Impact of Intron and Retransformation on Transgene Expression in Leaf and Fruit Tissues of Field-Grown Pear Trees
Abstract
:1. Introduction
2. Results
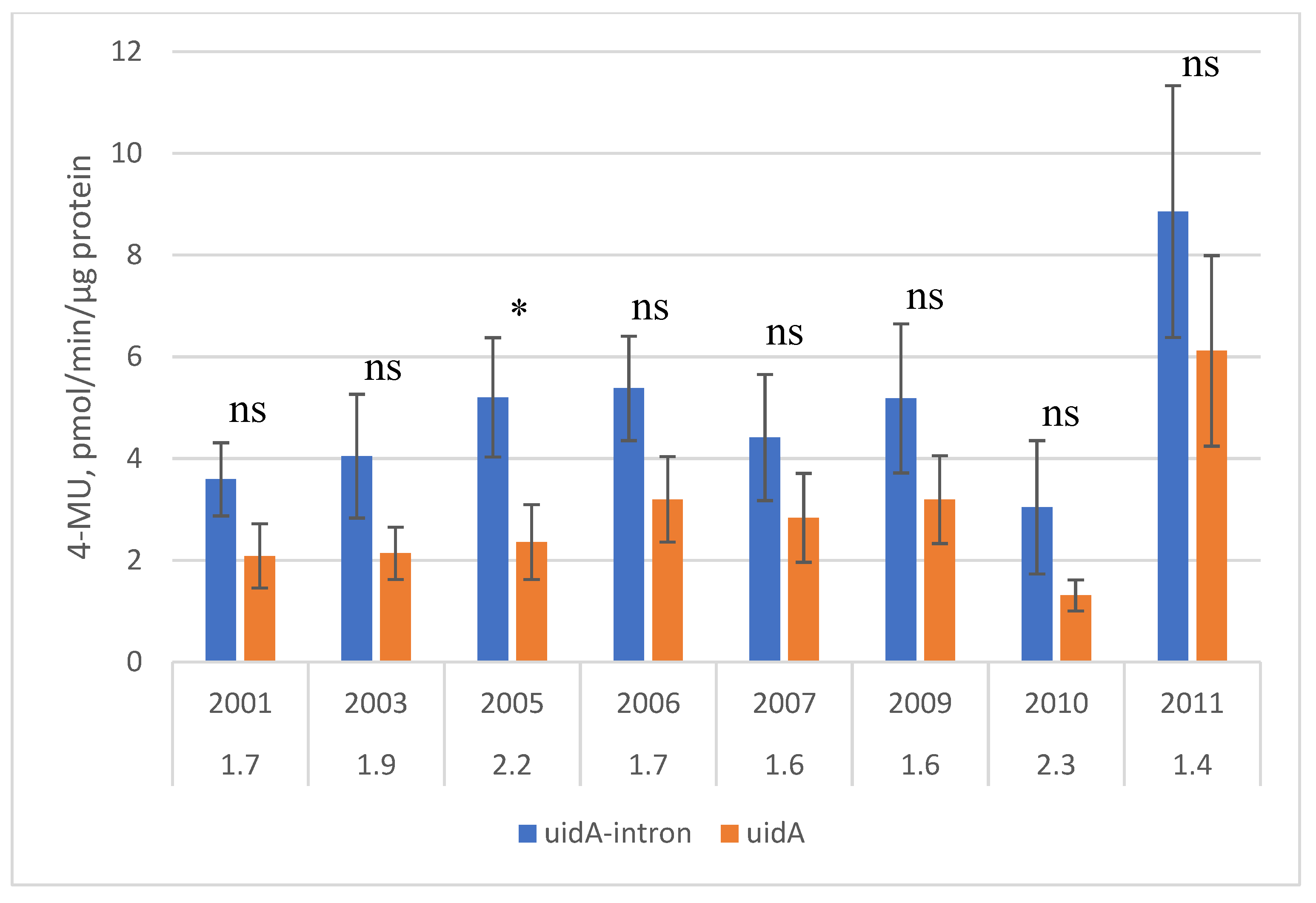
2.1. Transgene Expression in Leaves
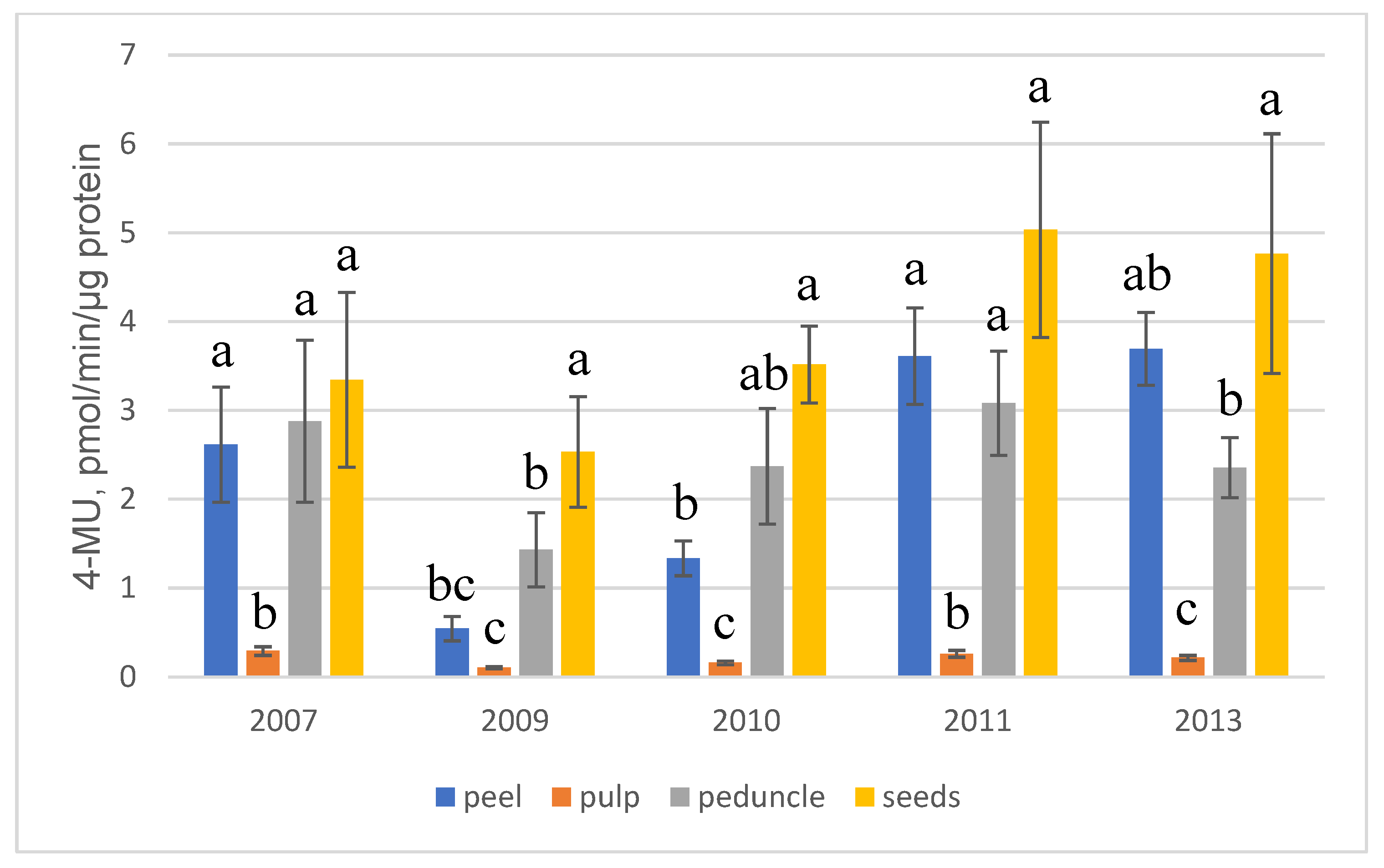
2.2. Transgene Expression in Fruits
2.3. Transgene Expression in Retransformants
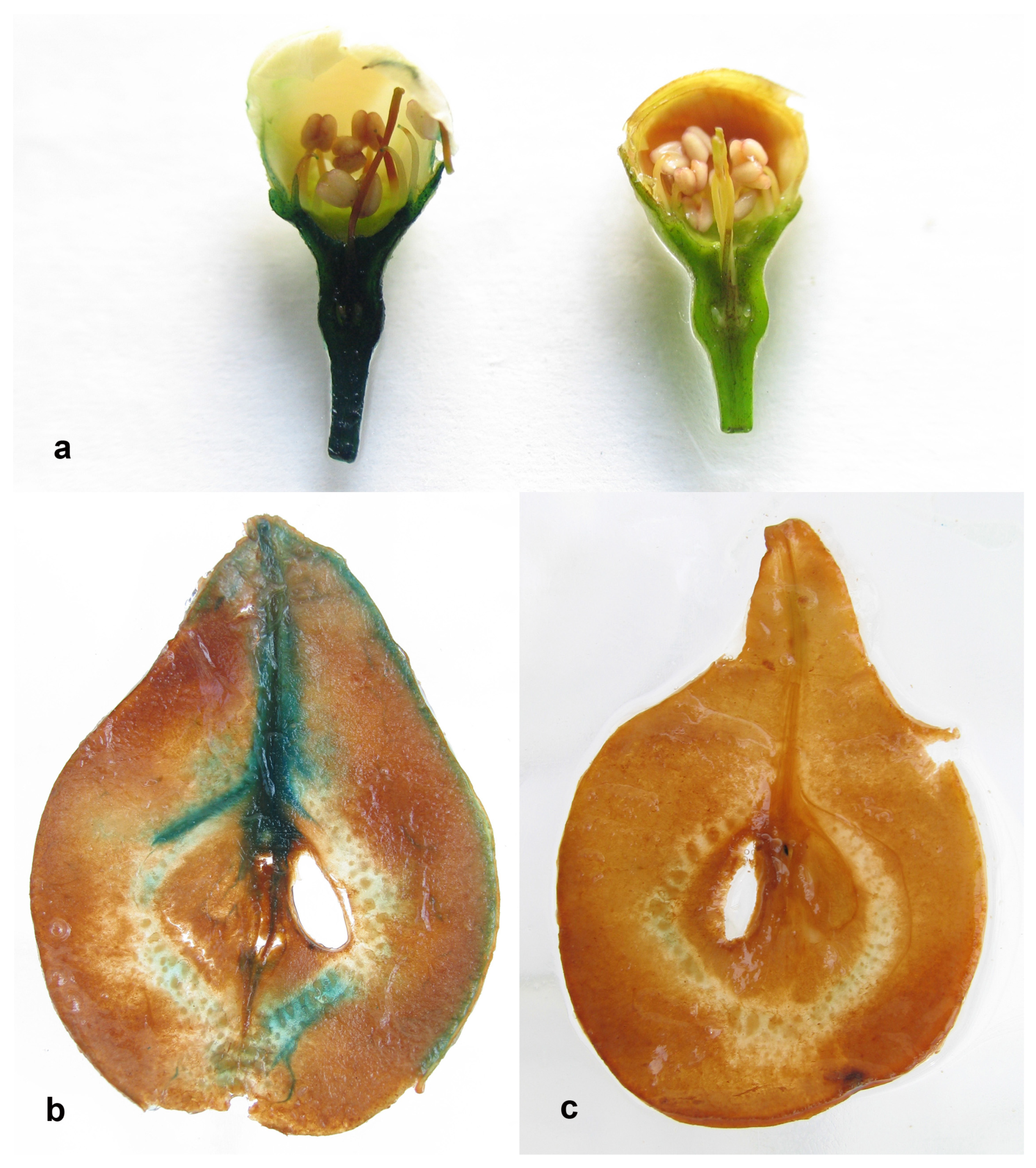
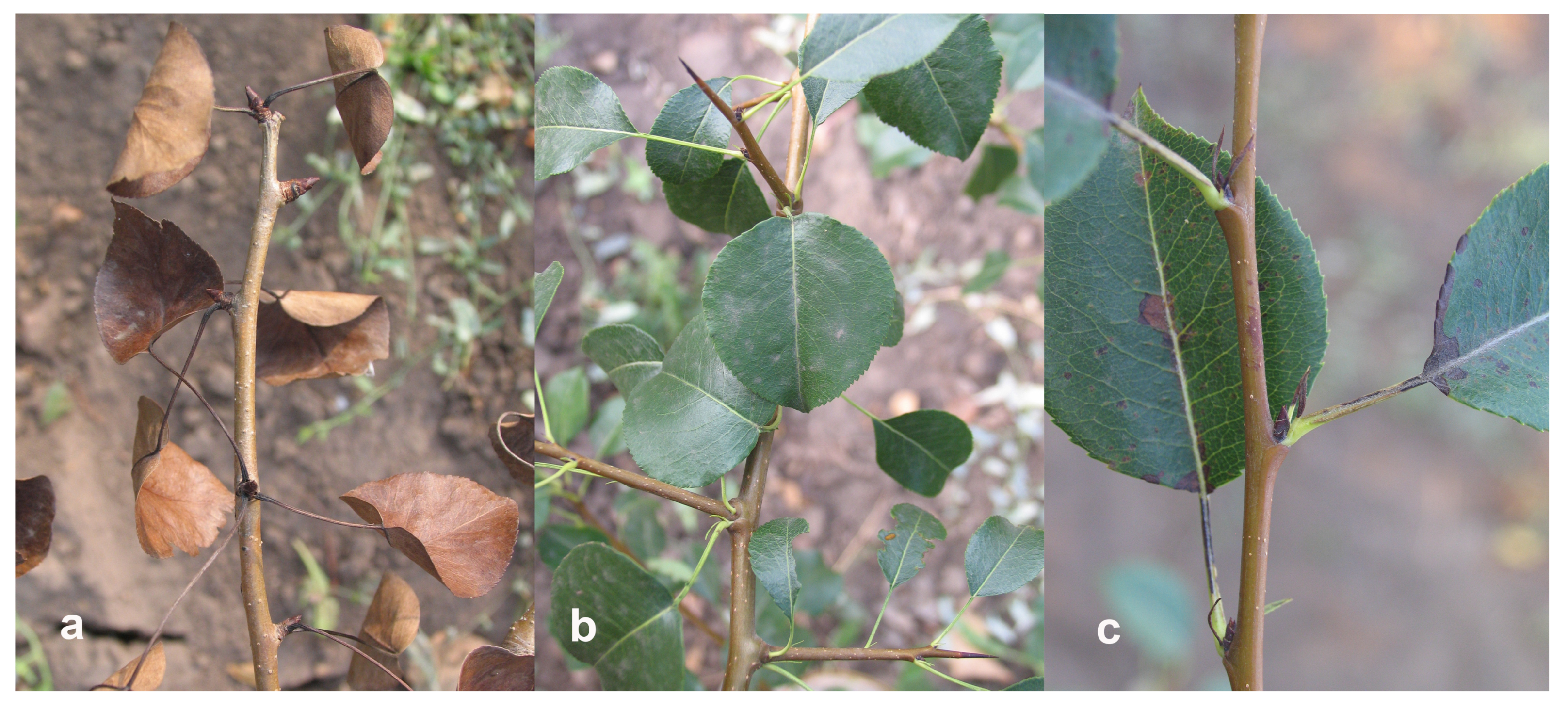
2.4. Identification of Potential Unintended Effects
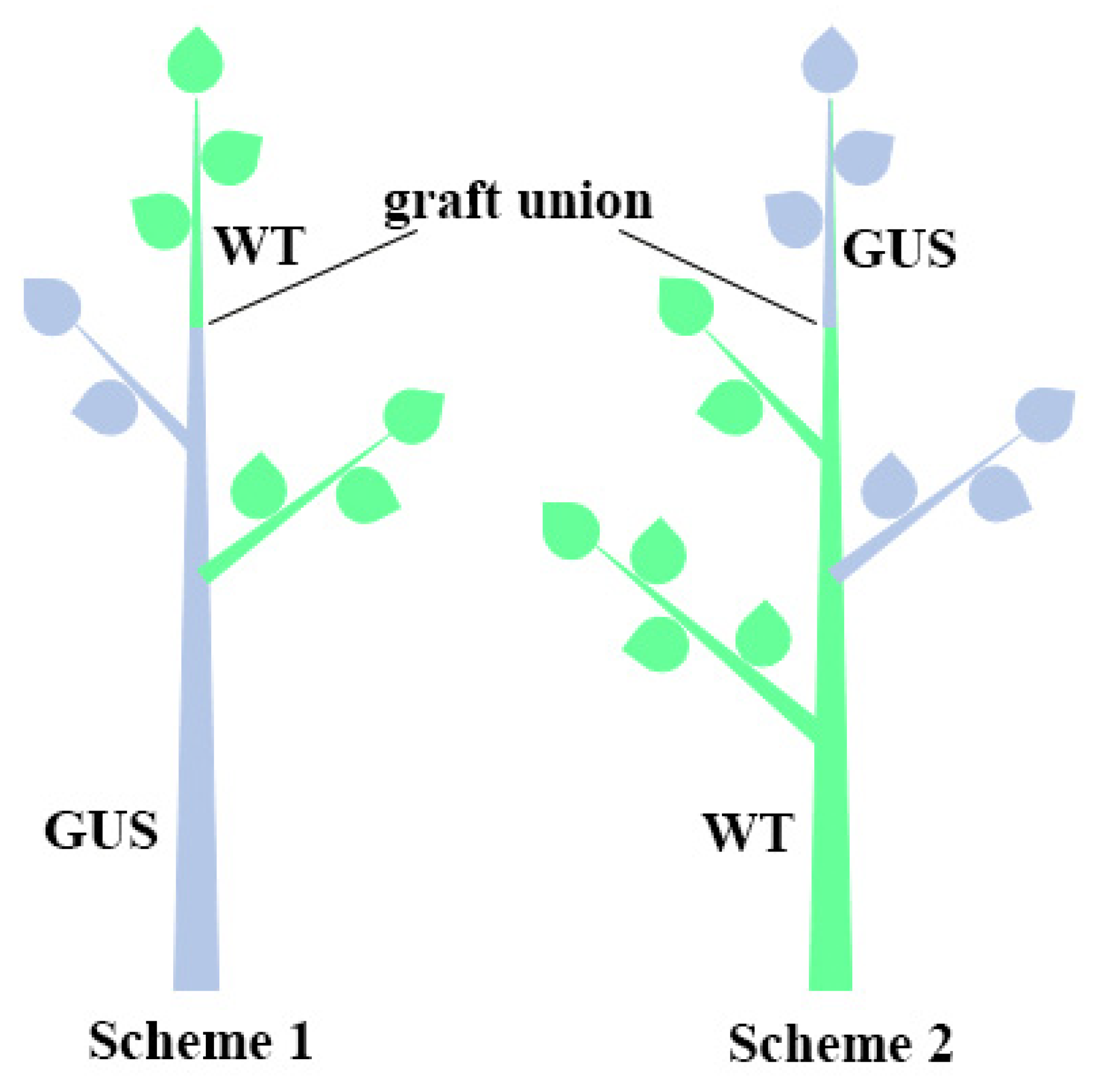
2.5. Protein Transport across Draft
3. Discussion
3.1. Expression Stability in Pear Leaves and Fruits
3.2. Intron-Mediated Enhancement in Pear Trees
3.3. Expression of Transgenes in Retransformants of Pear
3.4. Potential of Unintended Effects in Phenotype of Transgenic Trees
3.5. Movement of Protein through Graft Union
4. Materials and Methods
4.1. Plant Materials
4.2. β-Glucuronidase (GUS) Assays
4.3. Tree and Leaf Phenotypic Assessment
4.4. Herbicide Application
4.5. Grafting Experiments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Brunner, A.M.; Meilan, R.; Strauss, S.H. Stability of transgenes in trees: Expression of two reporter genes in poplar over three field seasons. Tree Physiol. 2009, 29, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Fladung, M.; Hoenicka, H.; Ahuja, M.R. Genomic stability and long-term transgene expression in poplar. Transgenic Res. 2013, 22, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Macaya-Sanz, D.; Chen, J.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic performance of Populus deltoides trees engineered for biofuel production. Biotechnol. Biofuels 2017, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.H.; Ma, C.; Ault, K.; Klocko, A.L. Lessons from two decades of field trials with genetically modified trees in the USA: Biology and regulatory compliance. In Biosafety of Forest Transgenic Trees; Vettori, C., Gallardo, F., Häggman, H., Kazana, V., Migliacci, F., Pilate, G., Fladung, M., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 101–124. [Google Scholar]
- Donev, E.N.; Derba-Maceluch, M.; Yassin, Z.; Gandla, M.L.; Pramod, S.; Heinonen, E.; Kumar, V.; Scheepers, G.; Vilaplana, F.; Johansson, U.; et al. Field testing of transgenic aspen from large greenhouse screening identifies unexpected winners. Plant Biotechnol. J. 2023, 21, 1005–1021. [Google Scholar] [CrossRef]
- Baulcombe, D.C.; Dean, C. Epigenetic regulation in plant responses to the environment. Cold Spring Harb. Perspect. Biol. 2014, 6, a019471. [Google Scholar] [CrossRef]
- Tricker, P.J. Transgenerational inheritance or resetting of stress-induced epigenetic modifications: Two sides of the same coin. Front. Plant Sci. 2015, 6, 699. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Shehryar, K.; Khan, R.; Iqbal, A.; Hussain, S.; Imdad, S.; Bibi, A.; Hamayun, L.; Nakamura, I. Transgene stacking as effective tool for enhanced disease resistance in plants. Mol. Biotechnol. 2020, 62, 1–7. [Google Scholar] [CrossRef]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019; ISAAA Brief No. 55; ISAAA: New York, NY, USA, 2019. [Google Scholar]
- Francois, I.E.J.A.; Broekaert, W.F.; Cammue, B.P.A. Different approaches for multi-transgene-stacking in plants. Plant Sci. 2002, 163, 281–295. [Google Scholar] [CrossRef]
- Mesnage, R.; Agapito-Tenfen, S.; Vilperte, V.; Renney, G.; Ward, M.; Séralini, G.-E.; Nodari, R.; Antoniou, M. An integrated multi-omics analysis of the NK603 Roundup-tolerant GM maize reveals metabolism disturbances caused by the transformation process. Sci. Rep. 2016, 6, 37855. [Google Scholar] [CrossRef]
- Ladics, G.S.; Bartholomaeus, A.; Bregitzer, P.; Doerrer, N.G.; Gray, A.; Holzhauser, T.; Jordan, M.; Keese, P.; Kok, E.; Macdonald, P.; et al. Genetic basis and detection of unintended effects in genetically modified crop plants. Transgenic Res. 2015, 24, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Arnone, J.T. Genomic considerations for the modification of Saccharomyces cerevisiae for biofuel and metabolite biosynthesis. Microorganisms 2020, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Callis, J.; Fromm, M.; Walbot, V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987, 1, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, X.; Li, D.; Zhang, H. Establishment of an efficient genome editing system in lettuce without sacrificing specificity. Front. Plant Sci. 2022, 13, 930592. [Google Scholar] [CrossRef]
- Back, G.; Walther, D. Identification of cis-regulatory motifs in first introns and the prediction of intronmediated enhancement of gene expression in Arabidopsis thaliana. BMC Genom. 2021, 22, 390. [Google Scholar] [CrossRef]
- Gaion, L.A.; Carvalho, R.F. Long-distance signaling: What grafting has revealed? J. Plant Growth Regul. 2018, 37, 694–704. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Harada, T. Grafting and RNA transport via phloem tissue in horticultural plants. Sci Hortic. 2010, 125, 545–550. [Google Scholar] [CrossRef]
- Haroldsen, V.M.; Szczerba, M.; Aktas, H.; Lopez-Baltazar, J.; Odias, M.; Chi-Ham, C.; Labavitch, J.; Bennett, A.; Powell, A. Mobility of transgenic nucleic acids and proteins within grafted rootstocks for agricultural improvement. Front. Plant Sci. 2012, 3, 39. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Wang, K.; Feng, J.; Sun, S.; Lu, X.; Liu, Z.; Zhao, D.; Li, L.; Wang, D. Transcriptome analysis of the effects of grafting interstocks on apple rootstocks and scions. Int. J. Mol. Sci. 2023, 24, 807. [Google Scholar] [CrossRef]
- Zhao, D.; Song, G.-Q. Rootstock-to-scion transfer of transgene-derived small interfering RNAs and their effect on virus resistance in nontransgenic sweet cherry. Plant Biotechnol. J. 2014, 12, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Weber, K.C.; Qiu, W.; Stanton, D.; Mahmoud, L.M.; Wu, H.; Huyck, P.; Zale, J.; Al Jasim, K.; Grosser, J.W.; et al. The vascular targeted citrus FLOWERING LOCUS T3 gene promotes non-inductive early flowering in transgenic Carrizo rootstocks and grafted juvenile scions. Sci Rep. 2020, 10, 21404. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shen, W.; Liu, C.; Tahir, M.; Li, X.; Zhou, S.; Ma, F.; Guan, Q. Engineering drought-tolerant apple by knocking down six GH3 genes and potential application of transgenic apple as a rootstock. Hort. Res. 2022, 9, uhac122. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Walworth, A.E.; Loescher, W.H. Grafting of genetically engineered plants. J. Am. Soc. Hortic. Sci. 2015, 140, 203–213. [Google Scholar] [CrossRef]
- Kodama, H.; Miyahara, T.; Oguchi, T.; Tsujimoto, T.; Ozeki, Y.; Ogawa, T.; Yamaguchi, Y.; Ohta, D. Effect of transgenic rootstock grafting on the omics profiles in tomato. Food Safety 2021, 9, 32–47. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Dedow, L.K.; Oren, E.; Braybrook, S.A. Fake news blues: A GUS staining protocol to reduce false negative. Plant Direct. 2022, 6, e367. [Google Scholar] [CrossRef]
- Borejsza-Wysocka, E.; Norelli, J.L.; Aldwinckle, H.S.; Malnoy, M. Stable expression and phenotypic impact of attacinE transgene in orchard grown apple trees over a 12 years period. BMC Biotechnol. 2010, 10, 41. [Google Scholar] [CrossRef]
- Zeller, S.L.; Kalinina, O.; Brunner, S.; Keller, B.; Schmid, B. Transgene × environment interactions in genetically modified wheat. PLoS ONE 2010, 5, e11405. [Google Scholar] [CrossRef]
- Trtikova, M.; Wikmark, O.G.; Zemp, N.; Widmer, A.; Hilbeck, A. Transgene expression and Bt protein content in transgenic Bt maize (MON810) under optimal and stressful environmental conditions. PLoS ONE 2015, 10, e0123011. [Google Scholar] [CrossRef]
- Dole, R.; Hoerling, M.; Perlwitz, J.; Eischeid, J.; Pegion, P.; Zhang, T.; Quan, X.-W.; Xu, T.; Murray, D. Was there a basis for anticipating the 2010 Russian heat wave? Geophys. Res. Lett. 2011, 38, L06702. [Google Scholar] [CrossRef]
- Cervera, M.; Pina, J.; Juárez, J.; Navarro, L.; Pena, L. A broad exploration of a transgenic population of citrus: Stability of gene expression and phenotype. Theor. Appl. Genet. 2000, 100, 670–677. [Google Scholar] [CrossRef]
- Que, Q.; Chilton, M.-D.M.; de Fontes, C.M.; He, C.; Nuccio, M.; Zhu, T.; Wu, Y.; Chen, J.S.; Shi, L. Trait stacking in transgenic crops: Challenges and opportunities. GM Crops 2010, 1, 220–229. [Google Scholar] [CrossRef]
- Li, J.; Meilan, R.; Ma, C.; Barish, M.; Strauss, S.H. Stability of herbicide resistance over 8 years of coppice in field-grown, genetically engineered poplars. West. J. Appl. For. 2008, 23, 89–93. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic compounds and chemical characteristics of pears (Pyrus communis L.). Int. J. Food Prop. 2015, 18, 536–546. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Ma, L.; Zhong, J.; Kayahara, H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in oriental pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef]
- Hudina, M.; Stampar, F.; Orazem, P.; Petkovsek, M.; Veberic, R. Phenolic compounds profile, carbohydrates and external fruit quality of the ‘Concorde’ pear (Pyrus communis L.) after bagging. Can. J. Plant Sci. 2012, 92, 67–75. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wroblewska, A.M.; Jablonska-Trypuc, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, T.; Yang, S.; Li, X.; Song, B.; Wang, Y.; Lin, Q.; Cao, J. Analysis of phenolic components and related biological activities of 35 apple (Malus pumila Mill.) cultivars. Molecules 2020, 25, 4153. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant properties of pulp, peel and seeds of phlegrean mandarin (Citrus reticulate Blanco) at different stages of fruit ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef]
- Laxa, M. Intron-mediated enhancement: A tool for heterologous gene expression in plants? Front. Plant Sci. 2017, 7, 1977. [Google Scholar] [CrossRef]
- Vancanneyt, G.; Schmidt, R.; O’Connor-Sanchez, A.; Wilmitzer, L.; Rocha-Sosa, M. Construction of an intron-containing marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. 1990, 220, 245–250. [Google Scholar] [CrossRef] [PubMed]
- De Bondt, A.; Eggermont, K.; Penninckx, I.; Goderis, I.; Broekaert, W.F. Agrobacterium-mediated transformation of apple (Malus × domestica Borkh.): An assessment of factors affecting regeneration of transgenic plants. Plant Cell Rep. 1996, 15, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Sink, K.C. Transformation of Montmorency sour cherry (Prunus cerasus L.) and Gisela 6 (P. cerasus × P. canescens) cherry rootstock mediated by Agrobacterium tumefaciens. Plant Cell Rep. 2006, 25, 117–123. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Dolgov, S.V. Stability of gus and bar gene expression in transgenic pear clonal rootstock plants during several years. Acta Hortic. 2008, 800, 373–382. [Google Scholar] [CrossRef]
- Parra, G.; Bradnam, K.; Pose, A.; Korf, I. Comparative and functional analysis of intron-mediated enhancement signals reveals conserved features among plants. Nucleic Acids Res. 2011, 39, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Feike, D.; Korolev, A.V.; Soumpourou, E.; Murakami, E.; Reid, D.; Breakspear, A.; Rogers, C.; Radutoiu, S.; Stougaard, J.; Harwood, W.A.; et al. Characterizing standard genetic parts and establishing common principles for engineering legume and cereal roots. Plant Biotechnol. J. 2019, 17, 2234–2245. [Google Scholar] [CrossRef]
- Lebedev, V. The rooting of stem cuttings and the stability of uidA gene expression in generative and vegetative progeny of transgenic pear rootstock in the field. Plants 2019, 8, 291. [Google Scholar] [CrossRef]
- Lebedev, V. Stability of transgene inheritance in progeny of field-grown pear trees over a 7-year period. Plants 2022, 11, 151. [Google Scholar] [CrossRef]
- Cervera, M.; Navarro, L.; Pena, L. Gene stacking in 1-year-cycling APETALA1 citrus plants for a rapid evaluation of transgenic traits in reproductive tissues. J. Biotechnol. 2009, 140, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.G.; Taran, S.A.; Shmatchenko, V.V.; Lunin, V.G.; Skryabin, K.G.; Dolgov, S.V. Molecular breeding of pear. Acta Hortic. 2002, 587, 217–223. [Google Scholar] [CrossRef]
- Bonadei, M.; Zelasco, S.; Giorcelli, A.; Gennaro, M.; Calligari, P.; Quattrini, E.; Balestrazzi, A. Transgene stability and agronomical performance of two transgenic Basta (R)-tolerant lines of Populus alba L. Plant Biosyst. 2012, 146, 33–40. [Google Scholar] [CrossRef]
- Miki, B.; Abdeen, A.; Manabe, Y.; MacDonald, P. Selectable marker genes and unintended changes to the plant transcriptome. Plant Biotechnol. J. 2009, 7, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pasonen, H.; Vihervuori, L.; Seppänen, S.; Lyytikäinen-Saarenmaa, P.; Ylioja, T.; von Weissenberg, K.; Pappinen, A. Field performance of chitinase transgenic silver birch (Betula pendula Roth): Growth and adaptive traits. Trees 2008, 22, 413–421. [Google Scholar] [CrossRef]
- Hagee, D.; Abu Hardan, A.; Botero, J.; Arnone, J.T. Genomic clustering within functionally related gene families in Ascomycota fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3267–3277. [Google Scholar] [CrossRef]
- Kutsokon, N.; Danchenko, M.; Skultety, L.; Kleman, J.; Rashydov, N. Transformation of hybrid black poplar with selective and reporter genes affects leaf proteome, yet without indication of a considerable environmental hazard. Acta Physiol. Plant. 2020, 42, 86. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, H.-m.; Liu, G.-f.; Li, H.-y.; Jiang, J. A label-free differential quantitative proteomics analysis of a TaLEA-introduced transgenic Populus simonii × Populus nigra dwarf mutant. Mol. Biol. Rep. 2012, 39, 7657–7664. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Lebedeva, T.N.; Shestibratov, K.A. Impact of transgenic birch with modified nitrogen metabolism on soil properties, microbial biomass and enzymes in 4-year study. Plant Soil 2023, 484, 627–643. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Faskhiev, V.N.; Kovalenko, N.P.; Schestibratov, K.A.; Miroshnikov, A.I. Testing transgenic aspen plants with bar gene for herbicide resistance under semi-natural conditions. Acta Naturae 2016, 8, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Tsukaya, H. Mechanism of leaf-shape determination. Annu. Rev. Plant Biol. 2006, 57, 477–496. [Google Scholar] [CrossRef]
- Srinivasan, C.; Liu, Z.; Scorza, R. Ectopic expression of class 1 KNOX genes induce adventitious shoot regeneration and alter growth and development of tobacco (Nicotiana tabacum L) and European plum (Prunus domestica L). Plant Cell Rep. 2011, 30, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kaku, T.; Baba, K.; Taniguchi, T.; Kurita, M.; Konagaya, K.-I.; Ishii, K.; Kondo, T.; Serada, S.; Iizuka, H.; Kaida, R.; et al. Analyses of leaves from open field-grown transgenic poplars overexpressing xyloglucanase. J. Wood Sci. 2012, 58, 281–289. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Malnoy, M.; Gribaudo, I. Breeding next generation tree fruits: Technical and legal challenges. Hortic Res. 2017, 4, 17067. [Google Scholar] [CrossRef]
- Kieffer, M.; Master, V.; Waites, R.; Davies, B. TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis. Plant J. 2011, 68, 147–158. [Google Scholar] [CrossRef]
- Waters, M.T.; Scaffidi, A.; Moulin, S.; Sun, Y.; Flematti, G.; Smith, S. A Selaginella moellendorffii Ortholog of KARRIKIN INSENSITIVE2 functions in Arabidopsis development but cannot mediate responses to karrikins or strigolactones. Plant Cell 2015, 27, 1925–1944. [Google Scholar] [CrossRef]
- Vidyagina, E.; Subbotina, N.; Belyi, V.; Lebedev, V.; Krutovsky, K.; Shestibratov, K. Various effects of the expression of the xyloglucanase gene from Penicillium canescens in transgenic aspen under semi-natural conditions. BMC Plant Biol. 2020, 20, 251. [Google Scholar] [CrossRef]
- Lebedev, V. Fruit characteristics of transgenic pear (Pyrus communis L.) trees during long-term field trials. Plant Foods Hum. Nutr. 2023, 78, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, W.; Huang, J.; Zhao, L.; Ma, C.; Hao, L.; Yuan, H.; Harada, T.; Li, T. KNOTTED1 mRNA undergoes long-distance transport and interacts with movement protein binding protein 2C in pear (Pyrus betulaefolia). Plant Cell Tiss. Organ Cult. 2015, 121, 109–119. [Google Scholar] [CrossRef]
- Wenzel, S.; Flachowsky, H.; Hanke, M.-V. The Fast-track breeding approach can be improved by heatinduced expression of the FLOWERING LOCUS T genes from poplar (Populus trichocarpa) in apple (Malus × domestica Borkh.). Plant Cell Tiss. Organ Cult. 2013, 115, 127–137. [Google Scholar] [CrossRef]
- Artlip, T.S.; Wisniewski, M.E.; Arora, R.; Norelli, J.L. An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic. Res. 2016, 3, 16006. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.K.; Kalariya, H.; Schnabel, G. The Gastrodia antifungal protein (GAFP-1) and its transcript are absent from scions of chimeric-grafted plum. HortScience 2010, 45, 188–192. [Google Scholar] [CrossRef]
- Wang, L.R.; Yang, M.; Akinnagbe, A.; Liang, H.; Wang, J.; Ewald, D. Bacillus thuringiensis protein transfer between rootstock and scion of grafted poplar. Plant Biol. 2012, 14, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Abelenda, J.A.; Cruz-Oro, E.; Cuellar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUST. Nature 2011, 478, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Martínez-García, P.; Nikbakht-Dehkordi, A.; Prudencio, Á.; Gómez, E.; Rodamilans, B.; Dicenta, F.; García, J.; Martínez-Gómez, P. Gene expression analysis of induced plum pox virus (Sharka) resistance in peach (Prunus persica) by almond (P. dulcis) grafting. Int. J. Mol. Sci. 2021, 22, 3585. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, Y.; Wu, X.; Xin, Y.; Guo, J.; Wu, F.; Yu, H.; Sun, Z.; Xu, C. Scion-to-rootstock mobile transcription factor CmHY5 positively modulates the nitrate uptake capacity of melon scion grafted on squash rootstock. Int. J. Mol. Sci. 2023, 24, 162. [Google Scholar] [CrossRef]
- Chen, P.F.; Ren, Y.C.; Zhang, J.; Wang, J.M.; Yang, M.S. Expression and transportation of Bt toxic protein in 8-year-old grafted transgenic poplar. Sci. Silvae Sin. 2016, 52, 46–52. [Google Scholar]
- Tang, M.; Bai, X.; Wang, J.; Chen, T.; Meng, X.; Deng, H.; Li, C.; Xu, Z.-F. Efficiency of graft-transmitted JcFT for floral induction in woody perennial species of the Jatropha genus depends on transport distance. Tree Physiol. 2022, 42, 189–201. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Popova, A.A.; Shestibratov, K.A. Genetic engineering and genome editing for improving nitrogen use efficiency in plants. Cells 2021, 10, 3303. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Dolgov, S.V. The effect of selective agents and a plant intron on transformation efficiency and expression of heterologous genes in pear Pyrus communis L. Russ. J. Genet. 2000, 36, 650–655. [Google Scholar]
- Eckes, P.; Rosahl, S.; Schell, J.; Willmitzer, L. Isolation and characterization of a light-inducible, organ-specific gene from potato and analysis of its expression after tagging and transfer into tobacco and potato shoots. Molec. Gen. Genet. 1986, 205, 14–22. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Skryabin, K.G.; Dolgov, S.V. Transgenic pear clonal rootstocks resistant to herbicide “Basta”. Acta Hortic. 2002, 596, 193–197. [Google Scholar] [CrossRef]
- Scott, R.; Draper, J.; Jefferson, R.; Dury, G.; Jacob, L. Analysis of gene organization and expression in plants. In Plant Genetic Transformation and Gene Expression: A Laboratory Manual; Draper, J., Scott, R., Armitage, P., Walden, R., Eds.; Blackwell: Oxford, UK, 1988; pp. 263–339. [Google Scholar]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability—Pear (Pyrus communis L.); TG/15/3; International Union for the Protection of New Varieties of Plants: Geneva, Switzerland, 2000. [Google Scholar]
- Bylesjö, M.; Segura, V.; Soolanayakanahally, R.; Rae, A.; Trygg, J.; Gustafsson, P.; Jansson, S.; Street, N. LAMINA: A tool for rapid quantification of leaf size and shape parameters. BMC Plant Biol. 2008, 8, 82. [Google Scholar] [CrossRef]

| Transgene | Line | 4-MU, pmol/min/µg Protein | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2003 | 2005 | 2006 | 2007 | 2009 | 2010 | 2011 | ||
| control | GP217 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| uidA-intron | HA-1 | 59 | 45 | 197 | 156 | 220 | 429 | 118 | 211 |
| HA-2 | 101 | 24 | 36 | 29 | 6 | 57 | 14 | 17 | |
| HA-3 | 66 | 84 | 184 | 89 | 164 | 387 | 716 | 194 | |
| HA-4 | 113 | 83 | 342 | 131 | 314 | 209 | 267 | 216 | |
| HA-5 | 59 | 55 | 211 | 141 | 132 | 367 | 95 | 226 | |
| HA-6 | 210 | 23 | 323 | 88 | 35 | 92 | 14 | 56 | |
| HA-7 | 28 | 10 | 23 | 11 | 5 | 8 | 6 | 5 | |
| HB-1 | 242 | 214 | 675 | 105 | 100 | 786 | 242 | 393 | |
| HB-2 | 157 | 30 | 560 | 256 | 133 | 199 | 103 | 72 | |
| HB-4 | 249 | 51 | 465 | 143 | 28 | 114 | 17 | 31 | |
| uidA | NI-1 | 10 | 6 | 37 | 3 | 3 | 10 | 16 | 3 |
| NII-1 | 44 | 66 | 213 | 163 | 234 | 349 | 87 | 266 | |
| NII-2 | 40 | 27 | 58 | 68 | 95 | 135 | 45 | 107 | |
| NII-3 | 238 | 60 | 443 | 108 | 53 | 240 | 106 | 195 | |
| NII-4 | 72 | 8 | 159 | 35 | 98 | 350 | 64 | 152 | |
| NIII-2 | 66 | 24 | 79 | 82 | 70 | 45 | 63 | 67 | |
| NIII-4 | 86 | 62 | 57 | 114 | 61 | 181 | 170 | 55 | |
| NIII-5 | 22 | 21 | 113 | 7 | 24 | 9 | 42 | 26 | |
| NIV-2 | 96 | 20 | 73 | 35 | 29 | 141 | 35 | 13 | |
| Line | Initial | 4-MU, pmol/min/µg Protein | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | 2002 | 2003 | 2005 | 2006 | 2007 | 2009 | 2010 | 2011 | |
| GP217 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P-BK | GP217 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| T-AP | NII-1 | 92 | 50 | 591 | 21 | 185 | 353 | 147 | 467 |
| T-BO | NII-1 | 8 | 4 | 21 | 3 | 8 | 7 | 5 | 15 |
| T-BT | NII-1 | 36 | 24 | 152 | 18 | 236 | 82 | 288 | 242 |
| T-CD | NI-1 | 3 | 3 | 5 | 4 | 6 | 3 | 2 | 15 |
| T-CF | NII-1 | 6 | 7 | 112 | 6 | 49 | 6 | 24 | 30 |
| T2-AF | NIII-2 | 5 | 4 | 11 | 3 | 8 | 12 | 9 | 20 |
| T2-BF | NIII-4 | 45 | 22 | 82 | 19 | 118 | 121 | 123 | 242 |
| T2-BR | NIV-2 | 33 | 39 | 109 | 36 | 82 | 129 | 64 | 287 |
| T2-BS | NIV-2 | 78 | 21 | 204 | 12 | 47 | 91 | 62 | 313 |
| T2-DE | NIV-2 | 6 | 3 | 7 | 4 | 5 | 7 | 5 | 19 |
| T2-ES | NIII-4 | 91 | 40 | 131 | 41 | 114 | 172 | 173 | 312 |
| Line | Greenhouse | Field | ||
|---|---|---|---|---|
| 2000 | 2001 | 2008 | 2009 | |
| GP217 | 0.09 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| HB-4 | 10.6 ± 1.5 | 5.5 ± 0.7 | 5.8 ± 1.9 | 5.6 ± 1.7 |
| NII-1 | 3.0 ± 0.5 | 8.0 ± 1.1 | 5.9 ± 1.8 | 8.2 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, V. Impact of Intron and Retransformation on Transgene Expression in Leaf and Fruit Tissues of Field-Grown Pear Trees. Int. J. Mol. Sci. 2023, 24, 12883. https://doi.org/10.3390/ijms241612883
Lebedev V. Impact of Intron and Retransformation on Transgene Expression in Leaf and Fruit Tissues of Field-Grown Pear Trees. International Journal of Molecular Sciences. 2023; 24(16):12883. https://doi.org/10.3390/ijms241612883
Chicago/Turabian StyleLebedev, Vadim. 2023. "Impact of Intron and Retransformation on Transgene Expression in Leaf and Fruit Tissues of Field-Grown Pear Trees" International Journal of Molecular Sciences 24, no. 16: 12883. https://doi.org/10.3390/ijms241612883
APA StyleLebedev, V. (2023). Impact of Intron and Retransformation on Transgene Expression in Leaf and Fruit Tissues of Field-Grown Pear Trees. International Journal of Molecular Sciences, 24(16), 12883. https://doi.org/10.3390/ijms241612883






