Selection Behavior and OBP-Transcription Response of Western Flower Thrips, Frankliniella occidentalis, to Six Plant VOCs from Kidney Beans
Abstract
:1. Introduction
2. Results
2.1. Selection Behavior Response of F. occidentalis for Six Single VOCs of P. vulgaris
2.2. Selection Behavior Response of F. occidentalis for Mixed VOCs of P. vulgaris
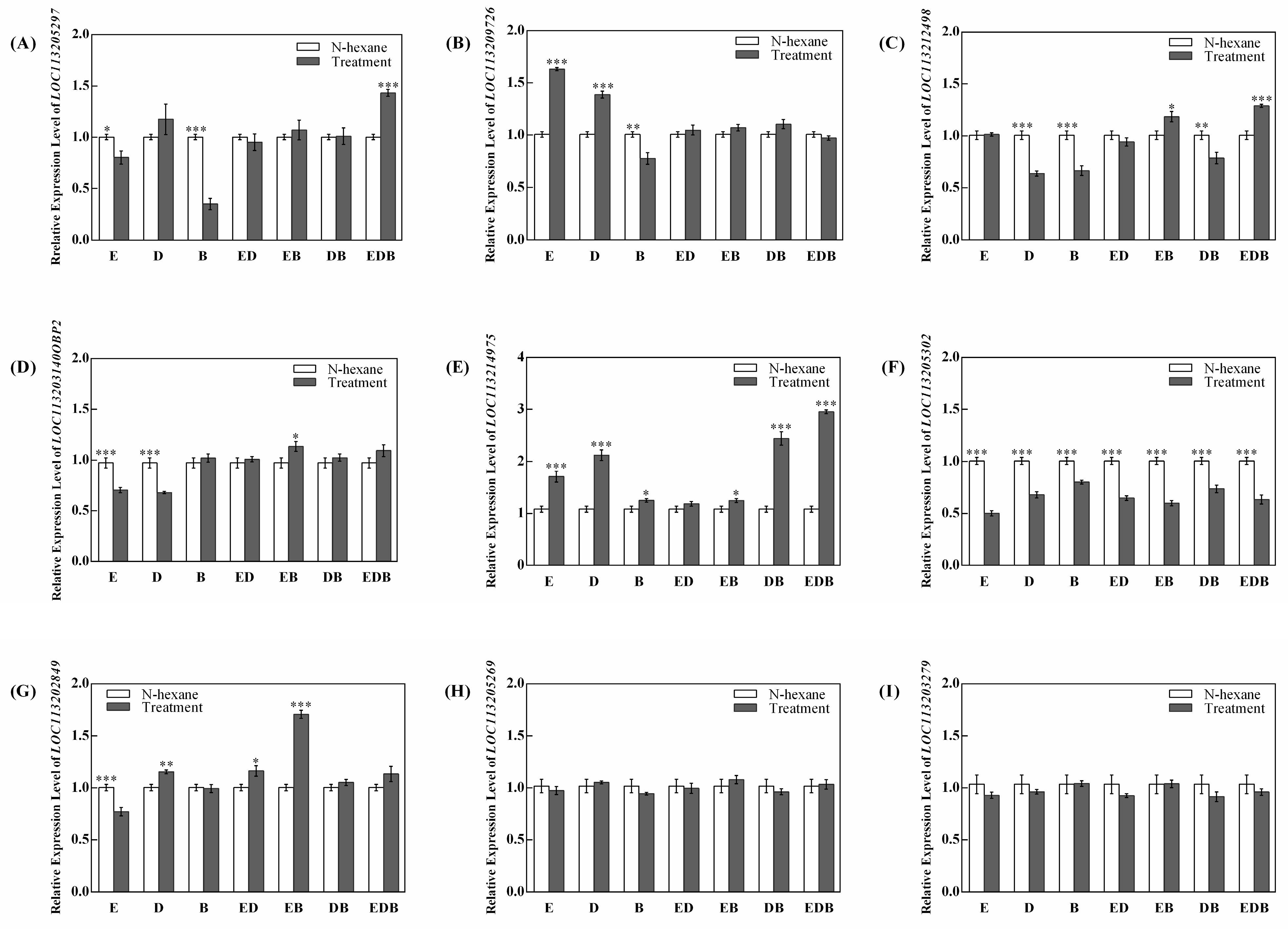
2.3. Effect of VOCs of P. vulgaris on the Transcription Level of OBP Genes in F. occidentalis
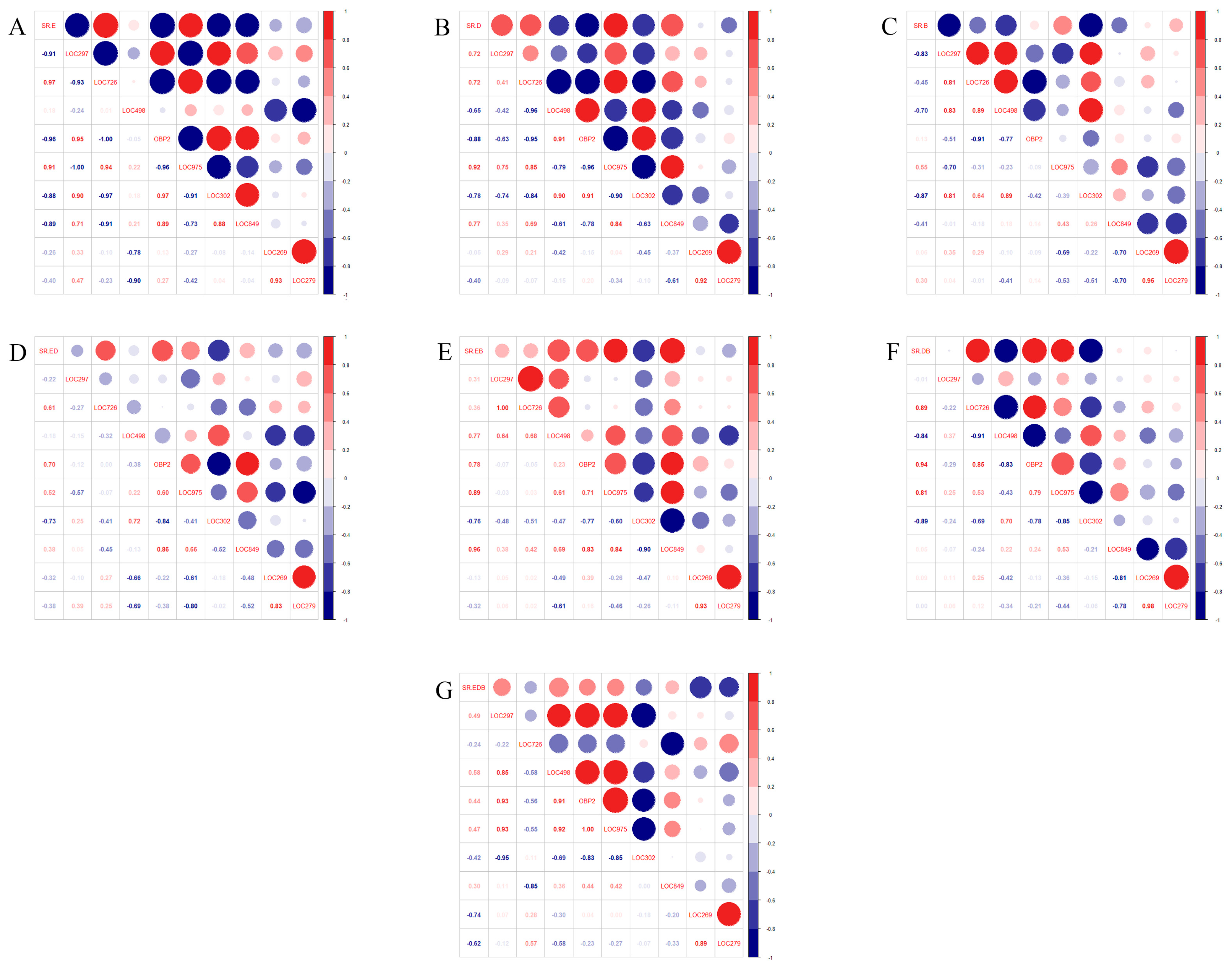
2.4. Correlation Analysis of the Selection Rate with the Transcript Expression Levels of OBP Genes in F. occidentalis Adults
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Testing Compounds Preparation
4.3. Preparation of Uni-Lure and Multi-Lure
4.4. Selection Assays of F. occidentalis Adults for the Special VOCs
4.5. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirk, W.D.J.; Terry, L.I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.; Hoddle, M.S.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.Y.; Xing, Z.L.; Ma, T.T.; Xu, D.W.; Li, Y.Y.; Lei, Z.R.; Gao, Y.L. Competitive interaction between Frankliniella occidentalis and locally present thrips species: A global review. J. Pest Sci. 2021, 94, 5–16. [Google Scholar] [CrossRef]
- Bielza, P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 2008, 64, 1131–1138. [Google Scholar] [CrossRef]
- Carroll, M.J.; Schmelz, E.A.; Meagher, R.L.; Teal, P. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol. 2006, 32, 1911–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Lei, Z.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, R.A. Western flower thrips (Thysanoptera: Thripidae) and insecticide resistance: An overview and strategies to mitigate insecticide resistance development. J. Entomol. Sci. 2016, 51, 257–273. [Google Scholar] [CrossRef]
- Demirozer, O.; Tyler-Julian, K.; Funderburk, J.; Leppla, N.; Reitz, S. Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Manag. Sci. 2012, 68, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Bielza, P.; Quinto, V.; Contreras, J.; Torné, M.; Martín, A.; Espinosa, P.J. Resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande), in greenhouses of south-eastern Spain. Pest Manag. Sci. 2007, 63, 682–687. [Google Scholar] [CrossRef]
- The Arthropod Pesticide Resistance Database. Available online: http://www.pesticideresistance.org (accessed on 17 July 2023).
- Zhang, K.; Yuan, J.J.; Wang, J.; Hua, D.K.; Zheng, X.B.; Tao, M.; Zhang, Z.; Wan, Y.R.; Wang, S.Y.; Zhang, Y.J.; et al. Susceptibility levels of field populations of Frankliniella occidentalis (Thysanoptera: Thripidae) to seven insecticides in China. Crop Protect. 2022, 153, 105886. [Google Scholar] [CrossRef]
- Bielza, P.; Quinto, V.; Grávalos, C.; Fernández, E.; Abellán, J.; Contreras, J. Stability of spinosad resistance in Frankliniella occidentalis (Pergande) under laboratory conditions. Bull. Entomol. Res. 2008, 98, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Kono, S.; Murai, T.; Miyata, T. Mechanisms of resistance to spinosad in the western flower thrip, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Insect Sci. 2008, 15, 125–132. [Google Scholar] [CrossRef]
- Das, A.; Lee, S.H.; Hyun, T.K.; Kim, S.W.; Kim, J.Y. Plant volatiles as method of communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Mitchell, V.J.; Suckling, D.M. 6-Pentyl-2H-pyran-2-one: A potent peach-derived kairomone for New Zealand flower thrips, Thrips obscuratus. J. Chem. Ecol. 2014, 40, 50–55. [Google Scholar] [CrossRef]
- Delphia, C.M.; Mescher, M.C.; De Moraes, C.M. Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J. Chem. Ecol. 2007, 33, 997–1012. [Google Scholar] [CrossRef]
- Jiang, N.N.; Mao, G.F.; Li, T.; Mo, J.C. The olfactory behavior response of Cytorhinus Lividipennis to single component of rice volatiles. Ecol. Environ. Sci. 2018, 27, 262–267. [Google Scholar]
- Lou, Y.G.; Cheng, J.A. Role of rice volatiles in the foraging behaviour of the predator Cyrtorhinus lividipennis for the rice brown planthopper Nilaparvata lugens. BioControl 2003, 48, 73–86. [Google Scholar] [CrossRef]
- Reddy, G.V.P. Recent Trends in the Olfactory Responses of Insect Natural Enemies to Plant Volatiles; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- James, D.G. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: Methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 2003, 29, 1601–1609. [Google Scholar] [CrossRef]
- Yu, H.L.; Zhang, Y.J.; Wyckhuys, K.A.G.; Wu, K.M.; Gao, X.W.; Guo, Y.Y. Electrophysiological and behavioral responses of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ. Entomol. 2010, 39, 600–609. [Google Scholar] [CrossRef]
- Meng, X.; Hu, J.J.; Li, Y.H.; Dai, J.Q.; Ouyang, G.C. Screening for effective odors through which Conopomorpha sinensis Bradley (Lepidoptera: Gracillariidae) locates its host. Chemoecology 2021, 31, 301–310. [Google Scholar] [CrossRef]
- Ninkovic, V.; Ahmed, E.; Glinwood, R.; Pettersson, J. Effects of two types of semiochemical on population development of the bird cherry oat aphid Rhopalosiphum padi in a barley crop. Agric. For. Entomol. 2015, 5, 27–34. [Google Scholar] [CrossRef]
- Hansson, B.S. Olfaction in Lepidoptera. Experientia 1995, 51, 1003–1027. [Google Scholar] [CrossRef]
- Dicke, M.; Loon, J. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Sugiura, H.; Mimura, H.; Kamiya, K.; Osaki, T.; Takeuchi, S. Highly sensitive VOC detectors using insect olfactory receptors reconstituted into lipid bilayers. Sci. Adv. 2021, 7, eabd2013. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhang, Y.; Fan, D.S.; Feng, J.N. Identification and expression profiling of odorant-binding proteins and chemosensory proteins of Daktulosphaira vitifoliae (Hemiptera: Phylloxeridae). J. Econ. Entomol. 2017, 110, 1812–1820. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Z.J.; Liu, H.; Liu, X.W.; Jin, Y.X.; Pokharel, S.S.; Chen, F.J. Elevated CO2-mediated plant VOCs change aggravates invasive thrips occurrence by altering their host-selection behaviour. J. Appl. Entomol. 2021, 145, 777–788. [Google Scholar] [CrossRef]
- Liu, X.W.; Liu, H.; Wang, Y.H.; Qian, L.; Chen, F.J. Elevated CO2 aggravates invasive thrip damage by altering its host plant nutrient and secondary metabolism. Environ. Pollut. 2022, 295, 118736. [Google Scholar] [CrossRef] [PubMed]
- Avellaneda, J.; Díaz, M.; Coy-Barrera, E.; Rodríguez, D.; Osorio, C. Rose volatile compounds allow the design of new control strategies for the western flower thrips (Frankliniella occidentalis). J. Pest Sci. 2021, 94, 129–142. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Hu, H.; Shang, X.X. Identification of volatiles in cucumber flowers and their effects on behavioral response of Frankliniella occidentalis. J. Hunan Agric. Univ. 2022, 48, 39–45. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J., Jr.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef] [PubMed]
- GebrezİHer, H.G. The role of herbivore-induced plant volatiles (HIPVs) as indirect plant defense mechanism in a diverse plant and herbivore species; a review. Int. J. Agric. Environ. Food Sci. 2018, 2, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects-Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Clarke, A.R. The “sequential cues hypothesis”: A conceptual model to explain host location and ranking by polyphagous herbivores. Insect Sci. 2019, 27, 1136–1147. [Google Scholar] [CrossRef]
- Teulon, D.A.J.; Davidson, M.M.; Perry, N.B.; Nielsen, M.C.; Castañé, C.; Bosch, D.; Riudavets, J.; van Tol, R.W.H.M.; de Kogel, W.J. Methyl isonicotinate–a non-pheromone thrips semiochemical–and its potential for pest management. Int. J. Trop. Insect Sci. 2017, 37, 50–56. [Google Scholar] [CrossRef]
- Binyameen, M.; Ejaz, M.; Shad, S.A.; Razaq, M.; Shah, R.M.; Schlyter, F. Eugenol, a plant volatile, synergizes the effect of the thrips attractant, ethyl isonicotinate. Environ. Entomol. 2018, 47, 1560–1564. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 10, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.X.; Gao, J.L.; Chen, Z.M. Research progress of behavioral orientation of phytophagous insects to their host plants. Chin. J. Trop. Agric. 2004, 24, 62–68. [Google Scholar]
- Wimalaratne, P.D.C.; Slessor, K.N.; Borden, J.H.; Chong, L.J.; Abate, T. Isolation and identification of house fly, Musca domestica L., repellents from pepper tree, Schinus molle L. J. Chem. Ecol. 1996, 22, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.R.; Zhou, P.Y.; Li, Z.Y.; Fu, R.J.; Yuan, S.Y.; Xiao, C. Analysis the volatile constituents of pine needles from health and debility Pinus yunnanensis. Nat. Prod. Res. Dev. 2010, 22, 1048–1052. [Google Scholar] [CrossRef]
- Tang, Y.C.; Zhou, C.L.; Chen, X.M.; Zheng, H. Foraging behavior of the dead leaf butterfly, Kallima inachus. J. Insect Sci. 2013, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.L.; Dong, J.F.; Ning, C.; Ding, P.P.; Sun, G.J.; Wang, C.Z. An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol. Biol. 2018, 28, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.J.; Chen, Y.X.; Chen, Y.; Chen, X.Q.; Lin, S.; Zhang, J.; Yang, G.; Wei, H. A mixture of ρ-anisaldehyde and ethyl nicotinate elicits positive antennal and behavioral responses in Frankliniella occidentalis. Entomol. Exp. Appl. 2022, 170, 603–611. [Google Scholar] [CrossRef]
- Altuzar, A.; Malo, E.A.; Gonzalez-Hernandez, H.; Rojas, J.C. Electrophysiological and behavioural responses of Scyphophorus acupunctatus (Col., Curculionidae) to Agave tequilana volatiles. J. Appl. Entomol. 2007, 131, 121–127. [Google Scholar] [CrossRef]
- Bedini, S.; Cosci, F.; Tani, C.; Pierattini, E.C.; Venturi, F.; Lucchi, A.; Ioriatti, C.; Ascrizzi, R.; Flamini, G.; Ferroni, G.; et al. Essential oils as post-harvest crop protectants against the fruit fly Drosophila suzukii: Bioactivity and organoleptic profile. Insects 2020, 11, 508. [Google Scholar] [CrossRef]
- Tasin, M.; Backman, A.C.; Coracini, M.; Casado, D.; Ioriatti, C.; Witzgall, P. Synergism and redundancy in a plant volatile blend attracting grapevine moth females. Phytochemistry 2007, 68, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Han, Y.; Tang, L.D.; Wu, J.H.; Shaukat, A. Behavioral responses of Megalurothrips usitatus (Thysanoptera:Thripoidae) to host plant and volatile compounds. J. Environ. Entomol. 2021, 43, 1566–1580. [Google Scholar] [CrossRef] [Green Version]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Adler, C. Behavioral responses of Callosobruchus maculatus to volatile organic compounds found in the headspace of dried green pea seeds. J. Pest Sci. 2015, 89, 107–116. [Google Scholar] [CrossRef]
- Landolt, P.J.; Todd, A.; Zack, R.S. Field response of alfalfa looper and cabbage looper moths (Lepidoptera: Noctuidae, Plusiinae) to single and binary blends of floral odorants. Environ. Entomol. 2006, 35, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell Mol. Life Sci. 2006, 63, 1658–1676. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C.; Zaremska, V.; Zhu, J.; Knoll, W.; Pelosi, P. Ligand-binding assays with OBPs and CSPs. Methods Enzymol. 2020, 642, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Nardiello, M.; Farina, D.; Scala, A.; Cammack, J.; Tomberlin, J.; Vogel, H.; Salvia, R.; Persaud, K.; Falabella, P.J.I. Hermetia illucens(L.) (Diptera: Stratiomyidae) odorant binding proteins and their interactions with selected volatile organic compounds: An in silico approach. Insects 2021, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Nardiello, M.; Scieuzo, C.; Salvia, R.; Farina, D.; Franco, A.; Cammack, J.A.; Tomberlin, J.K.; Falabella, P.; Persaud, K. Odorant binding proteins from Hermetia illucens: Potential sensing elements for detecting volatile aldehydes involved in early stages of organic decomposition. Nanotechnology 2022, 33, 205501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, M.; Eleftherianos, I.; Mohamed, A.; Cao, Y.; Song, B.; Zang, L.; Jia, C.; Bian, J.; Nemat Keyhani, O.; et al. An odorant binding protein is involved in counteracting detection-avoidance and Toll-pathway innate immunity. J. Adv. Res. 2022, 48, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, R.Z.; Wang, X.H.; Liu, X.W.; Chen, F.J. Elevated CO2 altered rice VOCs aggravate population occurrence of brown planthoppers by improving host selection ability. Biology 2022, 11, 882. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Butt, T.M. Preferences of the peripheral olfactory system of western flower thrips, Frankliniella occidentalis towards stereoisomers of common plant volatiles. Chemoecology 2014, 25, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
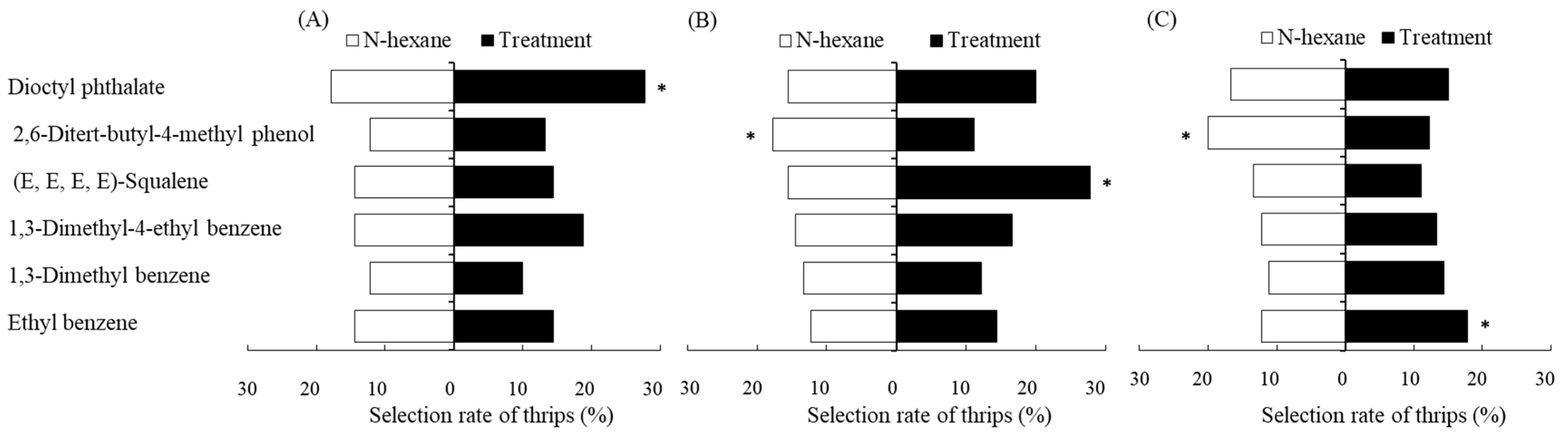
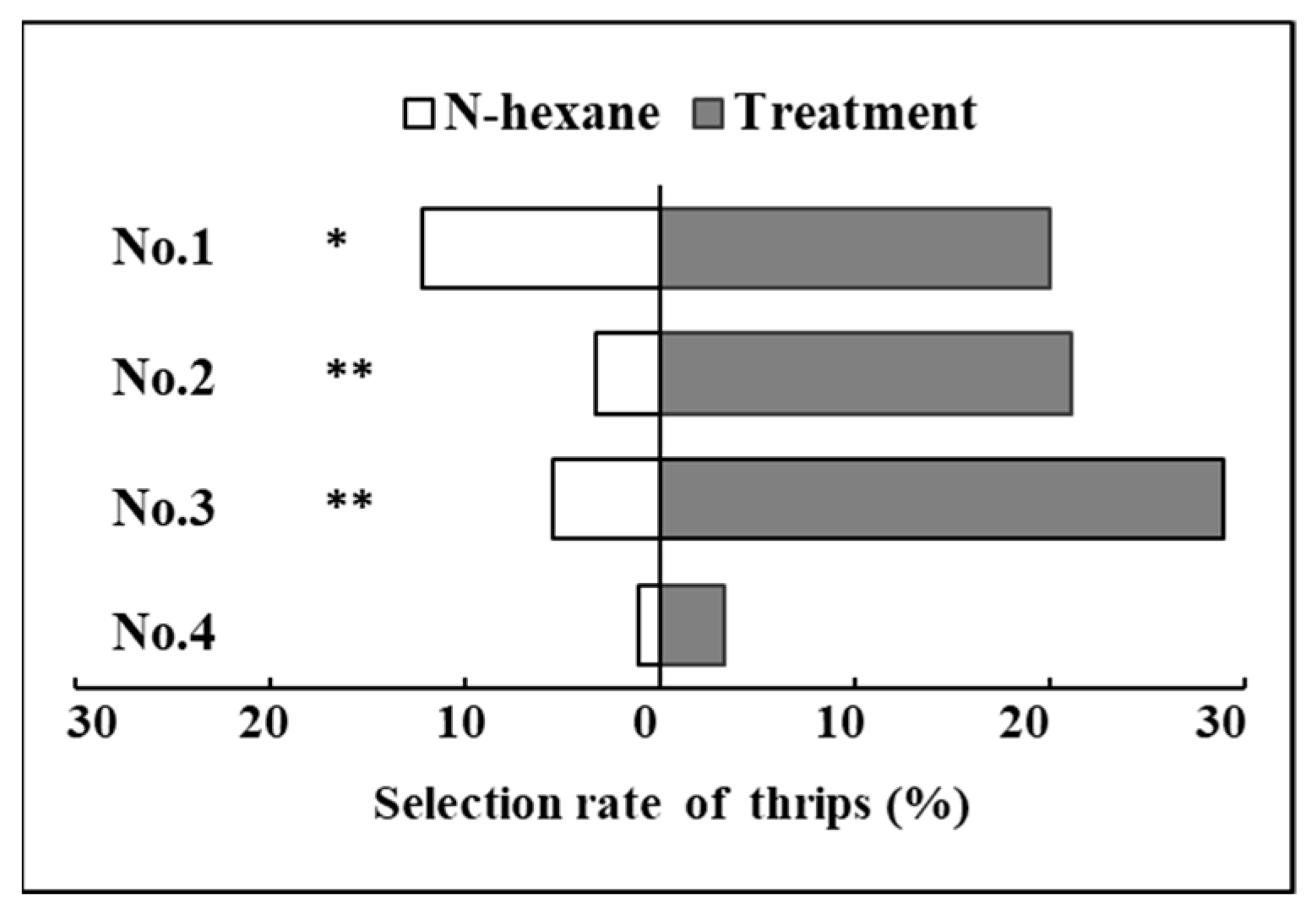
| Treatments | 12.7 μL/mL (E, E, E, E)-Squalene | 3.2 μL/mL Dioctyl Phthalate | 82.2 μL/mL Ethyl Benzene |
|---|---|---|---|
| No. 1 | + | + | − |
| No. 2 | + | − | + |
| No. 3 | − | + | + |
| No. 4 | + | + | + |
| Volatile Types | VOCs | Structure Formula | CAS Number | Concentration (%) | ||
|---|---|---|---|---|---|---|
| Low | Medium | High | ||||
| Aromatic hydrocarbons | Ethyl benzene |  | 100-41-4 | 5.28 | 6.75 | 8.22 |
| 1,3-Dimethyl benzene | 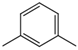 | 108-38-3 | 36.57 | 40.71 | 44.85 | |
| 1,3-Dimethyl-4-ethyl benzene | 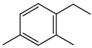 | 874-41-9 | 1.32 | 1.89 | 2.46 | |
| Olefins | (E, E, E, E)-Squalene | 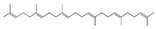 | 111-02-4 | 0.26 | 1.27 | 2.27 |
| Phenols | 2,6-Ditert-butyl-4-methyl phenol |  | 128-37-0 | 0 | 1.07 | 2.14 |
| Esters | Dioctyl phthalate | 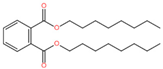 | 117-81-7 | 0.32 | 0.52 | 0.72 |
| Primers | Sequence | Description | |
|---|---|---|---|
| LOC113205297 | Forward | AGCTAACCCCCTCACTGCTA | Odorant-binding protein genes |
| Reverse | AGGATGCACTTGACGTAGCC | ||
| LOC113209726 | Forward | CATGGCTGAGTACCAGACGG | |
| Reverse | GCCTGTAGCTCCTCCCAAAG | ||
| LOC113212498 | Forward | CACTCCCATCCCGACAAAGG | |
| Reverse | AACCTCTGTCGCCACGATTT | ||
| LOC113203140OBP2 | Forward | AGTCTGATTCCGAGCTCCCT | |
| Reverse | GTCTCTGTCTGCAGCGAAGT | ||
| LOC113214975 | Forward | GGTTGACGCCGATATGTTGC | |
| Reverse | CTGCATCCCCTTAGACGACC | ||
| LOC113205302 | Forward | GCTACTGTCGCCAGTGAACA | |
| Reverse | TTGGAAGCCTCTCTCTTTCGC | ||
| LOC113202849 | Forward | GCGTCGAAATAGAGCCCAGT | |
| Reverse | CACCTGTCGCTTATGCCTGA | ||
| LOC113205269 | Forward | ATGAACCACGATGCCTGTGT | |
| Reverse | CGTCATCCGTCATAGTGCCA | ||
| LOC113203279 | Forward | ATCAAAGAGTGCGCAGACCA | |
| Reverse | CTTCCGAATGCTTCAGCACG | ||
| β-actin | Forward | ACGACTTACAACTCCATCA | Housekeeping genes |
| Reverse | AGTGCCTCCAGACAAAA | ||
| rpl32 | Forward | CTGGCGTAAACCTAAGGGTATTGA | |
| Reverse | AAGCACCTTCTTGAACCCAGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, X.; Jin, Y.; Duan, R.; Gu, Y.; Liu, X.; Qian, L.; Chen, F. Selection Behavior and OBP-Transcription Response of Western Flower Thrips, Frankliniella occidentalis, to Six Plant VOCs from Kidney Beans. Int. J. Mol. Sci. 2023, 24, 12789. https://doi.org/10.3390/ijms241612789
Wang Y, Zhu X, Jin Y, Duan R, Gu Y, Liu X, Qian L, Chen F. Selection Behavior and OBP-Transcription Response of Western Flower Thrips, Frankliniella occidentalis, to Six Plant VOCs from Kidney Beans. International Journal of Molecular Sciences. 2023; 24(16):12789. https://doi.org/10.3390/ijms241612789
Chicago/Turabian StyleWang, Yanhui, Xiaobing Zhu, Yixuan Jin, Ruichuan Duan, Yunkai Gu, Xiaowei Liu, Lei Qian, and Fajun Chen. 2023. "Selection Behavior and OBP-Transcription Response of Western Flower Thrips, Frankliniella occidentalis, to Six Plant VOCs from Kidney Beans" International Journal of Molecular Sciences 24, no. 16: 12789. https://doi.org/10.3390/ijms241612789
APA StyleWang, Y., Zhu, X., Jin, Y., Duan, R., Gu, Y., Liu, X., Qian, L., & Chen, F. (2023). Selection Behavior and OBP-Transcription Response of Western Flower Thrips, Frankliniella occidentalis, to Six Plant VOCs from Kidney Beans. International Journal of Molecular Sciences, 24(16), 12789. https://doi.org/10.3390/ijms241612789







