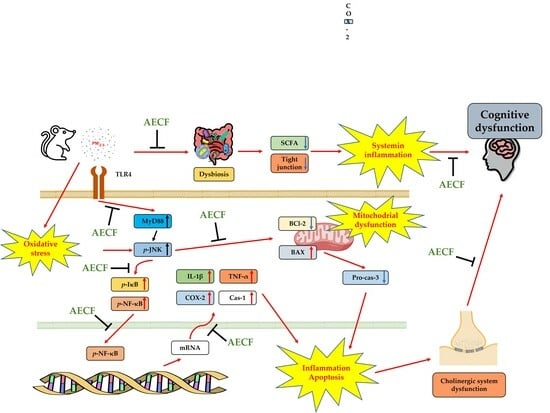
Codium fragile Suppresses PM2.5-Induced Cognitive Dysfunction by Regulating Gut–Brain Axis via TLR-4/MyD88 Pathway
Abstract
:1. Introduction
2. Results
2.1. Identification of Compound Using UPLC-QTOF/MSE
2.2. Saccharide and Sulfate Analysis
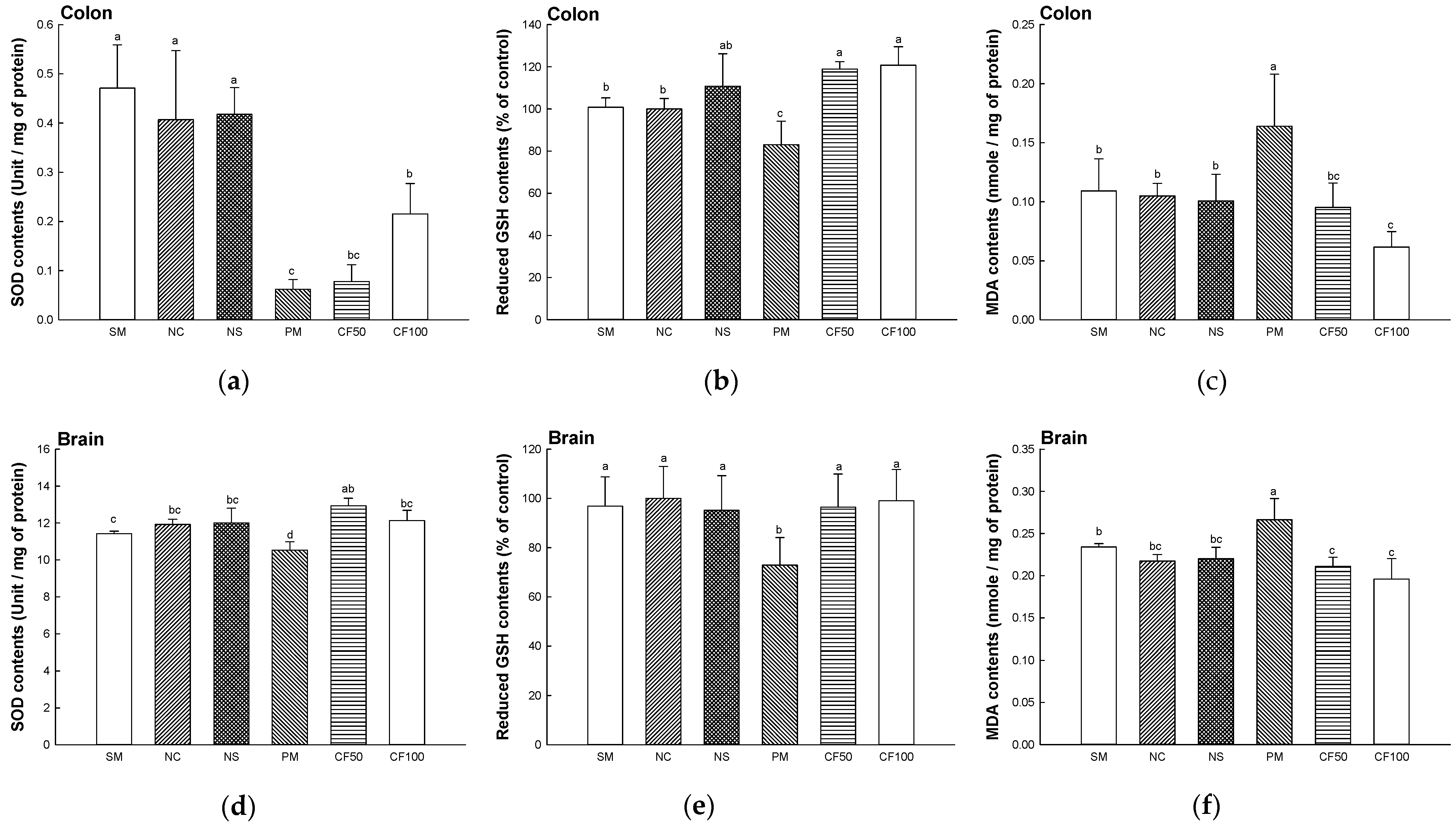
2.3. Antioxidant System Test
2.3.1. Superoxide Dismutase (SOD) Contents
2.3.2. Reduced Glutathione (GSH) Contents
2.3.3. Malondialdehyde (MDA) Contents
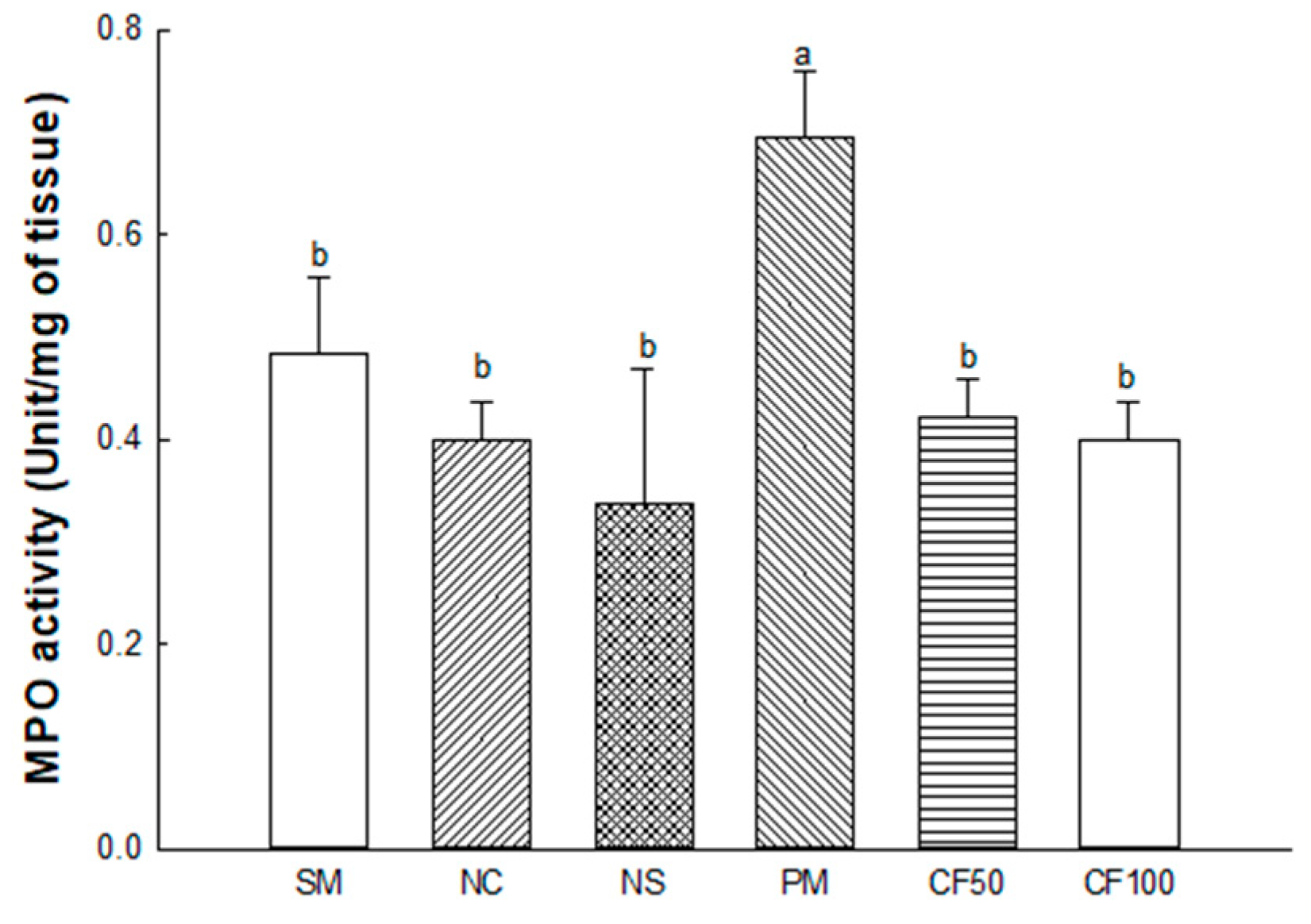
2.3.4. Myeloperoxidase (MPO) Activity
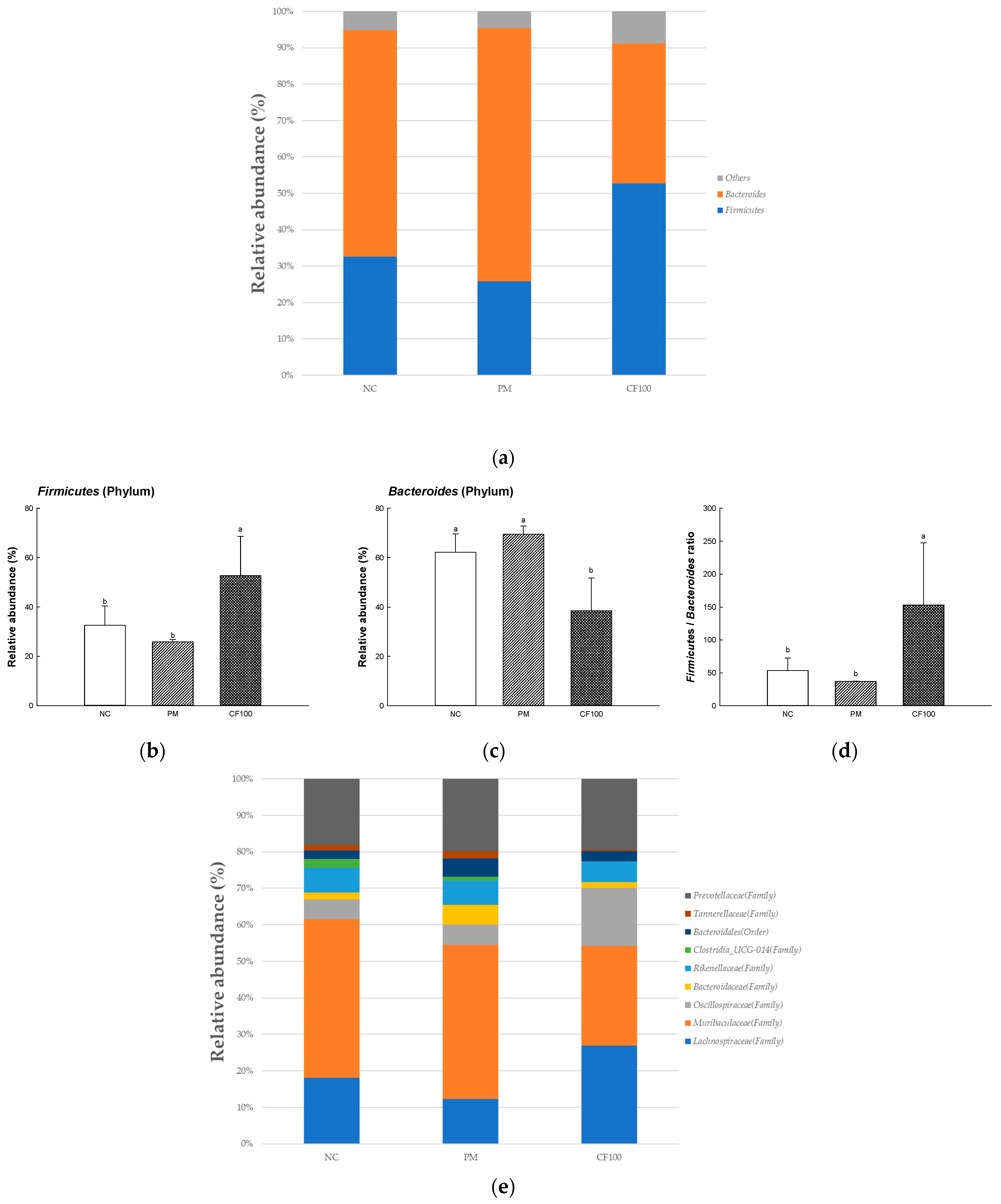
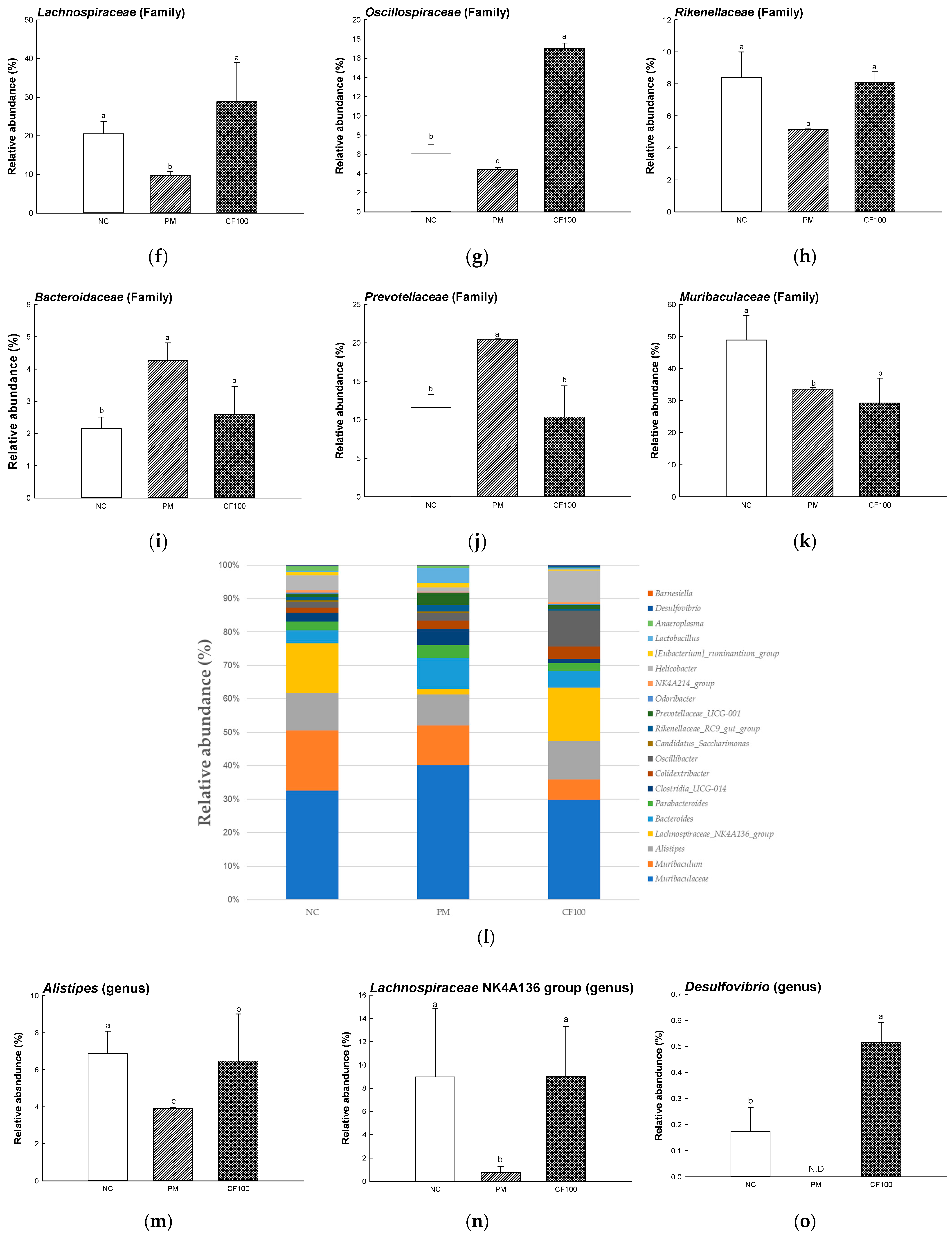
2.4. The Regulation Effect of AECF on Gut Microbiome Abundance
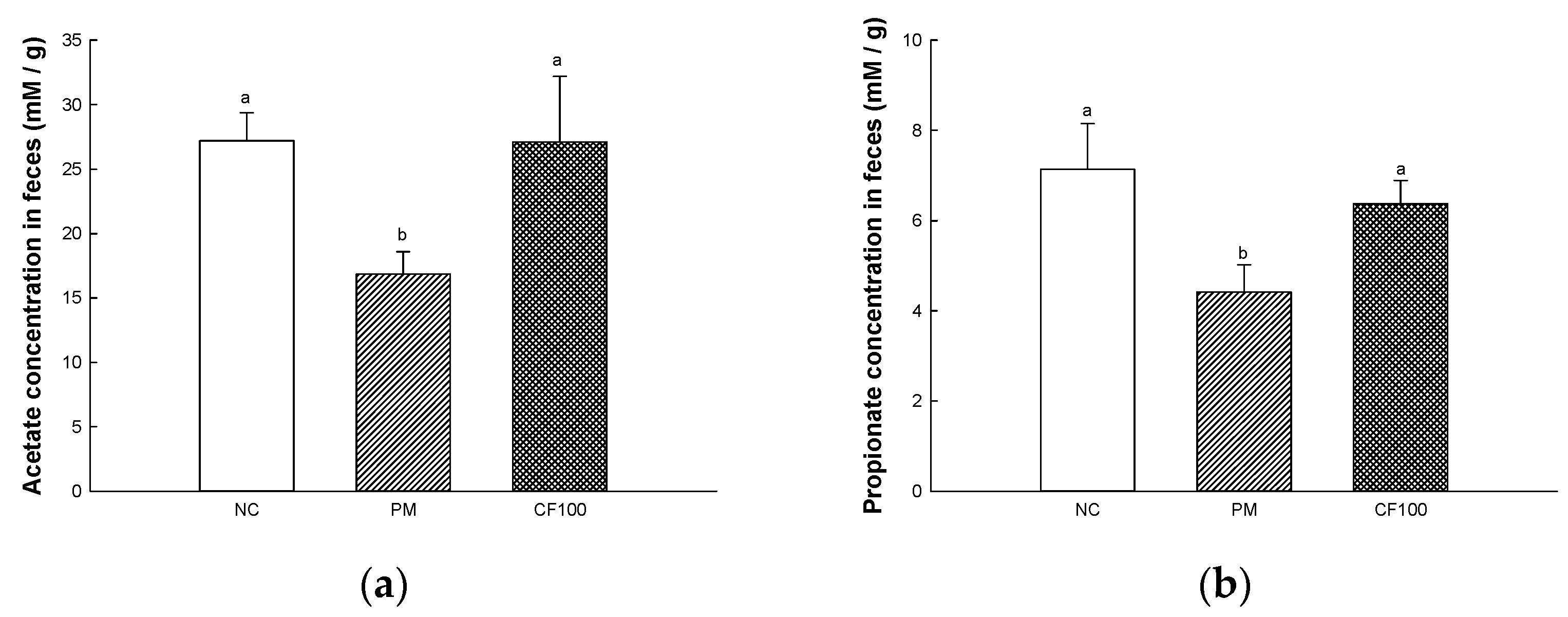
2.5. The Regulation Effect of AECF on SCFA Contents
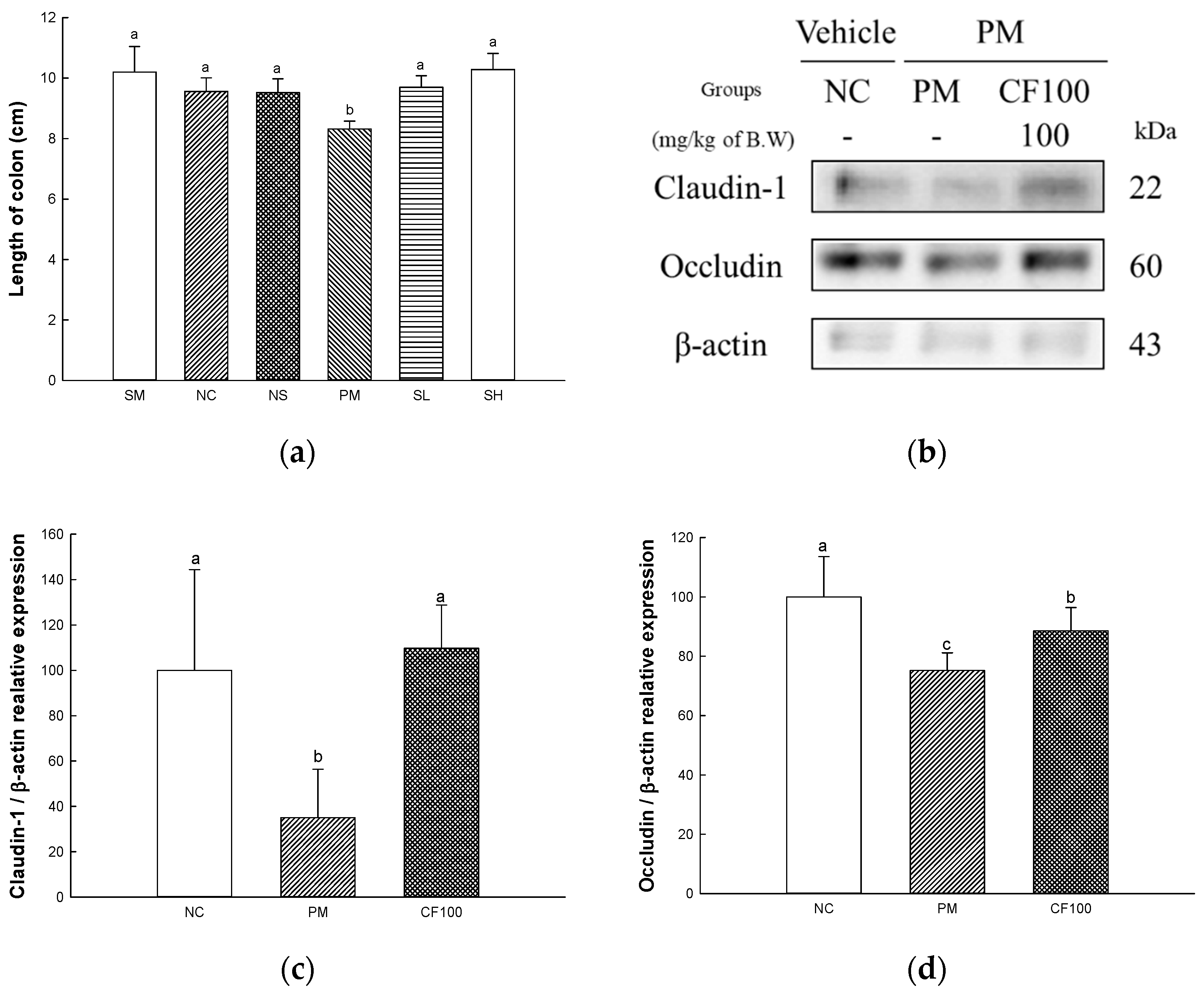
2.6. The Regulation Effect of AECF on PM2.5-Induced Gut Barrier Dysfunction
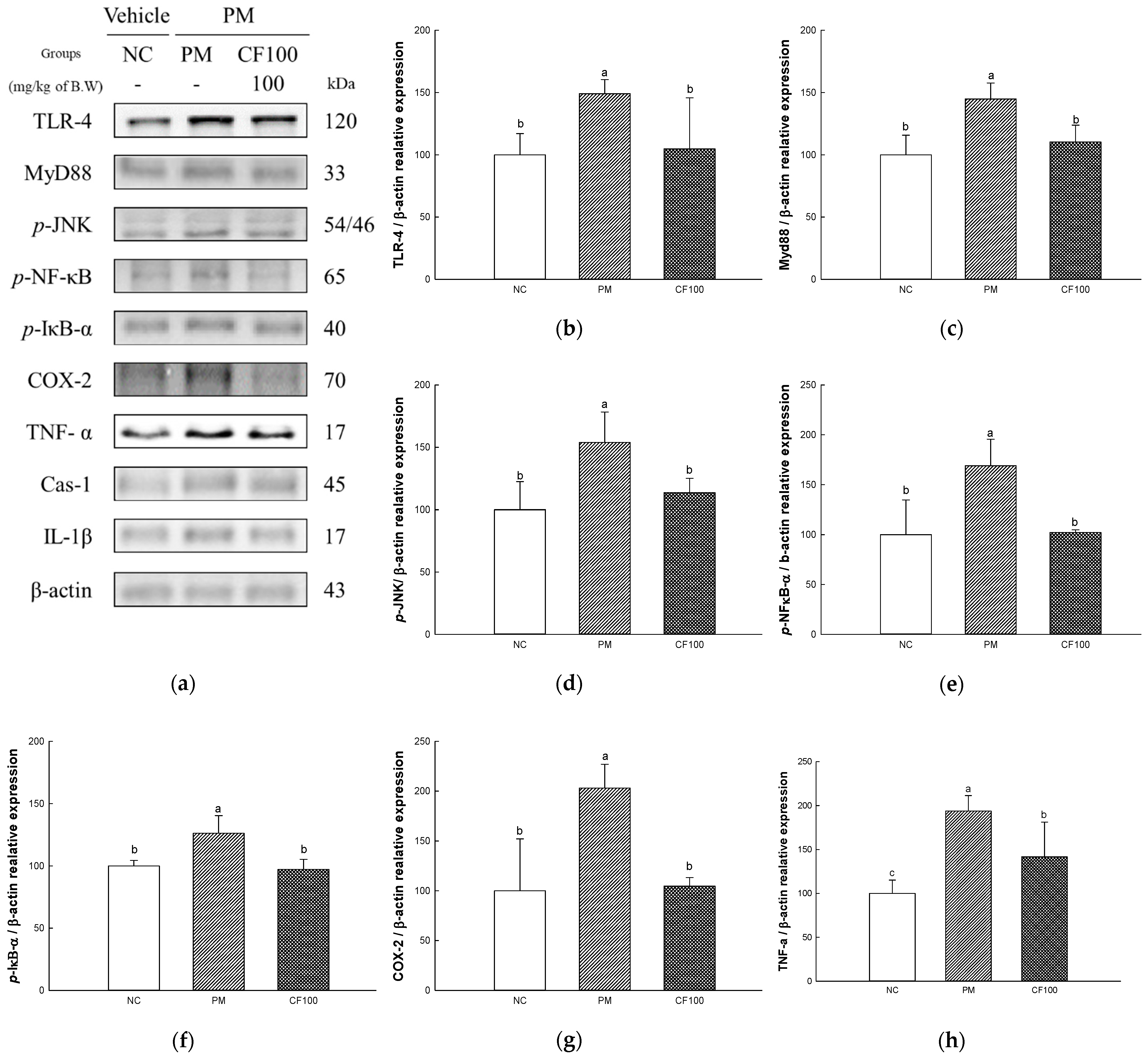
2.7. The Regulation Effect of AECF on PM2.5-Induced TLR-4/MyD88 Signaling Inflammation in Colon Tissues
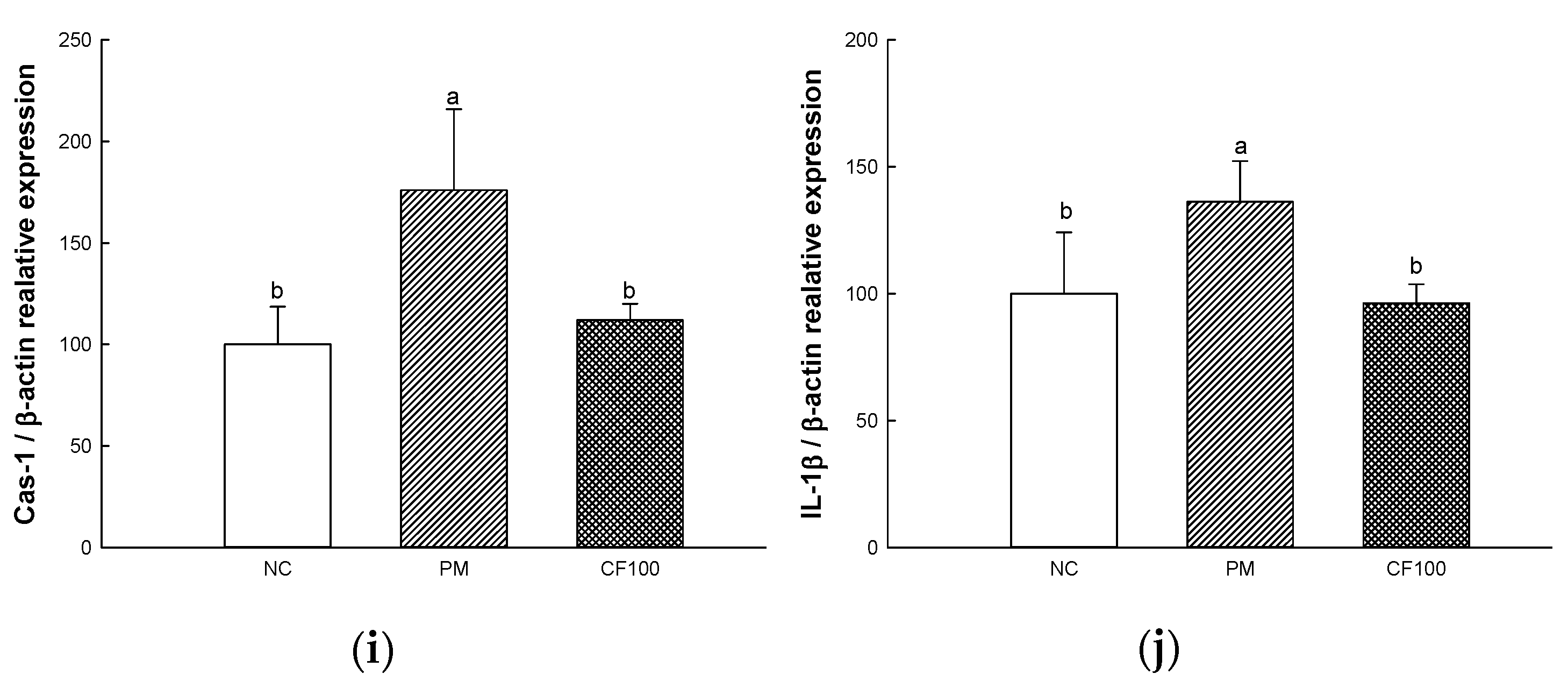
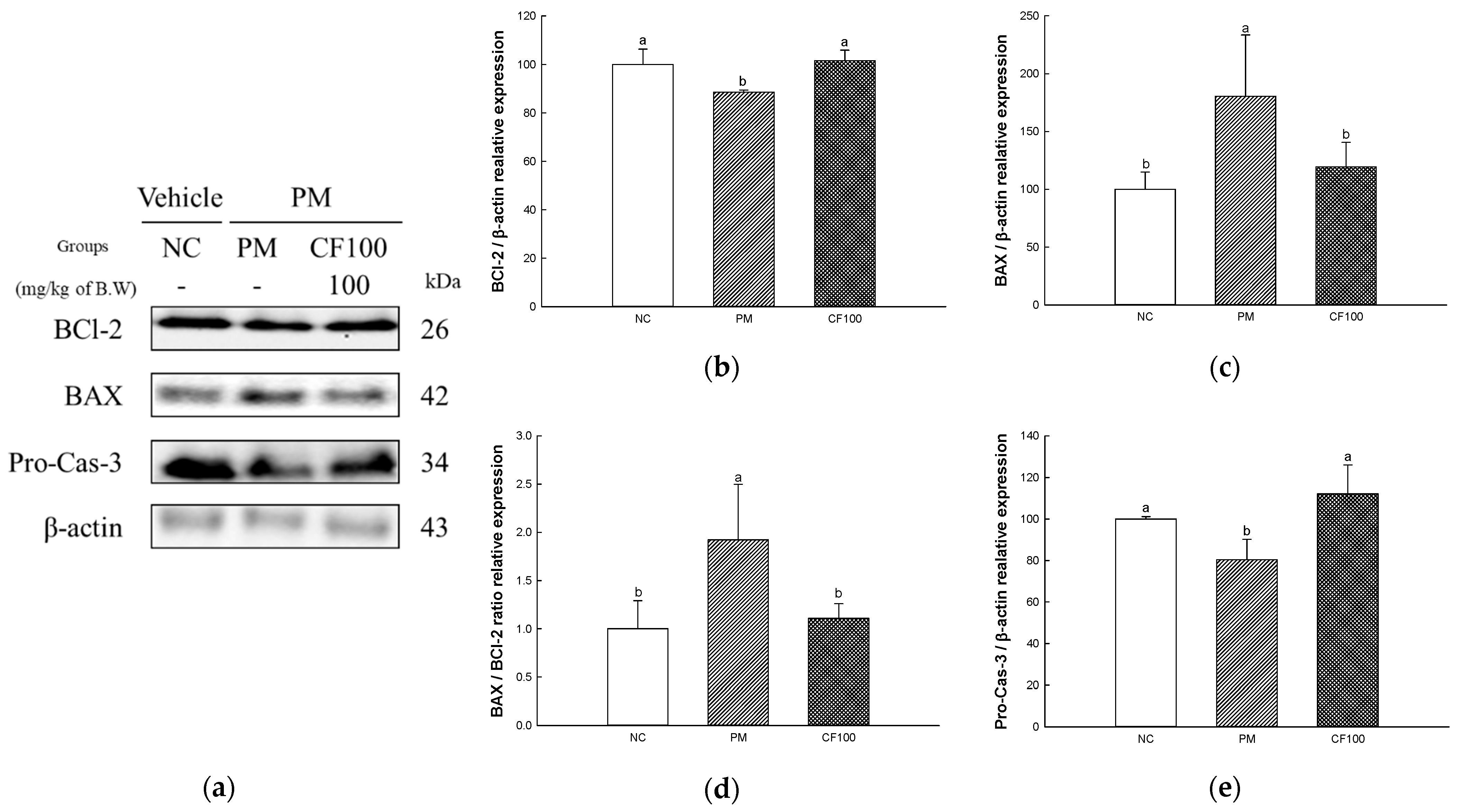
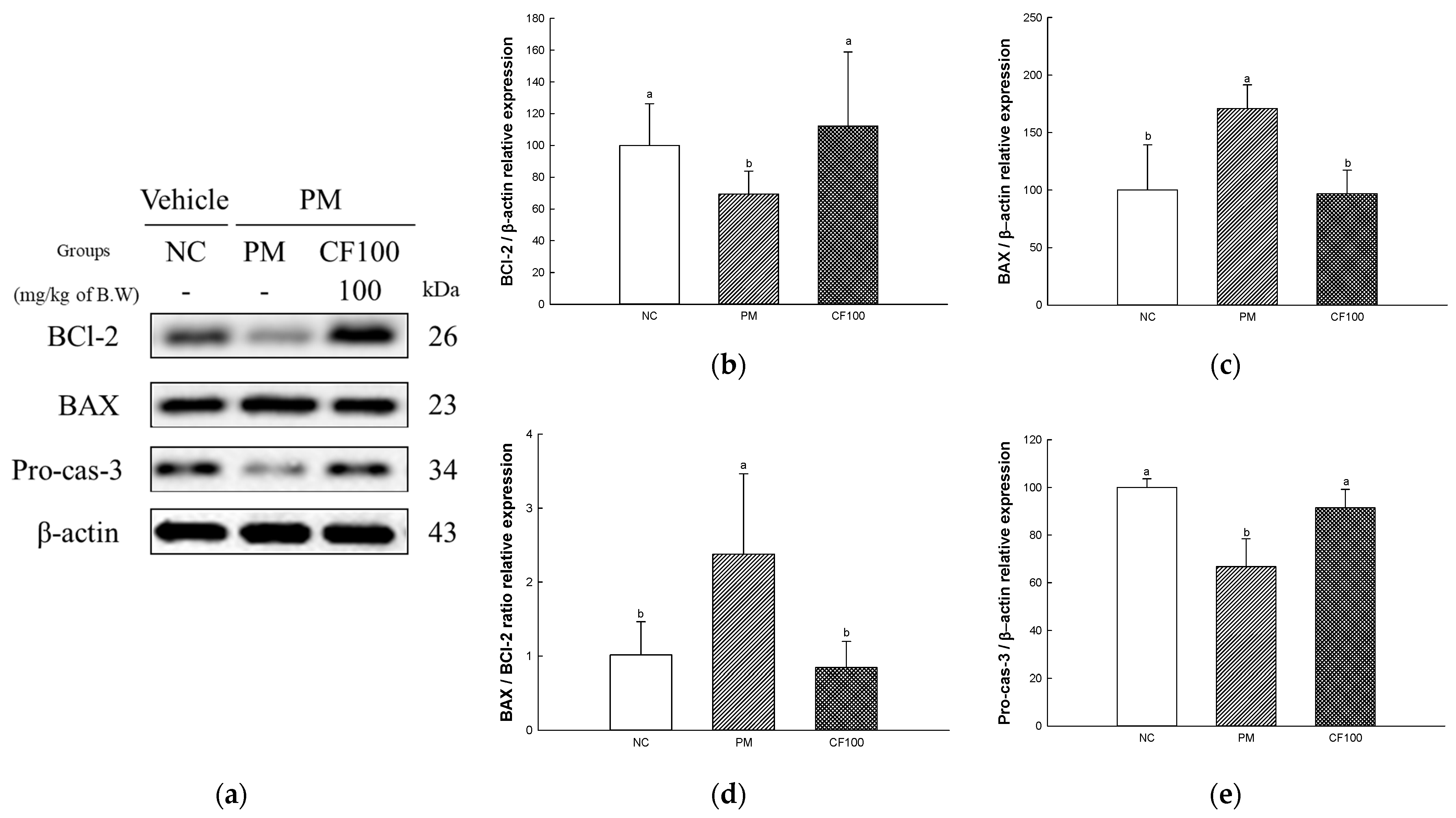
2.8. The Regulation Effect of AECF on PM2.5-Induced Apoptosis in Colon
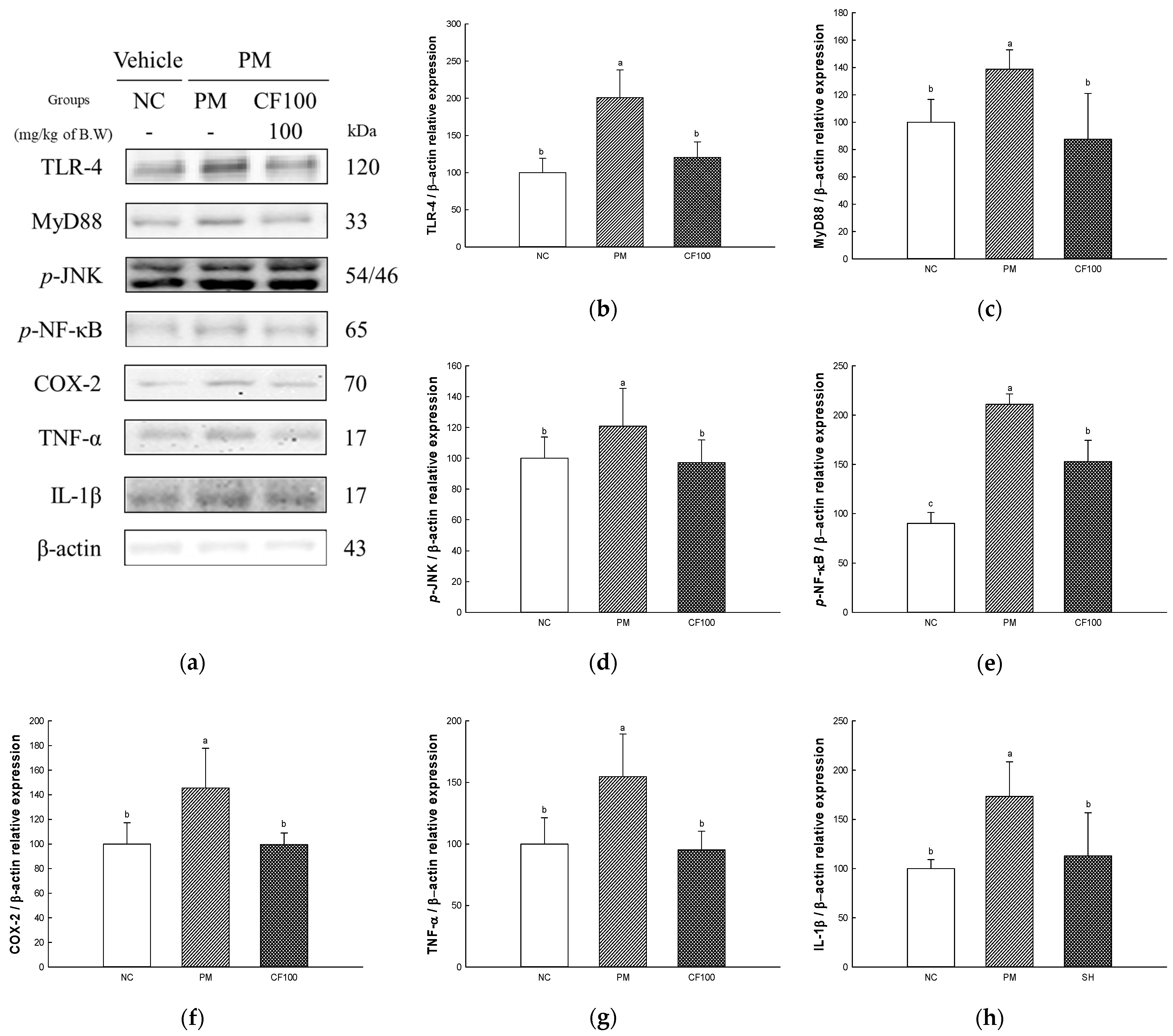
2.9. The Regulation Effect of AECF on PM2.5-Induced TLR-4/MyD88 Signaling Inflammation in Brain Tissues
2.10. The Regulation Effect of AECF on PM2.5-Induced Apoptosis in Brain
2.11. Mitochondrial Activity
2.11.1. Mitochondrial ROS Levels
2.11.2. Mitochondrial Membrane Potential (MMP) Levels
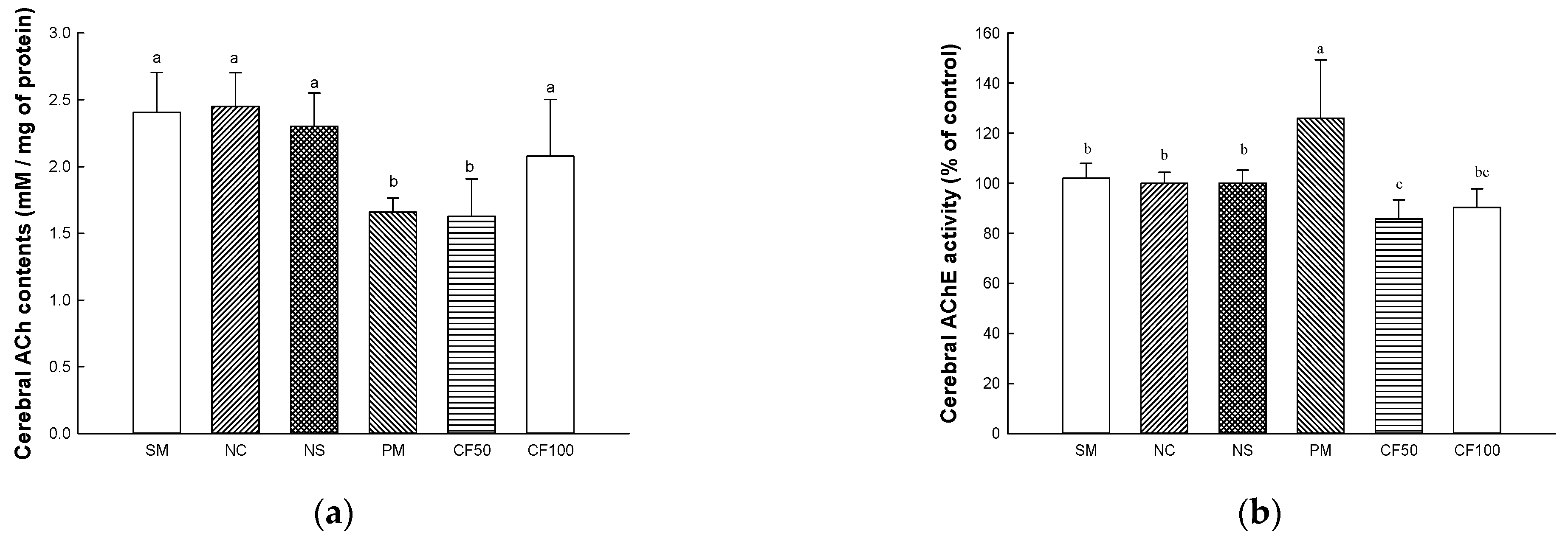
2.12. Cholinergic System
2.12.1. Acetylcholine (ACh) Contents
2.12.2. Acetylcholinesterase (AChE) Activity
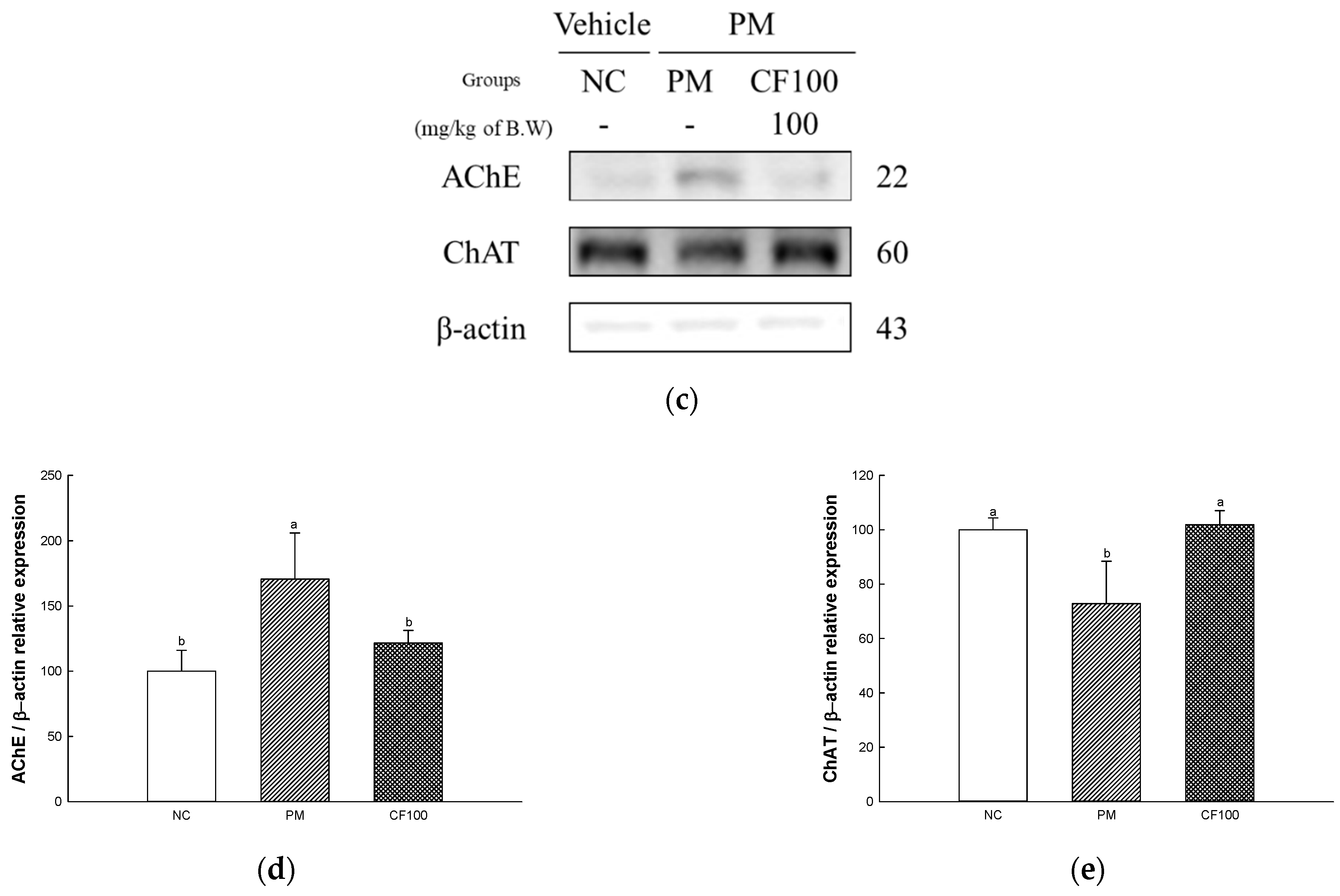
2.12.3. Protein Expression Levels of Cholinergic System
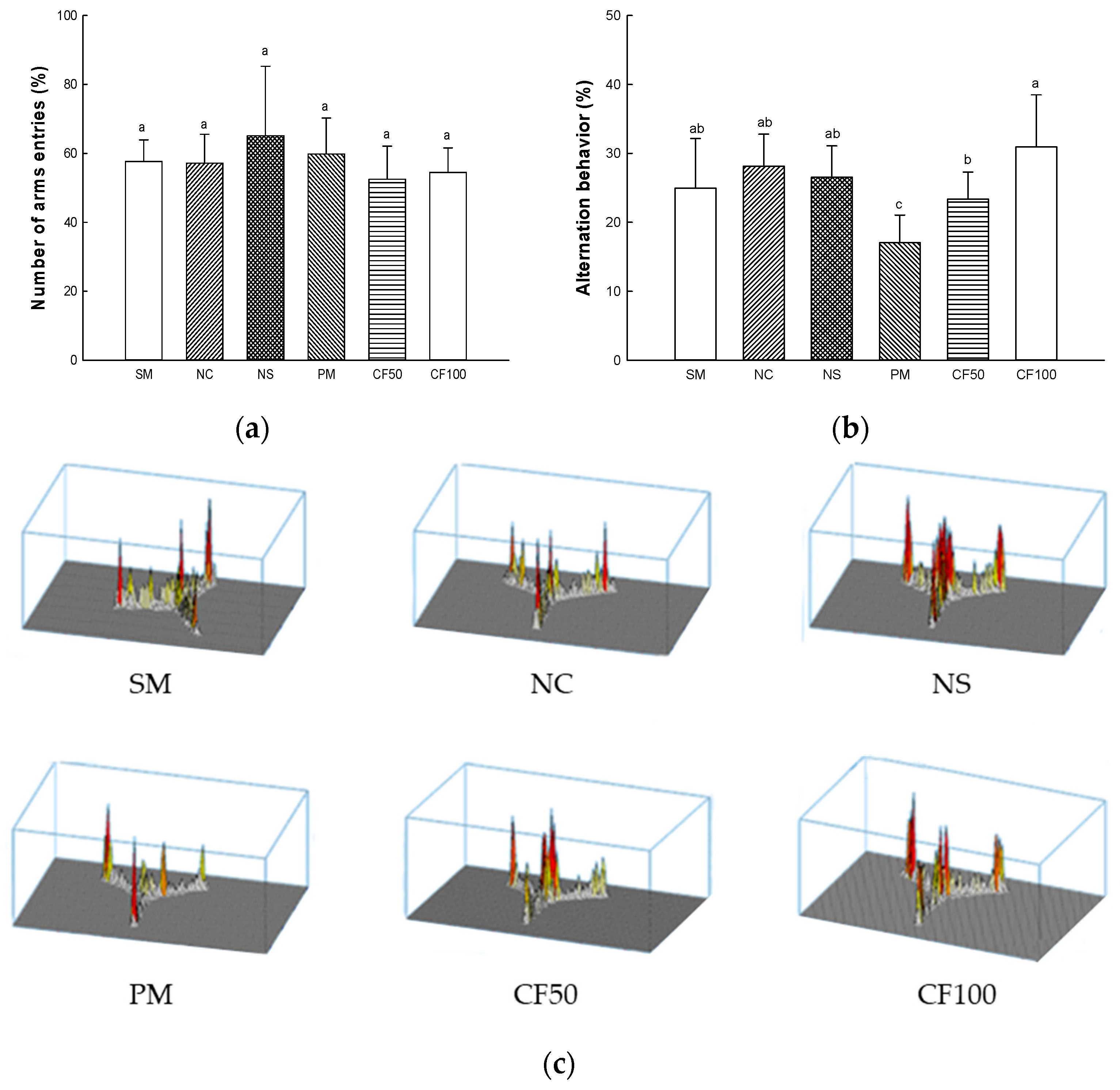
2.13. Behavior Test
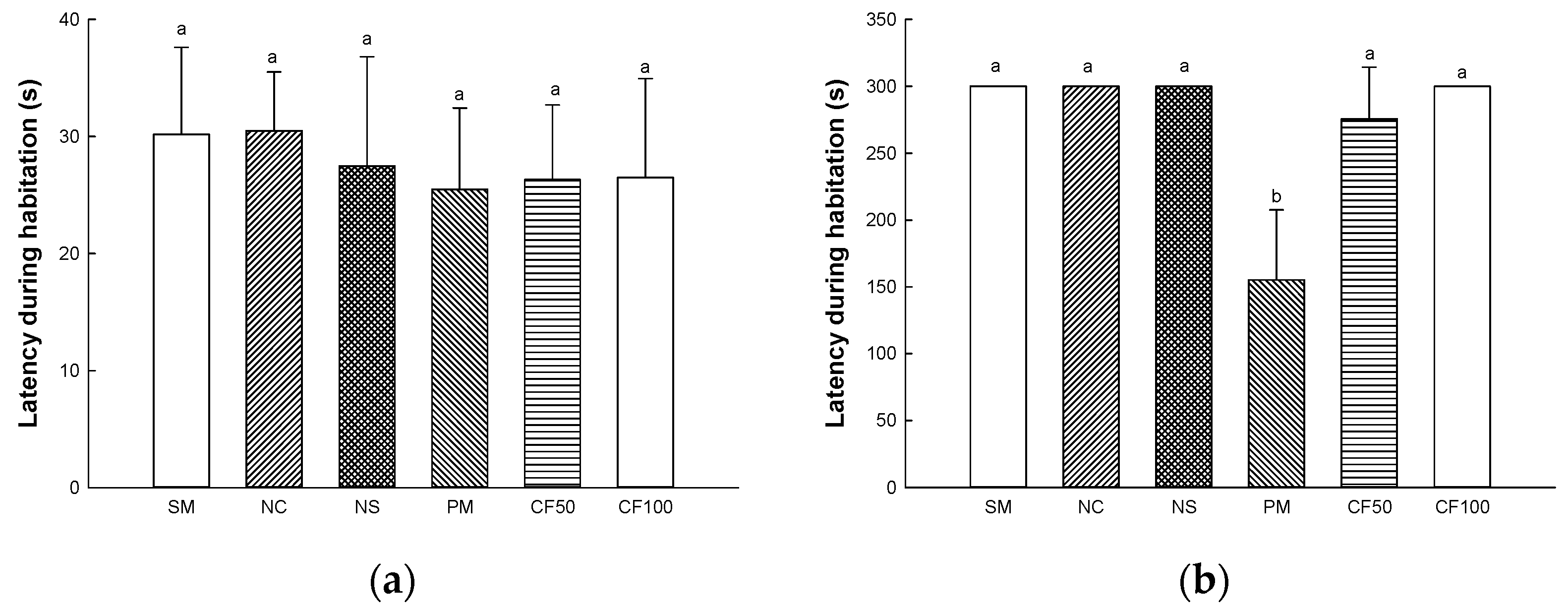
2.13.1. Y-Maze Test
2.13.2. Passive Avoidance Test
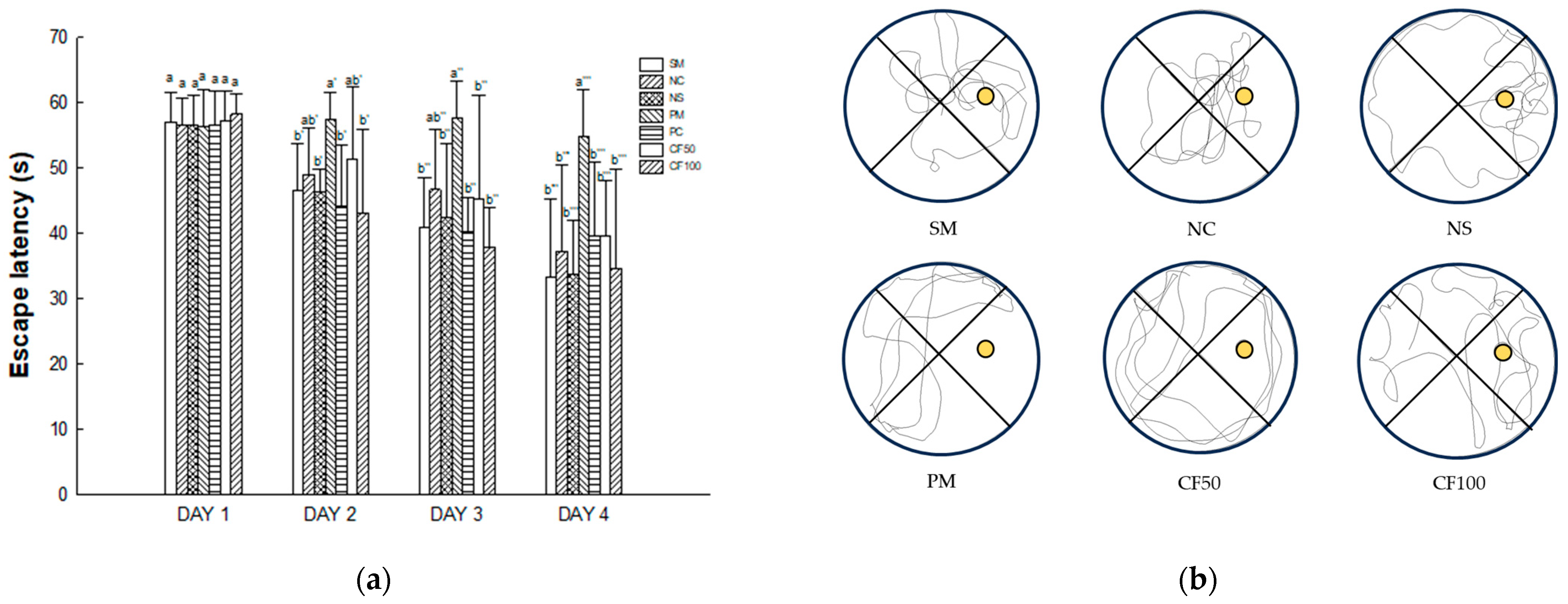
2.13.3. Morris Water Maze Test
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Bioactive Compound Analysis
4.3.1. UPLC Q-TOF MSE Analysis
4.3.2. Total Polysaccharide Contents
4.3.3. Total Sulfate Contents
4.3.4. Monosaccharide Composition
4.4. In Vivo Mouse Experimental Design
4.5. Tissue Preparation
4.6. Antioxidant System
4.6.1. SOD Contents
4.6.2. Reduced GSH Contents
4.6.3. MDA Contents
4.6.4. MPO Activity in Colon Tissues
4.7. Western Blot
4.8. 16S rRNA Sequencing
4.9. SCFA Concentartion Analysis
4.10. Mitochondrial Activity
4.10.1. Mitochondrial Isolation
4.10.2. Mitochondrial ROS Contents
4.10.3. MMP Levels
4.11. Cholinergic System Test
4.11.1. ACh Contents
4.11.2. AChE Activity
4.12. Behavial Test
4.12.1. Y-Maze Test
4.12.2. Passive Avoidance Test
4.12.3. Morris Water Maze Test
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer's disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, X.; Wang, A.; Wang-Li, L.; Yang, M.; Xiao, H.; He, Y.; Wang, K. Size-segregated physicochemical properties of inhalable particulate matter in a tunnel-ventilated layer house in China. Environ. Res. 2022, 204, 112064. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, K.; Liu, J.; Wu, S.; Shen, D.; Dai, P.; Li, C. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-κB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere 2019, 236, 124373. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kang, J.Y.; Kim, J.M.; Moon, J.H.; Lee, H.L.; Jeong, H.R.; Go, M.J.; Lee, U.; Heo, H.J. Effect of Ethyl Acetate Fraction from Eucommia ulmoides Leaves on PM2.5-Induced Inflammation and Cognitive Dysfunction. Oxid. Med. Cell. Longev. 2022, 2022, 7157444. [Google Scholar] [CrossRef]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Gänzle, M.; Rioux, K.P.; Thiesen, A.; Barkema, H.W.; Wine, E. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C. The impact of chronic intestinal inflammation on brain disorders: The microbiota-gut-brain axis. Mol. Neurobiol. 2019, 56, 6941–6951. [Google Scholar] [CrossRef]
- Kim, H.Y.; Bae, C.H.; Kim, J.; Lee, Y.; Jeon, H.; Kim, H.; Kim, S. Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease. Antioxidants 2022, 11, 141. [Google Scholar] [CrossRef]
- Chen, X.; D’Souza, R.; Hong, S.T. The role of gut microbiota in the gut-brain axis: Current challenges and perspectives. Protein Cell 2013, 4, 403–414. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, gut microbiome, and Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 8243–8250. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.-J.; Heo, H.J. Water Extract of Ecklonia cava Protects against Fine Dust (PM2.5)-Induced Health Damage by Regulating Gut Health. J. Microbiol. Biotechnol. 2022, 32, 927. [Google Scholar] [CrossRef] [PubMed]
- Koz, F.F.Y.; Yavasoglu, N.U.K.; Demirel, Z. Antioxidant and antimicrobial activities of Codium fragile (Suringar) Hariot (Chlorophyta) essential oil and extracts. Asian J. Chem. 2009, 21, 1197. [Google Scholar]
- Lee, J.B.; Ohta, Y.; Hayashi, K.; Hayashi, T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr. Res. 2010, 345, 1452–1454. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.; Jung, K.; Pham, T.N.A.; Yang, S.; Ahn, B. Potential Prebiotic and Anti-Obesity Effects of Codium fragile Extract. Appl. Sci. 2022, 12, 959. [Google Scholar] [CrossRef]
- Lee, S.A.; Moon, S.M.; Choi, Y.H.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, Y.H.; Kim, D.K.; Kim, C.S. Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed. Pharmacother. 2017, 93, 1055–1064. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.A.; Park, C.I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, Y.G.; Lee, S.H.; Park, C.B.; Choi, H.G.; Jang, H.J.; Lee, Y.S.; Kwon, D.Y. Codium fragile Ethanol Extraction Inhibited Inflammatory Response through the Inhibition of JNK Phosphorylation. Prev. Nutr. Food Sci. 2010, 15, 206–212. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Shin, E.J.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Heo, H.J. Protective effect of Codium fragile extract on fine dust (PM2.5)-induced toxicity in nasal cavity, lung, and brain cells. Korean J. Food Sci. Technol. 2021, 53, 223–229. [Google Scholar]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Kim, J.C.; Heo, H.J. Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice. Antioxidants 2021, 10, 1932. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kim, J.M.; Moon, J.H.; Kim, M.J.; Jeong, H.R.; Go, M.J.; Kim, H.-J.; Eo, H.J.; Lee, U.; Heo, H.J. Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice. Int. J. Mol. Sci. 2023, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Keskinkaya, H.B.; Deveci, E.; Güneş, E.; Okudan, E.Ş.; Akköz, C.; Gümüş, N.E.; Karakurt, S. Chemical Composition, In Vitro Antimicrobial and Antioxidant Activities of Marine Macroalgae Codium fragile (Suringar) Hariot. Commagene J. Biol. 2022, 6, 94–104. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Fatemi, I.; Kalantari, H.; Hosseini Tabatabaei, S.M.T.; Mehrabani, M.; Gholamine, B.; Kalantar, M.; Mehrzadi, S.; Goudarzi, M. Ameliorative effects of gallic acid on gentamicin-induced nephrotoxicity in rats. J. Asian Nat. Prod. Res. 2018, 20, 1182–1193. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef]
- Li, Y.; Lai, W.; Zheng, C.; Babu, J.R.; Xue, C.; Ai, Q.; Huggins, K.W. Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells. Antioxidants 2022, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.S.; Ko, J.Y.; Fu, X.; Jeon, Y.J. Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Seidman, M. Polyunsaturated Phosphatidylcholine in NT Factor Improves Mitochondrial Function, Auditory Sensitivity and May Slow Some of the Aging Processes; Anti-Aging Medical News: Boca Raton, FL, USA, 2001. [Google Scholar]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.; Stratton, G.; Gadd, G. Cadmium transport, resistance, and toxicity in bacteria, algae, and fungi. Can. J. Microbiol. 1986, 32, 447–464. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.-J.; Chung, M.-Y.; Heo, H.J. Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function. Mar. Drugs 2022, 20, 439. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, S.; Tatum, R.; Garg, P.M.; Herco, M.; Shekhawat, P.S.; Chen, Y.H. Necrotizing enterocolitis leads to disruption of tight junctions and increase in gut permeability in a mouse model. BMC Pediatr. 2018, 18, 372. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, X.; Liu, J.; Sun, J.; Wang, X.; Fan, G.; Meng, X.; Zhang, J.; Zhang, Y. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr. Opin. Pharmacol. 2022, 62, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Q.; Dong, Z.; Yan, Y.; Fu, Y.; Liu, X.; Zhao, B.; Duan, X. Phosphatidylcholine ameliorates lps-induced systemic inflammation and cognitive impairments via mediating the gut–brain axis balance. J. Agric. Food Chem. 2020, 68, 14884–14895. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Yao, H.; Geng, F.; Nie, S. Interaction between four galactans with different structural characteristics and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 63, 3653–3663. [Google Scholar] [CrossRef]
- Devi, P.B.; Kavitake, D.; Jayamanohar, J.; Shetty, P.H. Preferential growth stimulation of probiotic bacteria by galactan exopolysaccharide from Weissella confusa KR780676. Food Res. Int. 2021, 143, 110333. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota–gut–brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am. J. Respir. Crit. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Haasbach, E.; Lueftenegger, D.; Heyken, W.T.; Ocker, M.; Planz, O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front. Immunol. 2020, 11, 598444. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 2015, 13, 51. [Google Scholar] [CrossRef]
- Sun, Q.; Scott, M.J. Caspase-1 as a multifunctional inflammatory mediator: Noncytokine maturation roles. J. Leukoc. Biol. 2016, 100, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xiao, H.; Wu, W.; Wang, Y.; Liu, X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem. Toxicol. 2013, 53, 62–68. [Google Scholar] [CrossRef]
- Gottlieb, E.; Vander Heiden, M.G.; Thompson, C.B. Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol. Cell Biol. 2000, 20, 5680–5689. [Google Scholar] [CrossRef]
- Chin, A.C.; Flynn, A.N.; Fedwick, J.P.; Buret, A.G. The role of caspase-3 in lipopolysaccharide-mediated disruption of intestinal epithelial tight junctions. Can. J. Physiol. 2006, 84, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ding, Y.; Wang, L.; Xiao, Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res. Bull. 2020, 164, 249–256. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.M.; Lee, S.A.; Han, S.H.; Park, B.-R.; Choi, M.S.; Kim, J.S.; Kim, S.G.; Kim, H.J.; Chun, H.S.; Kim, D.K. Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharmacother. 2018, 97, 264–270. [Google Scholar] [CrossRef]
- Yoon, H.D.; Jeong, E.J.; Choi, J.W.; Lee, M.S.; Park, M.; Yoon, N.Y.; Kim, Y.K.; Cho, D.M.; Kim, J.I.; Kim, H.R. Anti-inflammatory effects of ethanolic extracts from Codium fragile on LPS-stimulated RAW 264.7 macrophages via nuclear factor kappaB inactivation. Fish. Aquat. Sci. 2011, 14, 267–274. [Google Scholar] [CrossRef]
- Yang, Y.; Lim, J.; Li, C.; Lee, S.; Hong, S. Effects of sulfated polysaccharides isolated from Codium fragile on inflammatory cytokine gene expression and Edwardsiella tarda infection in rockfish, Sebastes schlegelii. Fish Shellfish. Immunol. 2021, 112, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Je, J.G.; Huang, C.; Oh, J.Y.; Fu, X.; Wang, K.; Ahn, G.; Xu, J.; Gao, X.; Jeon, Y.J. Anti-inflammatory effect of sulfated polysaccharides isolated from Codium fragile in vitro in RAW 264.7 macrophages and in vivo in zebrafish. Mar. Drugs 2022, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Lee, J.H.; Kim, J.E.; Ha, J.; Kang, I. Oleamide suppresses lipopolysaccharide-induced expression of iNOS and COX-2 through inhibition of NF-κB activation in BV2 murine microglial cells. Neurosci. Lett. 2010, 474, 148–153. [Google Scholar] [CrossRef]
- Josephine, P.; Akila, V.; Nandhini, K.; Muninathan, N. Anti-apoptotic effect of sulphated polysaccharides through downregulating the expression of Cytochrome C and Caspase 3 in Cyclosporine A-induced apoptosis. Eur.Chem. Bull. 2023, 12, 1607–1613. [Google Scholar]
- Yang, J.Y.; Abe, K.; Xu, N.J.; Matsuki, N.; Wu, C.F. Oleamide attenuates apoptotic death in cultured rat cerebellar granule neurons. Neurosci. Lett. 2002, 328, 165–169. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, M.J.; Ahn, J.; Ha, T.Y.; Jung, C.H.; Seo, H.D.; Jang, Y.J. Identifying Codium fragile extract components and their effects on muscle weight and exercise endurance. Food Chem. 2021, 353, 129463. [Google Scholar] [CrossRef]
- Uemura, M.; Manabe, H.; Yoshida, N.; Fujita, N.; Ochiai, J.; Matsumoto, N.; Takagi, T.; Naito, Y.; Yoshikawa, T. α-Tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002, 456, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, R.; Nishi, K.; Gunasekaran, V.P.; Annamalai, G.; Agilan, B.; Ganeshan, M. p-Coumaric acid ameliorates ethanol–induced kidney injury by inhibiting inflammatory cytokine production and NF–κB signaling in rats. Asian Pac. J. Trop. Biomed. 2019, 9, 188. [Google Scholar]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Van Remmen, H.; Richardson, A. Oxidative damage to mitochondria and aging. Exp. Gerontol. 2001, 36, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free. Radic. Biol. Med. 1999, 26, 463–471. [Google Scholar] [CrossRef]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, U.; Kang, J.Y.; Kim, J.M.; Shin, E.J.; Heo, H.J. Ameliorative effect of onion (Allium Cepa L.) flesh and peel on amyloid-β-induced cognitive dysfunction via mitochondrial activation. Korean J. Food Sci. Technol. 2020, 52, 263–273. [Google Scholar]
- Reyes-Soto, C.Y.; Villaseca-Flores, M.; Ovalle-Noguez, E.A.; Nava-Osorio, J.; Galván-Arzate, S.; Rangel-López, E.; Maya-López, M.; Retana-Márquez, S.; Túnez, I.; Tinkov, A.A. Oleamide Reduces Mitochondrial Dysfunction and Toxicity in Rat Cortical Slices Through the Combined Action of Cannabinoid Receptors Activation and Induction of Antioxidant Activity. Neurotox. Res. 2022, 40, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Khairallah, R.J.; Dabkowski, E.R. Update on lipids and mitochondrial function: Impact of dietary n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 122–126. [Google Scholar] [CrossRef]
- Kassan, M.; Kwon, Y.; Munkhsaikhan, U.; Sahyoun, A.M.; Ishrat, T.; Galán, M.; Gonzalez, A.A.; Abidi, A.H.; Kassan, A.; Ait-Aissa, K. Protective Role of Short-Chain Fatty Acids against Ang-II-Induced Mitochondrial Dysfunction in Brain Endothelial Cells: A Potential Role of Heme Oxygenase 2. Antioxidants 2023, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Lu, B.; Monroe, R.; Ward, S.; Halvorsen, S. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J. Neurochem. 2005, 92, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Mumaw, C.L.; Levesque, S.; McGraw, C.; Robertson, S.; Lucas, S.; Stafflinger, J.E.; Campen, M.J.; Hall, P.; Norenberg, J.P.; Anderson, T. Microglial priming through the lung–brain axis: The role of air pollution–induced circulating factors. FASEB J. 2016, 30, 1880. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, Y.J.; Kim, J.-H.; Jeong, S.-J. Ficus erecta Thunb leaves alleviate memory loss induced by scopolamine in mice via regulation of oxidative stress and cholinergic system. Mol. Neurobiol. 2021, 58, 3665–3676. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.B. Choline and phosphatidylcholine may maintain cognitive performance by multiple mechanisms. Am. J. Clin. Nutr. 2019, 110, 1268–1269. [Google Scholar] [CrossRef]
- Carroll, P.T. Evidence to suggest that extracellular acetate is accumulated by rat hippocampal cholinergic nerve terminals for acetylcholine formation and release. Brain Res. 1997, 753, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jindal, D.K.; Dhingra, M.S.; Kumar, A.; Parle, M.; Dhingra, S. Protective effect of gallic acid in experimental model of ketamine-induced psychosis: Possible behaviour, biochemical, neurochemical and cellular alterations. Inflammopharmacology 2018, 26, 413–424. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflammation 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, L.; Tang, M. Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 2017, 37, 644–667. [Google Scholar] [CrossRef]
- Rashno, M.; Gholipour, P.; Salehi, I.; Komaki, A.; Rashidi, K.; Khoshnam, S.E.; Ghaderi, S. p-Coumaric acid mitigates passive avoidance memory and hippocampal synaptic plasticity impairments in aluminum chloride-induced Alzheimer’s disease rat model. J. Funct. Foods 2022, 94, 105117. [Google Scholar] [CrossRef]
- Heo, H.J.; Park, Y.J.; Suh, Y.M.; Choi, S.J.; Kim, M.J.; Cho, H.Y.; Chang, Y.J.; Hong, B.; Kim, H.K.; Kim, E. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Fatty acids of green macrophytic algae from the sea of Japan. Phytochemistry 1993, 32, 1203–1207. [Google Scholar] [CrossRef]
- Das, U. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 2003, 19, 62. [Google Scholar] [CrossRef]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The effects of a 6-month high dose omega-3 and omega-6 polyunsaturated fatty acids and antioxidant vitamins supplementation on cognitive function and functional capacity in older adults with mild cognitive impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Fatty acids of species in the genus Codium. Bot. Mar. 2003, 46, 456–460. [Google Scholar] [CrossRef]
- Mohammed, Z.Y.; Alsamarrae, K.W.; Hamza, S.J. Quantitative analysis of total polysaccharides and total carotene from Lycium barbarum fruit. Int. J. Mod. Biol. Med. 2013, 4, 204–215. [Google Scholar]
- Souza, B.W.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.J.; Heo, H.J. Ecklonia cava attenuates PM2.5-induced cognitive decline through mitochondrial activation and anti-inflammatory effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green tea seed oil suppressed Aβ1–42-induced behavioral and cognitive deficit via the Aβ-related Akt pathway. Int. J. Mol. Sci. 2019, 20, 1865. [Google Scholar] [CrossRef]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- MC, V. Colorimetric determination of acetylcholine by the Hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Fr. 1958, 16, 179–185. [Google Scholar]
- Van der Borght, K.; Havekes, R.; Bos, T.; Eggen, B.J.; Van der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324. [Google Scholar] [CrossRef]
- Newman, J.P.; Kosson, D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986, 95, 252. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
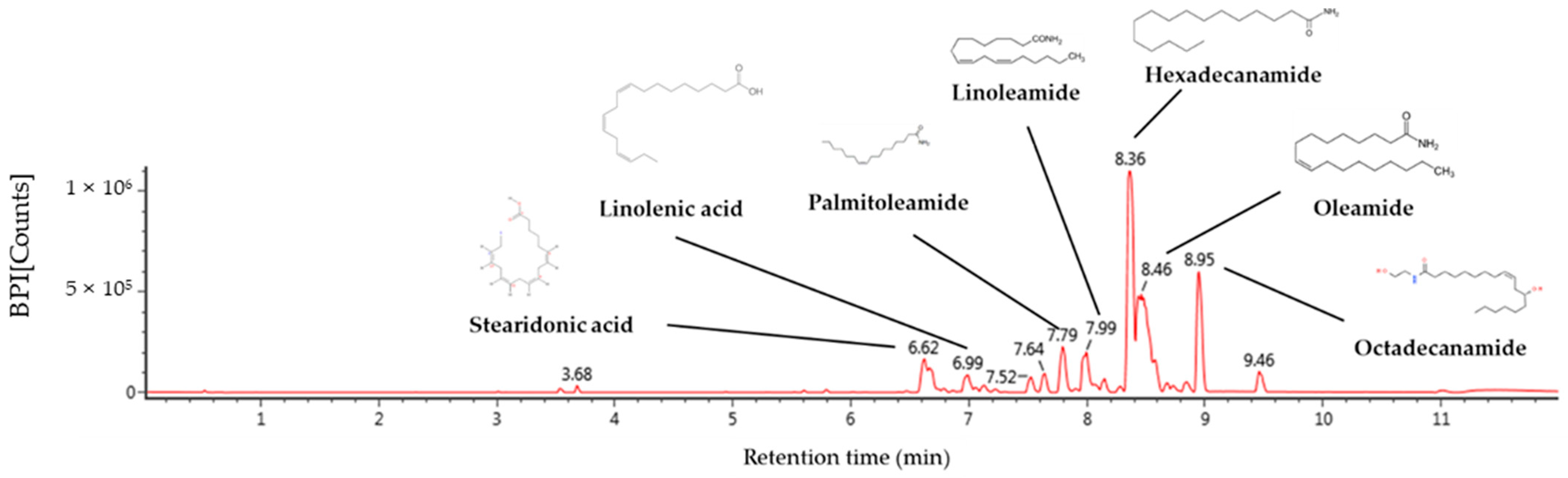


| No. | RT a (min) | Parent Ion b (m/z) | Fragment Ion (m/z) | Identified Compounds |
|---|---|---|---|---|
| 1 | 6.62 | 277 | 107, 93, 91, 79, 67 | Stearidonic acid |
| 2 | 6.99 | 279 | 227, 157, 95, 81 | Linolenic acid |
| 3 | 7.79 | 254 | 237, 219, 149, 135 | Palmitoleamide |
| 4 | 7.99 | 280 | 263, 245, 175, 113, 95, 81 | Linoleamide |
| 5 | 8.36 | 256 | 116, 102, 88, 74, 57 | Hexadecanamide |
| 6 | 8.46 | 282 | 283, 265, 247, 240 | Oleamide |
| 7 | 8.95 | 284 | 285. 102, 88 | Octadecanamide |
| Total Polysaccharide (%) | Sulfate (%) | Relative Area (%) | |||||
|---|---|---|---|---|---|---|---|
| Galactose | Arabinose | Glucose | Xylose | Fucose | Rhamnose | ||
| 31.18 ± 1.20 | 31.05 ± 2.81 | 48.81 ± 0.51 | 21.35 ± 0.17 | 20.27 ± 0.02 | 7.84 ± 0.31 | 0.89 ± 0.04 | 0.80 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.Y.; Kim, J.M.; Lee, H.L.; Go, M.J.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Lee, D.Y.; Kim, H.-J.; Heo, H.J. Codium fragile Suppresses PM2.5-Induced Cognitive Dysfunction by Regulating Gut–Brain Axis via TLR-4/MyD88 Pathway. Int. J. Mol. Sci. 2023, 24, 12898. https://doi.org/10.3390/ijms241612898
Kim TY, Kim JM, Lee HL, Go MJ, Joo SG, Kim JH, Lee HS, Lee DY, Kim H-J, Heo HJ. Codium fragile Suppresses PM2.5-Induced Cognitive Dysfunction by Regulating Gut–Brain Axis via TLR-4/MyD88 Pathway. International Journal of Molecular Sciences. 2023; 24(16):12898. https://doi.org/10.3390/ijms241612898
Chicago/Turabian StyleKim, Tae Yoon, Jong Min Kim, Hyo Lim Lee, Min Ji Go, Seung Gyum Joo, Ju Hui Kim, Han Su Lee, Dong Yeol Lee, Hyun-Jin Kim, and Ho Jin Heo. 2023. "Codium fragile Suppresses PM2.5-Induced Cognitive Dysfunction by Regulating Gut–Brain Axis via TLR-4/MyD88 Pathway" International Journal of Molecular Sciences 24, no. 16: 12898. https://doi.org/10.3390/ijms241612898
APA StyleKim, T. Y., Kim, J. M., Lee, H. L., Go, M. J., Joo, S. G., Kim, J. H., Lee, H. S., Lee, D. Y., Kim, H.-J., & Heo, H. J. (2023). Codium fragile Suppresses PM2.5-Induced Cognitive Dysfunction by Regulating Gut–Brain Axis via TLR-4/MyD88 Pathway. International Journal of Molecular Sciences, 24(16), 12898. https://doi.org/10.3390/ijms241612898