Toxicol. 2021, 22, 53. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 2021. [Google Scholar] [CrossRef]
- Rajesh, N.A.; Drishya, L.; Ambati, M.M.R.; Narayanan, A.L.; Alex, M.; Kumar, K.; Abraham, J.J.; Vijayakumar, T.M. Safety and Efficacy of Saroglitazar in Nonalcoholic Fatty Liver Patients with Diabetic Dyslipidemia—A Prospective, Interventional, Pilot Study. J. Clin. Exp. Hepatol. 2022, 12, 61–67. [Google Scholar] [CrossRef]
- Padole, P.; Arora, A.; Sharma, P.; Chand, P.; Verma, N.; Kumar, A. Saroglitazar for Nonalcoholic Fatty Liver Disease: A Single Centre Experience in 91 Patients. J. Clin. Exp. Hepatol. 2022, 12, 435–439. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Dutta, A.; Chakraborty, S.B.D. Efficacy and Safety of Saroglitazar in Real-World Patients of Non-Alcoholic Fatty Liver Disease with or without Diabetes Including Compensated Cirrhosis: A Tertiary Care Center Experience. JGH Open 2023, 7, 215–220. [Google Scholar] [CrossRef]
- Hazra, S.; Xiong, S.; Wang, J.; Rippe, R.A.; Krishna, V.; Chatterjee, K.; Tsukamoto, H. Peroxisome Proliferator-Activated Receptor γ Induces a Phenotypic Switch from Activated to Quiescent Hepatic Stellate Cells. J. Biol. Chem. 2004, 279, 11392–11401. [Google Scholar] [CrossRef]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A Placebo-Controlled Trial of Pioglitazone in Subjects with Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients with Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Honda, Y.; Ogawa, Y.; Kessoku, T.; Kobayashi, T.; Imajo, K.; Ozaki, A.; Nogami, A.; Taguri, M.; Yamanaka, T.; et al. Comparing the Effects of Tofogliflozin and Pioglitazone in Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus (ToPiND Study): A Randomized Prospective Open-Label Controlled Trial. BMJ Open Diabetes Res. Care 2021, 9, e001990. [Google Scholar] [CrossRef]
- Della Pepa, G.; Russo, M.; Vitale, M.; Carli, F.; Vetrani, C.; Masulli, M.; Riccardi, G.; Vaccaro, O.; Gastaldelli, A.; Rivellese, A.A.; et al. Pioglitazone Even at Low Dosage Improves NAFLD in Type 2 Diabetes: Clinical and Pathophysiological Insights from a Subgroup of the TOSCA.IT Randomised Trial. Diabetes Res. Clin. Pract. 2021, 178, 108984. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Sabatini, S.; Carli, F.; Gaggini, M.; Bril, F.; Belfort-DeAguiar, R.; Positano, V.; Barb, D.; Kadiyala, S.; Harrison, S.; et al. PPAR-γ-Induced Changes in Visceral Fat and Adiponectin Levels Are Associated with Improvement of Steatohepatitis in Patients with NASH. Liver Int. 2021, 41, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Jacques, V.; Bolze, S.; Hallakou-Bozec, S.; Czarnik, A.W.; Divakaruni, A.S.; Fouqueray, P.; Murphy, A.N.; Van Der Ploeg, L.H.T.; Dewitt, S. Deuterium-Stabilized (R)-Pioglitazone (PXL065) Is Responsible for Pioglitazone Efficacy in NASH yet Exhibits Little to No PPARγ Activity. Hepatol. Commun. 2021, 5, 2021. [Google Scholar] [CrossRef]
- Harrison, S.A.; Thang, C.; Bolze, S.; Dewitt, S.; Hallakou-Bozec, S.; Dubourg, J.; Bedossa, P.; Cusi, K.; Ratziu, V.; Grouin, J.-M.; et al. Evaluation of PXL065–Deuterium-Stabilized (R)-Pioglitazone in Patients with NASH: A Phase II Randomized Placebo-Controlled Trial (DESTINY-1). J. Hepatol. 2023, 78, 914–925. [Google Scholar] [CrossRef]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; de Muynck, K.; Heymann, F.; Adarbes, V.; Defrêne, E.; et al. Differential Effects of Selective- and Pan-PPAR Agonists on Experimental Steatohepatitis and Hepatic Macrophages☆. J. Hepatol. 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Sven, M.F.; Pierre, B.; Manal, F.A.; Quentin, M.A.; Elisabetta, B.; Vlad, R.; Philippe, H.M.; Bruno, S.; Jean-Louis, J.; Pierre, B.; et al. A Randomised, Double-Blind, Placebo-Controlled, Multi-Centre, Dose-Range, Proof-of-Concept, 24-Week Treatment Study of Lanifibranor in Adult Subjects with Non-Alcoholic Steatohepatitis: Design of the NATIVE Study. Contemp. Clin. Trials 2020, 98, 106170. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Panzitt, K.; Wagner, M. FXR in Liver Physiology: Multiple Faces to Regulate Liver Metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166133. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile Acids Lower Triglyceride Levels via a Pathway Involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Claudel, T.; Sturm, E.; Duez, H.; Torra, I.P.; Sirvent, A.; Kosykh, V.; Fruchart, J.-C.; Dallongeville, J.; Hum, D.W.; Kuipers, F.; et al. Bile Acid-Activated Nuclear Receptor FXR Suppresses Apolipoprotein A-I Transcription via a Negative FXR Response Element. J. Clin. Investig. 2002, 109, 961–971. [Google Scholar] [CrossRef]
- Claudel, T.; Inoue, Y.; Barbier, O.; Duran-Sandoval, D.; Kosykh, V.; Fruchart, J.; Fruchart, J.C.; Gonzalez, F.J.; Staels, B. Farnesoid X Receptor Agonists Suppress Hepatic Apolipoprotein CIII Expression. Gastroenterology 2003, 125, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Monte, M.J.; Dominguez, M.; Muntané, J.; Marin, J.J.G. Differential Activation of the Human Farnesoid X Receptor Depends on the Pattern of Expressed Isoforms and the Bile Acid Pool Composition. Biochem. Pharmacol. 2013, 86, 926–939. [Google Scholar] [CrossRef]
- Ramos Pittol, J.M.; Milona, A.; Morris, I.; Willemsen, E.C.L.; van der Veen, S.W.; Kalkhoven, E.; van Mil, S.W.C. FXR Isoforms Control Different Metabolic Functions in Liver Cells via Binding to Specific DNA Motifs. Gastroenterology 2020, 159, 1853–1865.e10. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Bowlus, C.L.; Levy, C.; Mayo, M.J.; Pratt, D.S.; Vuppalanchi, R.; Younossi, Z.M. Application of the Latest Advances in Evidence-Based Medicine in Primary Biliary Cholangitis. Am. J. Gastroenterol. 2023, 118, 232–242. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Gilglioni, E.H.; de Boer, B.A.; Runge, J.H.; de Waart, D.R.; Salgueiro, C.L.; Ishii-Iwamoto, E.L.; Oude Elferink, R.P.J.; Gaemers, I.C. Bile Acid Receptor Agonists INT747 and INT777 Decrease Oestrogen Deficiency-Related Postmenopausal Obesity and Hepatic Steatosis in Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 2054–2062. [Google Scholar] [CrossRef]
- Briand, F.; Brousseau, E.; Quinsat, M.; Burcelin, R.; Sulpice, T. Obeticholic Acid Raises LDL-Cholesterol and Reduces HDL-Cholesterol in the Diet-Induced NASH (DIN) Hamster Model. Eur. J. Pharmacol. 2018, 818, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Gui, T.; Alecu, I.; Lone, M.A.; Hornemann, T.; Chen, Q.; Visentin, M.; Hiller, C.; Hausler, S.; Kullak-Ublick, G.A. Farnesoid X Receptor Activation Induces the Degradation of Hepatotoxic 1-Deoxysphingolipids in Non-Alcoholic Fatty Liver Disease. Liver Int. 2020, 40, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Huang, C.C.; Yang, Y.Y.; Huang, S.F.; Lee, T.Y.; Li, T.H.; Hou, M.C.; Lin, H.C. Obeticholic Acid Treatment Ameliorates the Cardiac Dysfunction in NASH Mice. PLoS ONE 2022, 17, e0276717. [Google Scholar] [CrossRef]
- Lin, C.; Yu, B.; Liu, X.; Chen, L.; Zhang, Z.; Ye, W.; Zhong, H.; Bai, W.; Yang, Y.; Nie, B. Obeticholic Acid Inhibits Hepatic Fatty Acid Uptake Independent of FXR in Mouse. Biomed. Pharmacother. 2022, 150, 112984. [Google Scholar] [CrossRef]
- Goto, T.; Itoh, M.; Suganami, T.; Kanai, S.; Shirakawa, I.; Sakai, T.; Asakawa, M.; Yoneyama, T.; Kai, T.; Ogawa, Y. Obeticholic Acid Protects against Hepatocyte Death and Liver Fibrosis in a Murine Model of Nonalcoholic Steatohepatitis. Sci. Rep. 2018, 8, 8157. [Google Scholar] [CrossRef] [PubMed]
- Tølbøl, K.S.; Kristiansen, M.N.; Hansen, H.H.; Veidal, S.S.; Rigbolt, K.T.; Gillum, M.P.; Jelsing, J.; Vrang, N.; Feigh, M. Metabolic and Hepatic Effects of Liraglutide, Obeticholic Acid and Elafibranor in Diet-Induced Obese Mouse Models of Biopsy-Confirmed Nonalcoholic Steatohepatitis. World J. Gastroeneterology 2018, 24, 179–194. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X Nuclear Receptor Ligand Obeticholic Acid for Non-Cirrhotic, Non-Alcoholic Steatohepatitis (FLINT): A Multicentre, Randomised, Placebo-Controlled Trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.; et al. Obeticholic Acid for the Treatment of Non-Alcoholic Steatohepatitis: Interim Analysis from a Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Rinella, M.E.; Dufour, J.F.; Anstee, Q.M.; Goodman, Z.; Younossi, Z.; Harrison, S.A.; Loomba, R.; Sanyal, A.J.; Bonacci, M.; Trylesinski, A.; et al. Non-Invasive Evaluation of Response to Obeticholic Acid in Patients with NASH: Results from the REGENERATE Study. J. Hepatol. 2022, 76, 536–548. [Google Scholar] [CrossRef]
- Song, K.H.; Li, T.; Owsley, E.; Strom, S.; Chiang, J.Y.L. Bile Acids Activate Fibroblast Growth Factor 19 Signaling in Human Hepatocytes to Inhibit Cholesterol 7α-Hydroxylase Gene Expression. Hepatology 2009, 49, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Dammron, H.A.; Hillgartner, F.B. Fibroblast Growth Factor-19, a Novel Factor That Inhibits Hepatic Fatty Acid Synthesis. J. Biol. Chem. 2009, 284, 10023–10033. [Google Scholar] [CrossRef]
- Schwabl, P.; Hambruch, E.; Budas, G.R.; Supper, P.; Burnet, M.; Liles, J.T.; Birkel, M.; Brusilovskaya, K.; Königshofer, P.; Peck-Radosavljevic, M.; et al. The Non-Steroidal FXR Agonist Cilofexor Improves Portal Hypertension and Reduces Hepatic Fibrosis in a Rat NASH Model. Biomedicines 2021, 9, 60. [Google Scholar] [CrossRef]
- Younis, I.R.; Kirby, B.J.; Billin, A.N.; Xiao, D.; Song, Q.; Watkins, T.R.; Othman, A.A. Pharmacokinetics, Pharmacodynamics, Safety and Tolerability of Cilofexor, a Novel Nonsteroidal Farnesoid X Receptor Agonist, in Healthy Volunteers. Clin. Transl. Sci. 2022, 16, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wai-Sun Wong, V.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Khoshdeli, M.; Peach, M.; Chuang, J.C.; Lin, J.; Tsai, W.W.; Mahadevan, S.; Minto, W.; Diehl, L.; et al. IL-31 Levels Correlate with Pruritus in Patients with Cholestatic and Metabolic Liver Diseases and Is Farnesoid X Receptor Responsive in NASH. Hepatology 2023, 77, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Tully, D.C.; Rucker, P.V.; Chianelli, D.; Williams, J.; Vidal, A.; Alper, P.B.; Mutnick, D.; Bursulaya, B.; Schmeits, J.; Wu, X.; et al. Discovery of Tropifexor (LJN452), a Highly Potent Non-Bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH). J. Med. Chem. 2017, 60, 9960–9973. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.D.; Zheng, L.; Kim, Y.; Fang, B.; Liu, B.; Valdez, R.A.; Dietrich, W.F.; Rucker, P.V.; Chianelli, D.; Schmeits, J.; et al. Tropifexor-Mediated Abrogation of Steatohepatitis and Fibrosis Is Associated with the Antioxidative Gene Expression Profile in Rodents. Hepatol. Commun. 2019, 3, 1085–1097. [Google Scholar] [CrossRef]
- Chen, J.; Gu, J.; Shah, B.; Stringer, R.; Reis da Silva Torrao, L.; Hackling, M.; Nidamarthy, P.K.; Prince, W.T.; Woessner, R. Pharmacokinetics of Tropifexor, a Potent Farnesoid X Receptor Agonist, in Participants with Varying Degrees of Hepatic Impairment. J. Clin. Pharmacol. 2022, 62, 520–531. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Lopez, P.; Lawitz, E.J.; Lucas, K.J.; Loeffler, J.; Kim, W.; Goh, G.B.B.; Huang, J.F.; Serra, C.; Andreone, P.; et al. Tropifexor for Nonalcoholic Steatohepatitis: An Adaptive, Randomized, Placebo-Controlled Phase 2a/b Trial. Nat. Med. 2023, 29, 392–400. [Google Scholar] [CrossRef]
- Naoumov, N.V.; Brees, D.; Loeffler, J.; Chng, E.; Ren, Y.; Lopez, P.; Tai, D.; Lamle, S.; Sanyal, A.J. Digital Pathology with Artificial Intelligence Analyses Provides Greater Insights into Treatment-Induced Fibrosis Regression in NASH. J. Hepatol. 2022, 77, 1399–1409. [Google Scholar] [CrossRef]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in Patients with NASH: A Phase II Double-Blind Placebo-Controlled Dose-Ranging Study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Lee, K.J.; Shim-Lopez, J.; Lee, J.; Wagner, B.; Smith, N.D.; Chen, H.C.; Lawitz, E.J. A Structurally Optimized FXR Agonist, MET409, Reduced Liver Fat Content over 12 Weeks in Patients with Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Loustaud-Ratti, V.; Bureau, C.; Lawitz, E.; Abdelmalek, M.; Alkhouri, N.; Francque, S.; Girma, H.; Darteil, R.; et al. Hepatic and Renal Improvements with FXR Agonist Vonafexor in Individuals with Suspected Fibrotic NASH. J. Hepatol. 2023, 78, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, S.; Biagioli, M.; Morretta, E.; Di Giorgio, C.; Roselli, R.; Bordoni, M.; Bellini, R.; Urbani, G.; Massa, C.; Monti, M.C.; et al. Combinatorial Therapy with BAR502 and UDCA Resets FXR and GPBAR1 Signaling and Reverses Liver Histopathology in a Model of NASH. Sci. Rep. 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Carino, A.; Cipriani, S.; Marchianò, S.; Biagioli, M.; Santorelli, C.; Donini, A.; Zampella, A.; Monti, M.C.; Fiorucci, S. BAR502, a Dual FXR and GPBAR1 Agonist, Promotes Browning of White Adipose Tissue and Reverses Liver Steatosis and Fibrosis. Sci. Rep. 2017, 7, 42801. [Google Scholar] [CrossRef]
- Hu, Y.B.; Liu, X.Y.; Zhan, W. Farnesoid X Receptor Agonist INT-767 Attenuates Liver Steatosis and Inflammation in Rat Model of Nonalcoholic Steatohepatitis. Drug Des. Devel Ther. 2018, 12, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Xie, C.; Libby, A.E.; Ranjit, S.; Levi, J.; Myakala, K.; Bhasin, K.; Jones, B.A.; Orlicky, D.J.; Takahashi, S.; et al. The Role of FXR and TGR5 in Reversing and Preventing Progression of Western Diet–Induced Hepatic Steatosis, Inflammation, and Fibrosis in Mice. J. Biol. Chem. 2022, 298, 102530. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rossi, S.J.; Paredes, A.H.; Trotter, J.F.; Bashir, M.R.; Guy, C.D.; Banerjee, R.; Jaros, M.J.; Owers, S.; Baxter, B.A.; et al. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients with Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; Younes, Z.; Trotter, J.F.; Gunn, N.T.; Moussa, S.E.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients with Nonalcoholic Steatohepatitis. Gastroenterology 2021, 160, 219–231.e1. [Google Scholar] [CrossRef]
- Loomba, R.; Noureddin, M.; Kowdley, K.V.; Kohli, A.; Sheikh, A.; Neff, G.; Raj Bhandari, B.; Gunn, N.; Caldwell, S.H.; Goodman, Z.; et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology 2020, 73, 2021. [Google Scholar] [CrossRef]
- Lawitz, E.J.; Bhandari, B.R.; Ruane, P.J.; Kohli, A.; Harting, E.; Ding, D.; Chuang, J.C.; Huss, R.S.; Chung, C.; Myers, R.P.; et al. Fenofibrate Mitigates Hypertriglyceridemia in Nonalcoholic Steatohepatitis Patients Treated with Cilofexor/Firsocostat. Clin. Gastroenterol. Hepatol. 2023, 21, 143–152.e3. [Google Scholar] [CrossRef]
- Quentin Anstee, A.M.; Lucas, K.J.; Francque, S.; Abdelmalek, M.F.; Sanyal, A.J.; Ratziu, V.; Gadano, A.C.; Rinella, M.; Charlton, M.; Loomba, R.; et al. Tropifexor plus Cenicriviroc Combination versus Monotherapy in Non-Alcoholic Steatohepatitis: Results from the Phase 2b TANDEM Study. Hepatology 2023. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zheng, K.I.; Wang, X.-D.; Qiao, J.; Li, Y.-Y.; Zhang, L.; Zheng, M.-H.; Wu, J. LC-MS-Based Lipidomic Analysis in Distinguishing Patients with Nonalcoholic Steatohepatitis from Nonalcoholic Fatty Liver. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 452–459. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Almaghamsi, A.M.; Sherbeeni, S.M.; Alqutub, A.N.; Aldosary, A.S.; Isnani, A.C.; Al-Daghri, N.; Taylor-Robinson, S.D.; Gul, R. Alterations in Circulating Lipidomic Profile in Patients with Type 2 Diabetes with or without Non-Alcoholic Fatty Liver Disease. Front. Mol. Biosci. 2023, 10, 1030661. [Google Scholar] [CrossRef]
- Mocciaro, G.; Allison, M.; Jenkins, B.; Azzu, V.; Huang-Doran, I.; Herrera-Marcos, L.V.; Hall, Z.; Murgia, A.; Susan, D.; Frontini, M.; et al. Non-Alcoholic Fatty Liver Disease Is Characterised by a Reduced Polyunsaturated Fatty Acid Transport via Free Fatty Acids and High-Density Lipoproteins (HDL). Mol. Metab. 2023, 73, 101728. [Google Scholar] [CrossRef]
- Mccullough, A.; Previs, S.F.; Dasarathy, J.; Lee, K.; Osme, A.; Kim, C.; Ilchenko, S.; Lorkowski, S.W.; Smith, J.D.; Dasarathy, S.; et al. HDL Flux Is Higher in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Physiol. Endocrinol. Metab. 2019, 317, 852–862. [Google Scholar] [CrossRef]
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol. Med. 2020, 26, 1086–1100. [Google Scholar] [CrossRef]
- Fadaei, R.; Poustchi, H.; Meshkani, R.; Moradi, N.; Golmohammadi, T.; Merat, S. Impaired HDL Cholesterol Efflux Capacity in Patients with Non-Alcoholic Fatty Liver Disease Is Associated with Subclinical Atherosclerosis. Sci. Rep. 2018, 8, 11691. [Google Scholar] [CrossRef]
- Zhao, Y. Association between Apolipoprotein B/A1 and the Risk of Metabolic Dysfunction Associated Fatty Liver Disease According to Different Lipid Profiles in a Chinese Population: A Cross-Sectional Study. Clin. Chim. Acta 2022, 534, 138–145. [Google Scholar] [CrossRef]
- Yang, M.H.; Sung, J.; Gwak, G.-Y. The Associations between Apolipoprotein B, A1, and the B/A1 Ratio and Nonalcoholic Fatty Liver Disease in Both Normal-Weight and Overweight Korean Population. J. Clin. Lipidol. 2016, 10, 289–298. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, C.; Wang, Y. Overexpression of Apolipoprotein A-I Alleviates Endoplasmic Reticulum Stress in Hepatocytes. Lipids Health Dis. 2017, 16, 105. [Google Scholar] [CrossRef]
- Liu, W.; Qin, L.; Yu, H.; Lv, F.; Wang, Y. Apolipoprotein A-I and Adenosine Triphosphate-Binding Cassette Transporter A1 Expression Alleviates Lipid Accumulation in Hepatocytes. J. Gastroenterol. Hepatol. 2014, 29, 614–622. [Google Scholar] [CrossRef]
- Ma, D.; Liu, W.; Wang, Y. ApoA-I or ABCA1 Expression Suppresses Fatty Acid Synthesis by Reducing 27-Hydroxycholesterol Levels. Biochimie 2014, 103, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Ruscica, M.; Calabresi, L.; Chiesa, G.; Giovannoni, R.; Badimon, J.J. HDL Therapy Today: From Atherosclerosis, to Stent Compatibility to Heart Failure. Ann. Med. 2019, 51, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Karavia, E.A.; Papachristou, D.J.; Liopeta, K.; Triantaphyllidou, I.E.; Dimitrakopoulos, O.; Kypreos, K.E. Apolipoprotein A-I Modulates Processes Associated with Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Mol. Med. 2012, 18, 901–912. [Google Scholar] [CrossRef]
- Schierwagen, R.; Maybüchen, L.; Zimmer, S.; Hittatiya, K.; Bäck, C.; Klein, S.; Uschner, F.E.; Reul, W.; Boor, P.; Nickenig, G.; et al. Seven Weeks of Western Diet in Apolipoprotein-E-Deficient Mice Induce Metabolic Syndrome and Non-Alcoholic Steatohepatitis with Liver Fibrosis. Sci. Rep. 2015, 5, 12931. [Google Scholar] [CrossRef]
- Romano, G.; Reggi, S.; Kutryb-Zajac, B.; Facoetti, A.; Chisci, E.; Pettinato, M.; Giuffrè, M.R.; Vecchio, F.; Leoni, S.; De Giorgi, M.; et al. APOA-1Milano Muteins, Orally Delivered via Genetically Modified Rice, Show Anti-Atherogenic and Anti-Inflammatory Properties in Vitro and in Apoe−/− Atherosclerotic Mice. Int. J. Cardiol. 2018, 271, 233–239. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, L.; La, X.; Wu, H.; Yang, R.; Li, H.; Li, Z. Ferulic Acid and P-Coumaric Acid Synergistically Attenuate Non-Alcoholic Fatty Liver Disease through HDAC1/PPARG-Mediated Free Fatty Acid Uptake. Int. J. Mol. Sci. 2022, 23, 15297. [Google Scholar] [CrossRef]
- Ran, L.S.; Wu, Y.Z.; Gan, Y.W.; Wang, H.L.; Wu, L.J.; Zheng, C.M.; Ming, Y.; Xiong, R.; Li, Y.L.; Lei, S.H.; et al. Andrographolide Ameliorates Hepatic Steatosis by Suppressing FATP2-Mediated Fatty Acid Uptake in Mice with Nonalcoholic Fatty Liver Disease. J. Nat. Med. 2023, 77, 73–86. [Google Scholar] [CrossRef]
- Dong, R.; Yang, X.; Wang, C.; Liu, K.; Liu, Z.; Ma, X.; Sun, H.; Huo, X.; Fu, T.; Meng, Q. Yangonin Protects against Non-Alcoholic Fatty Liver Disease through Farnesoid X Receptor. Phytomedicine 2019, 53, 134–142. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Jeddi, S.; Ebrahimi-Kalan, A.; Alipour, M.R. Trans-Chalcone Prevents Insulin Resistance and Hepatic Inflammation and Also Promotes Hepatic Cholesterol Efflux in High-Fat Diet-Fed Rats: Modulation of MiR-34a-, MiR-451-, and MiR-33a-Related Pathways. Food Funct. 2018, 9, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, Q.; Xuan, C.X.; Wang, X.; Luo, H.Q.; Liu, J.E.; Wang, L.L.; Li, T.T.; Chen, Y.P.; Liu, J. 3-Acetyl-Oleanolic Acid Ameliorates Non-Alcoholic Fatty Liver Disease in High Fat Diet-Treated Rats by Activating AMPK-Related Pathways. Acta Pharmacol. Sin. 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Xia, S.-F.; Shao, J.; Zhao, S.-Y.; Qiu, Y.-Y.; Teng, L.-P.; Huang, W.; Wang, S.-S.; Cheng, X.-R.; Jiang, Y.-Y. Niga-Ichigoside F1 Ameliorates High-Fat Diet-Induced Hepatic Steatosis in Male Mice by Nrf2 Activation. Food Funct. 2018, 9, 906–916. [Google Scholar] [CrossRef]
- Duan, X.; Meng, Q.; Wang, C.; Liu, Z.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Huo, X.; Peng, J.; et al. Calycosin Attenuates Triglyceride Accumulation and Hepatic Fibrosis in Murine Model of Non-Alcoholic Steatohepatitis via Activating Farnesoid X Receptor. Phytomedicine 2017, 25, 83–92. [Google Scholar] [CrossRef]
- Duan, X.; Meng, Q.; Wang, C.; Liu, Z.; Sun, H.; Huo, X.; Sun, P.; Ma, X.; Peng, J.; Liu, K. Effects of Calycosin against High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. J. Gastroenterol. Hepatol. 2018, 33, 533–542. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, Z.T.; Huang, S.; Ning, M.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Alisol B Alleviates Hepatocyte Lipid Accumulation and Lipotoxicity via Regulating RARα-PPARγ-CD36 Cascade and Attenuates Non-Alcoholic Steatohepatitis in Mice. Nutrients 2022, 14, 2411. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Han, N.; Liu, Z.; Xiao, B.; Yin, J. Beneficial Effects of Neomangiferin on High Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Rats. Int. Immunopharmacol. 2015, 25, 218–228. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-Functionalized Lipid-Polymer Hybrid Nanoparticles for Oral Delivery of Silymarin and Enhanced Lipid-Lowering Effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef]
- Nobili, V.; Manco, M.; Devito, R.; Ciampalini, P.; Piemonte, F.; Marcellini, M. Effect of Vitamin E on Aminotransferase Levels and Insulin Resistance in Children with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2006, 24, 1553–1561. [Google Scholar] [CrossRef]
- Zang, S.; Chen, J.; Song, Y.; Bai, L.; Chen, J.; Chi, X.; He, F.; Sheng, H.; Wang, J.; Xie, S.; et al. Haptoglobin Genotype and Vitamin E Versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis in China: A Multicenter, Randomized, Placebo-Controlled Trial Design. Adv. Ther. 2018, 35, 218–231. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Anastasilakis, A.D.; Makras, P.; Hawa, G.; Sonnleitner, L.; Missbichler, A.; Doulberis, M.; Katsinelos, P.; Terpos, E. Noggin Levels in Nonalcoholic Fatty Liver Disease: The Effect of Vitamin E Treatment. Hormones 2018, 17, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Podszun, M.C.; Alawad, A.S.; Lingala, S.; Morris, N.; Huang, W.C.A.; Yang, S.; Schoenfeld, M.; Rolt, A.; Ouwerkerk, R.; Valdez, K.; et al. Vitamin E Treatment in NAFLD Patients Demonstrates That Oxidative Stress Drives Steatosis through Upregulation of De-Novo Lipogenesis. Redox Biol. 2020, 37, 101710. [Google Scholar] [CrossRef]
- Kheong, C.W.; Mustapha, N.R.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949.e8. [Google Scholar] [CrossRef]
- Navarro, V.J.; Belle, S.H.; D’Amato, M.; Adfhal, N.; Brunt, E.M.; Fried, M.W.; Rajender Reddy, K.; Wahed, A.S.; Harrison, S. Silymarin in Non-Cirrhotics with Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo Controlled Trial. PLoS ONE 2019, 14, e0221683. [Google Scholar] [CrossRef]
- De Avelar, C.R.; Nunes, B.V.C.; da Silva Sassaki, B.; dos Santos Vasconcelos, M.; de Oliveira, L.P.M.; Lyra, A.C.; Bueno, A.A.; de Jesus, R.P. Efficacy of Silymarin in Patients with Non-Alcoholic Fatty Liver Disease—The Siliver Trial: A Study Protocol for a Randomized Controlled Clinical Trial. Trials 2023, 24, 177. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol Supplementation Improves Inflammatory Biomarkers in Patients with Nonalcoholic Fatty Liver Disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Nellemann, B.; Bibby, B.M.; Stødkilde-Jørgensen, H.; Pedersen, S.B.; Grønbæk, H.; Nielsen, S. No Effect of Resveratrol on VLDL-TG Kinetics and Insulin Sensitivity in Obese Men with Nonalcoholic Fatty Liver Disease. Diabetes Obes. Metab. 2018, 20, 2504–2509. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and Inflammation in Non-Alcoholic Fatty Liver Disease: A Randomized, Placebo Controlled Clinical Trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The Effect of Curcumin on Hepatic Fat Content in Individuals with Obesity. Diabetes Obes. Metab. 2022, 24, 2192–2202. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short Term Effects of Coffee Components Consumption on Gut Microbiota in Patients with Non-Alcoholic Fatty Liver and Diabetes: A Pilot Randomized Placebo-Controlled, Clinical Trial. EXCLI J. 2020, 19, 241–250. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of Supplementation with Main Coffee Components Including Caffeine and/or Chlorogenic Acid on Hepatic, Metabolic, and Inflammatory Indices in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha Supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Mol. Nutr. Food Res. 2021, 65, 2001178. [Google Scholar] [CrossRef] [PubMed]
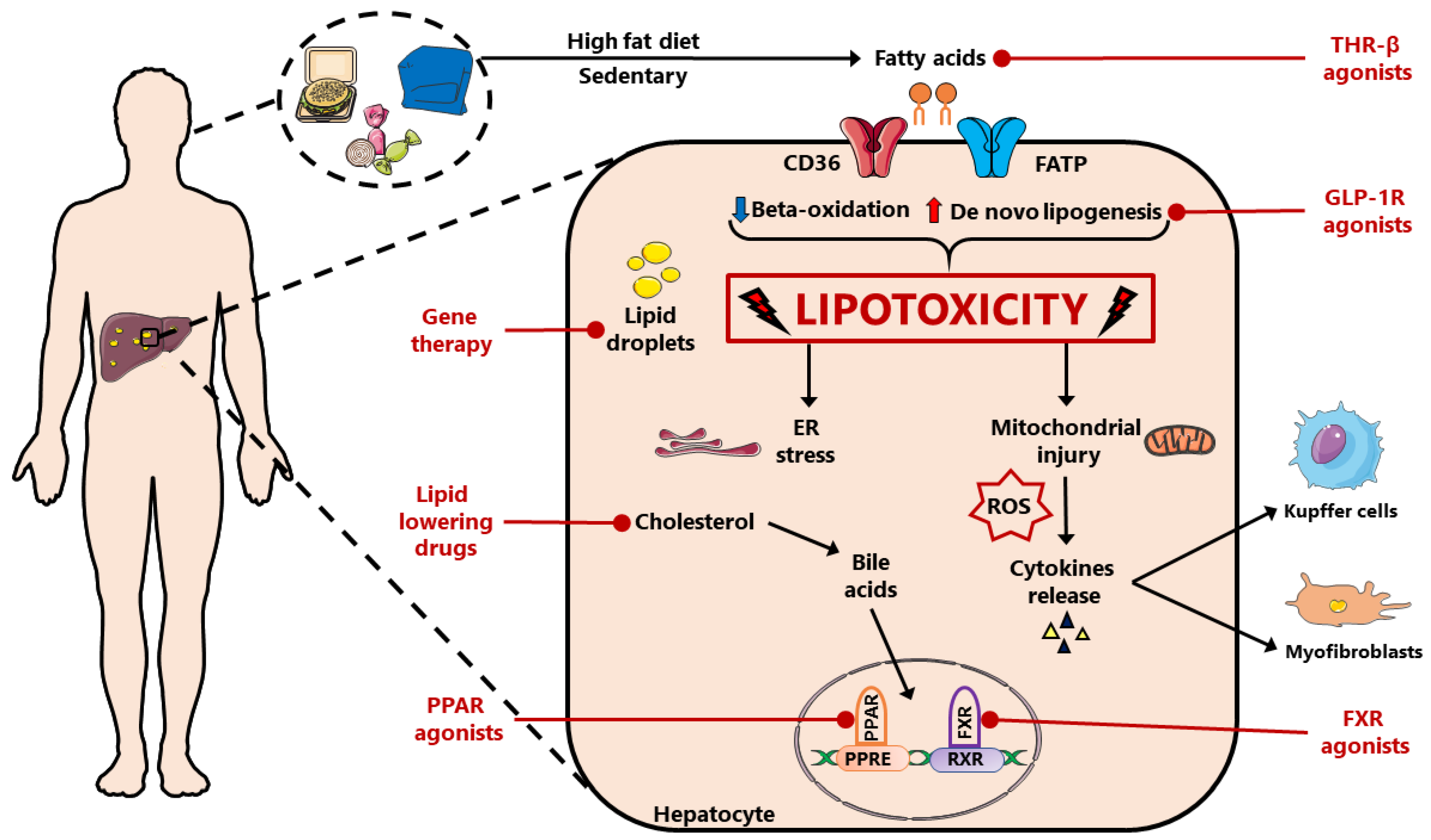
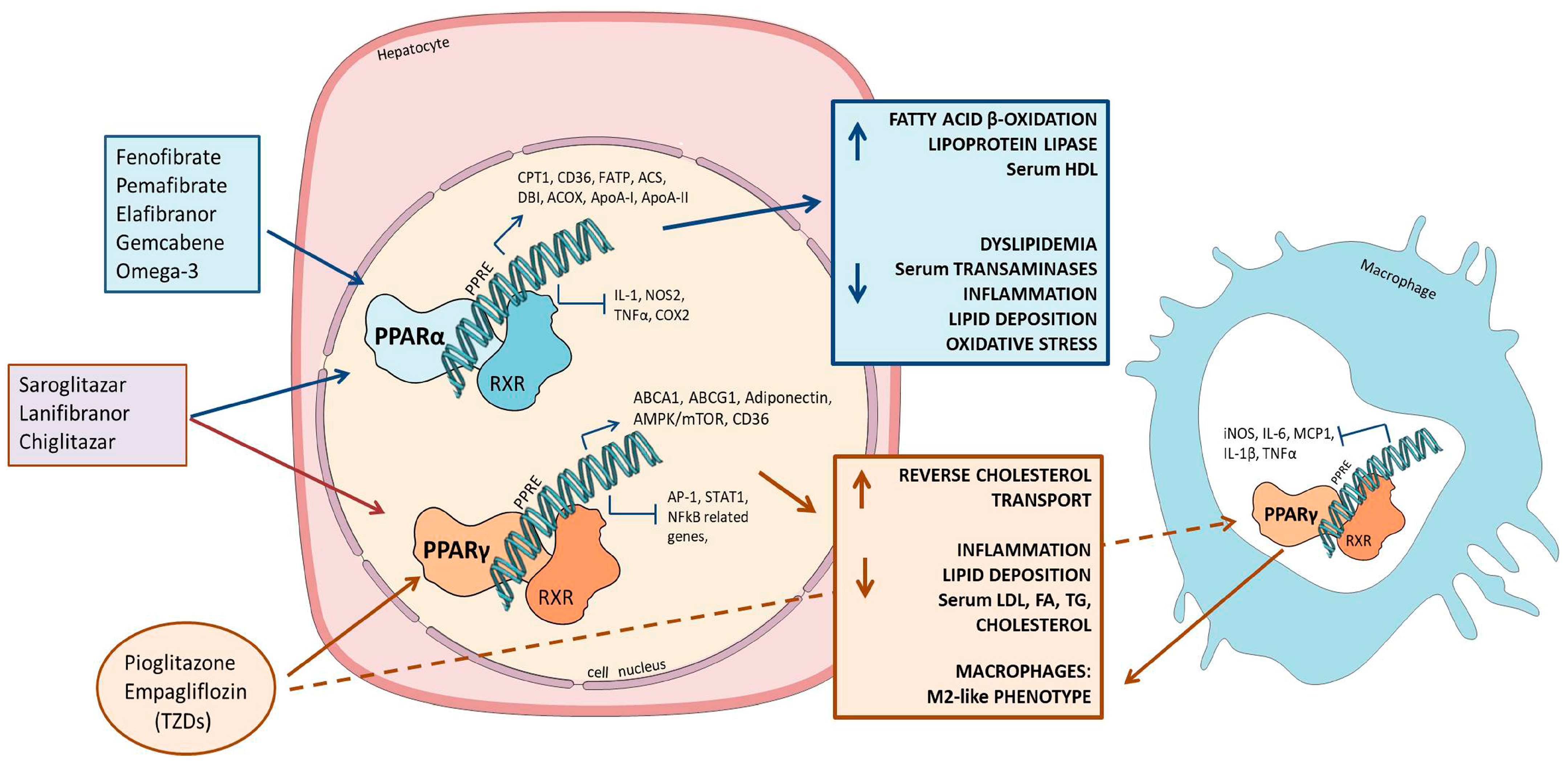
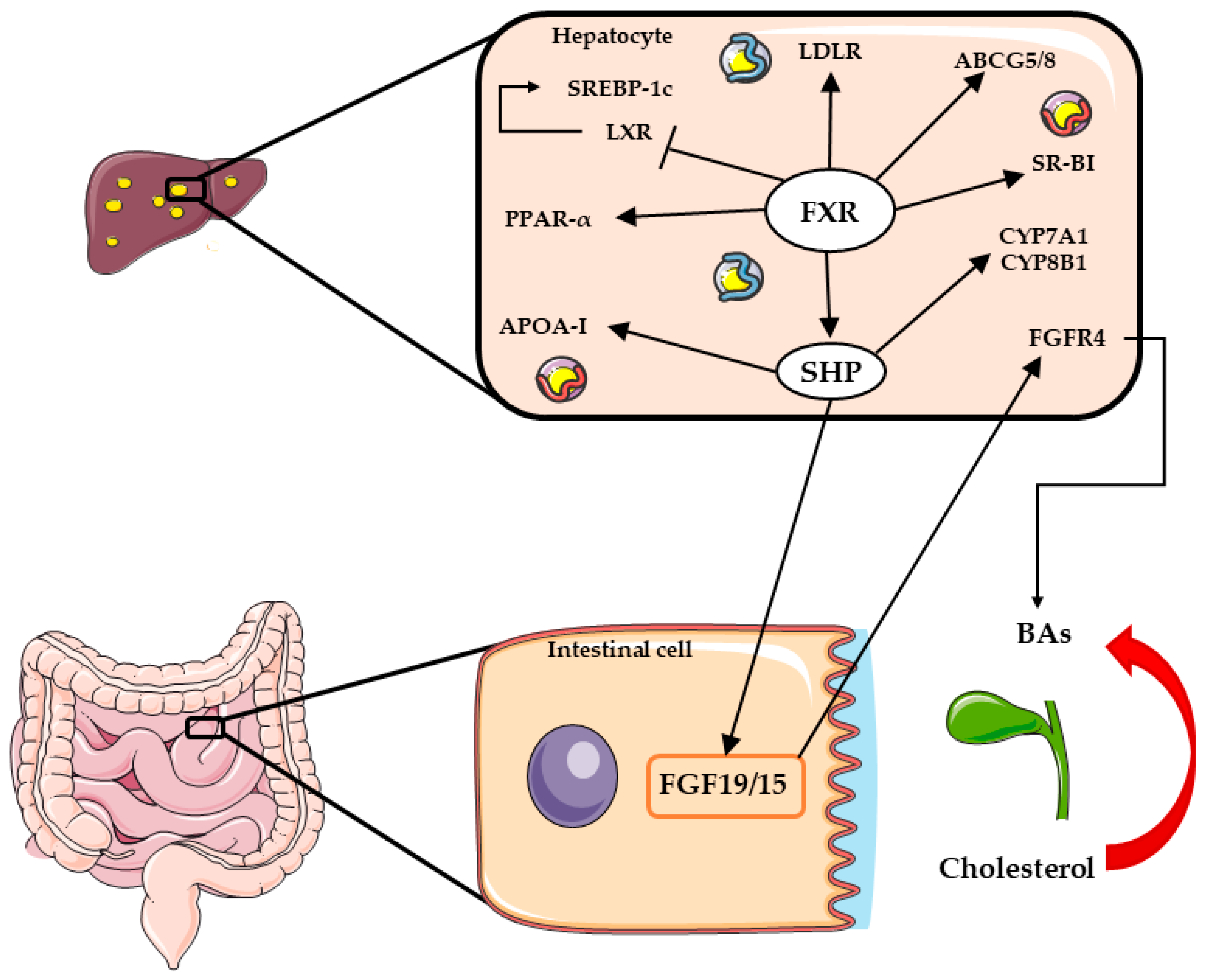
| Target | Identifier Trial | Condition | Drug | Phase | Status |
|---|---|---|---|---|---|
| pan-PPARs | NCT03459079 | T2D and NAFLD | Lanifibranor | Phase 2 | Recruiting |
| NCT04849728 | Fibrosis and NASH | Lanifibranor | Phase 3 | Recruiting | |
| NCT05232071 | T2M and NASH | Lanifibranor with SGLT2 | Phase 2 | Recruiting | |
| NCT03008070 | NASH | Lanifibranor (IVA337) | Phase 2 | Completed | |
| NCT05193916 | NASH | Chiglitazar | Phase 2 | Recruiting | |
| PPAR α/γ agonists | NCT03639623 | NASH | Saroglitazar | Phase 2 | Recruiting |
| NCT03617263 | NAFLD + PCOS | Saroglitazar | Phase 2 | Recruiting | |
| NCT05011305 | Fibrosis and NASH | Saroglitazar | Phase 2 | Recruiting | |
| NCT03061721 | NAFLD + NASH | Saroglitazar | Phase 2 | Completed | |
| NCT03863574 | NASH | Saroglitazar | Phase 2 | Completed | |
| PPAR α/δ agonists | NCT01694849 | NASH | Elafibranor (GFT505) | Phase 2 | Completed |
| NCT02704403 (RESOLVE-IT Trial) | NASH | Elafibranor | Phase 3 | Terminated | |
| NCT03883607 | NASH | Elafibranor | Phase 2 | Terminated | |
| NCT03953456 | NAFLD | Elafibranor | Phase 2 | Terminated | |
| PPAR δ agonist | NCT03551522 | NASH | Seladelpar | Phase 2 | Terminated |
| PPAR α agonists | NCT03350165 | NAFLD | Pemafibrate | Phase 2 | Completed |
| NCT05327127 | Fibrosis and NASH | Pemafibrate (K-877-ER) with SGLT inhibitor | Phase 2 | Recruiting |
| Target | Identifier Trial | Condition | Drug | Phase | Status |
|---|---|---|---|---|---|
| Nonsteroidal FXR agonists | NCT01999101 | NAFLD | Cilofexor (Px-104) | Phase 2 | Completed |
| NCT02854605 | NASH | Cilofexor (GS-9674) | Phase 2 | Completed | |
| NCT02781584 | NASH | Cilofexor with selonsertib and firsocostat | Phase 2 | Completed | |
| NCT03449446 | Cirrhosis (NASH) | Cilofexor with selonsertib and firsocostat | Phase 2 | Completed | |
| NCT03987074 | NASH | Cilofexor with semaglutide and firsocostat | Phase 2 | Completed | |
| NCT03681457 | NAFLD | Tropifexor (LJN452) | Phase 1 | Completed | |
| NCT02855164 | NASH | Tropifexor (LJN452) | Phase 2 | Terminated | |
| NCT03517540 | Fibrosis and NASH | Tropifexor with cenicriviroc | Phase 2 | Completed | |
| NCT04065841 | Fibrosis and NASH | Tropifexor with licogliflozin | Phase 2 | Recruiting | |
| NCT03812029 | NASH | Vonafexor (EYP001a) | Phase 2 | Completed | |
| NCT03976687 | Healthy and NASH | Vonafexor (EYP001a) | Phase 2 | Completed | |
| NCT02913105 | NASH | Nidufexor (LMB763) | Phase 2 | Terminated | |
| NCT05397379 | NASH | HEC96719 | Phase 2 | Recruiting | |
| NCT04328077 | NASH | TERN-101 | Phase 2 | Completed | |
| NCT05338034 | NASH | HPG1860 | Phase 2 | Active, not recruiting | |
| NCT04773964 | NASH | MET642 | Phase 2 | Active, not recruiting | |
| NCT04702490 | T2D and NASH | MET409 | Phase 2 | Active, not recruiting | |
| Steroidal FXR agonists | NCT05573204 | NASH | Obeticholic acid and vitamin E | Phase 2 | Active, not recruiting |
| NCT02548351 | Fibrosis (NASH) | Obeticholic acid | Phase 3 | Active, not recruiting | |
| NCT03836937 | NAFLD | Obeticholic acid | Not applicable | Completed | |
| NCT01265498 (FLINT trial) | NASH | Obeticholic acid | Phase 2 | Completed | |
| NCT02633956 (CONTROL trial) | Fibrosis (NASH) | Obeticholic acid | Phase 2 | Completed | |
| NCT00501592 | T2D (NAFLD) | Obeticholic acid (INT-747) | Phase 2 | Completed | |
| NCT03439254 (REVERSE trial) | Cirrhosis (NASH) | Obeticholic acid | Phase 3 | Completed | |
| NCT04378010 | NASH | EDP-305 | Phase 2 | Recruiting | |
| NCT02918929 | NAFLD and healthy | EDP-305 | Phase 1 | Completed | |
| NCT03421431 | NASH | EDP-305 | Phase 2 | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitulo, M.; Gnodi, E.; Rosini, G.; Meneveri, R.; Giovannoni, R.; Barisani, D. Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. Int. J. Mol. Sci. 2023, 24, 12748. https://doi.org/10.3390/ijms241612748
Vitulo M, Gnodi E, Rosini G, Meneveri R, Giovannoni R, Barisani D. Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. International Journal of Molecular Sciences. 2023; 24(16):12748. https://doi.org/10.3390/ijms241612748
Chicago/Turabian StyleVitulo, Manuela, Elisa Gnodi, Giulia Rosini, Raffaella Meneveri, Roberto Giovannoni, and Donatella Barisani. 2023. "Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD" International Journal of Molecular Sciences 24, no. 16: 12748. https://doi.org/10.3390/ijms241612748
APA StyleVitulo, M., Gnodi, E., Rosini, G., Meneveri, R., Giovannoni, R., & Barisani, D. (2023). Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. International Journal of Molecular Sciences, 24(16), 12748. https://doi.org/10.3390/ijms241612748







