Axonal Regrowth of Olfactory Sensory Neurons In Vitro
Abstract
:1. Introduction
2. Results
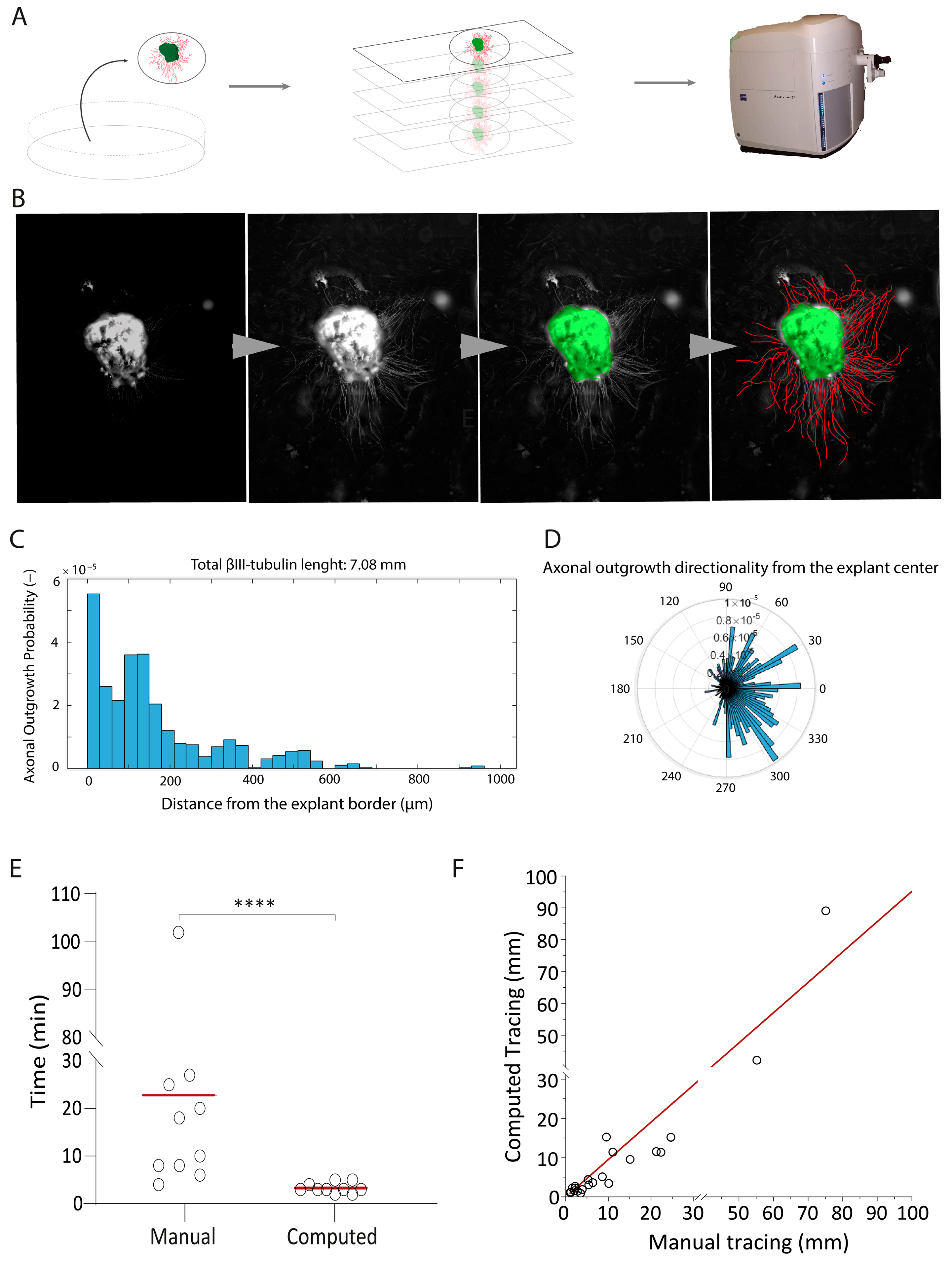
2.1. Development of a High-Throughput Automated Quantification Tool to Measure Axonal Length
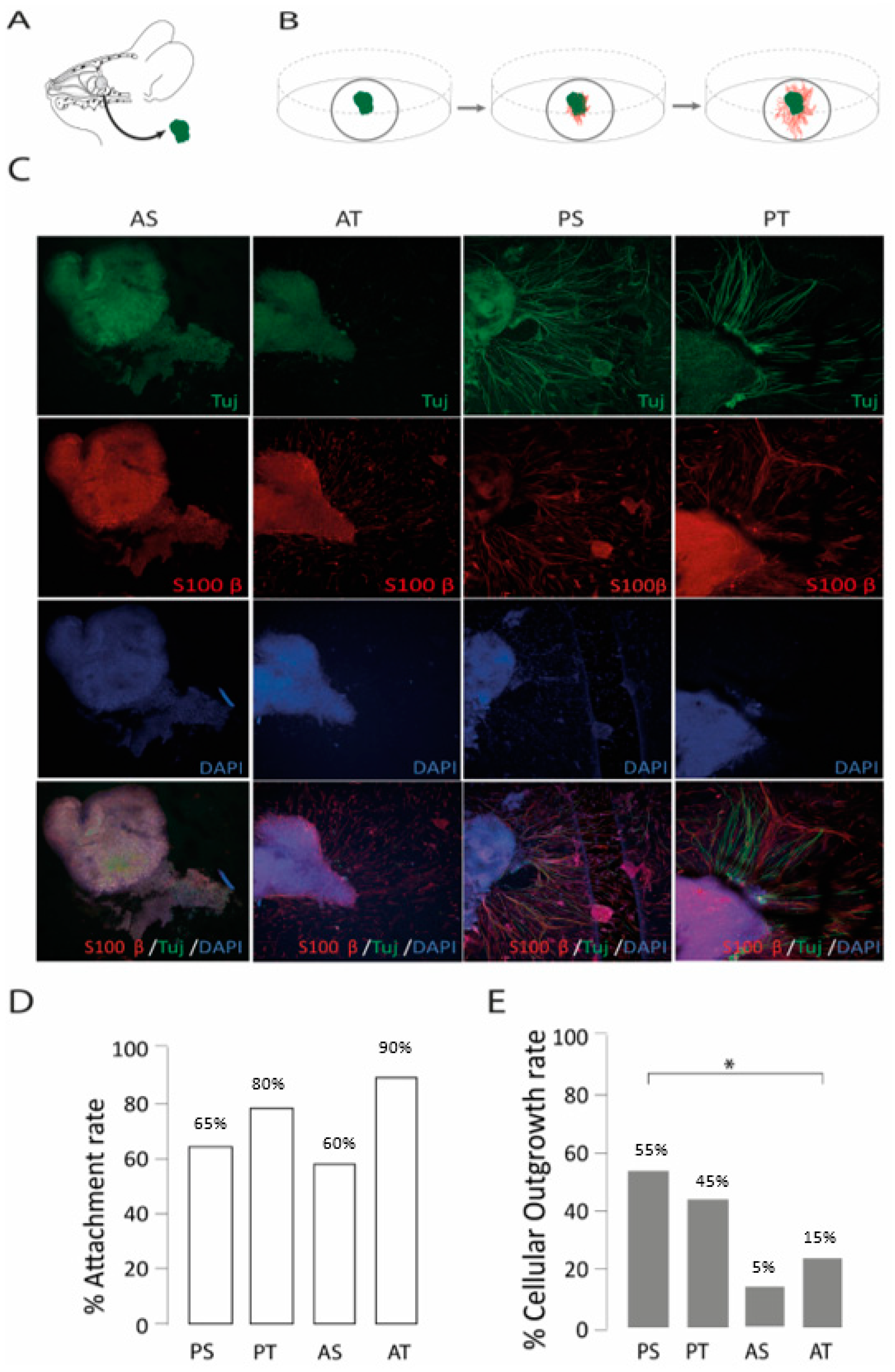
2.2. The Posterior Septum and Posterior Turbinate Regions Yielded the Highest Outgrowth Rate in an Organotypic Model of Olfactory Regeneration
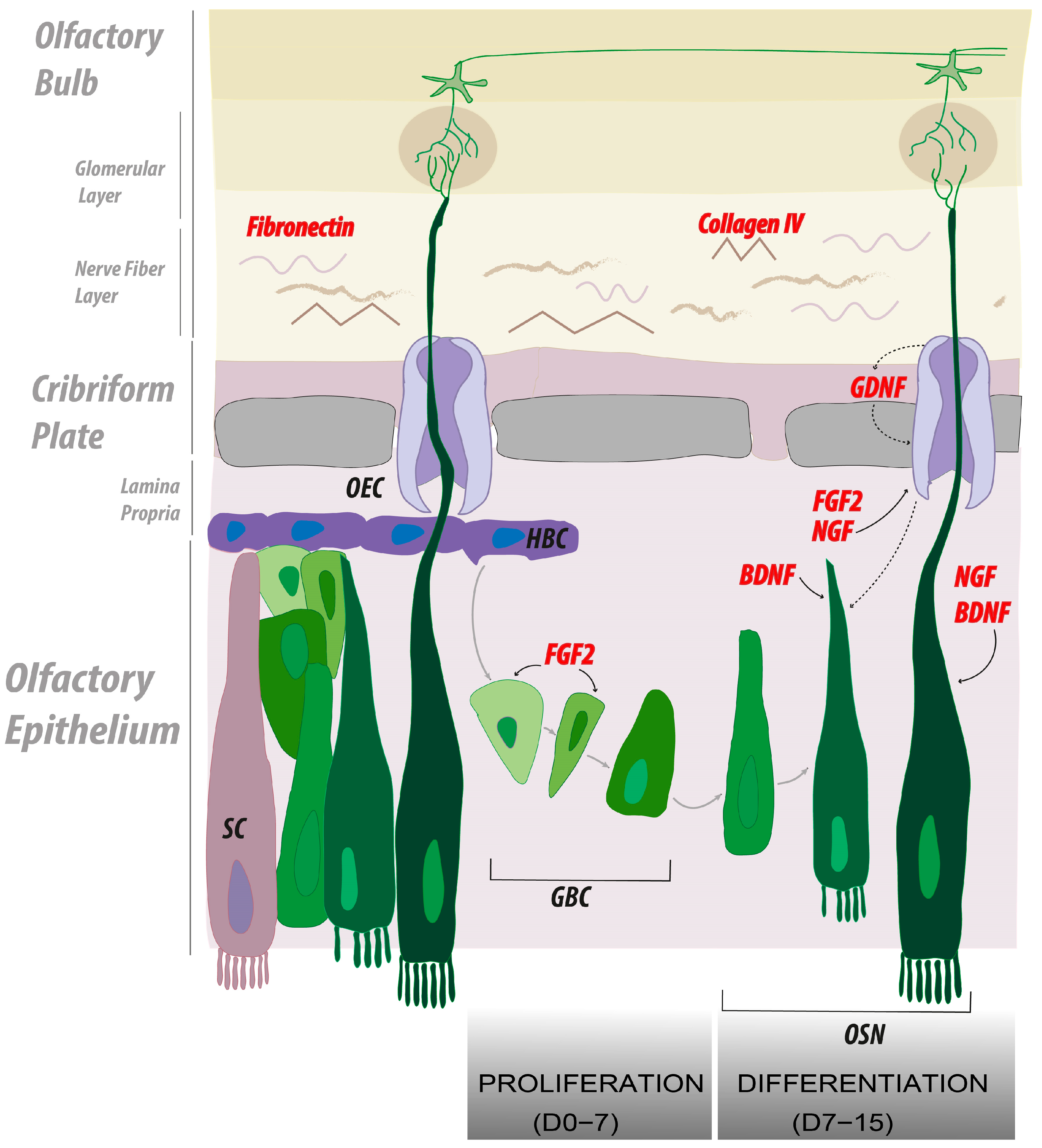
2.3. Collagen IV and Fibronectin Coatings Enhance Axonal Elongation
2.4. FGF2, NGF, and GDNF Enhance Neuroproliferation in Olfactory Explants
2.5. FGF2 Alone or in Combination with NGF and BDNF Enhances Axonal Elongation
3. Discussion
4. Materials and Methods
4.1. Culture Media
4.2. Dissection Technique to Harvest Murine Olfactory Epithelium (OE)
4.3. Coating
4.4. Growth Factor Treatments
4.5. Growth Factors Administration Paradigm
4.6. Immunostaining
4.7. Imaging Process
4.8. Data Collection and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landis, B.N.; Hummel, T. New Evidence for High Occurrence of Olfactory Dysfunctions within the Population. Am. J. Med. 2006, 119, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Scherer, P.W.; Hajiloo, S.A.; Dalton, P. Effect of Anatomy on Human Nasal Air Flow and Odorant Transport Patterns: Implications for Olfaction. Chem. Senses 2004, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Nordin, S. Olfactory disorders and their consequences for quality of life. Acta Oto Laryngol. 2005, 125, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Malaspina, D. Hidden consequences of olfactory dysfunction: A patient report series. BMC Ear Nose Throat Disord. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zamparo, I.; Francia, S.; Franchi, S.A.; Redolfi, N.; Costanzi, E.; Kerstens, A.; Fukutani, Y.; Battistutta, R.; de Laureto, P.P.; Munck, S.; et al. Axonal Odorant Receptors Mediate Axon Targeting. Cell Rep. 2019, 29, 4334–4348.e7. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial Receptor Codes for Odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Mori, K.; Nagao, H.; Yoshihara, Y. The Olfactory Bulb: Coding and Processing of Odor Molecule Information. Science 1999, 286, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Moriizumi, T.; Horikawa, I.; Uramoto, N.; Ishimaru, T.; Nishimura, T.; Furukawa, M. Role of nerve growth factor in the olfactory system. Microsc. Res. Tech. 2002, 58, 197–203. [Google Scholar] [CrossRef]
- Uranagase, A.; Katsunuma, S.; Doi, K.; Nibu, K.-I. BDNF expression in olfactory bulb and epithelium during regeneration of olfactory epithelium. Neurosci. Lett. 2012, 516, 45–49. [Google Scholar] [CrossRef]
- Frontera, J.L.; Cervino, A.S.; Jungblut, L.D.; Paz, D.A. Brain-derived neurotrophic factor (BDNF) expression in normal and regenerating olfactory epithelium of Xenopus laevis. Ann. Anat. Anat. Anz. 2015, 198, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Goldstein, B.J. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tanos, T.; Saibene, A.M.; Pipolo, C.; Battaglia, P.; Felisati, G.; Rubio, A. Isolation of putative stem cells present in human adult olfactory mucosa. PLoS ONE 2017, 12, e0181151. [Google Scholar] [CrossRef]
- Liang, F. Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes 2020, 11, 493. [Google Scholar] [CrossRef]
- Liberia, T.; Martin-Lopez, E.; Meller, S.J.; Greer, C.A. Sequential Maturation of Olfactory Sensory Neurons in the Mature Olfactory Epithelium. eNeuro 2019, 6, 26. [Google Scholar] [CrossRef]
- Wetzig, A.; Mackay-Sim, A.; Murrell, W. Characterization of Olfactory Stem Cells. Cell Transplant. 2011, 20, 1673–1691. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, E.; Moreno-Flores, M.T.; Avila, J.; Wandosell, F.; Minichiello, L.; Diaz-Nido, J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem. Int. 2007, 50, 491–498. [Google Scholar] [CrossRef]
- Woodhall, E.; West, A.K.; Chuah, M.I. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Mol. Brain Res. 2001, 88, 203–213. [Google Scholar] [CrossRef]
- Meyers, E.A.; Kessler, J.A. TGF-beta Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb. Perspect. Biol. 2017, 9, a022244. [Google Scholar] [CrossRef]
- Lakard, S.; Lesniewska, E.; Michel, G.; Lakard, B.; Morrand-Villeneuve, N.; Versaux-Botteri, C. In vitro induction of differentiation by retinoic acid in an immortalized olfactory neuronal cell line. Acta Histochem. 2007, 109, 111–121. [Google Scholar] [CrossRef]
- Tisay, K.T.; Key, B. The Extracellular Matrix Modulates Olfactory Neurite Outgrowth on Ensheathing Cells. J. Neurosci. 1999, 19, 9890–9899. [Google Scholar] [CrossRef] [PubMed]
- Doucette, R. Immunohistochemical localization of laminin, fibronectin and collagen type IV in the nerve fiber layer of the olfactory bulb. Int. J. Dev. Neurosci. 1996, 14, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Windus, L.C.E.; Chehrehasa, F.; Lineburg, K.E.; Claxton, C.; Mackay-Sim, A.; Key, B.; John, J.A.S. Stimulation of olfactory ensheathing cell motility enhances olfactory axon growth. Cell. Mol. Life Sci. 2011, 68, 3233–3247. [Google Scholar] [CrossRef] [PubMed]
- Temmel, A.F.P.; Quint, C.; Schickinger-Fischer, B.; Klimek, L.; Stoller, E.; Hummel, T. Characteristics of Olfactory Disorders in Relation to Major Causes of Olfactory Loss. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 635–641. [Google Scholar] [CrossRef]
- Moran, D.T.; Jafek, B.W.; Rowley, J.C.; Eller, P.M. Electron Microscopy of Olfactory Epithelia in Two Patients With Anosmia. JAMA Otolaryngol. Neck Surg. 1985, 111, 122–126. [Google Scholar] [CrossRef]
- Jafek, B.W.; Eller, P.M.; Esses, B.A.; Moran, D.T. Post-traumatic anosmia. Ultrastructural correlates. Arch. Neurol. 1989, 46, 300–304. [Google Scholar] [CrossRef]
- Kobayashi, M.; Costanzo, R.M. Olfactory Nerve Recovery Following Mild and Severe Injury and the Efficacy of Dexamethasone Treatment. Chem. Senses 2009, 34, 573–580. [Google Scholar] [CrossRef]
- Li, Q.; Siri, T.; Bressan, C.; de Koninck, Y.; Saghatelyan, A. Developmental Potential and Plasticity of Olfactory Epithelium Stem Cells Revealed by Heterotopic Grafting in the Adult Brain. Stem Cell Rep. 2020, 14, 692–702. [Google Scholar] [CrossRef]
- Ren, W.; Wang, L.; Zhang, X.; Feng, X.; Zhuang, L.; Jiang, N.; Xu, R.; Li, X.; Wang, P.; Sun, X.; et al. Expansion of murine and human olfactory epithelium/mucosa colonies and generation of mature olfactory sensory neurons under chemically defined conditions. Theranostics 2021, 11, 684–699. [Google Scholar] [CrossRef]
- Ayala-Grosso, C.; Pieruzzini, R.; Vargas-Saturno, L.; Cardier, J.E. Human olfactory mesenchymal stromal cells co-expressing horizontal basal and ensheathing cell proteins in culture. Biomedica 2020, 40, 72–88. [Google Scholar] [CrossRef]
- Minovi, A.; Aguado, A.; Brunert, D.; Kurtenbach, S.; Dazert, S.; Hatt, H.; Conrad, H. Isolation, culture optimization and functional characterization of stem cell neurospheres from mouse neonatal olfactory bulb and epithelium. European archives of oto-rhino-laryngology: Official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology-Head and Neck Surgery. Eur. Arch. Oto Rhino Laryngol. 2017, 274, 3071–3085. [Google Scholar]
- Krolewski, R.C.; Jang, W.; Schwob, J.E. The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo. Exp. Neurol. 2011, 229, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E.; Jacob, M.; Sarria, J.C.; Steiner, P.; Hirling, H.; Unser, E.M. Design and Validation of a Tool for Neurite Tracing and Analysis in Fluorescence Microscopy Images. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Li, Z.; Canete, P.; Strobl, H.; Dulin, J.; Kadoya, K.; Gibbs, D.; Poplawski, G.H.D. AxonTracer: A novel ImageJ plugin for automated quantification of axon regeneration in spinal cord tissue. BMC Neurosci. 2018, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Sophia, L.B.; Oliveira, M.M.P.; Cheffer, A.; Lameu, C.; Schwindt, T.T.; Ulrich, H. Functions of Neurotrophins and Growth Factorsin Neurogenesis and Brain Repair. Spec. Issue Cytom. Stem Cell Res. 2013, 83, 76–89. [Google Scholar]
- MacDonald, K.P.A.; Murrell, W.G.; Bartlett, P.F.; Bushell, G.R.; Mackay-Sim, A. FGF2 promotes neuronal differentiation in explant cultures of adult and embryonic mouse olfactory epithelium. J. Neurosci. Res. 1996, 44, 27–39. [Google Scholar] [CrossRef]
- Newman, R.; Winans, S.S. An experimental study of the ventral striatum of the golden hamster. II. Neuronal connections of the olfactory tubercle. J. Comp. Neurol. 1980, 191, 193–212. [Google Scholar] [CrossRef]
- Azin, M.; Mirnajafi-Zadeh, J.; Javan, M. Fibroblast Growth Factor-2 Enhanced the Recruitment of Progenitor Cells and Myelin Repair in Experimental Demyelination of Rat Hippocampal Formations. Cell J. 2015, 17, 540–546. [Google Scholar] [CrossRef]
- Bakardjiev, A. Biosynthesis of carnosine in primary cultures of rat olfactory bulb. Neurosci. Lett. 1997, 227, 115–118. [Google Scholar] [CrossRef]
- Williams, S.K.; Franklin, R.J.; Barnett, S.C. Response of olfactory ensheathing cells to the degeneration and regeneration of the peripheral olfactory system and the involvement of the neuregulins. J. Comp. Neurol. 2004, 470, 50–62. [Google Scholar] [CrossRef]
- Miwa, I.H.T. TrkA Expression in Mouse Olfactory Tract following Axotomy of Olfactory Nerves. Acta Oto Laryngol. 1998, 118, 79–82. [Google Scholar] [CrossRef]
- Fornaro, M.; Giovannelli, A.; Foggetti, A.; Muratori, L.; Geuna, S.; Novajra, G.; Perroteau, I. Role of neurotrophic factors in enhancing linear axonal growth of ganglionic sensory neurons in vitro. Neural Regen. Res. 2020, 15, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.-G.; Han, L.-Y.; Rawson, N.E.; Mirza, N.; Borgmann-Winter, K.; Lenox, R.H.; Arnold, S.E. In vivo and in vitro neurogenesis in human olfactory epithelium. J. Comp. Neurol. 2005, 483, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Féron, F.; Perry, C.; McGrath, J.J.; Mackay-Sim, A. New Techniques for Biopsy and Culture of Human Olfactory Epithelial Neurons. Arch. Otolaryngol. Neck Surg. 1998, 124, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.D.; Devéze, A.; Nivet, E.; Gepner, B.; Roman, F.S.; Féron, F. Isolating nasal olfactory stem cells from rodents or humans. J. Vis. Exp. JoVE 2011, 54, e2762. [Google Scholar]
- Newman, M.; Féron, F.; Mackay-Sim, A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience 2000, 99, 343–350. [Google Scholar] [CrossRef]
- Beasley, T.M.; Schumacker, R.E. Multiple Regression Approach to Analyzing Contingency Tables: Post Hoc and Planned Comparison Procedures. J. Exp. Educ. 1995, 64, 79–93. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Transformations, means, and confidence intervals. BMJ 1996, 312, 1079. [Google Scholar] [CrossRef]
- Rousset, F.; Schmidbauer, D.; Fink, S.; Adel, Y.; Obexer, B.; Müller, M.; Glueckert, R.; Löwenheim, H.; Senn, P. Phoenix auditory neurons as 3R cell model for high throughput screening of neurogenic compounds. Hear. Res. 2021, 414, 108391. [Google Scholar] [CrossRef]

| Name | Reference Number | Supplier | Concentration |
|---|---|---|---|
| Fibroblast growth factor 2 (FGF2) | CYT-218 | Prospec | 40 ng/mL [36] |
| Retinoic acid (RA) | R2625-50MG | Sigma-Aldrich | 10 ng/mL [20] |
| Nerve growth factor (NGF) | N6009-4X25UG | Sigma-Aldrich | 50 ng/mL |
| Brain-derived neurotrophic factor (BDNF) | B3795-10UG | Sigma-Aldrich | 50 ng/mL [17] |
| Glial cell-derived neurotrophic factor (GDNF) | 450-10 | Perotech (Chapel Hill, NC, USA) | 20 ng/mL [23] |
| Transforming growth factor β(TGFβ) | T2815-2UG | Sigma-Aldrich | 10 ng/mL [46] |
| Antibody Name | Reference Number | Supplier | Working Dilution |
|---|---|---|---|
| S-100 beta | S2532-100UL | Sigma-Aldrich (Merck) | 1/800 |
| Tubuline beta 3 (TUJ1) | 802001 | Biolegend (San Diego, CA, USA) | 1/1000 |
| Antibody Name | Reference Number | Supplier | Working Dilution |
|---|---|---|---|
| Alexa fluor 488 Donkey | A32766 | Life Technologies (Carlsbad, CA, USA) | 1/1000 |
| Alexa fluor 555 Donkey | A31572 | Life Technologies | 1/1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipione, R.; Liaudet, N.; Rousset, F.; Landis, B.N.; Hsieh, J.W.; Senn, P. Axonal Regrowth of Olfactory Sensory Neurons In Vitro. Int. J. Mol. Sci. 2023, 24, 12863. https://doi.org/10.3390/ijms241612863
Sipione R, Liaudet N, Rousset F, Landis BN, Hsieh JW, Senn P. Axonal Regrowth of Olfactory Sensory Neurons In Vitro. International Journal of Molecular Sciences. 2023; 24(16):12863. https://doi.org/10.3390/ijms241612863
Chicago/Turabian StyleSipione, Rebecca, Nicolas Liaudet, Francis Rousset, Basile N. Landis, Julien Wen Hsieh, and Pascal Senn. 2023. "Axonal Regrowth of Olfactory Sensory Neurons In Vitro" International Journal of Molecular Sciences 24, no. 16: 12863. https://doi.org/10.3390/ijms241612863
APA StyleSipione, R., Liaudet, N., Rousset, F., Landis, B. N., Hsieh, J. W., & Senn, P. (2023). Axonal Regrowth of Olfactory Sensory Neurons In Vitro. International Journal of Molecular Sciences, 24(16), 12863. https://doi.org/10.3390/ijms241612863






