The Molecular Regulatory Mechanism in Multipotency and Differentiation of Wharton’s Jelly Stem Cells
Abstract
:1. Introduction
2. Epigenetic Regulation
2.1. DNA Methylation
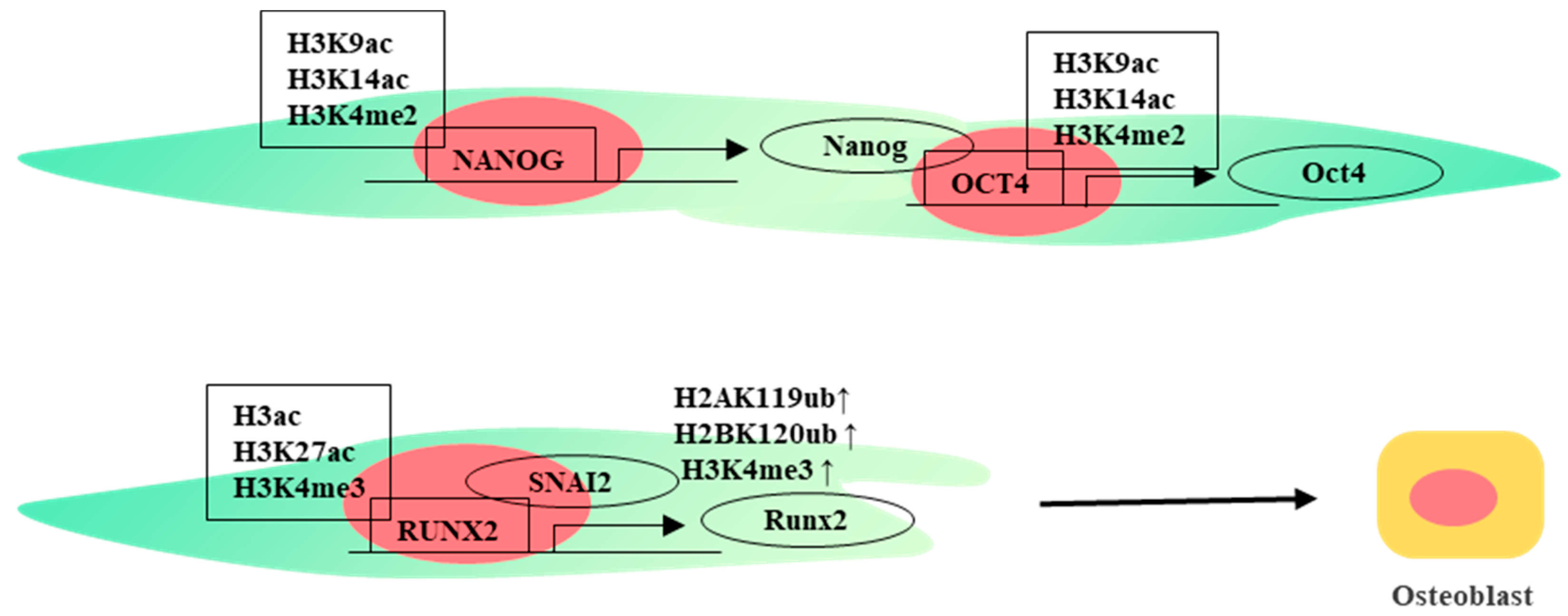
2.2. Histone Modifications
2.3. Non-Coding RNA
3. Transcriptional Regulation
4. Signaling Pathways
5. Other Important Molecules in Differentiation of WJ-MSCs
6. Combined Factors Mediate Osteogenic Differentiation of WJ-MSCs
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mcelreavey, K.D.; Irvine, A.I.; Ennis, K.T.; Mclean, W.I. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef]
- Bakhshi, T.; Zabriskie, R.C.; Bodie, S.; Kidd, S.; Ramin, S.; Paganessi, L.A.; Gregory, S.A.; Fung, H.C.; Christopherson, K.W., 2nd. Mesenchymal stem cells from the Wharton’s jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long-term ex vivo culture. Transfusion 2008, 48, 2638–2644. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Lan, S.; Li, P.; Wang, L.; Kou, J.; Qi, X.; Fan, R.; Hao, D.; Wu, C.; et al. Large-scale expansion of Wharton’s jelly-derived mesenchymal stem cells on gelatin microbeads, with retention of self-renewal and multipotency characteristics and the capacity for enhancing skin wound healing. Stem Cell Res. Ther. 2015, 6, 38. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Cetrulo, K.; Cetrulo, C. Wharton’s Jelly stem cells: Future clinical applications. Placenta 2011, 32, S311–S315. [Google Scholar] [CrossRef]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C.V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Lunyak, V.V.; Rosenfeld, M.G. Epigenetic regulation of stem cell fate. Hum. Mol. Genet. 2008, 17, R28–R36. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Schöler, H.R. Directing reprogramming to pluripotency by transcription factors. Curr. Opin. Genet. Dev. 2012, 22, 416–422. [Google Scholar] [CrossRef]
- Graf, T.; Enver, T. Forcing cells to change lineages. Nature 2009, 462, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Janke, R.; Dodson, A.E.; Rine, J. Metabolism and Epigenetics. Annu. Rev. Cell Dev. Biol. 2015, 31, 473–496. [Google Scholar] [CrossRef]
- He, Y.-F.; Li, B.-Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-Mediated Formation of 5-Carboxylcytosine and Its Excision by TDG in Mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Sasajima, Y.; Tanaka, H.; Miyake, S.; Yuasa, Y. A novel EID family member, EID-3, inhibits differentiation and forms a homodimer or heterodimer with EID-2. Biochem. Biophys. Res. Commun. 2005, 333, 969–975. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.-J.; Yin, J.Q.; Xu, R.-X. EID3 directly associates with DNMT3A during transdifferentiation of human umbilical cord mesenchymal stem cells to NPC-like cells. Sci. Rep. 2017, 7, 40463. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Y.; Gao, J.; Yuan, S.; Chu, Y.; Li, Y.; Yuan, W.; Wang, X. Tet2 regulates the function of mesenchymal stem cells. Bao Chin. J. Biotechnol. 2019, 35, 142–149. [Google Scholar]
- Yang, R.; Yu, T.; Kou, X.; Gao, X.; Chen, C.; Liu, D.; Zhou, Y.; Shi, S. Tet1 and Tet2 maintain mesenchymal stem cell homeostasis via demethylation of the P2rX7 promoter. Nat. Commun. 2018, 9, 2143. [Google Scholar] [CrossRef] [PubMed]
- Cakouros, D.; Hemming, S.; Gronthos, K.; Liu, R.; Zannettino, A.; Shi, S.; Gronthos, S. Specific functions of TET1 and TET2 in regulating mesenchymal cell lineage determination. Epigenet. Chromatin 2019, 12, 3. [Google Scholar] [CrossRef]
- Mahaira, L.G.; Katsara, O.; Pappou, E.; Iliopoulou, E.G.; Fortis, S.; Antsaklis, A.; Fotinopoulos, P.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. IGF2BP1 Expression in Human Mesenchymal Stem Cells Significantly Affects Their Proliferation and Is Under the Epigenetic Control of TET1/2 Demethylases. Stem Cells Dev. 2014, 23, 2501–2512. [Google Scholar] [CrossRef]
- Yannarelli, G.; Pacienza, N.; Cuniberti, L.; Medin, J.; Davies, J.; Keating, A. Brief Report: The Potential Role of Epigenetics on Multipotent Cell Differentiation Capacity of Mesenchymal Stromal Cells. Stem Cells 2013, 31, 215–220. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Timoskainen, S.; West, F.D.; Vekterud, K.; Boquest, A.C.; Ährlund-Richter, L.; Stice, S.L.; Collas, P.; Ghanjati, F.; Santourlidis, S.; et al. Lineage-Specific Promoter DNA Methylation Patterns Segregate Adult Progenitor Cell Types. Stem Cells Dev. 2010, 19, 1257–1266. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Jacobsen, B.M.; Reiner, A.H.; Andersen, I.S.; Collas, P. Promoter DNA Methylation Patterns of Differentiated Cells Are Largely Programmed at the Progenitor Stage. Mol. Biol. Cell 2010, 21, 2066–2077. [Google Scholar] [CrossRef]
- Govarthanan, K.; Gupta, P.K.; Ramasamy, D.; Kumar, P.; Mahadevan, S.; Verma, R.S. DNA methylation microarray uncovers a permissive methylome for cardiomyocyte differentiation in human mesenchymal stem cells. Genomics 2019, 112, 1384–1395. [Google Scholar] [CrossRef]
- Leow, S.C.; Poschmann, J.; Too, P.G.; Yin, J.; Joseph, R.; McFarlane, C.; Dogra, S.; Shabbir, A.; Ingham, P.W.; Prabhakar, S.; et al. The transcription factor SOX6 contributes to the developmental origins of obesity by promoting adipogenesis. Development 2016, 143, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, Y.; Liu, Z.; Zhao, W.; Huang, L.; Wu, T.; Mu, Y. Integrated analysis of DNA methylome and transcriptome reveals the differences in biological characteristics of porcine mesenchymal stem cells. BMC Genet. 2021, 22, 56. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Yan, H.; Qi, H.; Deng, C.; Ye, T.; Zhou, S.; Li, F.-R. Role of histone deacetylase inhibitors in the aging of human umbilical cord mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 2231–2239. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.-Y.; Pockwinse, S.M. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef]
- Rojas, A.; Aguilar, R.; Henriquez, B.; Lian, J.B.; Stein, J.L.; Stein, G.S.; van Wijnen, A.J.; van Zundert, B.; Allende, M.L.; Montecino, M. Epigenetic Control of the Bone-master Runx2 Gene during Osteoblast-lineage Commitment by the Histone Demethylase JARID1B/KDM5B. J. Biol. Chem. 2015, 290, 28329–28342. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, H.; Aguilar, R.; Prieto, C.P.; Bustos, F.; Aedo, S.; Lattus, J.; van Zundert, B.; Palma, V.; Montecino, M. Epigenetic Signatures at the RUNX2-P1 and Sp7 Gene Promoters Control Osteogenic Lineage Commitment of Umbilical Cord-Derived Mesenchymal Stem Cells. J. Cell. Physiol. 2017, 232, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Ghazimoradi, M.H.; Farivar, S. The role of DNA demethylation in induction of stem cells. Prog. Biophys. Mol. Biol. 2020, 153, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Sarnowska, A.; Siedlecka, P.; Szablowska-Gadomska, I.; Wielgos, M.; Jurga, M.; Lukomska, B.; Domanska-Janik, K. Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy 2014, 16, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.-R.; Seo, M.-S.; Roh, K.-H.; Park, S.-B.; Hwang, J.-W.; Sun, B.; Seo, K.; Lee, Y.-S.; Kang, S.-K.; et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009, 42, 711–720. [Google Scholar] [CrossRef]
- Karantzali, E.; Schulz, H.; Hummel, O.; Hubner, N.; Hatzopoulos, A.; Kretsovali, A. Histone deacetylase inhibition accelerates the early events of stem cell differentiation: Transcriptomic and epigenetic analysis. Genome Biol. 2008, 9, R65. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A.; Warrier, S. Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Res. Ther. 2017, 8, 185. [Google Scholar] [CrossRef]
- Belame Shivakumar, S.; Bharti, D.; Baregundi Subbarao, R.; Park, J.-M.; Son, Y.-B.; Ullah, I.; Choe, Y.-H.; Lee, H.-J.; Park, B.-W.; Lee, S.-L.; et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell. Physiol. 2019, 234, 3933–3947. [Google Scholar] [CrossRef] [PubMed]
- Cannell, I.G.; Kong, Y.W.; Bushell, M. How do microRNAs regulate gene expression? Biochem. Soc. Trans. 2008, 36, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Balzano, F.; Campesi, I.; Cruciani, S.; Garroni, G.; Bellu, E.; Giudici, S.D.; Angius, A.; Oggiano, A.; Rallo, V.; Capobianco, G.; et al. Epigenetics, Stem Cells, and Autophagy: Exploring a Path Involving miRNA. Int. J. Mol. Sci. 2019, 20, 5091. [Google Scholar] [CrossRef]
- Balzano, F.; Cruciani, S.; Basoli, V.; Santaniello, S.; Facchin, F.; Ventura, C.; Maioli, M. MiR200 and miR302: Two Big Families Influencing Stem Cell Behavior. Molecules 2018, 23, 282. [Google Scholar] [CrossRef]
- Han, X.; Yang, H.; Liu, H.; Zhang, C.; Cao, Y.; Fan, Z.; Shi, R. miR-196b-5p inhibits proliferation of Wharton’s jelly umbilical cord stem cells. FEBS Open Bio 2021, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Yang, H.; Cao, Y.; Yu, D.; Zhao, Y.; Cao, Y. MicroRNA-196a-5p overexpression in Wharton’s jelly umbilical cord stem cells promotes their osteogenic differentiation and new bone formation in bone defects in the rat calvarium. Cell Tissue Res. 2022, 390, 245–260. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, R.; Zhang, S.; Shu, Q.; Zhang, D.; Xu, G. Altered expression of microRNAs in the neuronal differentiation of human Wharton’s Jelly mesenchymal stem cells. Neurosci. Lett. 2015, 600, 69–74. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Fam, S.-Z.; Chong, C.-E.; Abdullah, S.; Cheah, P.-S.; Nordin, N.; Ling, K.-H. miR-3099 promotes neurogenesis and inhibits astrogliogenesis during murine neural development. Gene 2019, 697, 201–212. [Google Scholar] [CrossRef]
- Wang, H.; Ban, W.; Wang, T.; Li, Z.; Dang, X. miR-20b/106a modulate Ngn2 gene expression during neural differentiation of human umbilical cord mesenchymal stem cells. Neuroreport 2017, 28, 1225–1231. [Google Scholar] [CrossRef]
- Chang, S.-J.; Weng, S.-L.; Hsieh, J.-Y.; Wang, T.-Y.; Chang, M.D.-T.; Wang, H.-W. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Med. Genom. 2011, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Khanna, A. Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 2016, 343, 237–247. [Google Scholar] [CrossRef]
- Hsieh, J.-Y.; Huang, T.-S.; Cheng, S.-M.; Lin, W.-S.; Tsai, T.-N.; Lee, O.K.; Wang, H.-W. miR-146a-5p circuitry uncouples cell proliferation and migration, but not differentiation, in human mesenchymal stem cells. Nucleic Acids Res. 2013, 41, 9753–9763. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tang, X.; Wan, J.; Zhang, X.; Liu, C.; Liu, T. Functions and mechanisms of circular RNAs in regulating stem cell differentiation. RNA Biol. 2021, 18, 2136–2149. [Google Scholar] [CrossRef]
- Ruan, Z.; Chen, G.; Zhang, R.; Zhu, L. Circular RNA expression profiles during the differentiation of human umbilical cord–derived mesenchymal stem cells into cardiomyocyte-like cells. J. Cell. Physiol. 2019, 234, 16412–16423. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Sun, B.; Wang, D.; Zhu, Y.; Zhao, X.; Yang, X.; Zhang, Y. Circ6401, a novel circular RNA, is implicated in repair of the damaged endometrium by Wharton’s jelly-derived mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B axis. Stem Cell Res. Ther. 2020, 11, 520. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Ding, Y.; Lu, X.; Zhang, G.; Yang, J.; Zheng, H.; Wang, H.; Jiang, Y.; Xu, L. LncRNA Structural Characteristics in Epigenetic Regulation. Int. J. Mol. Sci. 2017, 18, 2659. [Google Scholar] [CrossRef]
- Xie, Z.-Y.; Wang, P.; Wu, Y.-F.; Shen, H.-Y. Long non-coding RNA: The functional regulator of mesenchymal stem cells. World J. Stem Cells 2019, 11, 167–179. [Google Scholar] [CrossRef]
- Liu, F.; Song, D.-Y.; Huang, J.; Yang, H.-Q.; Di You, D.; Ni, J.-D. Long non-coding RNA CIR inhibits chondrogenic differentiation of mesenchymal stem cells by epigenetically suppressing ATOH8 via methyltransferase EZH2. Mol. Med. 2021, 27, 12. [Google Scholar] [CrossRef]
- Al-Obaide, M.; Ishmakej, A.; Brown, C.; Mazzella, M.; Agosta, P.; Perez-Cruet, M.; Chaudhry, G.R. The potential role of integrin alpha 6 in human mesenchymal stem cells. Front. Genet. 2022, 13, 968228. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Su, P.-F.; Huang, Y.-F.; Yew, T.-L.; Hung, S.-C. Oct4 and Nanog Directly Regulate Dnmt1 to Maintain Self-Renewal and Undifferentiated State in Mesenchymal Stem Cells. Mol. Cell 2012, 47, 169–182. [Google Scholar] [CrossRef]
- Vaquerizas, J.M.; Kummerfeld, S.K.; Teichmann, S.A.; Luscombe, N.M. A census of human transcription factors: Function, expression and evolution. Nat. Rev. Genet. 2009, 10, 252–263. [Google Scholar] [CrossRef]
- Fong, C.-Y.; Chak, L.-L.; Biswas, A.; Tan, J.-H.; Gauthaman, K.; Chan, W.-K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Rodda, D.J.; Chew, J.-L.; Lim, L.-H.; Loh, Y.-H.; Wang, B.; Ng, H.-H.; Robson, P. Transcriptional Regulation of Nanog by OCT4 and SOX2. J. Biol. Chem. 2005, 280, 24731–24737. [Google Scholar] [CrossRef]
- Loh, Y.-H.; Wu, Q.; Chew, J.-L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef]
- Gao, L.R.; Zhang, N.K.; Ding, Q.A.; Chen, H.Y.; Hu, X.; Jiang, S.; Li, T.C.; Chen, Y.; Wang, Z.G.; Ye, Y.; et al. Common Expression of Stemness Molecular Markers and Early Cardiac Transcription Factors in Human Wharton’s Jelly-Derived Mesenchymal Stem Cells and Embryonic Stem Cells. Cell Transplant. 2013, 22, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Shivakumar, S.B.; Ullah, I.; Subbarao, R.B.; Park, J.-S.; Lee, S.-L.; Park, B.-W.; Rho, G.-J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef]
- Ng, H.-H.; Surani, M.A. The transcriptional and signalling networks of pluripotency. Nature 2011, 13, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.J.; Liu, K.; Rameshwar, P. Functional Similarities Among Genes Regulated by Oct4 in Human Mesenchymal and Embryonic Stem Cells. Stem Cells 2007, 25, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef]
- Gil-Kulik, P.; Świstowska, M.; Krzyżanowski, A.; Petniak, A.; Kwaśniewska, A.; Płachno, B.J.; Galkowski, D.; Bogucka-Kocka, A.; Kocki, J. Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker. Int. J. Mol. Sci. 2022, 23, 7630. [Google Scholar] [CrossRef] [PubMed]
- Schaal, C.M.; Bora-Singhal, N.; Kumar, D.M.; Chellappan, S.P. Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer. Mol. Cancer 2018, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Świstowska, M.; Gil-Kulik, P.; Krzyżanowski, A.; Bielecki, T.; Czop, M.; Kwaśniewska, A.; Kocki, J. Potential Effect of SOX2 on the Cell Cycle of Wharton’s Jelly Stem Cells (WJSCs). Oxidative Med. Cell. Longev. 2019, 2019, 5084689. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Chang, C.W.; Duan, K.; Poidinger, M.; Ng, K.L.; Chong, Y.S.; Gluckman, P.D.; Stünkel, W. E2F1 Orchestrates Transcriptomics and Oxidative Metabolism in Wharton’s Jelly-Derived Mesenchymal Stem Cells from Growth-Restricted Infants. PLoS ONE 2016, 11, e0163035. [Google Scholar] [CrossRef]
- Jeong, B.-C.; Kang, I.-H.; Hwang, Y.-C.; Kim, S.-H.; Koh, J.-T. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014, 5, e1532. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, X.-F.; Hubbell, K.; Cui, X. NR2F2 regulates chondrogenesis of human mesenchymal stem cells in bioprinted cartilage. Biotechnol. Bioeng. 2017, 114, 208–216. [Google Scholar] [CrossRef]
- Ma, L.; Huang, M.; Liao, X.; Cai, X.; Wu, Q. NR2F2 Regulates Cell Proliferation and Immunomodulation in Whartons’ Jelly Stem Cells. Genes 2022, 13, 1458. [Google Scholar] [CrossRef]
- Velletri, T.; Xie, N.; Wang, Y.; Huang, Y.; Yang, Q.; Chen, X.; Chen, Q.; Shou, P.; Gan, Y.; Cao, G.; et al. P53 functional abnormality in mesenchymal stem cells promotes osteosarcoma development. Cell Death Dis. 2016, 7, e2015. [Google Scholar] [CrossRef]
- Armesilla-Diaz, A.; Elvira, G.; Silva, A. p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp. Cell Res. 2009, 315, 3598–3610. [Google Scholar] [CrossRef]
- Velletri, T.; Huang, Y.; Wang, Y.; Li, Q.; Hu, M.; Xie, N.; Yang, Q.; Chen, X.; Chen, Q.; Shou, P.; et al. Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin. Cell Death Differ. 2021, 28, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.; Mao, J.; Wen, H.; Xu, L.; Ren, Y.; Du, H.; Yang, H. Mouse bone marrow mesenchymal stem cells with distinct p53 statuses display differential characteristics. Mol. Med. Rep. 2020, 21, 2051–2062. [Google Scholar] [CrossRef]
- He, Y.; de Castro, L.F.; Shin, M.H.; Dubois, W.; Yang, H.H.; Jiang, S.; Mishra, P.J.; Ren, L.; Gou, H.; Lal, A.; et al. p53 Loss Increases the Osteogenic Differentiation of Bone Marrow Stromal Cells. Stem Cells 2015, 33, 1304–1319. [Google Scholar] [CrossRef]
- Artigas, N.; Gámez, B.; Cubillos-Rojas, M.; Diego, C.S.-D.; Valer, J.A.; Pons, G.; Rosa, J.L.; Ventura, F. p53 inhibits SP7/Osterix activity in the transcriptional program of osteoblast differentiation. Cell Death Differ. 2017, 24, 2022–2031. [Google Scholar] [CrossRef]
- Zhou, W.; Gross, K.M.; Kuperwasser, C. Molecular regulation of Snai2 in development and disease. J. Cell Sci. 2019, 132, jcs235127. [Google Scholar] [CrossRef]
- Tang, Y.; Weiss, S.J. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle 2017, 16, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, E.; Lisignoli, G.; Manferdini, C.; Lambertini, E.; Penolazzi, L.; Vecchiatini, R.; Gabusi, E.; Chieco, P.; Facchini, A.; Gambari, R.; et al. Role of Slug transcription factor in human mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 740–751. [Google Scholar] [CrossRef]
- Hang, H.; Yu, Y.; Wu, N.; Huang, Q.; Xia, Q.; Bian, J. Induction of Highly Functional Hepatocytes from Human Umbilical Cord Mesenchymal Stem Cells by HNF4α Transduction. PLoS ONE 2014, 9, e104133. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Wu, N.; Xia, L.; Cao, J.; Xia, Q.; Zhao, J.; Zhang, J.; Hang, H. HNF4α overexpression enhances the therapeutic potential of umbilical cord mesenchymal stem/stromal cells in mice with acute liver failure. FEBS Lett. 2022, 596, 3176–3190. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pruitt, K. Wnt Pathway: An Integral Hub for Developmental and Oncogenic Signaling Networks. Int. J. Mol. Sci. 2020, 21, 8018. [Google Scholar] [CrossRef]
- Ling, L.; Nurcombe, V.; Cool, S.M. Wnt signaling controls the fate of mesenchymal stem cells. Gene 2009, 433, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Batsali, A.K.; Pontikoglou, C.; Koutroulakis, D.; Pavlaki, K.I.; Damianaki, A.; Mavroudi, I.; Alpantaki, K.; Kouvidi, E.; Kontakis, G.; Papadaki, H.A. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton’s jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 102. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Liu, Y.; Jia, Z.; Li, Y.; Zhang, C.; Chen, P.; Ma, K.; Affara, N.; Zhou, C. WNT signaling promotes Nkx2.5 expression and early cardiomyogenesis via downregulation of Hdac1. Biochim. Biophys. Acta BBA Mol. Cell Res. 2009, 1793, 300–311. [Google Scholar] [CrossRef]
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2009, 30, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Berlier, J.L.; Rigutto, S.; Valle, A.D.; Lechanteur, J.; Soyfoo, M.S.; Gangji, V.; Rasschaert, J. Adenosine Triphosphate Prevents Serum Deprivation-Induced Apoptosis in Human Mesenchymal Stem Cells via Activation of the MAPK Signaling Pathways. Stem Cells 2015, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Q.-H.; Huang, L.-H.; Dong, H.-Y.; Lin, L.-J.; Tan, J.-M. Correlation of CDC42 Activity with Cell Proliferation and Palmitate-Mediated Cell Death in Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stromal Cells. Stem Cells Dev. 2017, 26, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, Y.-L.; Tyan, Y.-S.; Chiu, Y.-H.; Liang, Y.-H.; Chen, C.-P.; Wu, J.-C.; Wang, H.-S. Interleukin-1β-induced matrix metalloproteinase-3 via ERK1/2 pathway to promote mesenchymal stem cell migration. PLoS ONE 2021, 16, e0252163. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Fan, Z.; Liu, D.; Wang, F.; Zhou, Y. IGFBP2 enhances adipogenic differentiation potentials of mesenchymal stem cells from Wharton’s jelly of the umbilical cord via JNK and Akt signaling pathways. PLoS ONE 2017, 12, e0184182. [Google Scholar] [CrossRef]
- Hiew, V.V.; Teoh, P.L. Collagen Modulates the Biological Characteristics of WJ-MSCs in Basal and Osteoinduced Conditions. Stem Cells Int. 2022, 2022, 2116367. [Google Scholar] [CrossRef]
- Zavala, G.; Prieto, C.P.; Villanueva, A.A.; Palma, V. Sonic hedgehog (SHH) signaling improves the angiogenic potential of Wharton’s jelly-derived mesenchymal stem cells (WJ-MSC). Stem Cell Res. Ther. 2017, 8, 203. [Google Scholar] [CrossRef]
- Goyal, U.; Ta, M. A novel role of vitronectin in promoting survival of mesenchymal stem cells under serum deprivation stress. Stem Cell Res. Ther. 2020, 11, 181. [Google Scholar] [CrossRef]
- Ju, D.; Van Thao, D.; Lu, C.; Ali, A.; Shibu, M.A.; Chen, R.; Day, C.H.; Shih, T.; Tsai, C.; Kuo, C.; et al. Protective effects of CHIP overexpression and Wharton’s jelly mesenchymal-derived stem cell treatment against streptozotocin-induced neurotoxicity in rats. Environ. Toxicol. 2022, 37, 1979–1987. [Google Scholar] [CrossRef]
- Jang, I.K.; Jung, H.J.; Noh, O.K.; Lee, D.; Lee, K.C.; Park, J.E. B7-H1-mediated immunosuppressive properties in human mesenchymal stem cells are mediated by STAT-1 and not PI3K/Akt signaling. Mol. Med. Rep. 2018, 18, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xiao, F.; Hao, H.; Hua, F.; Luo, Z.; Huang, Z.; Li, Q.; Chen, S.; Cheng, X.; Zhang, X.; et al. JARID1B promotes colorectal cancer proliferation and Wnt/β-catenin signaling via decreasing CDX2 level. Cell Commun. Signal. 2020, 18, 169. [Google Scholar] [CrossRef]
- Uzan, B.; Figeac, F.; Portha, B.; Movassat, J. Mechanisms of KGF Mediated Signaling in Pancreatic Duct Cell Proliferation and Differentiation. PLoS ONE 2009, 4, e4734. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, Y.; Xu, M.; Ma, K.; Fu, X.; Zhang, M.; Wang, G. Role of Keratinocyte Growth Factor in the Differentiation of Sweat Gland-Like Cells From Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Transl. Med. 2016, 5, 106–116. [Google Scholar] [CrossRef]
- Bracci-Laudiero, L.; De Stefano, M.E. NGF in Early Embryogenesis, Differentiation, and Pathology in the Nervous and Immune Systems. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2015; Volume 29, pp. 125–152. [Google Scholar] [CrossRef]
- Morys, J.; Borkowska, P.; Zielinska, A.; Kowalski, J. Study of the influence of NGF-β gene overexpression in human mesenchymal stem cells on the expression level of SOX1 and neural pathway genes. Mol. Biol. Rep. 2022, 49, 4435–4441. [Google Scholar] [CrossRef]
- Borkowska, P.; Morys, J.; Zielinska, A.; Sadlocha, M.; Kowalski, J. Survival and Neurogenesis-Promoting Effects of the Co-Overexpression of BCLXL and BDNF Genes on Wharton’s Jelly-Derived Mesenchymal Stem Cells. Life 2022, 12, 1406. [Google Scholar] [CrossRef]
- Langhnoja, J.; Buch, L.; Pillai, P. Potential role of NGF, BDNF, and their receptors in oligodendrocytes differentiation from neural stem cell: An in vitro study. Cell Biol. Int. 2020, 45, 432–446. [Google Scholar] [CrossRef]
- Bustos, F.; Sepúlveda, H.; Prieto, C.P.; Carrasco, M.; Díaz, L.; Palma, J.; Lattus, J.; Montecino, M.; Palma, V. Runt-Related Transcription Factor 2 Induction During Differentiation of Wharton’s Jelly Mesenchymal Stem Cells to Osteoblasts Is Regulated by Jumonji AT-Rich Interactive Domain 1B Histone Demethylase. Stem Cells 2017, 35, 2430–2441. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Celil, A.B.; Campbell, P.G. BMP-2 and Insulin-like Growth Factor-I Mediate Osterix (Osx) Expression in Human Mesenchymal Stem Cells via the MAPK and Protein Kinase D Signaling Pathways. J. Biol. Chem. 2005, 280, 31353–31359. [Google Scholar] [CrossRef]
- Ono, M.; Inkson, C.A.; Kilts, T.M.; Young, M.F. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Miner. Res. 2011, 26, 193–208. [Google Scholar] [CrossRef]
- Fallah, A.; Alipour, M.; Jamali, Z.; Farjadfar, A.; Roshangar, L.; Nasr, M.P.; Hashemi, P.; Aghazadeh, M. Overexpression Effects of miR-424 and BMP2 on the Osteogenesis of Wharton’s Jelly-Derived Stem Cells. BioMed. Res. Int. 2021, 2021, 7031492. [Google Scholar] [CrossRef]
- He, S.; Yang, S.; Zhang, Y.; Li, X.; Gao, D.; Zhong, Y.; Cao, L.; Ma, H.; Liu, Y.; Li, G.; et al. LncRNA ODIR1 inhibits osteogenic differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis. 2019, 10, 947. [Google Scholar] [CrossRef]
- Yang, L.; Bin, Z.; Hui, S.; Rong, L.; You, B.; Wu, P.; Han, X.; Qian, H.; Xu, W. The Role of CDR1as in Proliferation and Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2019, 2019, 2316834. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zheng, X.; He, Y.; Long, L.; Chen, W. Transcriptomic profiling and functional prediction reveal aberrant expression of circular RNAs during osteogenic differentiation in human umbilical cord mesenchymal stromal cells. Sci. Rep. 2021, 11, 19881. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; He, X.; Wu, Q. The Molecular Regulatory Mechanism in Multipotency and Differentiation of Wharton’s Jelly Stem Cells. Int. J. Mol. Sci. 2023, 24, 12909. https://doi.org/10.3390/ijms241612909
Ma L, He X, Wu Q. The Molecular Regulatory Mechanism in Multipotency and Differentiation of Wharton’s Jelly Stem Cells. International Journal of Molecular Sciences. 2023; 24(16):12909. https://doi.org/10.3390/ijms241612909
Chicago/Turabian StyleMa, Li, Xuguang He, and Qiang Wu. 2023. "The Molecular Regulatory Mechanism in Multipotency and Differentiation of Wharton’s Jelly Stem Cells" International Journal of Molecular Sciences 24, no. 16: 12909. https://doi.org/10.3390/ijms241612909




