Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis
Abstract
:1. What Is Multiple Sclerosis?
Ongoing Basic and Clinical Research
- Interferon beta medications: These synthetic injectable drugs mimic the naturally occurring protein interferon-β, which helps regulate the immune system and reduce inflammation.
- Glatiramer acetate: A synthetic mixture formulated with four different amino acids that replicates the structure of MBP. Upon its injection, it functions by redirecting the immune response away from myelin to prevent further damage.
- Teriflunomide: An oral medication that inhibits the proliferation of immune cells involved in MS through the inhibition of an enzyme called dihydroorotate dehydrogenase (DHODH).
- Dimethyl fumarate: An oral medication with anti-inflammatory and antioxidant properties that might exert its function by activating the Nrf2 pathway.
- Fingolimod: An oral medication acting as a sphingosine-1-phosphate receptor modulator. It traps immune cells in the lymph nodes, reducing their ability to attack the central nervous system.
- Natalizumab: An intravenous monoclonal antibody preventing immune cells from crossing the BBB and entering the central nervous system.
- Alemtuzumab: Intravenously infused monoclonal antibody that targets and depletes T and B cells involved in MS.
- Ocrelizumab: An intravenous monoclonal antibody specifically targeting and depleting B cells, mitigating the immune attack on myelin.
- Siponimod: An oral sphingosine-1-phosphate receptor modulator, similar to fingolimod. It prevents immune cells from reaching the CNS by trapping them in the lymph nodes.
- Cladribine: An oral medication primarily affecting lymphocytic function, leading to a reduction of certain immune cells.
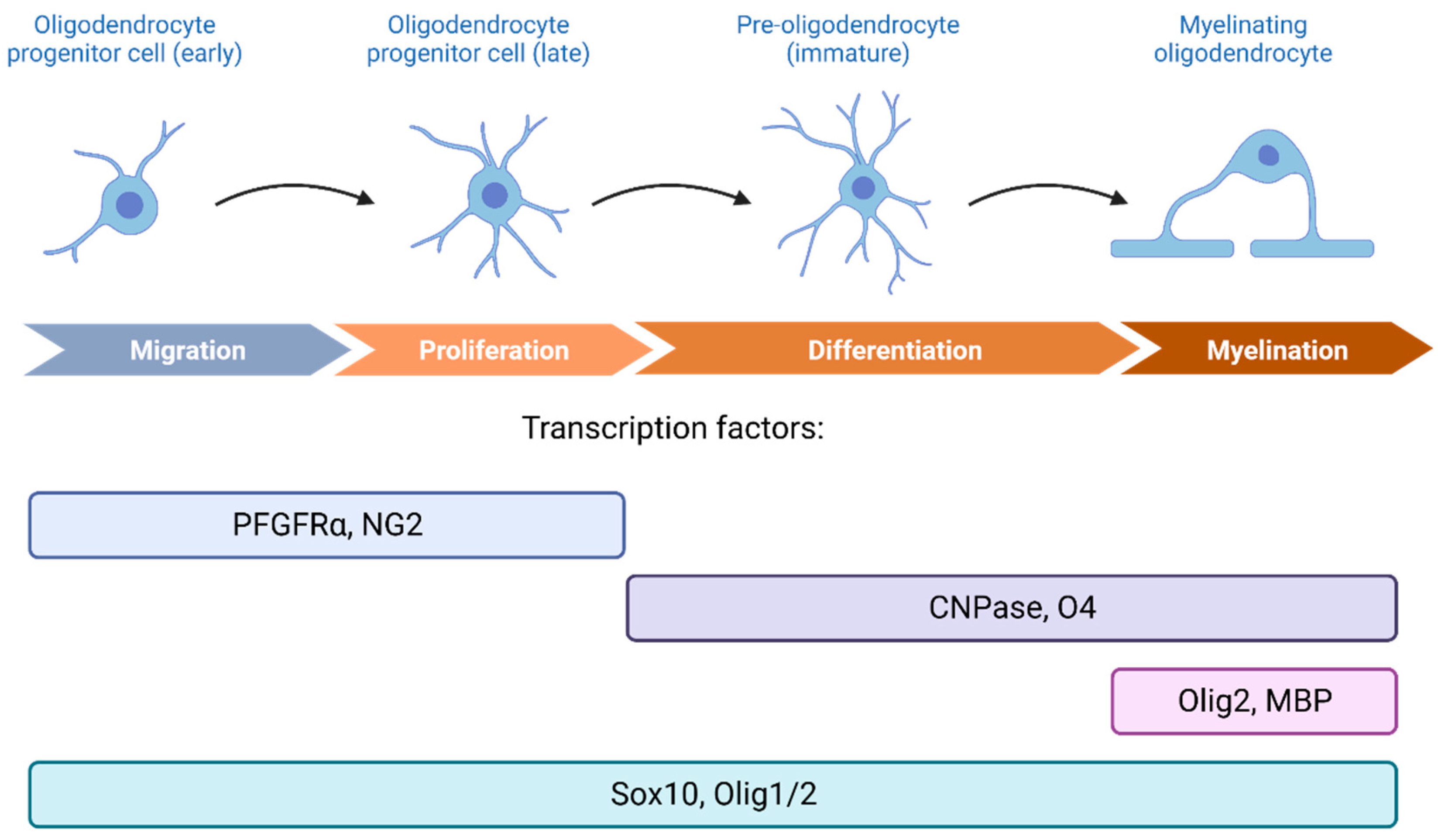
2. Oligodendroglia: Biology and Function
- Oligodendrocyte Precursor Cells: A Lookout into Their Functionality
- b.
- Oligodendrocytes
3. Myelin in the CNS
- Myelin Composition: Lipids
| Group | Overall Percentage |
|---|---|
| Cholesterol | 40% |
| Phospholipid | 38% |
| a. Phosphatidylcholine | 7% |
| b. Phosphaditylethanolamine | 7% |
| c. Plasmalogen | 13% |
| d. Sphingomyelin | 6% |
| b. Glycolipids | 20% |
| b.1. Galactosylceramide | 17% |
| b.2. Sulfatide | 3% |
| Fatty acids | 2% |
| a. Linoleic acid (18:2) | |
| b. Arachidic acid (20:0) | |
| c. Palmitic acid (16:0) | |
| d. Oleic acid (18:1) | |
| e. Eicosenoic acid (20:1) | |
| f. Arachidonic acid (20:4) | |
| g. Docosatetraenoic acid (22:4) | |
| h. Docosahexaenoic acid (22:6) |
- b.
- Myelin Composition: Proteins
4. Metabolic Profile of Oligodendroglia and Myelin: A Brief Insight into Lipid Metabolism
5. Altered Bioenergetics in Multiple Sclerosis
- Mitochondrial Dysfunction and Oxidative Stress
- b.
- Inflammation and Bioenergetics Interplay
- c.
- Glucose Metabolism in MS
- d.
- Dysregulation of Lipid Metabolism in MS
6. A Tentative Hypothesis on How Altered Bioenergetics May Affect MS Progression
7. Concluding Paragraph
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.G.; Meuth, S.G.; Martinez-Lapiscina, E.H.; Albrecht, P.; Menge, T. Treatment of Patients with Multiple Sclerosis Transitioning Between Relapsing and Progressive Disease. CNS Drugs 2023, 37, 69–92. [Google Scholar] [CrossRef]
- Kantarci, O.H. Phases and Phenotypes of Multiple Sclerosis. Contin. Lifelong Learn. Neurol. 2019, 25, 636. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Lo, C.H.; Gauthier, S.A.; Hickman, R.A.; Longbrake, E.; Airas, L.M.; Mao-Draayer, Y.; Riley, C.; De Jager, P.L.; Wesley, S.; et al. Toward Precision Phenotyping of Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200025. [Google Scholar] [CrossRef]
- Fernández, O.; Fernández, V.E.; Guerrero, M. Esclerosis múltiple. Med.-Programa Form. Médica Contin. Acreditado 2015, 11, 4610–4621. [Google Scholar] [CrossRef]
- Cagol, A.; Schaedelin, S.; Barakovic, M.; Benkert, P.; Todea, R.-A.; Rahmanzadeh, R.; Galbusera, R.; Lu, P.-J.; Weigel, M.; Melie-Garcia, L.; et al. Association of Brain Atrophy with Disease Progression Independent of Relapse Activity in Patients with Relapsing Multiple Sclerosis. JAMA Neurol. 2022, 79, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Multiple sclerosis: Role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol. 2023, 44, 266–275. [Google Scholar] [CrossRef]
- Stys, P.K.; Tsutsui, S. Recent advances in understanding multiple sclerosis. F1000Research 2019, 8, 2100. [Google Scholar] [CrossRef]
- Garg, N.; Smith, T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci 2018, 96, 1021–1042. [Google Scholar] [CrossRef]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; John, N.A.; Brownlee, W.J. Disease-modifying therapies for multiple sclerosis. BMJ 2018, k4674. [Google Scholar] [CrossRef]
- Winkelmann, A.; Loebermann, M.; Reisinger, E.C.; Hartung, H.-P.; Zettl, U.K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat. Rev. Neurol. 2016, 12, 217–233. [Google Scholar] [CrossRef]
- Piehl, F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J. Intern. Med. 2021, 289, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Travers, B.S.; Tsang, B.K.-T.; Barton, J.L. Multiple sclerosis: Diagnosis, disease-modifying therapy and prognosis. Aust. J. Gen. Pract. 2022, 51, 199–206. [Google Scholar] [CrossRef]
- Stangel, M.; Kuhlmann, T.; Matthews, P.M.; Kilpatrick, T.J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 742–754. [Google Scholar] [CrossRef]
- Nave, K.-A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef]
- Amin, M.; Hersh, C.M. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener. Dis. Manag. 2023, 13, 47–70. [Google Scholar] [CrossRef]
- Jolanda Münzel, E.; Williams, A. Promoting Remyelination in Multiple Sclerosis—Recent Advances. Drugs 2013, 73, 2017–2029. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.-Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov 2017, 16, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; Wee Yong, V.; Koch, M.W.; Mackie, A.; Potvin, S.; Patten, S.B.; Metz, L.M. Quetiapine fumarate for the treatment of multiple sclerosis: Focus on myelin repair. CNS Neurosci. Ther. 2013, 19, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, C.; McGinley, M.P. Advances in the Treatment of Multiple Sclerosis. Neurol. Clin. 2021, 39, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Göttle, P.; Flores-Rivera, J.; Hartung, H.-P.; Küry, P. Remyelination in multiple sclerosis: From concept to clinical trials. Curr. Opin. Neurol. 2019, 32, 378. [Google Scholar] [CrossRef]
- Zhao, X.; Jacob, C. Mechanisms of Demyelination and Remyelination Strategies for Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 6373. [Google Scholar] [CrossRef]
- López-Muguruza, E.; Villar-Gómez, N.; Matias-Guiu, J.A.; Selma-Calvo, B.; Moreno-Jiménez, L.; Sancho-Bielsa, F.; Lopez-Carbonero, J.; Benito-Martín, M.S.; García-Flores, S.; Bonel-García, N.; et al. The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination. Life 2022, 12, 474. [Google Scholar] [CrossRef]
- Smith, J.A.; Nicaise, A.M.; Ionescu, R.-B.; Hamel, R.; Peruzzotti-Jametti, L.; Pluchino, S. Stem Cell Therapies for Progressive Multiple Sclerosis. Front. Cell Dev. Biol. 2021, 9, 696434. [Google Scholar] [CrossRef]
- Alanazi, A.; Alassiri, M.; Jawdat, D.; Almalik, Y. Mesenchymal stem cell therapy: A review of clinical trials for multiple sclerosis. Regen. Ther. 2022, 21, 201–209. [Google Scholar] [CrossRef]
- Bae, D.-K.; Park, D.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Shin, K.; Choi, E.-K.; Kim, G.; Hong, J.T.; et al. Comparative Effects of Human Neural Stem Cells and Oligodendrocyte Progenitor Cells on the Neurobehavioral Disorders of Experimental Autoimmune Encephalomyelitis Mice. Stem Cells Int. 2016, 2016, 4079863. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, C.; Radzikowska, E.; Maciejewski, R. Neural stem cell therapy—Brief review. Clin. Neurol. Neurosurg. 2018, 173, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yellen, G. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 2018, 217, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; Marchi, E.D.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343. [Google Scholar] [CrossRef]
- Ski, B. Emerging evidence for compromised axonal bioenergetics and axoglial metabolic coupling as drivers of neurodegeneration. Neurobiol. Dis. 2022, 170, 105751. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Meyer, N.; Rinholm, J.E. Mitochondria in Myelinating Oligodendrocytes: Slow and Out of Breath? Metabolites 2021, 11, 359. [Google Scholar] [CrossRef]
- Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Matute, C. Pío del Río Hortega and the discovery of the oligodendrocytes. Front. Neuroanat. 2015, 9, 92. [Google Scholar] [CrossRef]
- Beiter, R.M.; Rivet-Noor, C.; Merchak, A.R.; Bai, R.; Johanson, D.M.; Slogar, E.; Sol-Church, K.; Overall, C.C.; Gaultier, A. Evidence for oligodendrocyte progenitor cell heterogeneity in the adult mouse brain. Sci. Rep. 2022, 12, 12921. [Google Scholar] [CrossRef]
- Raff, M.C.; Miller, R.H.; Noble, M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983, 303, 5916. [Google Scholar] [CrossRef]
- Skaper, S.D. Chapter 4—Oligodendrocyte precursor cells as a therapeutic target for demyelinating diseases. In Progress in Brain Research; Sharma, A., Sharma, H.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 245, pp. 119–144. [Google Scholar]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-Expressing Progenitors in the Gray and White Matter of the Adult Mouse Cerebral Cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Gao, M.-Y.; Gao, R.; Zhao, K.-H.; Zhang, Y.; Li, X. Oligodendrocyte lineage cells: Advances in development, disease, and heterogeneity. J. Neurochem. 2023, 164, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Shimizu, T.; Sherafat, A.; Richardson, W.D. Life-long oligodendrocyte development and plasticity. Semin. Cell Dev. Biol. 2021, 116, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Akay, L.A.; Effenberger, A.H.; Tsai, L.-H. Cell of all trades: Oligodendrocyte precursor cells in synaptic, vascular, and immune function. Genes Dev. 2021, 35, 180–198. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Nanescu, S.E.; Psachoulia, K.; Huang, J.K. Oligodendrocyte regeneration: Its significance in myelin replacement and neuroprotection in multiple sclerosis. Neuropharmacology 2016, 110, 633–643. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef]
- Skoff, R.P.; Benjamins, J.A. Oligodendrocytes. In Encyclopedia of the Neurological Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 643–647. ISBN 978-0-12-385158-1. [Google Scholar]
- Butt, A.M.; Fern, R.F.; Matute, C. Neurotransmitter signaling in white matter. Glia 2014, 62, 1762–1779. [Google Scholar] [CrossRef]
- Marisca, R.; Hoche, T.; Agirre, E.; Hoodless, L.J.; Barkey, W.; Auer, F.; Castelo-Branco, G.; Czopka, T. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 2020, 23, 363–374. [Google Scholar] [CrossRef]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2019, 24, 1837–1844. [Google Scholar] [CrossRef]
- Yi, C.; Verkhratsky, A.; Niu, J. Pathological potential of oligodendrocyte precursor cells: Terra incognita. Trends Neurosci. 2023, 46, 581–596. [Google Scholar] [CrossRef]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.; Wegner, M. Using the lineage determinants Olig2 and Sox10 to explore transcriptional regulation of oligodendrocyte development. Dev. Neurobiol. 2021, 81, 892–901. [Google Scholar] [CrossRef]
- Suminaite, D.; Lyons, D.A.; Livesey, M.R. Myelinated axon physiology and regulation of neural circuit function. Glia 2019, 67, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Paez, P.M.; Lyons, D.A. Calcium Signaling in the Oligodendrocyte Lineage: Regulators and Consequences. Annu. Rev. Neurosci. 2020, 43, 163–186. [Google Scholar] [CrossRef]
- Funfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Mobius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Williamson, J.M.; Lyons, D.A. Myelin Dynamics Throughout Life: An Ever-Changing Landscape? Front. Cell. Neurosci. 2018, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Attwell, D. The Energetics of CNS White Matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef]
- Kister, A.; Kister, I. Overview of myelin, major myelin lipids, and myelin-associated proteins. Front. Chem. 2023, 10, 1041961. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Montani, L. Lipids in regulating oligodendrocyte structure and function. Semin. Cell Dev. Biol. 2021, 112, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dimas, P.; Montani, L.; Pereira, J.A.; Moreno, D.; Trötzmüller, M.; Gerber, J.; Semenkovich, C.F.; Köfeler, H.C.; Suter, U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. eLife 2019, 8, e44702. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.; König, E. Novel diagnostic tools and solutions for multiple sclerosis treatment: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Barnes-Vélez, J.A.; Aksoy Yasar, F.B.; Hu, J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innovation 2023, 4, 100360. [Google Scholar] [CrossRef]
- Lee, A.G. Myelin: Delivery by raft. Curr. Biol. 2001, 11, R60–R62. [Google Scholar] [CrossRef]
- Buscham, T.J.; Eichel, M.A.; Siems, S.B.; Werner, H.B. Turning to myelin turnover. Neural Regen. Res. 2019, 14, 2063–2066. [Google Scholar] [CrossRef]
- Aber, E.R.; Griffey, C.J.; Davies, T.; Li, A.M.; Yang, Y.J.; Croce, K.R.; Goldman, J.E.; Grutzendler, J.; Canman, J.C.; Yamamoto, A. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 2022, 41, 111480. [Google Scholar] [CrossRef]
- Chrast, R.; Saher, G.; Nave, K.-A.; Verheijen, M. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J. Lipid Res. 2010, 52, 419–434. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Awasthi, A.; Belkaid, W.; De Faria, O., Jr.; Liazoghli, D.; Colman, D.R.; Dhaunchak, A.S. Lipidome and proteome map of myelin membranes. J. Neurosci. 2013, 91, 321–334. [Google Scholar] [CrossRef]
- Han, H.; Myllykoski, M.; Ruskamo, S.; Wang, C.; Kursula, P. Myelin-specific proteins: A structurally diverse group of membrane-interacting molecules. BioFactors 2013, 39, 233–241. [Google Scholar] [CrossRef]
- Ruskamo, S.; Raasakka, A.; Pedersen, J.S.; Martel, A.; Škubník, K.; Darwish, T.; Porcar, L.; Kursula, P. Human myelin proteolipid protein structure and lipid bilayer stacking. Cell. Mol. Life Sci. 2022, 79, 419. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. CMLS 2006, 63, 1945–1961. [Google Scholar] [CrossRef]
- Jahn, O.; Siems, S.B.; Kusch, K.; Hesse, D.; Jung, R.B.; Liepold, T.; Uecker, M.; Sun, T.; Werner, H.B. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front. Cell. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Narine, M.; Colognato, H. Current Insights Into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell. Neurosci. 2022, 16, 892968. [Google Scholar] [CrossRef]
- Rone, M.B.; Cui, Q.-L.; Fang, J.; Wang, L.-C.; Zhang, J.; Khan, D.; Bedard, M.; Almazan, G.; Ludwin, S.K.; Jones, R.; et al. Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 4698–4707. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z.-H. Oligodendrocyte-derived transcellular signaling regulates axonal energy metabolism. Curr. Opin. Neurobiol. 2023, 80, 102722. [Google Scholar] [CrossRef]
- Mot, A.I.; Depp, C.; Nave, K.-A. An emerging role of dysfunctional axon-oligodendrocyte coupling in neurodegenerative diseases. Dialogues Clin. Neurosci. 2018, 20, 283–292. [Google Scholar] [CrossRef]
- Tepavčević, V. Oligodendroglial Energy Metabolism and (re)Myelination. Life 2021, 11, 238. [Google Scholar] [CrossRef]
- Simons, M.; Nave, K.-A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef]
- Gil, M.; Gama, V. Emerging Mitochondrial-Mediated Mechanisms Involved in Oligodendrocyte Development. J. Neurosci. Res. 2023, 101, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Rivkees, S.A. Hypoglycemia influences oligodendrocyte development and myelin formation. Neuroreport 2006, 17, 55–59. [Google Scholar] [CrossRef]
- Rinholm, J.; Hamilton, N.; Kessaris, N.; Richardson, W.; Bergersen, L.; Attwell, D. Regulation of Oligodendrocyte Development and Myelination by Glucose and Lactate. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Fern, R.; Davis, P.; Waxman, S.G.; Ransom, B.R. Axon Conduction and Survival in CNS White Matter During Energy Deprivation: A Developmental Study. J. Neurophysiol. 1998, 79, 95–105. [Google Scholar] [CrossRef]
- McMullen, E.; Hertenstein, H.; Strassburger, K.; Deharde, L.; Brankatschk, M.; Schirmeier, S. Glycolytically impaired Drosophila glial cells fuel neural metabolism via β-oxidation. Nat. Commun. 2023, 14, 1. [Google Scholar] [CrossRef]
- Schulz, J.G.; Laranjeira, A.; Van Huffel, L.; Gärtner, A.; Vilain, S.; Bastianen, J.; Van Veldhoven, P.P.; Dotti, C.G. Glial β-Oxidation regulates Drosophila Energy Metabolism. Sci. Rep. 2015, 5, 7805. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F. Brain Metabolism during Fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- McGarry, J.D.; Foster, D.W. Regulation of Hepatic Fatty Acid Oxidation and Ketone Body Production. Annu. Rev. Biochem. 1980, 49, 395–420. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Mantha, O.L.; Schor, J.; Pascual, A.; Plaçais, P.-Y.; Pavlowsky, A.; Preat, T. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat. Metab. 2022, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; Baes, M.; et al. Myelin lipids as nervous system energy reserves. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Frahm, J.; et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef] [PubMed]
- Kassmann, C.M. Myelin peroxisomes—Essential organelles for the maintenance of white matter in the nervous system. Biochimie 2014, 98, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Terlecky, S.R.; Walton, P.A. The Biogenesis and Cell Biology of Peroxisomes in Human Health and Disease. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Wanders, R.J.A.; Vaz, F.M.; Waterham, H.R.; Ferdinandusse, S. Fatty Acid Oxidation in Peroxisomes: Enzymology, Metabolic Crosstalk with Other Organelles and Peroxisomal Disorders. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Lizard, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 55–70. ISBN 978-3-030-60204-8. [Google Scholar]
- Andersen, S.L.; Briggs, F.B.S.; Winnike, J.H.; Natanzon, Y.; Maichle, S.; Knagge, K.J.; Newby, L.K.; Gregory, S.G. Metabolome-based signature of disease pathology in MS. Mult. Scler. Relat. Disord. 2019, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Anthony, D.C. Metabolomics in multiple sclerosis disease course and progression. Mult. Scler. J. 2020, 26, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Waters, J.; Rui, B. Metabolomics as a promising tool for improving understanding of multiple sclerosis: A review of recent advances. Biomed. J. 2022, 45, 594–606. [Google Scholar] [CrossRef]
- Zahoor, I.; Rui, B.; Khan, J.; Datta, I.; Giri, S. An emerging potential of metabolomics in multiple sclerosis: A comprehensive overview. Cell. Mol. Life Sci. 2021, 78, 3181–3203. [Google Scholar] [CrossRef]
- de Oliveira, E.M.L.; Montani, D.A.; Oliveira-Silva, D.; Rodrigues-Oliveira, A.F.; de Andrade Matas, S.L.; Fernandes, G.B.P.; da Silva, I.D.C.G.; Lo Turco, E.G. Multiple sclerosis has a distinct lipid signature in plasma and cerebrospinal fluid. Arq. Neuropsiquiatr. 2019, 77, 696–704. [Google Scholar] [CrossRef]
- Villoslada, P.; Alonso, C.; Agirrezabal, I.; Kotelnikova, E.; Zubizarreta, I.; Pulido-Valdeolivas, I.; Saiz, A.; Comabella, M.; Montalban, X.; Villar, L.; et al. Metabolomic signatures associated with disease severity in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e321. [Google Scholar] [CrossRef]
- Stys, P.K. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005, 233, 3–13. [Google Scholar] [CrossRef]
- Sadeghian, M.; Mastrolia, V.; Rezaei Haddad, A.; Mosley, A.; Mullali, G.; Schiza, D.; Sajic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci. Rep. 2016, 6, 33249. [Google Scholar] [CrossRef]
- Butler, R.; Bradford, D.; Rodgers, K.E. Analysis of shared underlying mechanism in neurodegenerative disease. Front. Aging Neurosci. 2022, 14, 1006089. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.G.; de Souza Angelo, Y.; Iglesias, A.H.; Peron, J.P.S. Unraveling the Link Between Mitochondrial Dynamics and Neuroinflammation. Front. Immunol. 2021, 12, 624919. [Google Scholar] [CrossRef]
- de Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, S.; Mohammadi, P.; Yarani, R.; Haghgoo, S.M.; Emami Aleagha, M.S. Carbohydrate and lipid metabolism in multiple sclerosis: Clinical implications for etiology, pathogenesis, diagnosis, prognosis, and therapy. Arch. Biochem. Biophys. 2021, 712, 109030. [Google Scholar] [CrossRef]
- Adiele, R.C.; Adiele, C.A. Metabolic defects in multiple sclerosis. Mitochondrion 2019, 44, 7–14. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Pluchino, S. Targeting Mitochondrial Metabolism in Neuroinflammation: Towards a Therapy for Progressive Multiple Sclerosis. Trends Mol. Med. 2018, 24, 838–855. [Google Scholar] [CrossRef]
- Trapp, B.D.; Stys, P.K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009, 8, 280–291. [Google Scholar] [CrossRef]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Miljković, D.; Spasojević, I. Multiple Sclerosis: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334. [Google Scholar] [CrossRef]
- Castellanos, D.B.; Martín-Jiménez, C.A.; Rojas-Rodríguez, F.; Barreto, G.E.; González, J. Brain lipidomics as a rising field in neurodegenerative contexts: Perspectives with Machine Learning approaches. Front. Neuroendocrinol. 2021, 61, 100899. [Google Scholar] [CrossRef]
- Rosko, L.; Smith, V.N.; Yamazaki, R.; Huang, J.K. Oligodendrocyte Bioenergetics in Health and Disease. Neuroscientist 2019, 25, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Regenold, W.T.; Phatak, P.; Makley, M.J.; Stone, R.D.; Kling, M.A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 2008, 275, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Fonalledas-Perelló, M.A.; Valero-Politi, J.; Lizarraga-Dallo, M.A.; Segura-Cardona, R. The cerebrospinal fluid lactate is decreased in early stages of multiple sclerosis. Puerto Rico Health Sci. J. 2008, 27, 2. [Google Scholar]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid rafts and neurodegeneration: Structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res. 2020, 61, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S. Pathogenesis and Progression of Multiple Sclerosis: The Role of Arachidonic Acid-mediated Neuroinflammation. Exon Publ. 2017, 111–123. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Neves, B.; Guerra, I.M.; Moreira, A.; Melo, T.; Paiva, A.; Domingues, M.R. An overview of lipidomic analysis in different human matrices of multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102189. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Melo, T.; Monteiro, A.; Paiva, A.; Domingues, P.; Domingues, M.R. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch. Biochem. Biophys. 2021, 697, 108672. [Google Scholar] [CrossRef]
- Nogueras, L.; Gonzalo, H.; Jové, M.; Sol, J.; Gil-Sanchez, A.; Hervás, J.V.; Valcheva, P.; Gonzalez-Mingot, C.; Solana, M.J.; Peralta, S.; et al. Lipid profile of cerebrospinal fluid in multiple sclerosis patients: A potential tool for diagnosis. Sci. Rep. 2019, 9, 11313. [Google Scholar] [CrossRef]
- Penkert, H.; Lauber, C.; Gerl, M.J.; Klose, C.; Damm, M.; Fitzner, D.; Flierl-Hecht, A.; Kümpfel, T.; Kerschensteiner, M.; Hohlfeld, R.; et al. Plasma lipidomics of monozygotic twins discordant for multiple sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 2461–2466. [Google Scholar] [CrossRef]
- Giussani, P.; Prinetti, A.; Tringali, C. The role of Sphingolipids in myelination and myelin stability and their involvement in childhood and adult demyelinating disorders. J. Neurochem. 2021, 156, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Ray, S.K. Diverse Biological Functions of Sphingolipids in the CNS: Ceramide and Sphingosine Regulate Myelination in Developing Brain but Stimulate Demyelination during Pathogenesis of Multiple Sclerosis. J. Neurol. Psychol. 2017, 5, 1000035. [Google Scholar] [CrossRef]
- Podbielska, M.; O’Keeffe, J.; Pokryszko-Dragan, A. New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7319. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Fellows, K.; Browne, R.W.; Khare, P.; Radhakrishnan, S.K.; Hagemeier, J.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. Interdependence of Oxysterols with Cholesterol Profiles in Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2017, 23, 792–801. [Google Scholar] [CrossRef]
- Vejux, A.; Ghzaiel, I.; Nury, T.; Schneider, V.; Charrière, K.; Sghaier, R.; Zarrouk, A.; Leoni, V.; Moreau, T.; Lizard, G. Oxysterols and multiple sclerosis: Physiopathology, evolutive biomarkers and therapeutic strategy. J. Steroid Biochem. Mol. Biol. 2021, 210, 105870. [Google Scholar] [CrossRef]
- Reale, M.; Sanchez-Ramon, S. Lipids at the Cross-road of Autoimmunity in Multiple Sclerosis. Curr. Med. Chem. 2017, 24, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Torra, I.; Siddique, S.; Waddington, K.E.; Farrell, R.; Jury, E.C. Disrupted Lipid Metabolism in Multiple Sclerosis: A Role for Liver X Receptors? Front. Endocrinol. 2021, 12, 639757. [Google Scholar] [CrossRef]
- Martinsen, V.; Kursula, P. Multiple sclerosis and myelin basic protein: Insights into protein disorder and disease. Amino Acids 2022, 54, 99–109. [Google Scholar] [CrossRef]
- Micu, I.; Plemel, J.R.; Caprariello, A.V.; Nave, K.-A.; Stys, P.K. Erratum: Axo-myelinic neurotransmission: A novel mode of cell signalling in the central nervous system. Nat. Rev. Neurosci. 2018, 19, 1. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. https://doi.org/10.3390/ijms241612912
López-Muguruza E, Matute C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. International Journal of Molecular Sciences. 2023; 24(16):12912. https://doi.org/10.3390/ijms241612912
Chicago/Turabian StyleLópez-Muguruza, Eneritz, and Carlos Matute. 2023. "Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis" International Journal of Molecular Sciences 24, no. 16: 12912. https://doi.org/10.3390/ijms241612912
APA StyleLópez-Muguruza, E., & Matute, C. (2023). Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. International Journal of Molecular Sciences, 24(16), 12912. https://doi.org/10.3390/ijms241612912






