Low Molecular Weight and High Deacetylation Degree Chitosan Batch Alleviates Pathogenesis, Toxin Accumulation, and Fusarium Gene Regulation in Barley Leaf Pathosystem
Abstract
:1. Introduction
2. Results and Discussion
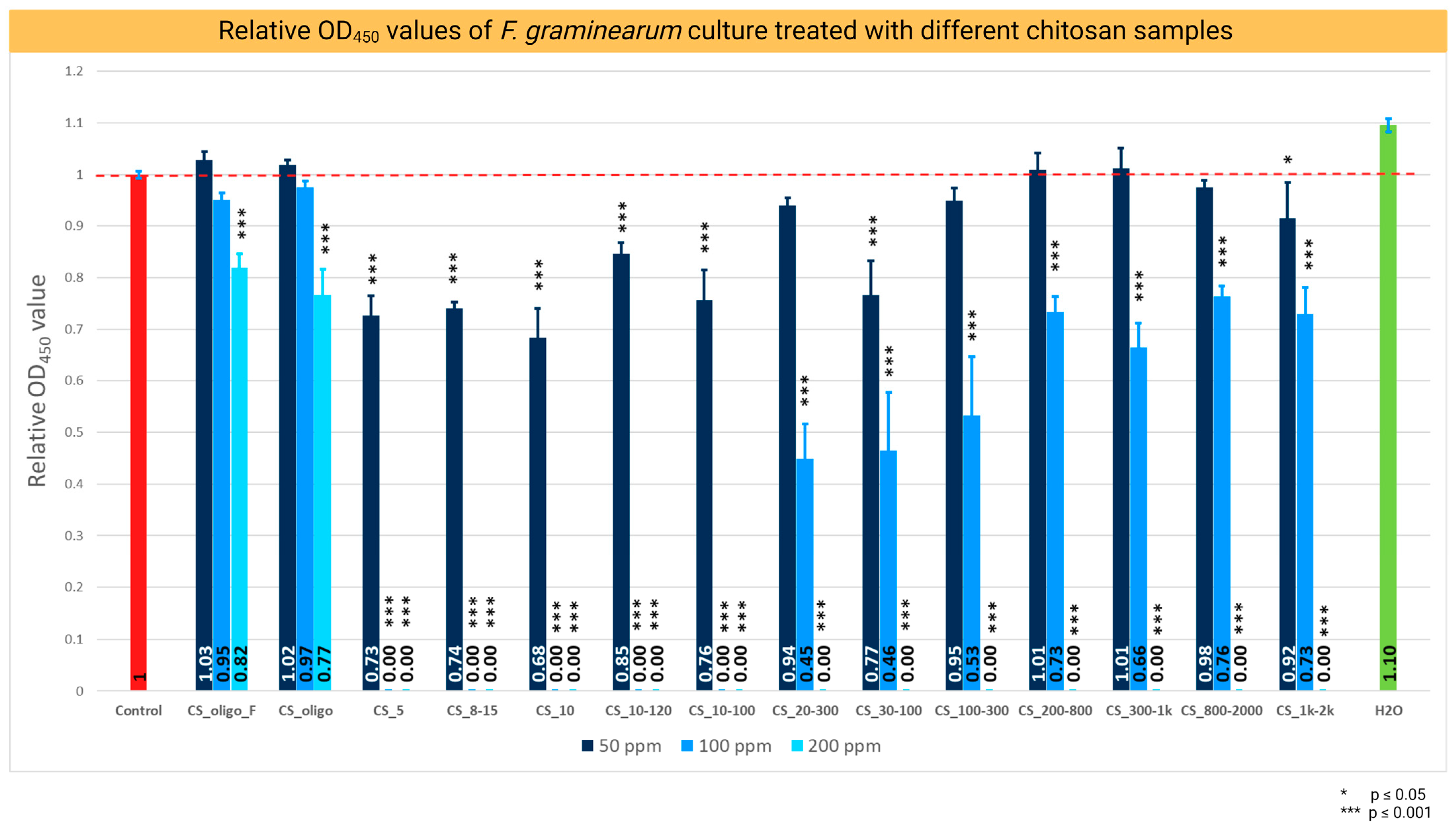
2.1. Chitosan Antifungal Activity and Minimum Inhibitory Concentration (MIC)
2.2. Identifying the Chitosan Application Time-Point for Transcriptomics
2.3. Chitosan Treatment Reduces the Accumulation of Mycotoxins of F. graminearum in Barley Leaves
2.4. Fg Transcriptomics in Planta and Chitosan Applications
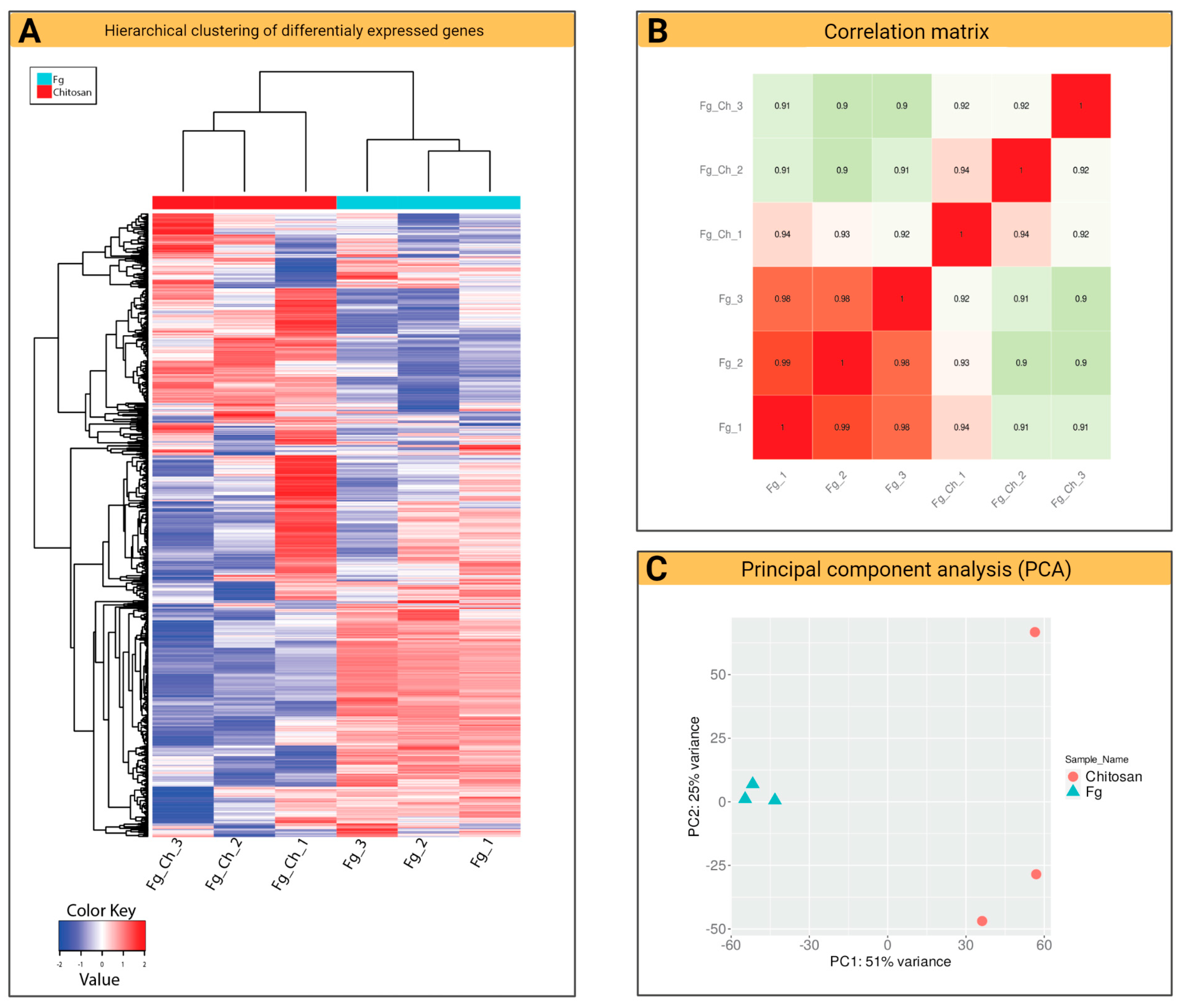
2.4.1. General Characteristics of F. graminearum Transcriptomes (RNA-Seq)
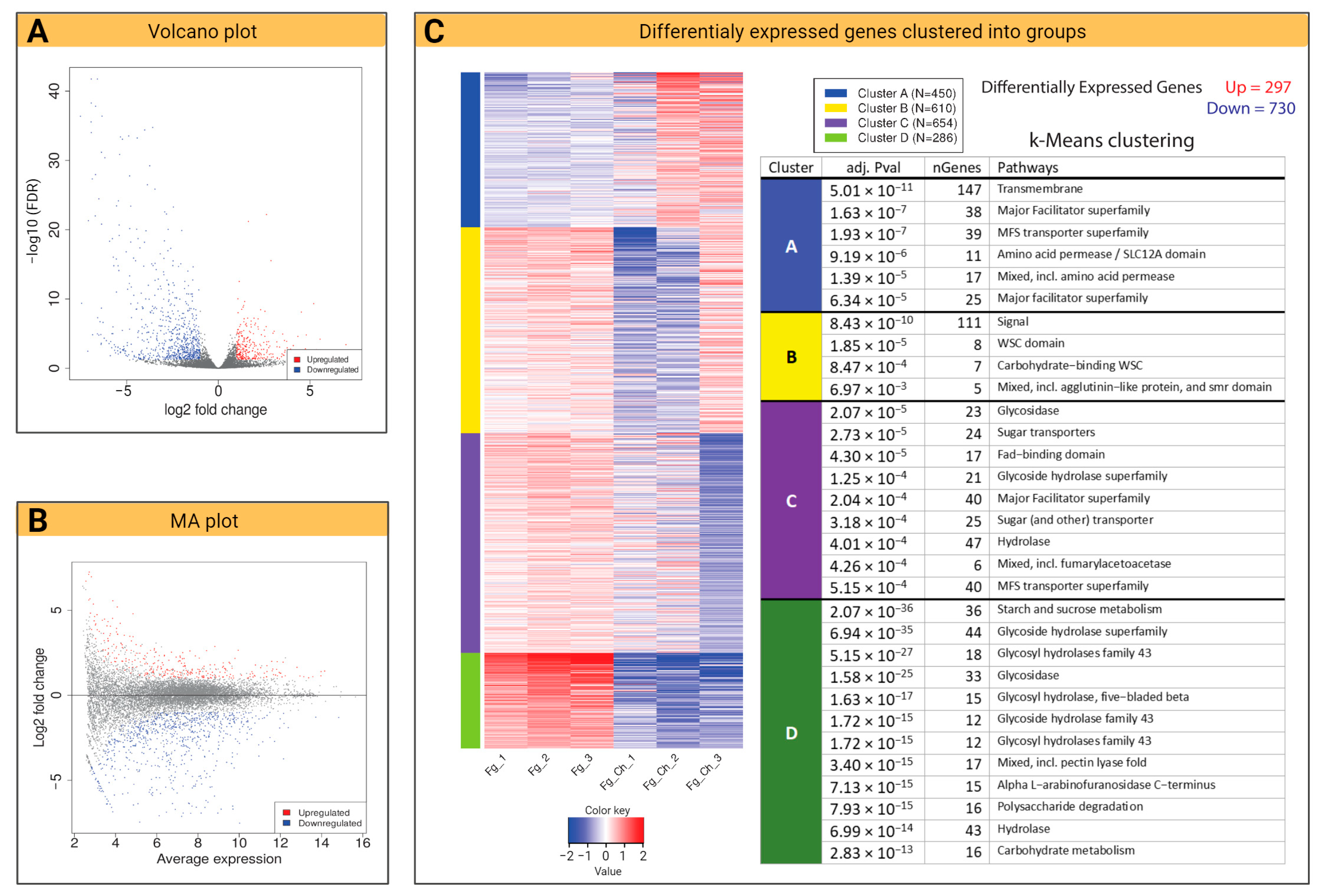
2.4.2. Volcano Plot and k-Means Clustering
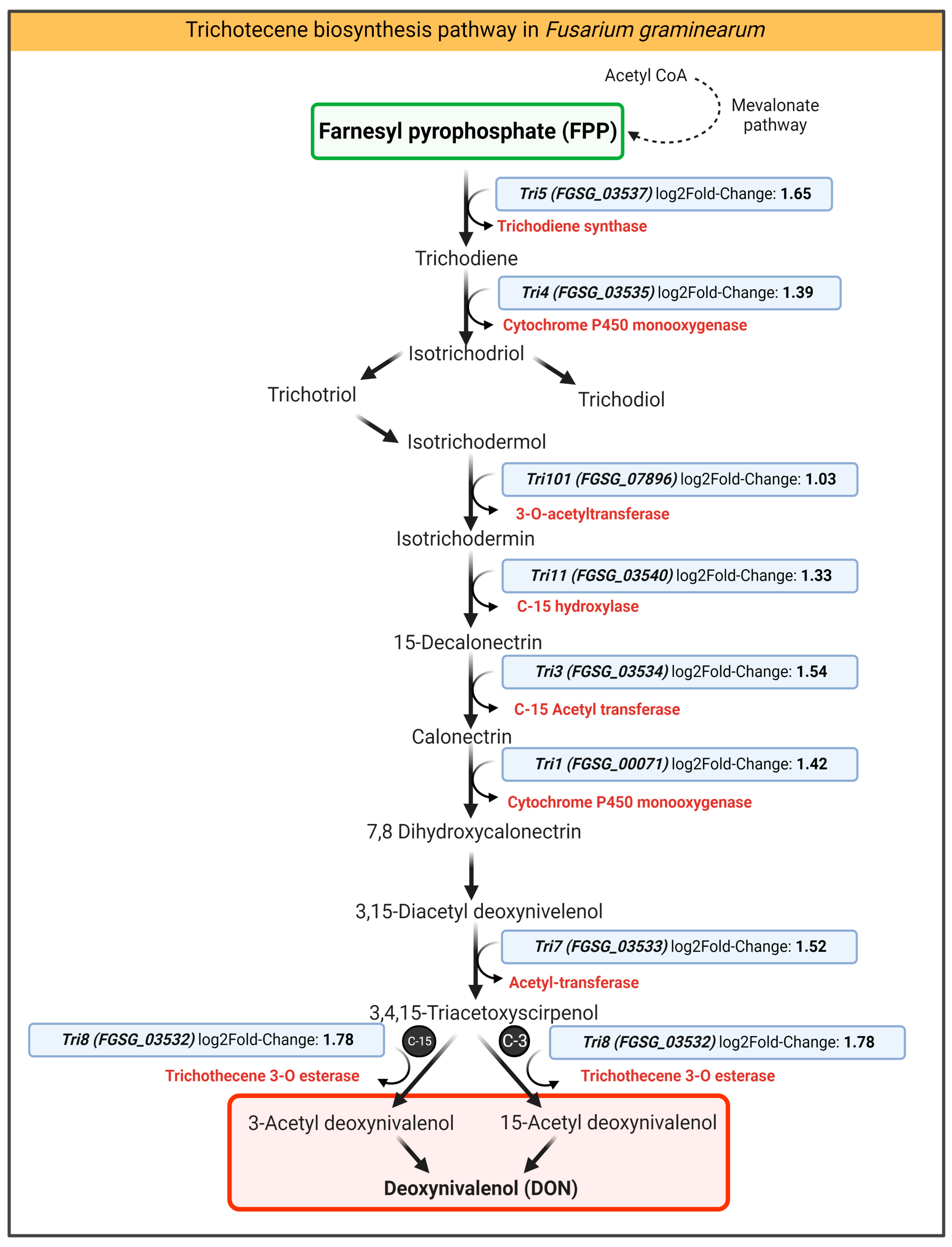
2.4.3. Chitosan Impact on DON-Related Transcripts of F. graminearum
2.4.4. DEG and KEGG Path Analysis
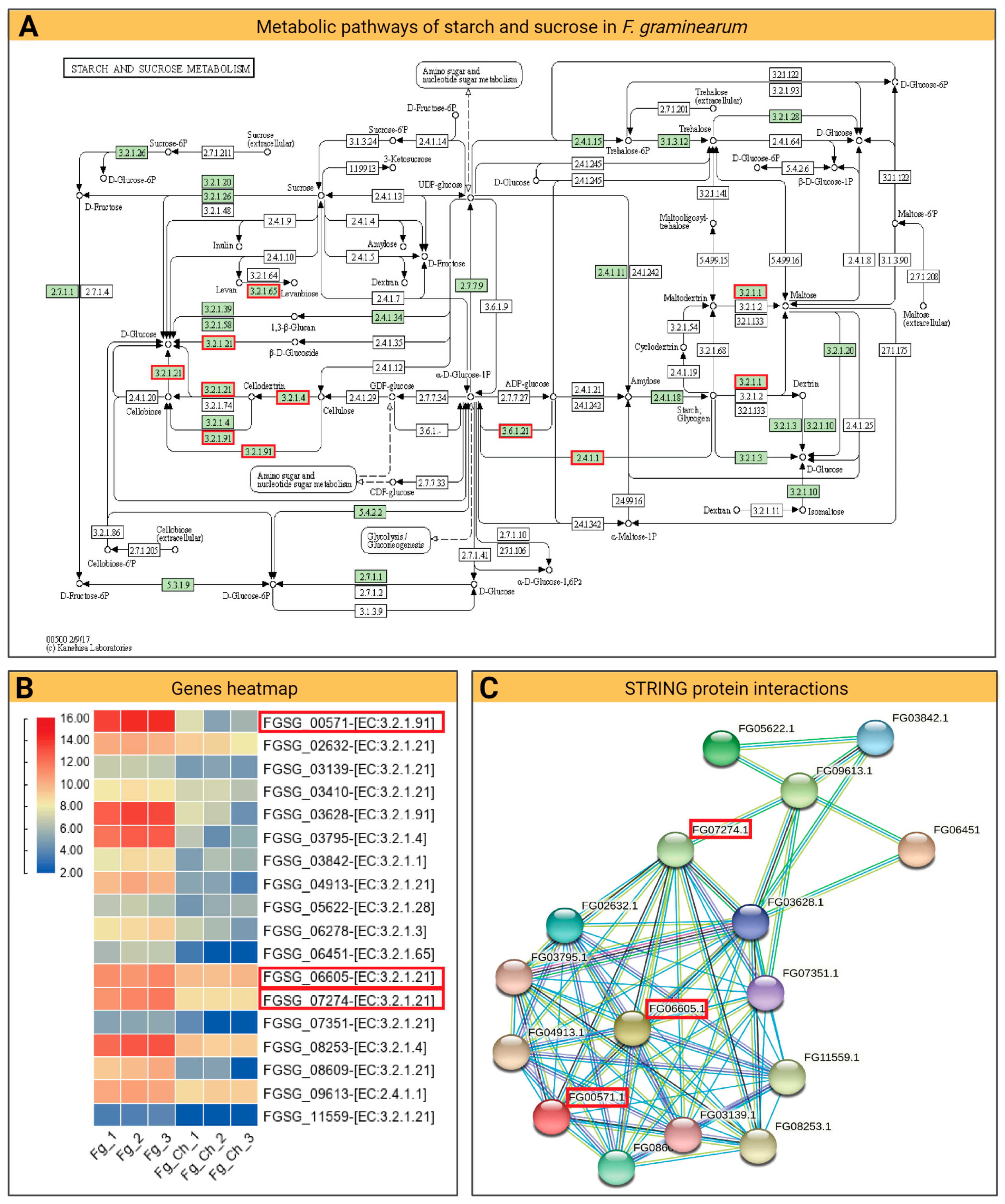
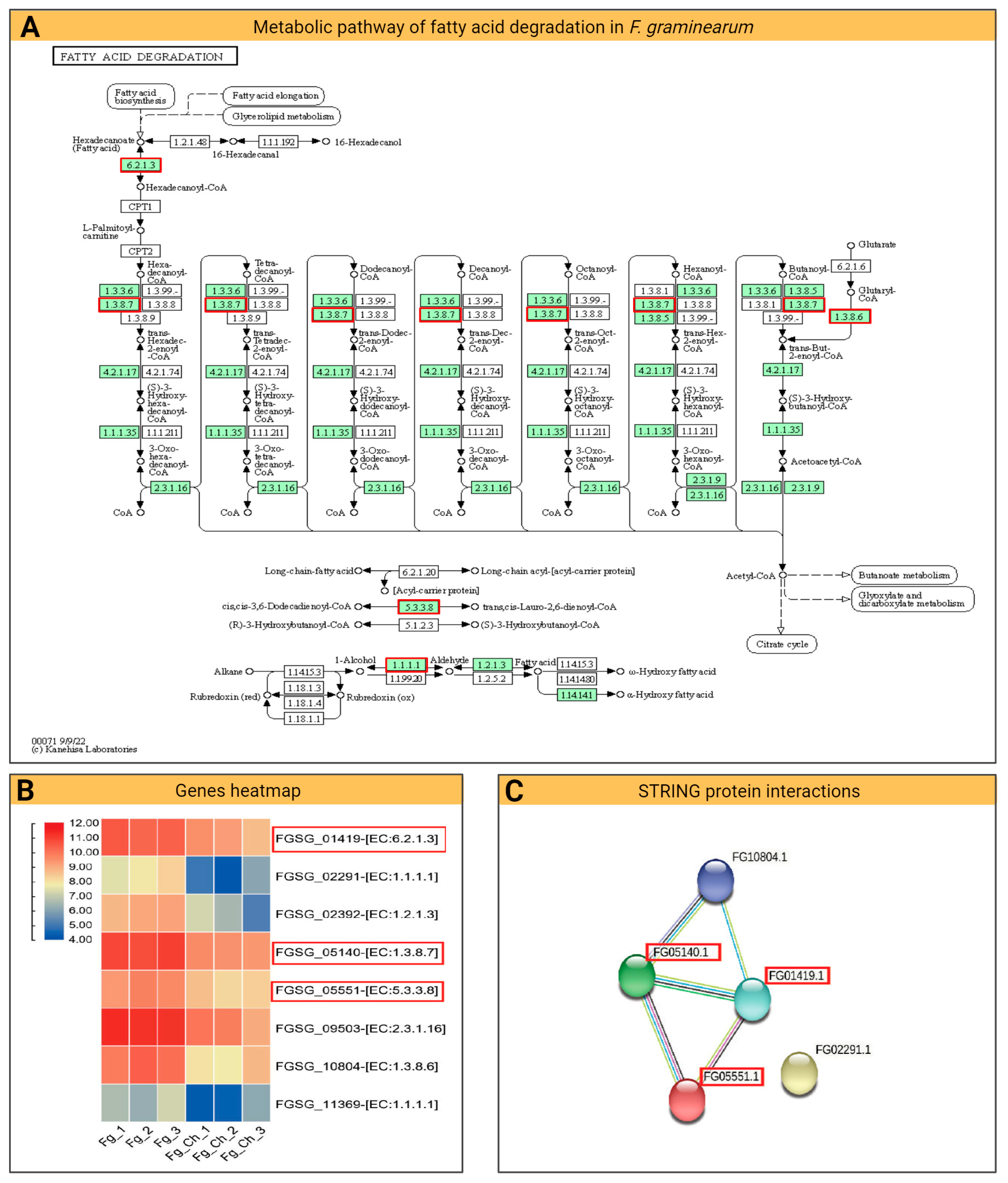
2.4.5. Fatty Acid Degradation Pathway
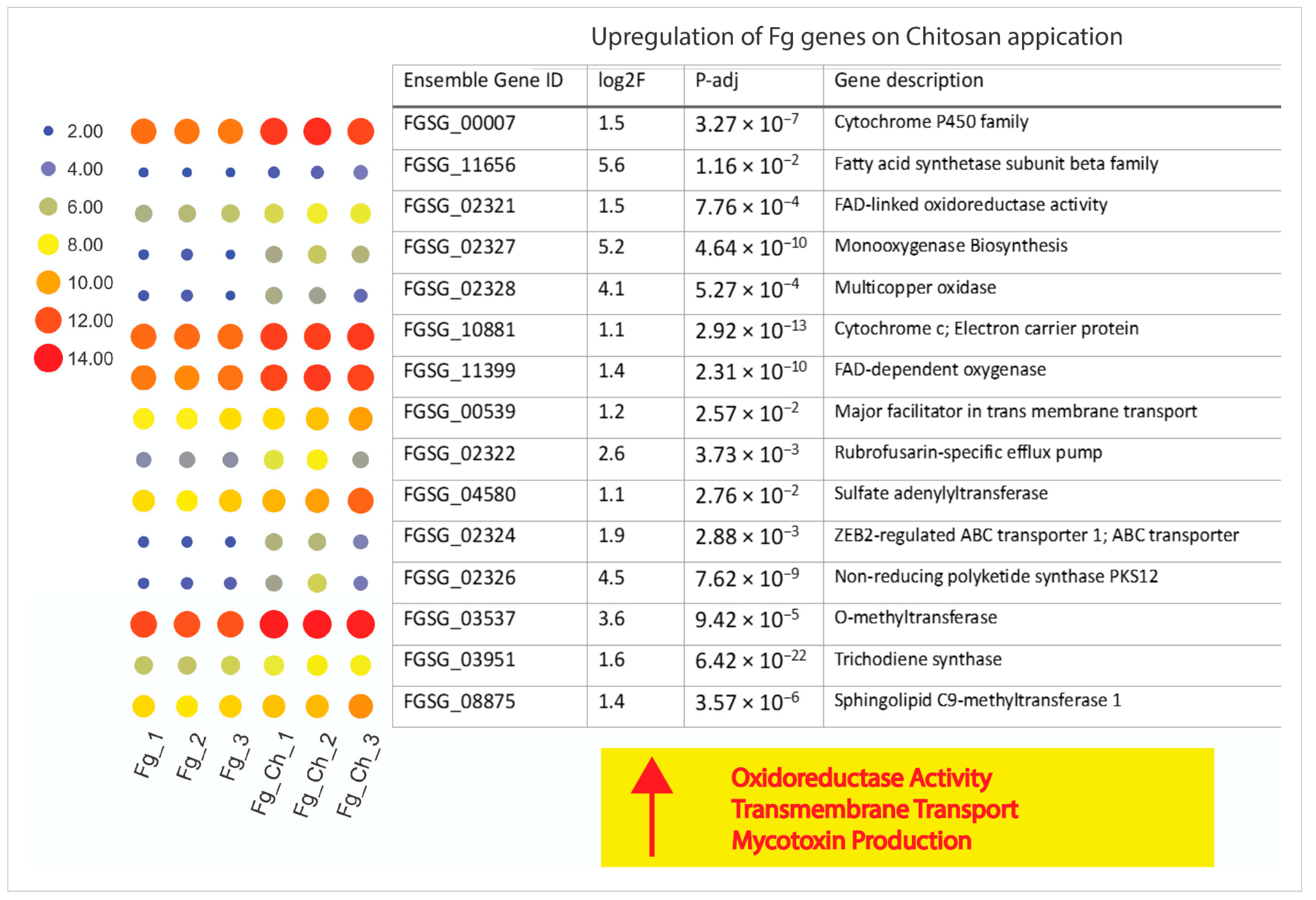
2.4.6. Chitosan Treatment Up-Regulates Stress-Responsive Genes of F. graminearum
2.4.7. qPCR Validation of RNA-Seq Data
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Chitosan Solution Preparation
3.2.2. Production of Macroconidia of F. graminearum
3.2.3. Determination of the Impact of Chitosan on F. graminearum Growth
- Antifungal activity of various batches of chitosan
- Petri plates assay
- Time-dependent response of Fg macroconidia and mycelium to chitosan application
- F. graminearum macroconidia Trypan blue viability test
- Statistical analysis
3.2.4. Inoculating Barley Leaves with F. graminearum
3.2.5. Deoxynivalenol Analysis in Barley Leaf Samples
3.3. RNA-Seq Data Analysis
3.3.1. RNA Extraction and mRNA Sequencing
3.3.2. The Quality Control and Mapping Sequence Read
3.3.3. Analyzing RNA-Seq Outputs
3.4. Quantitative Real-Time PCR (qRT-PCR) of Selected F. graminearum Genes
3.5. Estimation of F. graminearum in Barley Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium head blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Alisaac, E.M.; Fusarium, A.-K. Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Wu, D.; Wan, J.; Lu, J.; Wang, X.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Chitosan coatings on lecithin stabilized emulsions inhibit mycotoxin production by Fusarium pathogens. Food Control 2018, 92, 276–285. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Francesconi, S.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Sulyok, M.; Balestra, G.M. Chitosan Hydrochloride Decreases Fusarium graminearum Growth and Virulence and Boosts Growth, Development and Systemic Acquired Resistance in Two Durum Wheat Genotypes. Molecules 2020, 25, 4752. [Google Scholar] [CrossRef] [PubMed]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal Activity of Chitosan Oligomers–Amino Acid Conjugate Complexes against Fusarium culmorum in Spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Al-Hetar, M.; Zainal Abidin, M.; Sariah, M.; Wong, M. Antifungal activity of chitosan against Fusarium oxysporum f. sp. Cubense. J. Appl. Polym. Sci. 2011, 120, 2434–2439. [Google Scholar] [CrossRef]
- Schenk, P.M.; Carvalhais, L.C.; Kazan, K. Unraveling plant–microbe interactions: Can multi-species transcriptomics help? Trends Biotechnol. 2012, 30, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.M.; Vogel, J. Differential RNA-seq: The approach behind and the biological insight gained. Curr. Opin. Microbiol. 2014, 19, 97–105. [Google Scholar] [CrossRef]
- Hameed, A.; Poznanski, P.; Noman, M.; Ahmed, T.; Iqbal, A.; Nadolska-Orczyk, A.; Orczyk, W. Barley Resistance to Fusarium graminearum Infections: From Transcriptomics to Field with Food Safety Concerns. J. Agric. Food Chem. 2022, 70, 14571–14587. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, M.-H.; Park, S.-C.; Cheong, H.-S.; Jang, M.-K.; Nah, J.-W.; Hahm, K.-S. Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. J. Microbiol. Biotechnol. 2008, 18, 1729–1734. [Google Scholar]
- El-Mohamedya, R.; El-Aziz, M.A.; Kamel, S. Antifungal activity of chitosan nanoparticles against some plant pathogenic fungi in vitro. Agric. Eng. Int. CIGR J 2019, 21, 201–209. [Google Scholar]
- Lopez-Moya, F.; Kowbel, D.; Nueda, M.J.; Palma-Guerrero, J.; Glass, N.L.; Lopez-Llorca, L.V. Neurospora crassa transcriptomics reveals oxidative stress and plasma membrane homeostasis biology genes as key targets in response to chitosan. Mol. Biosyst. 2016, 12, 391–403. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Fang, Q.; Li, Y.; Zheng, X.; Zhang, Z. SNARE protein FgVam7 controls growth, asexual and sexual development, and plant infection in Fusarium graminearum. Mol. Plant Pathol. 2016, 17, 108–119. [Google Scholar] [CrossRef]
- Deshaies, M.; Lamari, N.; Ng, C.K.Y.; Ward, P.; Doohan, F.M. The impact of chitosan on the early metabolomic response of wheat to infection by Fusarium graminearum. BMC Plant Biol. 2022, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245. [Google Scholar] [CrossRef]
- McDermaid, A.; Monier, B.; Zhao, J.; Liu, B.; Ma, Q. Interpretation of differential gene expression results of RNA-seq data: Review and integration. Brief. Bioinform. 2019, 20, 2044–2054. [Google Scholar] [CrossRef]
- Weirauch, M.T.; Yang, A.; Albu, M.; Cote, A.G.; Montenegro-Montero, A.; Drewe, P.; Najafabadi, H.S.; Lambert, S.A.; Mann, I.; Cook, K. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 2014, 158, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Merhej, J.; Urban, M.; Dufresne, M.; Hammond-Kosack, K.E.; Richard-Forget, F.; Barreau, C. The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum. Mol. Plant Pathol. 2012, 13, 363–374. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; O’Donnell, K.; Ward, T.J.; Fujimura, M.; Hamamoto, H.; Shibata, T.; Yamaguchi, I. The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett. 2003, 539, 105–110. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Wu, K.-T.; Yang, P.; Hou, F.; Rajput, S.A.; Qi, D.-S.; Wang, S. Transcriptomics Reveals the Effect of Thymol on the Growth and Toxin Production of Fusarium graminearum. Toxins 2022, 14, 142. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Zhu, G.; Zhao, Z.; Wang, H.; Zhou, M.; Zhao, X.; Wu, A. Identification of Candidate Genes Associated with Trichothecene Biosynthesis in Fusarium graminearum Species Complex Combined with Transcriptomic and Proteomic Analysis. Microorganisms 2022, 10, 1479. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Li, P.; Wang, Y.; Hu, Q.; Shang, Y.; Chen, Y.; Zhu, X.; Feng, H.; Zhang, C. Transcriptome Profile of Fusarium graminearum Treated by Putrescine. J. Fungi 2022, 9, 60. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Mering, C.V.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Son, H.; Lee, J.; Choi, G.J.; Kim, J.-C.; Lee, Y.-W. Peroxisome function is required for virulence and survival of Fusarium graminearum. Mol. Plant-Microbe Interact. 2012, 25, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Guenther, J.C.; Hallen-Adams, H.E.; Bücking, H.; Shachar-Hill, Y.; Trail, F. Triacylglyceride metabolism by Fusarium graminearum during colonization and sexual development on wheat. Mol. Plant-Microbe Interact. 2009, 22, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Liang, Y.; Yu, J. FgPEX4 is involved in development, pathogenicity, and cell wall integrity in Fusarium graminearum. Curr. Genet. 2019, 65, 747–758. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, H.; Wang, X.; van Der Lee, T.; Waalwijk, C.; Van Diepeningen, A.; Brankovics, B.; Xu, J.; Xu, J.; Chen, W. FgPex3, a peroxisome biogenesis factor, is involved in regulating vegetative growth, conidiation, sexual development, and virulence in Fusarium graminearum. Front. Microbiol. 2019, 10, 2088. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Shao, W.; Zheng, Z.; Zhou, M.; Chen, C. The septins FaCdc3 and FaCdc12 are required for cytokinesis and affect asexual and sexual development, lipid metabolism and virulence in Fusarium asiaticum. Mol. Plant Pathol. 2017, 18, 1282–1294. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, T.; Jian, Y.; Ma, Z. The very long-chain fatty acid elongase FgElo2 governs tebuconazole sensitivity and virulence in Fusarium graminearum. Environ. Microbiol. 2022, 24, 5362–5377. [Google Scholar] [CrossRef]
- Montibus, M.; Ducos, C.; Bonnin-Verdal, M.N.; Bormann, J.; Ponts, N.; Richard-Forget, F.; Barreau, C. The bZIP transcription factor Fgap1 mediates oxidative stress response and trichothecene biosynthesis but not virulence in Fusarium graminearum. PLoS ONE 2013, 8, e83377. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.-H.; Guo, Y.; Wang, Y.-Z.; Zhang, D.; Xu, L.; Tang, W.-H. A cytoplasmic Cu-Zn superoxide dismutase SOD1 contributes to hyphal growth and virulence of Fusarium graminearum. Fungal Genet. Biol. 2016, 91, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Yuan, M.; Zhang, M.; Abubakar, Y.S.; Chen, X.; Zhong, H.; Zheng, W.; Zheng, H.; Zhou, J. FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum. J. Fungi 2010, 9, 145. [Google Scholar] [CrossRef]
- Puri, K.D.; Yan, C.; Leng, Y.; Zhong, S. RNA-Seq revealed differences in transcriptomes between 3ADON and 15ADON populations of Fusarium graminearum in vitro and in planta. PLoS ONE 2016, 11, e0163803. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Bolger, A.M.; MLohse; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level—The DESeq Package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012; Volume 10, p. f1000research. [Google Scholar]
- Ge, S.X.; ESon, W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Saito, R.; Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.-L.; Lotia, S.; Pico, A.R.; Bader, G.D.; Ideker, T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef]
- Harris, L.J.; Balcerzak, M.; Johnston, A.; Schneiderman, D.; Ouellet, T. Host-preferential Fusarium graminearum gene expression during infection of wheat, barley, and maize. Fungal Biol. 2016, 120, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-S.; Li, H.-P.; Zhang, J.-B.; Song, B.; Huang, T.; Du, X.-M.; Gong, A.-D.; Liu, Y.-K.; Feng, Y.-N.; Agboola, R.S. Trehalose 6-phosphate phosphatase is required for development, virulence and mycotoxin biosynthesis apart from trehalose biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2014, 63, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Bass, C.; Baldwin, T.K.; Chen, H.; Massot, F.; Carion, P.W.; Urban, M.; van de Meene, A.M.; Hammond-Kosack, K.E. Characterisation of the Fusarium graminearum-Wheat Floral Interaction. J. Pathog. 2011, 2011, 626345. [Google Scholar] [CrossRef]
- Kim, H.-K.; Yun, S.-H. Evaluation of Potential Reference Genes for Quantitative RT-PCR Analysis in Fusarium graminearum under Different Culture Conditions. Plant Pathol. J. 2011, 27, 301–309. [Google Scholar] [CrossRef]


| No. | Name of the Sample | Viscosity [cps] | Molecular Weight [kDa] | Degree of Deacetylation [%] | Origin | Provider |
|---|---|---|---|---|---|---|
| 1 | CS_oligo_F | NP * | ≤5 | NP * | Fungal | Pol-Aura |
| 2 | CS_oligo_ | NP * | ≤5 | ≥72 | Shrimp | Pol-Aura |
| 3 | CS_5 | 5 | 20 | ≥90 | Shrimp | Glentham |
| 4 | CS_10 | 10 | 30 | ≥90 | Shrimp | HMC+ |
| 5 | CS_8-15 | 8–15 | 20–100 | 87.6–92.5 | Shrimp | Pol-Aura |
| 6 | CS_10-120 | 10–120 | NP * | ≥85 | Aspergillus niger | Pol-Aura |
| 7 | CS_10-100 | 10–100 | 580 | ≥90 | Squid | Pol-Aura |
| 8 | CS_20-300 | 200–300 | 50-190 | 75–85 | Shrimp | Sigma-Aldrich |
| 9 | CS_30-100 | 30–100 | 250 | ≥90 | Shrimp | Pol-Aura |
| 10 | CS_100-300 | 100–300 | 890 | ≥90 | Shrimp | Pol-Aura |
| 11 | CS_200-800 | 200–800 | NP * | 75–85 | Shrimp | Sigma-Aldrich |
| 12 | CS_300-1k | 300–1000 | 1250 | ≥90 | Shrimp | Pol-Aura |
| 13 | CS_800-2k | 800–2000 | 1500 | ≥72 | Shrimp | Sigma-Aldrich |
| 14 | CS_1k-2k | 1000–2000 | 1500 | ≥90 | Shrimp | Pol-Aura |
| Direction | Adj. p Value | nGenes | Pathways |
|---|---|---|---|
| Enriched Pathways in DEGs for GO Molecular Functions | |||
| Down-regulated | 3.2 × 10−44 | 77 | Hydrolase activity, hydrolyzing O-glycosyl compounds |
| 1.4 × 10−25 | 316 | Catalytic activity | |
| 4.4 × 10−11 | 20 | Carbohydrate binding | |
| 7.4 × 10−10 | 100 | Oxidoreductase activity | |
| Up-regulated | 1.4 × 10−15 | 59 | Transporter activity |
| 4.2 × 10−6 | 17 | Tetrapyrrole binding | |
| 4.2 × 10−6 | 17 | Heme-binding | |
| 9.7 × 10−5 | 11 | Monooxygenase activity | |
| Enriched pathways in DEGs for GO Biological Process | |||
| Down-regulated | 3.5 × 10−39 | 90 | Carbohydrate metabolic process |
| 1.8 × 10−14 | 17 | Polysaccharide catabolic process | |
| 1.8 × 10−12 | 12 | Xylan metabolic process | |
| 3.9 × 10−12 | 16 | Cell wall organization or biogenesis | |
| Up-regulated | 2.9 × 10−17 | 62 | Transmembrane transport |
| 1.5 × 10−12 | 64 | Establishment of localization | |
| 5.1 × 10−4 | 14 | Ion transport | |
| 8.4 × 10−5 | 5 | Secondary metabolic process | |
| Enriched pathways in DEGs for GO Cellular Component | |||
| Down-regulated | 4.7 × 10−14 | 20 | Extracellular region |
| Up-regulated | 3.7 × 10−7 | 48 | Membrane |
| 9.8 × 10−6 | 31 | An integral component of the membrane | |
| 1.8 × 10−3 | 2 | Fatty acid synthase complex | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poznanski, P.; Hameed, A.; Dmochowska-Boguta, M.; Bryla, M.; Orczyk, W. Low Molecular Weight and High Deacetylation Degree Chitosan Batch Alleviates Pathogenesis, Toxin Accumulation, and Fusarium Gene Regulation in Barley Leaf Pathosystem. Int. J. Mol. Sci. 2023, 24, 12894. https://doi.org/10.3390/ijms241612894
Poznanski P, Hameed A, Dmochowska-Boguta M, Bryla M, Orczyk W. Low Molecular Weight and High Deacetylation Degree Chitosan Batch Alleviates Pathogenesis, Toxin Accumulation, and Fusarium Gene Regulation in Barley Leaf Pathosystem. International Journal of Molecular Sciences. 2023; 24(16):12894. https://doi.org/10.3390/ijms241612894
Chicago/Turabian StylePoznanski, Pawel, Amir Hameed, Marta Dmochowska-Boguta, Marcin Bryla, and Waclaw Orczyk. 2023. "Low Molecular Weight and High Deacetylation Degree Chitosan Batch Alleviates Pathogenesis, Toxin Accumulation, and Fusarium Gene Regulation in Barley Leaf Pathosystem" International Journal of Molecular Sciences 24, no. 16: 12894. https://doi.org/10.3390/ijms241612894
APA StylePoznanski, P., Hameed, A., Dmochowska-Boguta, M., Bryla, M., & Orczyk, W. (2023). Low Molecular Weight and High Deacetylation Degree Chitosan Batch Alleviates Pathogenesis, Toxin Accumulation, and Fusarium Gene Regulation in Barley Leaf Pathosystem. International Journal of Molecular Sciences, 24(16), 12894. https://doi.org/10.3390/ijms241612894







