Expression of Prostaglandin Genes and β-Catenin in Whole Blood as Potential Markers of Muscle Degeneration
Abstract
:1. Introduction
2. Results
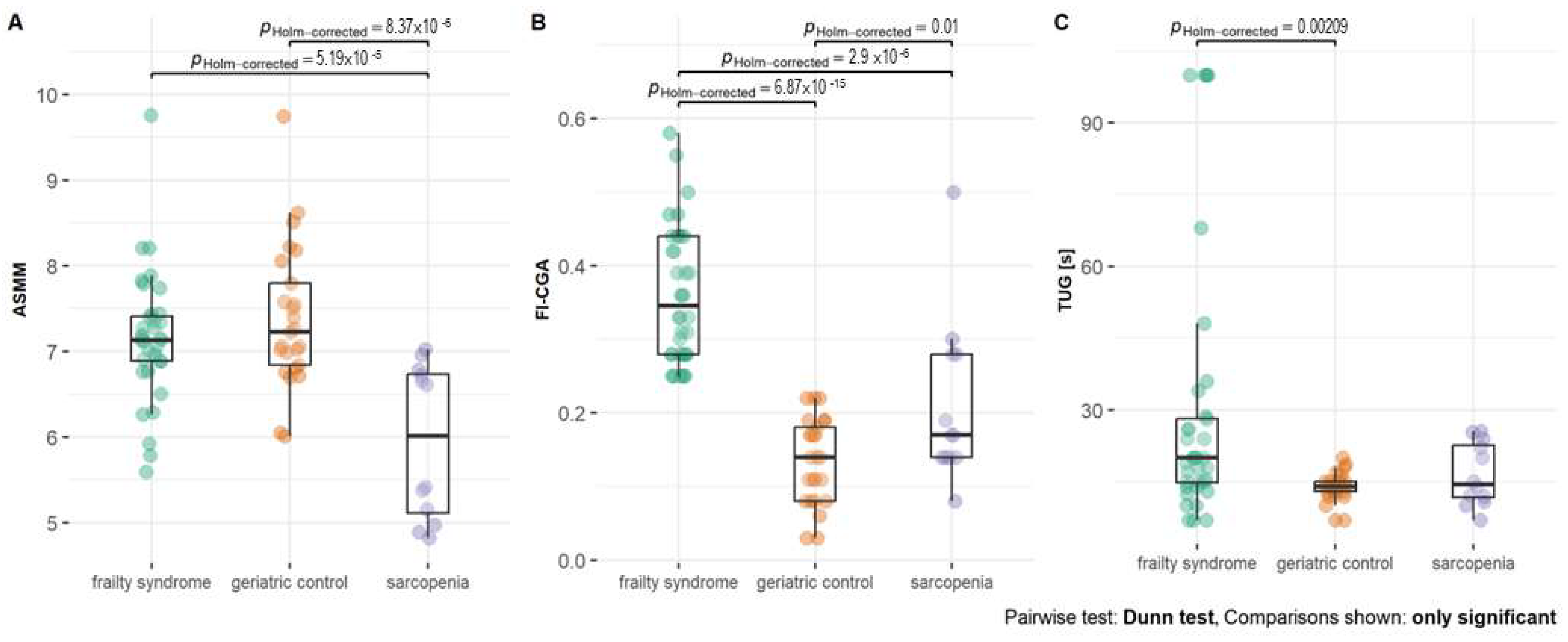
2.1. Clinical Characteristics of Study Groups
2.2. Significant Impact of Age on the Expression of the PTGER4 Gene
2.3. Serum Cytokines Level
2.4. Negative Trend between ASSM and the Expression of PTGER4
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. RNA Extraction
4.3. Gene Expression
4.4. Serum Cytokine Level
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty Syndrome: Implications and Challenges for Health Care Policy. Risk Manag. Healthcare Policy 2019, 12, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of Prostaglandin E2 in Tissue Repair and Regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, J.M.; Martin, C.; Morelli, M.P.; Schander, J.A.; Tateosian, N.L.; Amiano, N.O.; Rollandelli, A.; Palmero, D.J.; Levi, A.; Ciallella, L.; et al. PGE2 Displays Immunosuppressive Effects during Human Active Tuberculosis. Sci. Rep. 2021, 11, 13559. [Google Scholar] [CrossRef]
- Mo, C.; Zhao, R.; Vallejo, J.; Igwe, O.; Bonewald, L.; Wetmore, L.; Brotto, M. Prostaglandin E2 Promotes Proliferation of Skeletal Muscle Myoblasts via EP4 Receptor Activation. Cell Cycle 2015, 14, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Jemiolo, B.; Lavin, K.M.; Lester, B.E.; Trappe, S.W.; Trappe, T.A. Prostaglandin E2/Cyclooxygenase Pathway in Human Skeletal Muscle: Influence of Muscle Fiber Type and Age. J. Appl. Physiol. 2016, 120, 546–551. [Google Scholar] [CrossRef]
- Cilli, F.; Khan, M.; Fu, F.; Wang, J.H.C. Prostaglandin E2 Affects Proliferation and Collagen Synthesis by Human Patellar Tendon Fibroblasts. Clin. J. Sport Med. 2004, 14, 232–236. [Google Scholar] [CrossRef]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring Metabolism of Myeloid Cells Reverses Cognitive Decline in Ageing. Nature 2021, 590, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Ravichandran, M.; Wang, Y.X.; Alexandrova, L.; Yang, A.V.; Kraft, P.; Holbrook, C.A.; Schürch, C.M.; Ho, A.T.V.; Blau, H.M. Inhibition of Prostaglandin-Degrading Enzyme 15-PGDH Rejuvenates Aged Muscle Mass and Strength. Science 2021, 371, eabc8059. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Fumagalli, A.; Conti, S.; Madotto, F.; Bini, F.; Harari, S.; Mondoni, M.; Oggionni, T.; Barisione, E.; Ceruti, P.; et al. Sarcopenia in Idiopathic Pulmonary Fibrosis: A Prospective Study Exploring Prevalence, Associated Factors and Diagnostic Approach. Respir. Res. 2022, 23, 228. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia Is Associated with Severe Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yao, Y.; Chen, Y.; Yue, C.; Chen, J.; Tong, J.; Jiang, Y.; Chen, T. Crosstalk between AhR and Wnt/β-Catenin Signal Pathways in the Cardiac Developmental Toxicity of PM2.5 in Zebrafish Embryos. Toxicology 2016, 355–356, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The Many Faces and Functions of β-Catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Saenz, A.; Atreya, C.E.; Wang, C.; Pan, B.; Dreyer, C.A.; Brunen, D.; Prahallad, A.; Muñoz, D.P.; Ramms, D.J.; Burghi, V.; et al. A Reversible SRC-Relayed COX2 Inflammatory Program Drives Resistance to BRAF and EGFR Inhibition in BRAF(V600E) Colorectal Tumors. Nat. Cancer 2023, 4, 240–256. [Google Scholar] [CrossRef]
- Sha, Y.; Hong, H.; Cai, W.; Sun, T. Single-Cell Transcriptomics of Endothelial Cells in Upper and Lower Human Esophageal Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 7680–7694. [Google Scholar] [CrossRef]
- Cai, S.; Gao, Z. Atorvastatin Inhibits Proliferation and Promotes Apoptosis of Colon Cancer Cells via COX-2/PGE2/β-Catenin Pathway. J. BUON 2021, 26, 1219–1225. [Google Scholar]
- Wu, L.; Amarachintha, S.; Xu, J.; Oley, F.J.; Du, W. Mesenchymal COX2-PG Secretome Engages NR4A-WNT Signalling Axis in Haematopoietic Progenitors to Suppress Anti-Leukaemia Immunity. Br. J. Haematol. 2018, 183, 445–456. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Pan, X.; Dai, X.; Pan, G.; Li, Z.; Mai, X.; Tian, Y.; Zhang, S.; Liu, B.; et al. Transferrin Receptor 1 Ablation in Satellite Cells Impedes Skeletal Muscle Regeneration through Activation of Ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Jiao, W.; Wang, Y.; Wang, X.; Zhao, Y.; Fan, X.; Tian, L.; Li, X.; Mi, J. Ferroptosis and Its Role in Skeletal Muscle Diseases. Front. Mol. Biosci. 2022, 9, 1051866. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Vaish, V.; Feng, M.; Field, K.; Chatzistamou, I.; Shim, M. Transgenic Expression of Cyclooxygenase-2 (COX2) Causes Premature Aging Phenotypes in Mice. Aging 2016, 8, 2392–2406. [Google Scholar] [CrossRef] [PubMed]
- Kinitz, R.; Heyne, E.; Koch, L.G.; Britton, S.L.; Thierbach, M.; Wildemann, B. The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. Int. J. Mol. Sci. 2022, 23, 79. [Google Scholar] [CrossRef]
- Yamamoto, R.; Chung, R.; Vazquez, J.M.; Sheng, H.; Steinberg, P.L.; Ioannidis, N.M.; Sudmant, P.H. Tissue-Specific Impacts of Aging and Genetics on Gene Expression Patterns in Humans. Nat. Commun. 2022, 13, 5803. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T. Overlaps between Frailty and Sarcopenia Definitions. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 65–69. [Google Scholar] [CrossRef]
- Trappe, T.A.; Ratchford, S.M.; Brower, B.E.; Liu, S.Z.; Lavin, K.M.; Carroll, C.C.; Jemiolo, B.; Trappe, S.W. COX Inhibitor Influence on Skeletal Muscle Fiber Size and Metabolic Adaptations to Resistance Exercise in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1289–1294. [Google Scholar] [CrossRef]
- Lan, T.; Wei, X. Inhibition of 15-PDGH: A Strategy to Rejuvenate Aged Muscles? Mol. Biomed. 2021, 2, 14. [Google Scholar] [CrossRef]
- Trappe, T.A.; Liu, S.Z. Effects of Prostaglandins and COX-Inhibiting Drugs on Skeletal Muscle Adaptations to Exercise. J. Appl. Physiol. 2013, 115, 909–919. [Google Scholar] [CrossRef]
- Acevedo, N.; Reinius, L.E.; Vitezic, M.; Fortino, V.; Söderhäll, C.; Honkanen, H.; Veijola, R.; Simell, O.; Toppari, J.; Ilonen, J.; et al. Age-Associated DNA Methylation Changes in Immune Genes, Histone Modifiers and Chromatin Remodeling Factors within 5 Years after Birth in Human Blood Leukocytes. Clin. Epigenet. 2015, 7, 34. [Google Scholar] [CrossRef]
- Ho, A.T.V.; Palla, A.R.; Blake, M.R.; Yucel, N.D.; Wang, Y.X.; Magnusson, K.E.G.; Holbrook, C.A.; Kraft, P.E.; Delp, S.L.; Blau, H.M. Prostaglandin E2 Is Essential for Efficacious Skeletal Muscle Stem-Cell Function, Augmenting Regeneration & Strength. Proc. Natl. Acad. Sci. USA 2017, 114, 6675. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M.; Dedkov, E.I.; Borisov, A.B.; Faulkner, J.A. Skeletal Muscle Regeneration in Very Old Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B224–B233. [Google Scholar] [CrossRef] [PubMed]
- Shefer, G.; Van de Mark, D.P.; Richardson, J.B.; Yablonka-Reuveni, Z. Satellite-Cell Pool Size Does Matter: Defining the Myogenic Potency of Aging Skeletal Muscle. Dev. Biol. 2006, 294, 50–66. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling during Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Florian, M.C.; Nattamai, K.J.; Dörr, K.; Marka, G.; Uberle, B.; Vas, V.; Eckl, C.; Andrä, I.; Schiemann, M.; Oostendorp, R.A.J.; et al. A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing. Nature 2013, 503, 392–396. [Google Scholar] [CrossRef]
- Panteghini, M. Serum Isoforms of Creatine Kinase Isoenzymes. Clin. Biochem. 1988, 21, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Y. Ferroptosis: An Iron-Dependent Cell Death Form Linking Metabolism, Diseases, Immune Cell and Targeted Therapy. Clin. Transl. Oncol. 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Xie, M.; Kang, R.; Fan, Y.; Niu, X.; Wang, H.; Cao, L.; Tang, D. HSPB1 as a Novel Regulator of Ferroptotic Cancer Cell Death. Oncogene 2015, 34, 5617–5625. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Li, K.; Yuan, D.; Yang, S.; Zhou, L.; Zhao, Y.; Miao, S.; Lv, C.; Zhao, J. COX-2/PGE2 Pathway Inhibits the Ferroptosis Induced by Cerebral Ischemia Reperfusion. Mol. Neurobiol. 2022, 59, 1619–1631. [Google Scholar] [CrossRef]
- Pasricha, S.R.S.; Flecknoe-Brown, S.C.; Allen, K.J.; Gibson, P.R.; McMahon, L.P.; Olynyk, J.K.; Roger, S.D.; Savoia, H.F.; Tampi, R.; Thomson, A.R.; et al. Diagnosis and Management of Iron Deficiency Anaemia: A Clinical Update. Med. J. Aust. 2010, 193, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin--a Regulator of Intestinal Iron Absorption and Iron Recycling by Macrophages. Best Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–580S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, W.; Zhu, L.; Li, J.; Yao, Y.; Chen, F.; Sun, M.; Zhang, J.; Shen, N.; Song, Y.; et al. Iron Drives T Helper Cell Pathogenicity by Promoting RNA-Binding Protein PCBP1-Mediated Proinflammatory Cytokine Production. Immunity 2018, 49, 80–92.e7. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Ogtata, Y.; Shimizu, E.; Yamazaki, M.; Furuyama, S.; Sugiya, H. Tumor Necrosis Factor Alpha (TNF-Alpha)-Induced Prostaglandin E2 Release Is Mediated by the Activation of Cyclooxygenase-2 (COX-2) Transcription via NFkappaB in Human Gingival Fibroblasts. Mol. Cell Biochem. 2002, 238, 11–18. [Google Scholar] [CrossRef]
- Fournier, T.; Fadok, V.; Henson, P.M. Tumor Necrosis Factor-Alpha Inversely Regulates Prostaglandin D2 and Prostaglandin E2 Production in Murine Macrophages. Synergistic Action of Cyclic AMP on Cyclooxygenase-2 Expression and Prostaglandin E2 Synthesis. J. Biol. Chem. 1997, 272, 31065–31072. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, R.; Chen, D.; Mao, J.; Shi, R.; Wu, Z.; Kang, J.; Tian, W.; Zhang, C. COX2 Is Involved in Hypoxia-Induced TNF-α Expression in Osteoblast. Sci. Rep. 2015, 5, 10020. [Google Scholar] [CrossRef]
- Ackerman, W.E., 4th; Summerfield, T.L.S.; Vandre, D.D.; Robinson, J.M.; Kniss, D.A. Nuclear Factor-Kappa B Regulates Inducible Prostaglandin E Synthase Expression in Human Amnion Mesenchymal Cells. Biol. Reprod. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Eicosanoid Pathway Expression in Bovine Endometrial Epithelial and Stromal Cells in Response to Lipopolysaccharide, Interleukin 1 Beta, and Tumor Necrosis Factor Alpha. Reprod. Biol. 2018, 18, 390–396. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J. Am. Geriatr Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “Ggplot2”. R Package 2020. Available online: https://wilkelab.org/cowplot/index.html (accessed on 16 August 2023).
- Wickham, H. Bryan Jennifer Readxl: Read Excel Files. R Package 2019. Available online: https://readxl.tidyverse.org/ (accessed on 16 August 2023).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package 2021. Available online: https://dplyr.tidyverse.org/ (accessed on 16 August 2023).
- DeWitt, P. Qwraps2: Quick Wraps 2. R Package 2021. Available online: https://cran.r-project.org/web/packages/qwraps2/qwraps2.pdf (accessed on 16 August 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. Springer Nature: New York, NY, USA, 2016; ISBN 978-0-387-98140-6. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: New York, NY, USA, 2019. [Google Scholar]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]




| Frailty Syndrome (N = 36) | Geriatric Control (N = 25) | Sarcopenia (N = 12) | p-Value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 80.50 ± 8.72 | 72.56 ± 7.86 | 77.33 ± 13.75 | 0.004 a |
| Gender: Women n (%) Men n (%) | 25 (69.44%) 11 (30.56%) | 17 (68.00%) 8 (32.00%) | 6 (50.00%) 6 (50.00%) | 0.45 c |
| mean ± sd | mean ± sd | mean ± sd | p-value a | |
| ASMM (kg) | 7.19 ± 0.74 | 7.47 ± 0.80 | 5.95 ± 0.90 | <0.0001 |
| FI-CGA | 0.36 ± 0.09 | 0.14 ± 0.06 | 0.21 ± 0.11 | 0.004 |
| BMI (kg/m2) | 27.61 ± 6.37 | 29.86 ± 5.28 | 23.18 ± 2.97 | 0.005 |
| Cholesterol (mg/dL) | 173.85 ± 41.31 | 204.24 ± 42.99 | 194.50 ± 42.75 | 0.022 |
| Triglycerides (mmol/L) | 138.74 ± 66.59 | 147.04 ± 60.18 | 105.58 ± 42.91 | 0.154 |
| LDL (mg/dL) | 91.06 ± 30.08 | 120.50 ± 37.65 | 100.65 ± 37.64 | 0.006 |
| Albumin (g/dL) | 3.98 ± 0.49 | 4.10 ± 0.32 | 4.17 ± 0.38 | 0.289 |
| Hemoglobin (g/dL) | 12.11 ± 1.73 | 13.29 ± 1.43 | 12.50 ± 1.60 | 0.023 |
| LDH (U/L) | 252.89 ± 103.51 | 229.81 ± 41.67 | 260.52 ± 61.35 | 0.443 |
| Creatinine (µmol/L) | 0.88 ± 0.24 | 0.89 ± 0.27 | 0.97 ± 0.29 | 0.605 |
| Vit. B12 (pg/mL) | 397.72 ± 187.11 | 426.20 ± 243.40 | 455.65 ± 245.43 | 0.704 |
| Serum Fe level (mcg/dL) | 61.16 ± 22.84 | 84.11 ± 22.97 | 79.96 ± 10.50 | <0.0001 |
| median (IQR) | median (IQR) | median (IQR) | p-value b | |
| TUG (s) | 20.00 (14.75, 26.50) | 14.00 (13.00, 15.00) | 14.50 (11.70, 21.97) | <0.0001 |
| ESR (mm/h) | 22.00 (12.50, 35.25) | 10.00 (8.00, 22.00) | 11.00 (8.00, 15.50) | 0.048 |
| CRP (mg/dL) | 7.00 (5.00, 25.50) | 5.00 (5.00, 12.00) | 5.00 (5.00, 6.00) | 0.062 |
| NT-proBNP (pg/mL) | 264.30 (163.67, 510.20) | 258.60 (164.60, 559.60) | 354.80 (277.88, 666.57) | 0.308 |
| CK (U/L) | 71.00 (45.25, 86.50) | 72.00 (56.00, 106.00) | 67.00 (55.00, 70.79) | 0.382 |
| n (%) | n (%) | n (%) | p-value c | |
| Osteopenia | 0.009 | |||
| Present | 4 (11.11%) | 2 (8.00%) | 6 (50.00%) | |
| Not present | 32 (88.89%) | 23 (92.00%) | 6 (50.00%) | |
| NYHA class | 0.153 | |||
| 0 | 27 (75.00%) | 23 (92.00%) | 7 (58.33%) | |
| I | 1 (2.78%) | 0 (0.00%) | 0 (0.00%) | |
| II | 6 (16.67%) | 1 (4.00%) | 3 (25.00%) | |
| II-III | 1 (2.78%) | 1 (4.00%) | 2 (16.67%) | |
| III | 1 (2.78%) | 0 (0.00%) | 0 (0.00%) | |
| Decrease | 0.213 | |||
| Yes | 7 (19.44%) | 2 (8.00%) | 0 (0.00%) | |
| No | 29 (80.56%) | 23 (92.00%) | 12 (100.00%) | |
| Polyarthritis | 0.574 | |||
| Present | 18 (50.00%) | 13 (52.00%) | 4 (33.33%) | |
| Not present | 18 (50.00%) | 12 (48.00%) | 8 (66.67%) | |
| Osteoporosis | 0.691 | |||
| Present | 9 (25.00%) | 4 (16.00%) | 3 (25.00%) | |
| Not present | 27 (75.00%) | 21 (84.00%) | 9 (75.00%) | |
| Degenerative disc disease | 0.945 | |||
| Present | 13 (36.11%) | 8 (32.00%) | 4 (33.33%) | |
| Not present | 23 (63.89%) | 17 (68.00%) | 8 (66.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajda, A.; Bogucka, D.; Stypińska, B.; Radkowski, M.J.; Targowski, T.; Dudek, E.; Kmiołek, T.; Modzelewska, E.; Paradowska-Gorycka, A. Expression of Prostaglandin Genes and β-Catenin in Whole Blood as Potential Markers of Muscle Degeneration. Int. J. Mol. Sci. 2023, 24, 12885. https://doi.org/10.3390/ijms241612885
Wajda A, Bogucka D, Stypińska B, Radkowski MJ, Targowski T, Dudek E, Kmiołek T, Modzelewska E, Paradowska-Gorycka A. Expression of Prostaglandin Genes and β-Catenin in Whole Blood as Potential Markers of Muscle Degeneration. International Journal of Molecular Sciences. 2023; 24(16):12885. https://doi.org/10.3390/ijms241612885
Chicago/Turabian StyleWajda, Anna, Diana Bogucka, Barbara Stypińska, Marcin Jerzy Radkowski, Tomasz Targowski, Ewa Dudek, Tomasz Kmiołek, Ewa Modzelewska, and Agnieszka Paradowska-Gorycka. 2023. "Expression of Prostaglandin Genes and β-Catenin in Whole Blood as Potential Markers of Muscle Degeneration" International Journal of Molecular Sciences 24, no. 16: 12885. https://doi.org/10.3390/ijms241612885





