Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions
Abstract
1. Introduction
2. Genomics of Cryptosporidium spp.
2.1. Exploring Cryptosporidium Diversity and Evolutionary History through Whole Genome Sequencing
2.1.1. Early Studies on the Cryptosporidium Genome Preceding the Genomic Era
2.1.2. The Cryptosporidium Genomic Era: Opening Paths for Future Analyses
2.1.3. Draft Genome Sequencing to Enhance Our Understanding of Cryptosporidium Parasites
2.1.4. Utilizing the Latest Genomic Advancements for Cryptosporidium Research
2.2. Unraveling Cryptosporidium’s Secrets through Comparative Genomics
2.3. Overcoming Challenges in Isolating Cryptosporidium DNA from Clinical Samples
3. Proteome
3.1. MALDI-MS/MS-Based Proteomics in Cryptosporidium Studies
3.1.1. MALDI-MS/MS-Based Proteomics Preceding the Genomic Era of Cryptosporidium
3.1.2. Application of MALDI-MS/MS-Based Proteomics for Clinical Samples
3.2. LC-MS/MS-Based Proteomics in Cryptosporidium Studies
3.2.1. LC-MS/MS-Based Proteomics: Unveiling Insights into the Biology of Cryptosporidium
3.2.2. LC-MS/MS-Based Proteomics: Characterizing Specific Cryptosporidium Fractions
3.2.3. LC-MS/MS-Based Proteomics: Host–Parasite Interactions
3.2.4. LC-MS/MS-Based Proteomics: Drug Targets and Diagnosis
4. Transcriptome
4.1. Hybridization-Based Characterization of the Cryptosporidium Transcriptome
4.2. Reverse-Transcription PCR (RT-PCR)-Based Transcriptomics
4.3. Sequenced-Based Characterization Cryptosporidium spp.
4.3.1. Host–Parasite Interaction
4.3.2. Drug and Therapeutic Targets
5. Discussion
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kváč, M.; McEvoy, J.; Stenger, B.; Clark, M. Cryptosporidiosis in Other Vertebrates. In Cryptosporidium: Parasite and Disease; Springer: Vienna, Austria, 2014; pp. 237–323. [Google Scholar]
- Pumipuntu, N.; Piratae, S. Cryptosporidiosis: A zoonotic disease concern. Vet. World 2018, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia–a 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.S.; Peralta, R.H.S.; Peralta, J.M. New insights into the detection and molecular characterization of Cryptosporidium with emphasis in Brazilian studies: A review. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e28. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R. Cryptosporidiosis and the challenges of chemotherapy. Drug Resist. Updat. 2002, 5, 47–57. [Google Scholar] [CrossRef]
- Strong, W.B.; Gut, J.; Nelson, R.G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15-and 45-kilodalton zoite surface antigen products. Infect. Immun. 2000, 68, 4117–4134. [Google Scholar] [CrossRef]
- Ifeonu, O.O.; Chibucos, M.C.; Orvis, J.; Su, Q.; Elwin, K.; Guo, F.; Zhang, H.; Xiao, L.; Sun, M.; Chalmers, R.M. Annotated draft genome sequences of three species of Cryptosporidium: Cryptosporidium meleagridis isolate UKMEL1, C. baileyi isolate TAMU-09Q1 and C. hominis isolates TU502_2012 and UKH1. FEMS Pathog. Dis. 2016, 74, ftw080. [Google Scholar] [CrossRef]
- Nash, J.H.; Robertson, J.; Elwin, K.; Chalmers, R.A.; Kropinski, A.M.; Guy, R.A. Draft genome assembly of a potentially zoonotic Cryptosporidium parvum isolate, UKP1. Microbiol. Resour. Announc. 2018, 7, e01291-18. [Google Scholar] [CrossRef]
- Xu, Z.; Li, N.; Guo, Y.; Feng, Y.; Xiao, L. Comparative genomic analysis of three intestinal species reveals reductions in secreted pathogenesis determinants in bovine-specific and non-pathogenic Cryptosporidium species. Microb. Genom. 2020, 6, e000379. [Google Scholar] [CrossRef]
- Guo, Y.; Li, N.; Lysén, C.; Frace, M.; Tang, K.; Sammons, S.; Roellig, D.M.; Feng, Y.; Xiao, L. Isolation and enrichment of Cryptosporidium DNA and verification of DNA purity for whole-genome sequencing. J. Clin. Microbiol. 2015, 53, 641–647. [Google Scholar] [CrossRef]
- Abrahamsen, M.S.; Templeton, T.J.; Enomoto, S.; Abrahante, J.E.; Zhu, G.; Lancto, C.A.; Deng, M.; Liu, C.; Widmer, G.; Tzipori, S. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 2004, 304, 441–445. [Google Scholar] [CrossRef]
- Widmer, G.; Lee, Y.; Hunt, P.; Martinelli, A.; Tolkoff, M.; Bodi, K. Comparative genome analysis of two Cryptosporidium parvum isolates with different host range. Infect. Genet. Evol. 2012, 12, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Vigdorovich, V.; Kapur, V.; Abrahamsen, M.S. A random survey of the Cryptosporidium parvum genome. Infect. Immun. 1999, 67, 3960–3969. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, K.; Rowe, L.A.; Li, N.; Roellig, D.M.; Knipe, K.; Frace, M.; Yang, C.; Feng, Y.; Xiao, L. Comparative genomic analysis reveals occurrence of genetic recombination in virulent Cryptosporidium hominis subtypes and telomeric gene duplications in Cryptosporidium parvum. BMC Genom. 2015, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, N.; Roellig, D.M.; Kelley, A.; Liu, G.; Amer, S.; Tang, K.; Zhang, L.; Xiao, L. Comparative genomic analysis of the IId subtype family of Cryptosporidium parvum. Int. J. Parasitol. 2017, 47, 281–290. [Google Scholar] [CrossRef]
- Arias-Agudelo, L.M.; Garcia-Montoya, G.; Cabarcas, F.; Galvan-Diaz, A.L.; Alzate, J.F. Comparative genomic analysis of the principal Cryptosporidium species that infect humans. PeerJ 2020, 8, e10478. [Google Scholar] [CrossRef]
- Andersson, S.; Sikora, P.; Karlberg, M.L.; Winiecka-Krusnell, J.; Alm, E.; Beser, J.; Arrighi, R.B. It’s a dirty job—A robust method for the purification and de novo genome assembly of Cryptosporidium from clinical material. J. Microbiol. Methods 2015, 113, 10–12. [Google Scholar] [CrossRef]
- Hadfield, S.J.; Pachebat, J.A.; Swain, M.T.; Robinson, G.; Cameron, S.J.; Alexander, J.; Hegarty, M.J.; Elwin, K.; Chalmers, R.M. Generation of whole genome sequences of new Cryptosporidium hominis and Cryptosporidium parvum isolates directly from stool samples. BMC Genom. 2015, 16, 650. [Google Scholar] [CrossRef]
- Xu, P.; Widmer, G.; Wang, Y.; Ozaki, L.S.; Alves, J.M.; Serrano, M.G.; Puiu, D.; Manque, P.; Akiyoshi, D.; Mackey, A.J. The genome of Cryptosporidium hominis. Nature 2004, 431, 1107–1112. [Google Scholar] [CrossRef]
- Widmer, G.; Sullivan, S. Genomics and population biology of Cryptosporidium species. Parasite Immunol. 2012, 34, 61–71. [Google Scholar] [CrossRef]
- Troell, K.; Hallström, B.; Divne, A.-M.; Alsmark, C.; Arrighi, R.; Huss, M.; Beser, J.; Bertilsson, S. Cryptosporidium as a testbed for single cell genome characterization of unicellular eukaryotes. BMC Genom. 2016, 17, 471. [Google Scholar] [CrossRef]
- Gilchrist, C.A.; Cotton, J.A.; Burkey, C.; Arju, T.; Gilmartin, A.; Lin, Y.; Ahmed, E.; Steiner, K.; Alam, M.; Ahmed, S. Genetic diversity of Cryptosporidium hominis in a Bangladeshi community as revealed by whole-genome sequencing. J. Infect. Dis. 2018, 218, 259–264. [Google Scholar] [CrossRef]
- Guo, Y.; Cebelinski, E.; Matusevich, C.; Alderisio, K.A.; Lebbad, M.; McEvoy, J.; Roellig, D.M.; Yang, C.; Feng, Y.; Xiao, L. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J. Clin. Microbiol. 2015, 53, 1648–1654. [Google Scholar] [CrossRef]
- Magnuson, M.L.; Owens, J.H.; Kelty, C.A. Characterization of Cryptosporidium parvum by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl. Environ. Microbiol. 2000, 66, 4720–4724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glassmeyer, S.T.; Ware, M.W.; SCHAEFER III, F.W.; Shoemaker, J.A.; Kryak, D.D. An improved method for the analysis of Cryptosporidium parvum oocysts by matrix-assisted laser desorption/ionization time of flight mass spectrometry. J. Eukaryot. Microbiol. 2007, 54, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Karpe, A.V.; Hutton, M.L.; Mileto, S.J.; James, M.L.; Evans, C.; Shah, R.M.; Ghodke, A.B.; Hillyer, K.E.; Metcalfe, S.S.; Liu, J.-W. Cryptosporidiosis modulates the gut microbiome and metabolism in a murine infection model. Metabolites 2021, 11, 380. [Google Scholar] [CrossRef]
- Snelling, W.J.; Lin, Q.; Moore, J.E.; Millar, B.C.; Tosini, F.; Pozio, E.; Dooley, J.S.; Lowery, C.J. Proteomics Analysis and Protein Expression during Sporozoite Excystation of Cryptosporidium parvum (Coccidia, Apicomplexa)* S. Mol. Cell. Proteom. 2007, 6, 346–355. [Google Scholar] [CrossRef]
- Sanderson, S.J.; Xia, D.; Prieto, H.; Yates, J.; Heiges, M.; Kissinger, J.C.; Bromley, E.; Lal, K.; Sinden, R.E.; Tomley, F. Determining the protein repertoire of Cryptosporidium parvum sporozoites. Proteomics 2008, 8, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, A.Z. Sporozoite proteome analysis of Cryptosporidium parvum by one-dimensional SDS-PAGE and liquid chromatography tandem mass spectrometry. J. Vet. Sci. 2013, 14, 107–114. [Google Scholar] [CrossRef]
- Chatterjee, A.; Banerjee, S.; Steffen, M.; O’Connor, R.M.; Ward, H.D.; Robbins, P.W.; Samuelson, J. Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot. Cell 2010, 9, 84–96. [Google Scholar] [CrossRef]
- Huang, Y.; Rongsheng, M.; Wei, C.; Peng, Z.; Kai, S.; Xiaojiao, Y.; Xiaojuan, W.; Xiangpei, W.; Zhaoguo, C. Isolation and Proteomic Analysis of Rhoptry-Enriched Fractions from Cryptosporidium parvum. Iran. J. Public Health 2015, 44, 1187. [Google Scholar]
- Li, D.; Cui, Z.; Wang, L.; Zhang, K.; Cao, L.; Zheng, S.; Zhang, L. TMT-based Proteomic Analysis and Protein Expression During Excystation of Cryptosporidium Anderson. Parasit Vectors 2021, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Kaçar, Y.; Baykal, A.T.; Aydin, L.; Batmaz, H. Evaluation of the efficacy of cow colostrum in the treatment and its effect on serum proteomes in calves with cryptosporidiosis. Vet. Immunol. Immunopathol. 2022, 248, 110429. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, H.; Jiang, N.; Wang, Y.; Wang, Y.; Zhang, J.; Shen, Y.; Cao, J. Comparative proteomics reveals Cryptosporidium parvum manipulation of the host cell molecular expression and immune response. PLoS Negl. Trop. Dis. 2021, 15, e0009949. [Google Scholar] [CrossRef]
- Gathercole, R.; Tranfield, E.; Xia, D.; Perez-Cordon, G.; Robinson, G.; Timofte, D.; Zendri, F.; Chalmers, R.M. Analysis of Cryptosporidium spp. from clinical samples by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. J. Appl. Microbiol. 2021, 131, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Mantione, K.J.; Kream, R.M.; Kuzelova, H.; Ptacek, R.; Raboch, J.; Samuel, J.M.; Stefano, G.B. Comparing bioinformatic gene expression profiling methods: Microarray and RNA-Seq. Med. Sci. Monit. Basic Res. 2014, 20, 138. [Google Scholar]
- Zhang, H.; Guo, F.; Zhou, H.; Zhu, G. Transcriptome analysis reveals unique metabolic features in the Cryptosporidium parvum Oocysts associated with environmental survival and stresses. BMC Genom. 2012, 13, 647. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, A.-Y.; Ma, S.; Chen, X.; Li, Y.; Su, C.-J.; Norall, D.; Chen, J.; Strauss-Soukup, J.K.; Chen, X.-M. Delivery of parasite RNA transcripts into infected epithelial cells during Cryptosporidium infection and its potential impact on host gene transcription. J. Infect. Dis. 2017, 215, 636–643. [Google Scholar] [CrossRef][Green Version]
- Sawant, M.; Benamrouz-Vanneste, S.; Mouray, A.; Bouquet, P.; Gantois, N.; Creusy, C.; Duval, E.; Mihalache, A.; Gosset, P.; Chabé, M. Persistent Cryptosporidium parvum infection leads to the development of the tumor microenvironment in an experimental mouse model: Results of a microarray approach. Microorganisms 2021, 9, 2569. [Google Scholar] [CrossRef]
- Mauzy, M.J.; Enomoto, S.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S. The Cryptosporidium parvum transcriptome during in vitro development. PLoS ONE 2012, 7, e31715. [Google Scholar] [CrossRef]
- Mirhashemi, M.E. Transcriptome analysis of Cryptosporidium parvum Development In Vitro. Master’s Thesis, Tufts University-Graduate School of Biomedical Sciences, Boston, MA, USA, 2016. [Google Scholar]
- Matos, L.V.; McEvoy, J.; Tzipori, S.; Bresciani, K.D.; Widmer, G. The transcriptome of Cryptosporidium oocysts and intracellular stages. Sci. Rep. 2019, 9, 7856. [Google Scholar] [CrossRef]
- Lippuner, C.; Ramakrishnan, C.; Basso, W.U.; Schmid, M.W.; Okoniewski, M.; Smith, N.C.; Hässig, M.; Deplazes, P.; Hehl, A.B. RNA-Seq analysis during the life cycle of Cryptosporidium parvum reveals significant differential gene expression between proliferating stages in the intestine and infectious sporozoites. Int. J. Parasitol. 2018, 48, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Baptista, R.P.; Sateriale, A.; Striepen, B.; Kissinger, J.C. Analysis of long non-coding RNA in Cryptosporidium parvum reveals significant stage-specific antisense transcription. Front. Cell. Infect. Microbiol. 2021, 10, 608298. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Xie, F.; Wu, S.; Shao, T.; Li, X.; Li, J.; Jian, F.; Zhang, S.; Ning, C. Whole transcriptome analysis of HCT-8 cells infected by Cryptosporidium parvum. Parasites Vectors 2022, 15, 441. [Google Scholar] [CrossRef]
- Ogbuigwe, P.; Roberts, J.M.; Knox, M.A.; Heiser, A.; Pita, A.; Haack, N.A.; Garcia-Ramirez, J.C.; Velathanthiri, N.; Biggs, P.J.; French, N.P. A novel, stain-free, natural auto-fluorescent signal, Sig M, identified from cytometric and transcriptomic analysis of infectivity of Cryptosporidium hominis and Cryptosporidium parvum. Front. Cell. Infect. Microbiol. 2023, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Lopez, G.M.; Mendoza Cavazos, C.; Tibabuzo Perdomo, A.M.; Garfoot, A.L.; O’Connor, R.M.; Knoll, L.J. Dual transcriptomics to determine gamma interferon-independent host response to intestinal Cryptosporidium parvum infection. Infect. Immun. 2022, 90, e00638-21. [Google Scholar] [CrossRef] [PubMed]
- Umejiego, N.N.; Gollapalli, D.; Sharling, L.; Volftsun, A.; Lu, J.; Benjamin, N.N.; Stroupe, A.H.; Riera, T.V.; Striepen, B.; Hedstrom, L. Targeting a prokaryotic protein in a eukaryotic pathogen: Identification of lead compounds against cryptosporidiosis. Chem. Biol. 2008, 15, 70–77. [Google Scholar] [CrossRef]
- Roberts, C.W.; Roberts, F.; Henriquez, F.L.; Akiyoshi, D.; Samuel, B.U.; Richards, T.A.; Milhous, W.; Kyle, D.; McIntosh, L.; Hill, G.C. Evidence for mitochondrial-derived alternative oxidase in the apicomplexan parasite Cryptosporidium parvum: A potential anti-microbial agent target. Int. J. Parasitol. 2004, 34, 297–308. [Google Scholar] [CrossRef]
- Lee, S.; Wei, L.; Zhang, B.; Goering, R.; Majumdar, S.; Wen, J.; Taliaferro, J.M.; Lai, E.C. ELAV/Hu RNA binding proteins determine multiple programs of neural alternative splicing. PLoS Genet. 2021, 17, e1009439. [Google Scholar] [CrossRef]
- Gissot, M.; Choi, S.-W.; Thompson, R.F.; Greally, J.M.; Kim, K. Toxoplasma gondii and Cryptosporidium parvum lack detectable DNA cytosine methylation. Eukaryot. Cell 2008, 7, 537–540. [Google Scholar] [CrossRef]
- Aliaga, B.; Bulla, I.; Mouahid, G.; Duval, D.; Grunau, C. Universality of the DNA methylation codes in Eucaryotes. Sci. Rep. 2019, 9, 173. [Google Scholar] [CrossRef]
- Lawton, P.; Hejl, C.; Mancassola, R.; Naciri, M.; Petavy, A.-F. Effects of purine nucleosides on the in vitro growth of Cryptosporidium parvum. FEMS Microbiol. Lett. 2003, 226, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, T.-L.; Fan, X.-C.; Li, Y.-H.; Yuan, Y.-J.; Yin, Y.-L.; Wang, X.-T.; Zhang, L.-X.; Zhao, G.-H. Expression profiles of mRNA and lncRNA in HCT-8 cells infected with Cryptosporidium parvum IId subtype. Front. Microbiol. 2018, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
| Species | Genomes ID | Platform | Genome Size (Mbp) | # of Contigs | # of Reads | Contig N50 (bp) | Average Coverage |
|---|---|---|---|---|---|---|---|
| C. hominis | TU502 237895 | Sanger Dideoxy Sequencing | 8.70 | 1422 | - | 48,000 | 12 |
| TU502 2012 | Illumina MiSeq | 9.10 | 119 | 1,810,060 | 238,509 | 96 | |
| 30976 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.05 | 53 | 35,360,353 | 470,636 | 511 | |
| 37999 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.05 | 78 | 16,569,87 | 406,678 | 367.4 | |
| 33537 | 454 GS-FLX Titanium | 9.60 | 1464 | 1,157,140 | 27,749 | 31 | |
| 30974 | 454 GS-FLX Titanium | 8.84 | 443 | 1,048,412 | 78,110 | 43 | |
| SWEH2 | Ion Torrent | 8.81 | 1629 | 1,791,829 | 9465 | 35.2 | |
| SWEH5 | Ion Torrent | 8.82 | 1342 | 2,058,197 | 14,514 | 42.4 | |
| UdeA01 | Illumina MiSeq | 9.04 | 8 | 1,080,44 | 1,103,974 | 53.4 | |
| UKH1 | Illumina MiSeq | 9.14 | 156 | 3,798,205 | 179,408 | 197.3 | |
| UKH3 | Illumina MiSeq | 9.07 | 179 | 1,238,762 | 167,737 | 35.8 | |
| UKH4 | Illumina HiSeq | 9.39 | 2164 | 11,895,367 | 48,766 | 321.5 | |
| UKH5 | Illumina HiSeq | 9.06 | 526 | 12,649,912 | 81,885 | 362.2 | |
| C. parvum | Iowa II 5807 | Sanger Dideoxy Sequencing | 9.10 | 18 | - | 1,014,526 | 13 |
| UKP1 | Illumina HiSeq | 8.88 | 14 | 26,000,000 | 1,092,230 | 600 | |
| 31727 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.08 | 337 | 13,074,496 | 76,396 | 116.0 | |
| 34902 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.11 | 1076 | 18,907,631 | 21,594 | 168.7 | |
| 35090 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.04 | 3256 | 14,188,762 | 4248 | 3.256 | |
| C. baileyi | TAMU-09Q1 | gDNA Illumina library fragment size (bp) 654 | 8.43 | 145 | 6,240,960 | 203,018 | 70.06 |
| C. muris | 5808 | 4.5× Sanger and 10× 454 | 9.25 | 97 | 520,347 | 10 | |
| C. chipmunk genotype I | 1280935 | Illumina Genome Analyzer IIx 100 bp paired-end | 9.05 | 50 | 9,509,783 | 117,886 | 200 |
| C. bovis | 310047 | Illumina HiSeq 250 bp paired-end | 9.11 | 59 | 7,080,000 | 444,382 | 196 |
| C. ryanae | 515981 | Illumina HiSeq 250 bp paired-end | 9.06 | 100 | 5,130,000 | 231,122 | 142.5 |
| C. meleagridis | UKMEL1 | Illumina MiSeq | 8.9 | 57 | 11,431,022 | 322,908 | 110.4 |
| Year | The Greatest Milestone | Genomic Approach | Outcome of Study | Reference |
|---|---|---|---|---|
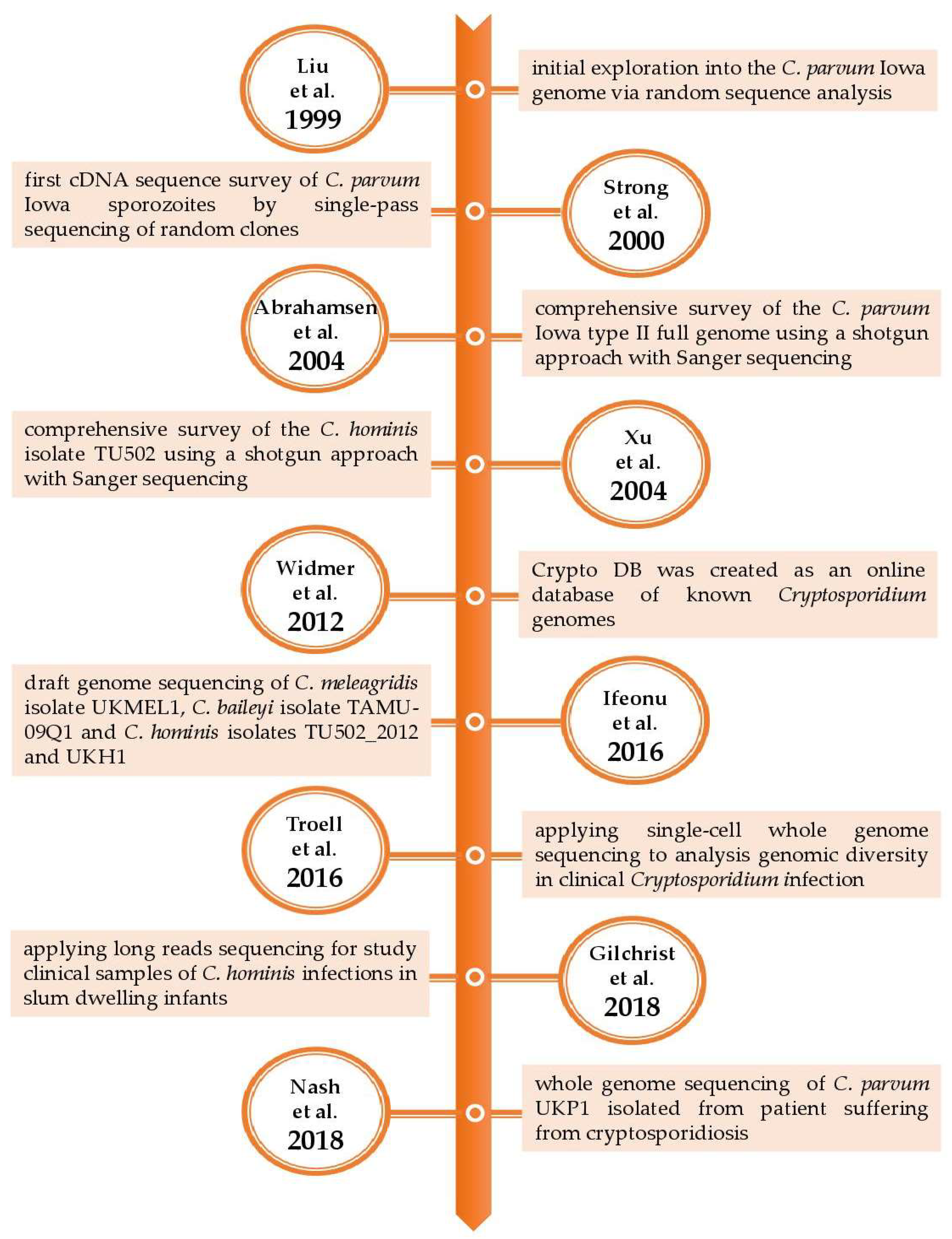
| 1999 | Initial genomic exploration into C. parvum Iowa strain | Random sequence analysis |
| [13] |
| 2000 | First cDNA sequence survey of C. parvum Iowa oocysts/sporozoites | Random sequence analysis with GSS approach to gene discovery |
| [6] |
| 2004 | Complete genome sequencing of C. parvum Iowa type II strain | Whole-genome with shotgun Sanger sequencing |
| [11] |
| 2004 | Complete genome sequencing C. hominis TU502 | Whole-genome with shotgun Sanger sequencing |
| [19] |
| 2012 | Comparative genome analysis of two C. parvum isolates (TU114 and C. parvum Iowa) | Whole-genome sequencing |
| [12] |
| 2015 | Sequencing of genomes C. chipmunk genotype I | Whole genome sequencing |
| [23] |
| 2015 | Comparative genome analysis of C. hominis and C. parvum | Whole genome sequencing |
| [14] |
| 2016 | Sequencing of genomes: C. meleagridis UKMEL1, C. baileyi TAMU-09Q1 and C. hominis TU502_2012 and UKH1 | Draft genome sequencing |
| [7] |
| 2016 | Genome sequencing of Cryptosporidium spp. in clinical samples | Single-cell sequencing |
| [21] |
| 2017 | Sequencing of the genomes of two specimens of C. parvum form China and Egypt | Whole genome sequencing |
| [15] |
| 2018 | Analysis of genetic diversity of C. hominis infections in slum-dwelling infants in Bangladesh | Long-read resequencing |
| [22] |
| 2018 | Analysis of a zoonotic isolate of C. parvum UKP1 isolated from a person with cryptosporidiosis | Draft genome sequencing |
| [8] |
| 2020 | Comparative analysis of Cryptosporidium species that infect humans | Whole-genome sequencing |
| [16] |
| 2020 | Sequencing of the genomes of C. bovis and C. ryanae | Whole-genome sequencing |
| [9] |
| Year | The Greatest Milestone | Outcome of Study | Reference |
|---|---|---|---|
| 2000 | Initial proteomic study of whole and freeze–thawed C. parvum oocysts and freeze–thawed C. muris |
| [24] |
| 2007 | Proteomic analysis of C. parvum oocysts |
| [25] |
| 2007 | Large-scale global proteomic analysis of non-excyted and excyted C. parvum sporozoites |
| [27] |
| 2008 | In-depth analysis of the expressed protein repertoire of C. parvum |
| [28] |
| 2010 | Proteome analysis for identifying the key components of the C. parvum oocyst wall |
| [29] |
| 2013 | Proteome analysis of C. parvum sporozoites |
| [30] |
| 2015 | Proteomic analysis of rhoptry-enriched fractions from C. parvum |
| [31] |
| 2021 | Proteomic analysis of C. andersoni oocysts before and after excystation |
| [32] |
| 2021 | Proteomic analysis of Cryptosporidium spp. from clinical samples |
| [35] |
| 2021 | Assessing the effectiveness of cow colostrum for treating cryptosporidiosis in calves and its impact on serum proteomes |
| [33] |
| 2021 | Characterize the changes to the proteome induced by C. parvum infection |
| [34] |
| 2021 | Investigation of the underlying biochemical interaction in C57BL/6J mice infected with C. parvum |
| [26] |
| Specimens | Strain | Technique | Search Engines | Reference Genome | Protein Coding Genes in Reference Genome |
|---|---|---|---|---|---|
| C. parvum and C.muris oocysts | Iowa and RN66 | MALDI-TOF peptide mass fingerprinting (PMF) | - | - | - |
| C. parvum sporozoites | Iowa | MALDI-TOF MS | - | - | - |
| C. parvum sporozoites non-excysted and excysted | ISSC162 | Combination of LC-MS/MS and iTRAQ isobaric labeling | ProQUANT software 1.1 (Applied Biosystems, Foster City, CA) | C. parvum Iowa type II | 3941 |
| C. parvum excysted oocyst/sporozoite | Iowa | Three independent platforms: 1-DE LC-MS/MS, 2-DE LC-MS/MS, and MudPIT | MASCOT search tool, SEQUEST algorithm version 27 | C. parvum Iowa type II | 3941 |
| C. parvum sporozoites | Iowa | SDS-PAGE and LC-MS/MS | MASCOT search tool | C. parvum Iowa type II C. hominis TU502 | 3941 3886 |
| C. parvum oocysts | Iowa | LC-MS/MS | SEQUEST search tool, NR database at the NCBI | C. parvum Iowa type II C. hominis TU502 | 3941 3886 |
| C. parvum Isolate | Iowa | SDS-PAGE and LC-MS/MS | MASCOT in the NCBI, CryptoDB v5.0, and EupathDB v2.16 databases | C. parvum Iowa type II | 3941 |
| C. andersoni oocysts | - 1 | SDS-PAGE and LC-MS/MS | MaxQuant search engine (v.1.5.2.8), UniProt database | C. andersoni 30847 | 3876 |
| Cryptosporidium spp. | - | MALDI-TOF MS | flexControl software on a microflex LT/SH MALDI-TOF (Bruker Daltonik GmbH) | C. parvum Iowa type II C. hominis TU502 | 3941 3886 |
| Cryptosporidium spp. | - | Label-free proteomic quantification techniques and LC-MS/MS | Maxquant search engine (v.1.5.2.8, Max Planck Institute of Biochemistry, Munich, Germany) | C. parvum Iowa type II C. hominis TU502 | 3941 3886 |
| C. parvum | - | Multi-omics approach: GC-MS and LC-HR-MS | The Protein Discoverer 2.2 (Thermo Fisher Scientific, Bremen, Germany) and Sequest HT search engines | C. parvum Iowa type II C. hominis TU502 | 3941 3886 |
| Year | The Greatest Milestone | Outcome of Study | Reference |
|---|---|---|---|
| 2011 | Construction and analysis of full-length cDNA library of C. parvum |
| [40] |
| 2012 | CpArray15K in profiling the gene expressions in the oocysts of C. parvum and their responses to UV irradiation |
| [37] |
| 2016 | New insights into the intracellular development of C. parvum |
| [41] |
| 2017 | Discovery of parasite RNA Transcripts delivery to infected epithelial cells during cryptosporidiosis |
| [38] |
| 2018 | RNA-Seq insights from intestinal proliferating stages to infectious sporozoites |
| [43] |
| 2019 | Comparison of gene expression in the sporozoite and intracellular stages of C. parvum by RNA-Seq |
| [42] |
| 2021 | Analysis of Long Non-Coding RNA in C. parvum |
| [44] |
| 2021 | Transcriptome analysis of the ileocecal tissue infected with C. parvum for exploring its potential to induce digestive adenocarcinoma in a rodent model |
| [39] |
| 2022 | Whole transcriptome analysis of HCT-8 cells infected by C. parvum |
| [45] |
| 2022 | Dual transcriptomics to determine IFN-g-independent transcriptomic response to C. parvum infection |
| [47] |
| 2023 | Transcriptomic analysis of infectivity of C. hominis and C. parvum |
| [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, J.; Sroka, J.; Cencek, T. Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions. Int. J. Mol. Sci. 2023, 24, 12867. https://doi.org/10.3390/ijms241612867
Dąbrowska J, Sroka J, Cencek T. Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions. International Journal of Molecular Sciences. 2023; 24(16):12867. https://doi.org/10.3390/ijms241612867
Chicago/Turabian StyleDąbrowska, Joanna, Jacek Sroka, and Tomasz Cencek. 2023. "Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions" International Journal of Molecular Sciences 24, no. 16: 12867. https://doi.org/10.3390/ijms241612867
APA StyleDąbrowska, J., Sroka, J., & Cencek, T. (2023). Investigating Cryptosporidium spp. Using Genomic, Proteomic and Transcriptomic Techniques: Current Progress and Future Directions. International Journal of Molecular Sciences, 24(16), 12867. https://doi.org/10.3390/ijms241612867








