CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
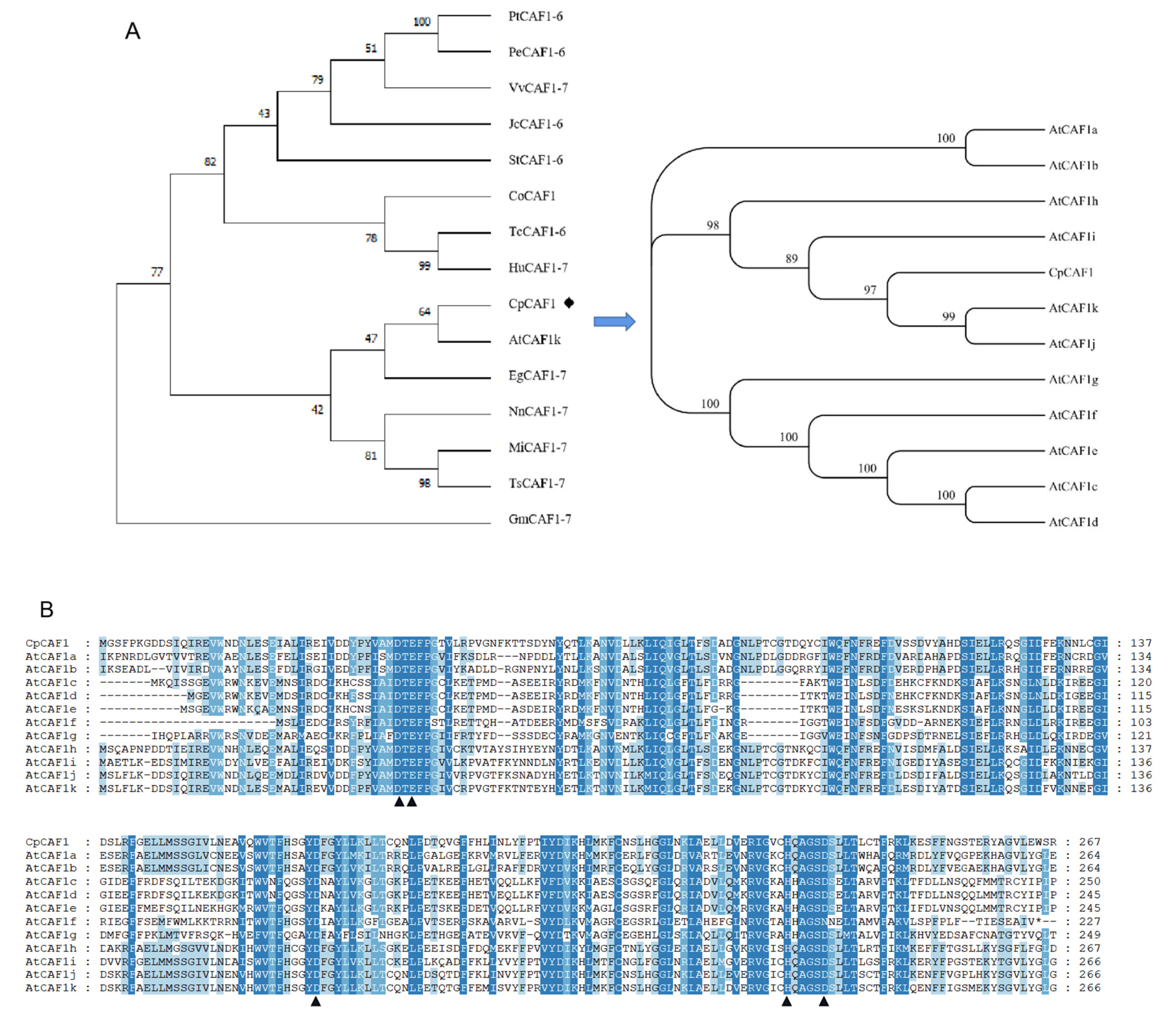
2.1. Sequence Analysis of CpCAF1
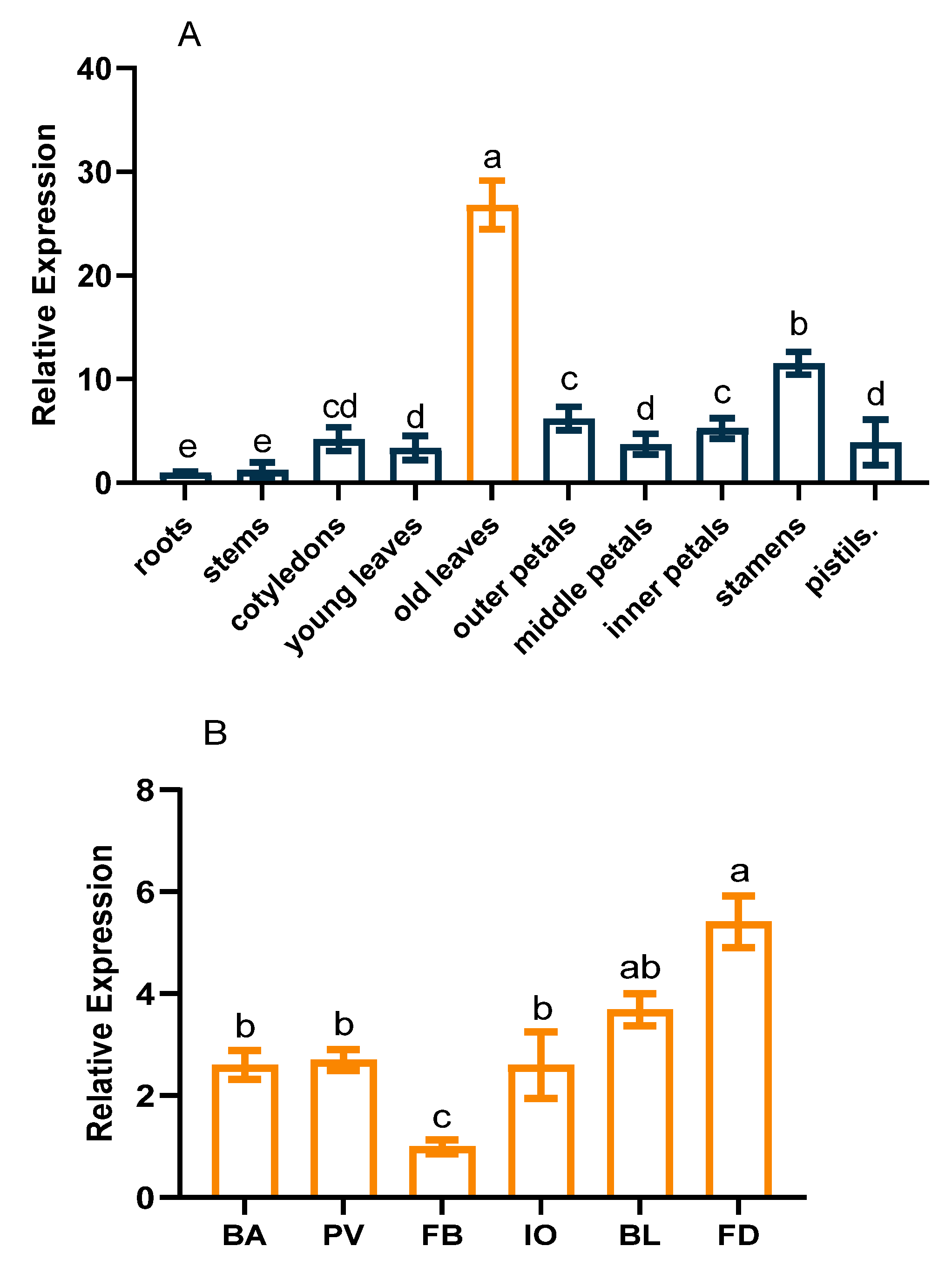
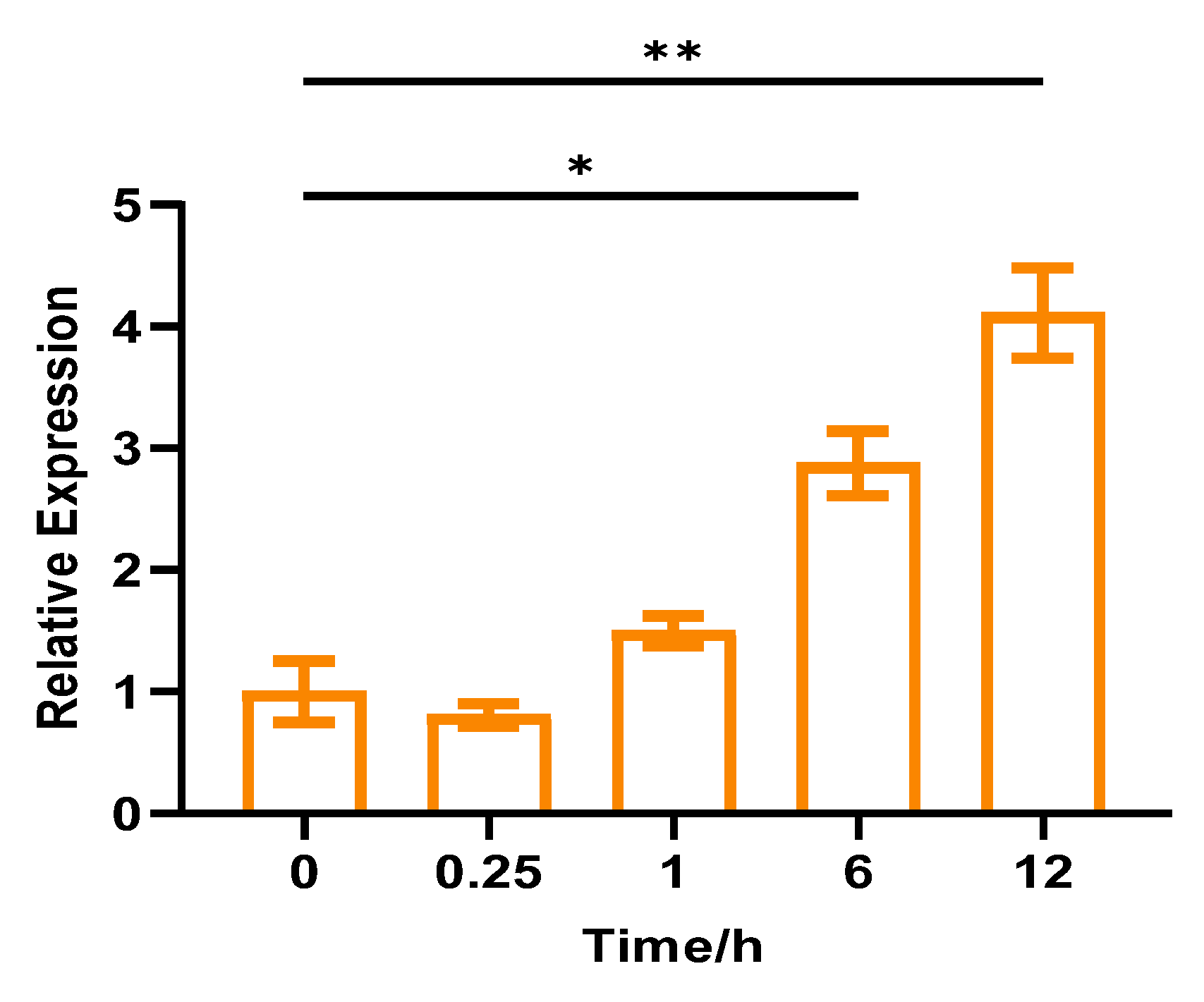
2.2. CpCAF1 Response to Chilling and Flower Development in Wintersweet
2.3. Overexpression of CpCAF1 in Arabidopsis
2.4. Biochemical Analysis of Transgenic Arabidopsis Overexpressing CpCAF1
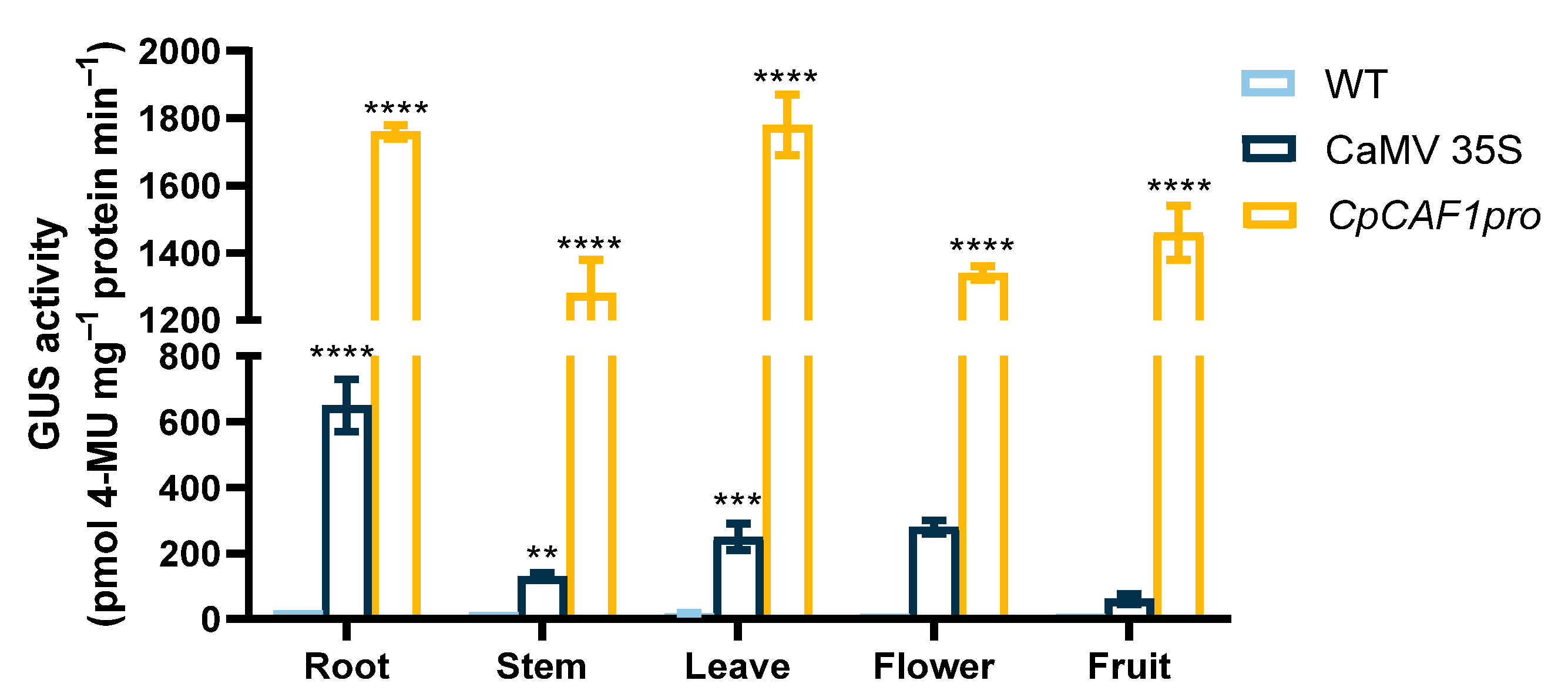
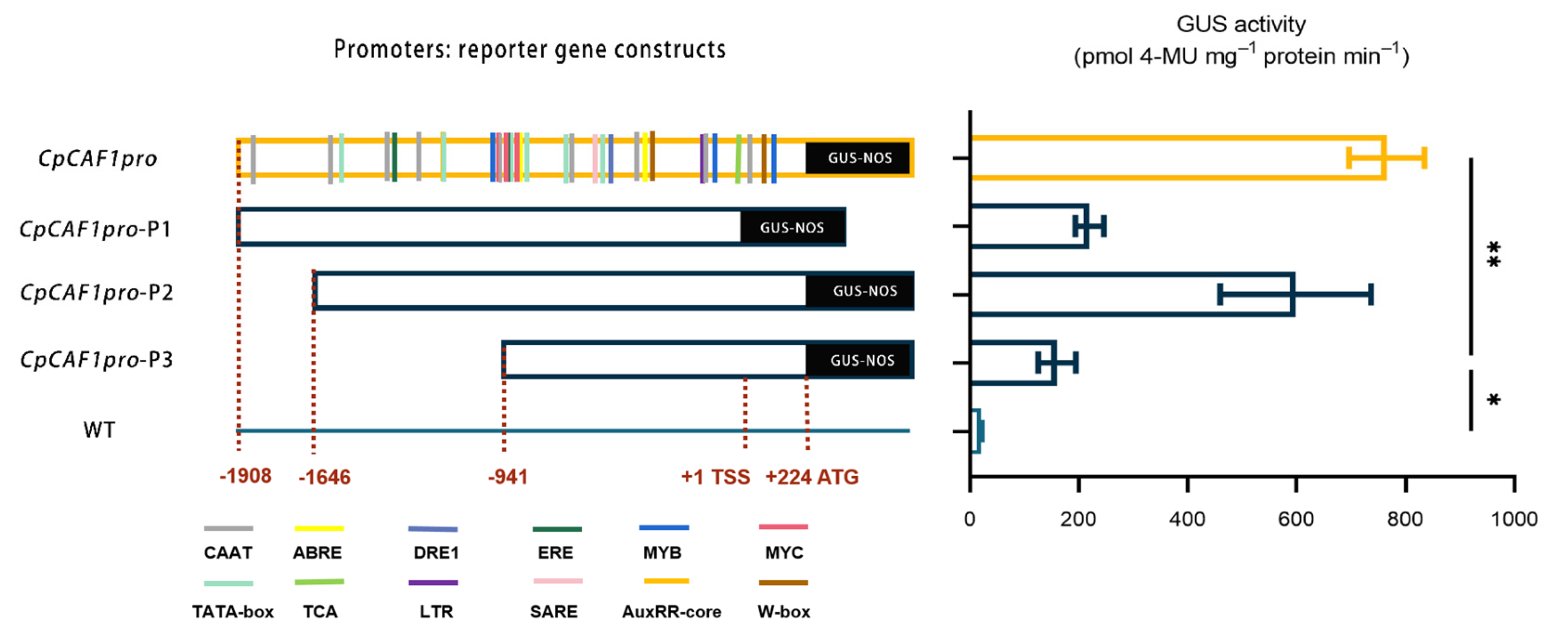
2.5. Cloning, Sequence Analysis, and Functional Characterization of the Promoter of CpCAF1
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Low-Temperature Treatment
4.2. Cloning of CpCAF1 and Bioinformatic Analysis
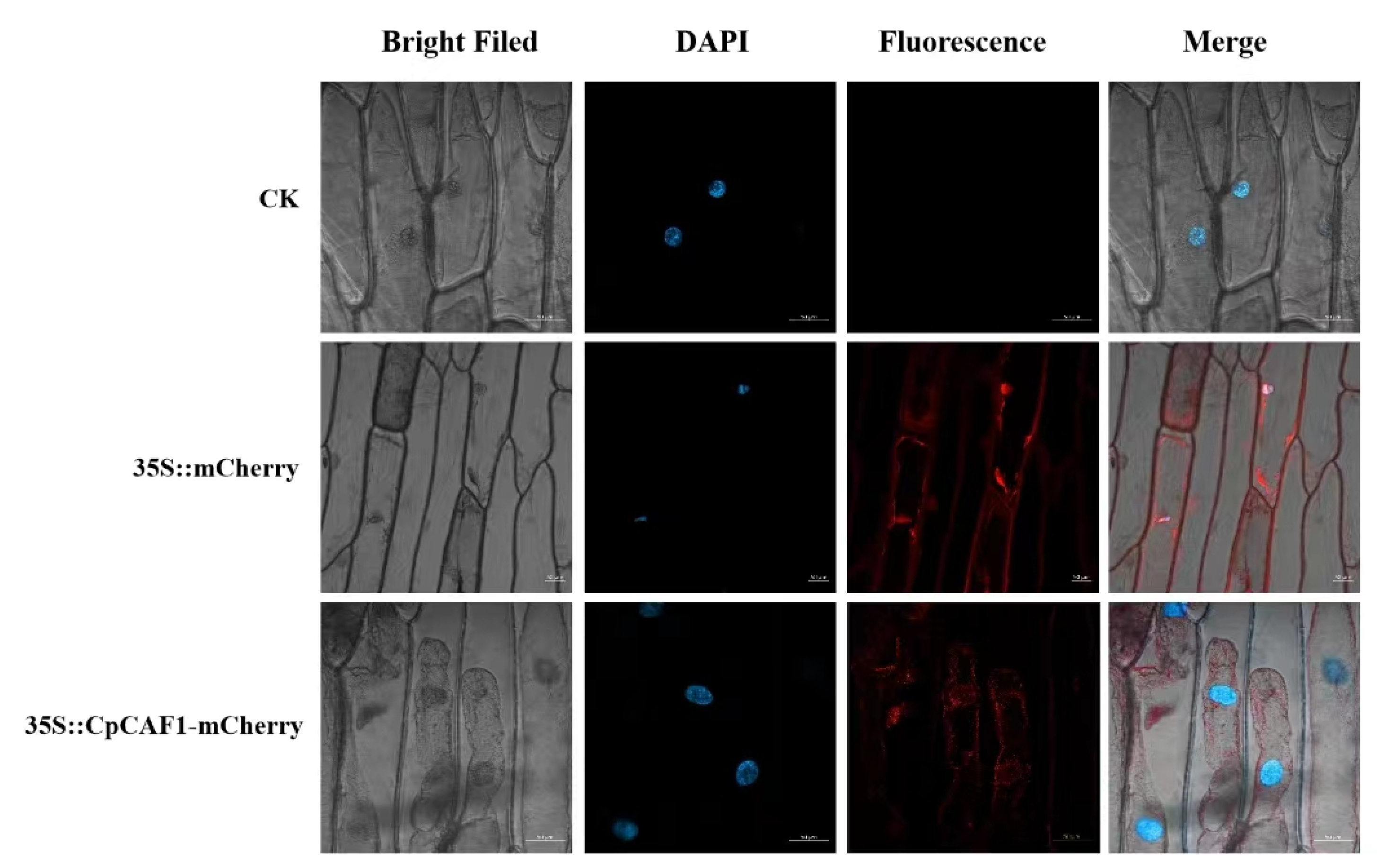
4.3. Subcellular Localization of CpCAF1
4.4. RNA Isolation and Quantitative Real-Time PCR Analysis of CpCAF1
4.5. Construction of Plant Overexpression Vectors for CpCAF1 and Transformation of Arabidopsis
4.6. Phenotypic Observation
4.7. Measurement of Physiological Indicators
4.8. Cloning and Analysis of the Upstream Sequence of CpCAF1
4.9. Construction and Transformation of CpCAF1 Promoter Deletions Fused to GUS
4.10. Histochemical and Fluorometric Analysis of GUS Activity
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. MRNA degradation by miRNAs and GW182 requires both CCR4: NOT deadenylase and DCP1: DCP2 decapping complexes. Gene Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Balu, B.; Maher, S.P.; Pance, A.; Chauhan, C.; Naumov, A.V.; Andrews, R.M.; Ellis, P.D.; Khan, S.M.; Lin, J.W.; Janse, C.J.; et al. CCR4-Associated Factor 1 Coordinates the Expression of Plasmodium falciparum Egress and Invasion Proteins. Eukaryot. Cell 2011, 10, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Molin, L.; Puisieux, A. C-elegans homologue of the Caf1 gene, which encodes a subunit of the CCR4-NOT complex, is essential for embryonic and larval development and for meiotic progression. Gene 2005, 358, 73–81. [Google Scholar] [CrossRef]
- Kopylov, P.K.; Platonov, M.E.; Ablamunits, V.G.; Kombarova, T.I.; Ivanov, S.A.; Kadnikova, L.A.; Somov, A.N.; Dentovskaya, S.V.; Uversky, V.N.; Anisimov, A.P. Yersinia pestis Caf1 Protein: Effect of Sequence Polymorphism on Intrinsic Disorder Propensity, Serological Cross-Reactivity and Cross-Protectivity of Isoforms. PLoS ONE 2016, 11, e0162308. [Google Scholar] [CrossRef]
- Niinuma, S.; Fukaya, T.; Tomari, Y. CCR4 and CAF1 deadenylases have an intrinsic activity to remove the post-poly(A) sequence. RNA 2016, 22, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.L.; Huang, L.F.; Fang, J.C.; Yeh, C.H.; Hong, C.Y.; Wu, S.J.; Lu, C.A. Divergence of the expression and subcellular localization of CCR4-associated factor 1 (CAF1) deadenylase proteins in Oryza sativa. Plant Mol. Biol. 2014, 85, 443–458. [Google Scholar] [CrossRef]
- Fang, J.C.; Tsai, Y.C.; Chou, W.L.; Liu, H.Y.; Chang, C.C.; Wu, S.J.; Lu, C.A. A CCR4-associated factor 1, OsCAF1B, confers tolerance of low-temperature stress to rice seedlings. Plant Mol. Biol. 2021, 105, 177–192. [Google Scholar] [CrossRef]
- Sarowar, S.; Oh, H.W.; Cho, H.S.; Baek, K.H.; Seong, E.S.; Joung, Y.H.; Choi, G.J.; Lee, S.; Choi, D. Capsicum annuum CCR4-associated factor CaCAF1 is necessary for plant development and defence response. Plant J. 2007, 51, 792–802. [Google Scholar] [CrossRef]
- Walley, J.W.; Kelley, D.R.; Nestorova, G.; Hirschberg, D.L.; Dehesh, K. Arabidopsis Deadenylases AtCAF1a and AtCAF1b Play Overlapping and Distinct Roles in Mediating Environmental Stress Responses. Plant Physiol. 2010, 152, 866–875. [Google Scholar] [CrossRef]
- Shimo, H.M.; Terassi, C.; Silva, C.C.L.; Zanella, J.D.; Mercaldi, G.F.; Rocco, S.A.; Benedetti, C.E. Role of the Citrus sinensis RNA deadenylase CsCAF1 in citrus canker resistance. Mol. Plant Pathol. 2019, 20, 1105–1118. [Google Scholar] [CrossRef]
- Liang, W.X.; Li, C.B.; Liu, F.; Jiang, H.L.; Li, S.Y.; Sun, J.Q.; Wu, X.Y.; Li, C.Y. The Arabidopsis homologs of CCR4-associated factor 1 show mRNA deadenylation activity and play a role in plant defence responses. Cell Res. 2009, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, R.; Wang, Y.; Wang, X.; Peng, J.; Nevo, E.; Ren, X.; Sun, D. New insights into the evolution of CAF1 family and utilization of TaCAF1Ia1 specificity to reveal the origin of the maternal progenitor for common wheat. J. Adv. Res. 2022, 42, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Nawaz, M.A.; Wei, N.N.; Cheng, F.; Bie, Z.L. Suboptimal Temperature Acclimation Enhances Chilling Tolerance by Improving Photosynthetic Adaptability and Osmoregulation Ability in Watermelon. Hortic. Plant J. 2020, 6, 49–60. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wu, S.H.; Wang, X.Q.; Mao, T.Y.; Bao, M.Z.; Zhang, J.W.; Zhang, J. Genome-wide identification and characterization of the bHLH gene family in an ornamental woody plant Prunus mume. Hortic. Plant J. 2022, 8, 531–544. [Google Scholar] [CrossRef]
- Liu, D.F.; Sui, S.Z.; Ma, J.; Li, Z.N.; Guo, Y.L.; Luo, D.P.; Yang, J.F.; Li, M.Y. Transcriptomic Analysis of Flower Development in Wintersweet (Chimonanthus praecox). PLoS ONE 2014, 9, e86976. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. Gus Fusions—Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher-Plants. Embo J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Li, Z.N.; Liu, N.; Zhang, W.; Wu, C.Y.; Jiang, Y.J.; Ma, J.; Li, M.Y.; Sui, S.Z. Integrated transcriptome and proteome analysis provides insight into chilling-induced dormancy breaking in Chimonanthus praecox. Hortic. Res. 2020, 7, 198. [Google Scholar] [CrossRef]
- Lin, J.; Liu, D.F.; Wang, X.; Ahmed, S.; Li, M.Y.; Kovinich, N.; Sui, S.Z. Transgene CpNAC68 from Wintersweet (Chimonanthus praecox) Improves Arabidopsis Survival of Multiple Abiotic Stresses. Plants 2021, 10, 1403. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Dai, S.; Wang, R.; Li, N.; Shen, X.; Zhou, X.; Lu, C.; Zheng, X.; Hu, Z.; et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.L.; Chung, Y.L.; Fang, J.C.; Lu, C.A. Novel interaction between CCR4 and CAF1 in rice CCR4-NOT deadenylase complex. Plant Mol. Biol. 2017, 93, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Collart, M.A. Global control of gene expression in yeast by the Ccr4-Not complex. Gene 2003, 313, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ramnarain, D.B.; Chiang, Y.C.; Ding, L.H.; McMahon, J.S.; Denis, C.L. Genome wide expression analysis of the CCR4-NOT complex indicates that it consists of three modules with the NOT module controlling SAGA-responsive genes. Mol. Genet. Genom. 2008, 279, 323–337. [Google Scholar] [CrossRef]
- Schwede, A.; Manful, T.; Jha, B.A.; Helbig, C.; Bercovich, N.; Stewart, M.; Clayton, C. The role of deadenylation in the degradation of unstable mRNAs in trypanosomes. Nucleic Acids Res. 2009, 37, 5511–5528. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.L.; Wei, H.; Sun, W.B.; Zhou, P.J.; Zhu, S.; Li, D.W.; Zhuge, Q. Genome-Wide and Comprehensive Analysis of the Multiple Stress-Related CAF1 (CCR4-Associated Factor 1) Family and Its Expression in Poplar. Plants 2021, 10, 981. [Google Scholar] [CrossRef]
- Stalberg, K.; Ellerstom, M.; Ezcurra, I.; Ablov, S.; Rask, L. Disruption of an overlapping E-box/ABRE motif abolished high transcription of the napA storage-protein promoter in transgenic Brassica napus seeds. Planta 1996, 199, 515–519. [Google Scholar] [CrossRef]
- Walley, J.W.; Kelley, D.R.; Savchenko, T.; Dehesh, K. Investigating the function of CAF1 deadenylases during plant stress responses. Plant Signal Behav. 2010, 5, 802–805. [Google Scholar] [CrossRef][Green Version]
- Lv, Q.D.; Qiu, J.; Liu, J.; Li, Z.; Zhang, W.T.; Wang, Q.; Fang, J.; Pan, J.J.; Chen, Z.D.; Cheng, W.L.; et al. The Chimonanthus salicifolius genome provides insight into magnoliid evolution and flavonoid biosynthesis. Plant J. 2020, 103, 1910–1923. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Molecular Basis of Plant Cold Acclimation: Insights Gained from Studying the CBF Cold Response Pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, G.; Hellebust, J.A. Regulation of Proline Content of Chlorella autotrophica in Response to Changes in Salinity. Can. J. Bot. 1989, 67, 1959–1965. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.H.; Hu, L.D.; Zhang, J.Q.; Jiang, Y.; Yang, Y.; Yan, Y.B. DsCaf1 is involved in environmental stress response of Dunaliella salina. Int. J. Biol. Macromol. 2016, 82, 369–374. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sc. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Kou, K.; Yang, H.; Li, H.; Fang, C.; Chen, L.; Yue, L.; Nan, H.; Kong, L.; Li, X.; Wang, F.; et al. A functionally divergent SOC1 homolog improves soybean yield and latitudinal adaptation. Curr. Biol. 2022, 32, 1728–1742.e6. [Google Scholar] [CrossRef]
- Crevillen, P.; Dean, C. Regulation of the floral repressor gene FLC: The complexity of transcription in a chromatin context. Curr. Opin. Plant Biol. 2011, 14, 38–44. [Google Scholar] [CrossRef]
- Hartmann, U.; Hohmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000, 21, 351–360. [Google Scholar] [CrossRef]
- Fukazawa, J.; Miyamoto, C.; Ando, H.; Mori, K.; Takahashi, Y. DELLA-GAF1 complex is involved in tissue-specific expression and gibberellin feedback regulation of GA20ox1 in Arabidopsis. Plant Mol. Biol. 2021, 107, 147–158. [Google Scholar] [CrossRef]
- Choudhary, M.; Jetley, U.K.; Khan, M.A.; Zutshi, S.; Fatma, T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol. Environ. Saf. 2007, 66, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hellebust, J.A. The Relationship between Inorganic Nitrogen-Metabolism and Proline Accumulation in Osmoregulatory Responses of 2 Euryhaline Microalgae. Plant Physiol. 1988, 88, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Seto, H.; Ohkubo, T. Determination of a Guanosine Malonaldehyde Adduct in Urine by High-Performance Liquid-Chromatography with a Thiobarbituric Acid Reaction Detector. J. Chromatogr. Biomed. 1991, 570, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal-Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beyer, W.; Imlay, J.; Fridovich, I. Superoxide Dismutases. Prog. Nucleic Acid Res. Mol. Biol. 1991, 40, 221–253. [Google Scholar] [PubMed]
- Yang, Y.; Lee, J.H.; Poindexter, M.R.; Shao, Y.H.; Liu, W.S.; Lenaghan, S.C.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N. Rational design and testing of abiotic stress-inducible synthetic promoters from poplar cis-regulatory elements. Plant Biotechnol. J. 2021, 19, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.S.; Shen, Q.R.; Guo, S.W. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Devi, B.S.R.; Kim, Y.J.; Selvi, S.K.; Gayathri, S.; Altanzul, K.; Parvin, S.; Yang, D.U.; Lee, O.R.; Lee, S.; Yang, D.C. Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ. J. Plant Physiol. 2012, 59, 318–325. [Google Scholar] [CrossRef]
- Gao, H.; Yang, W.J.; Li, C.X.; Zhou, X.G.; Gao, D.M.; Rahman, M.K.U.; Li, N.H.; Wu, F.Z. Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature. Plants 2020, 9, 65. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.C.J.; Wang, P.Y.; Zhang, B.F.; Li, M.; Chen, S.; Shi, R.; Tunlaya-Anukit, S.; Liu, X.Y.; Wang, Z.F.; et al. The AREB1 Transcription Factor Influences Histone Acetylation to Regulate Drought Responses and Tolerance in Populus trichocarpa. Plant Cell 2019, 31, 663–686. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Yang, N.; Chen, L.Q. Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J. 2022, 8, 221–229. [Google Scholar] [CrossRef]
- Mornya, P.M.P.; Cheng, F.Y. Effect of Combined Chilling and GA(3) Treatment on Bud Abortion in Forced ‘Luoyanghong’ Tree Peony (Paeonia suffruticosa Andr.). Hortic. Plant J. 2018, 4, 250–256. [Google Scholar] [CrossRef]
- Tao, H.X.; Sun, H.Q.; Wang, Y.F.; Wang, X.; Guo, Y.P. Effects of water stress on quality and sugar metabolism in ‘Gala’ apple fruit. Hortic. Plant J. 2023, 9, 60–72. [Google Scholar] [CrossRef]
- Murashige, T. Citation Classic—Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue-Cultures. Curr. Contents 1978, 10, 473–497. [Google Scholar] [CrossRef]
- Sui, S.Z.; Luo, J.H.; Ma, J.; Zhu, Q.L.; Lei, X.H.; Li, M.Y. Generation and Analysis of Expressed Sequence Tags from Chimonanthus praecox (Wintersweet) Flowers for Discovering Stress-Responsive and Floral Development-Related Genes. Comp. Funct. Genom. 2012, 2012, 134596. [Google Scholar] [CrossRef]
- Michiels, A.; Van den Ende, W.; Tucker, M.; Van Riet, L.; Van Laere, A. Extraction of high-quality genomic DNA from latex-containing plants. Anal. Biochem. 2003, 315, 85–89. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]

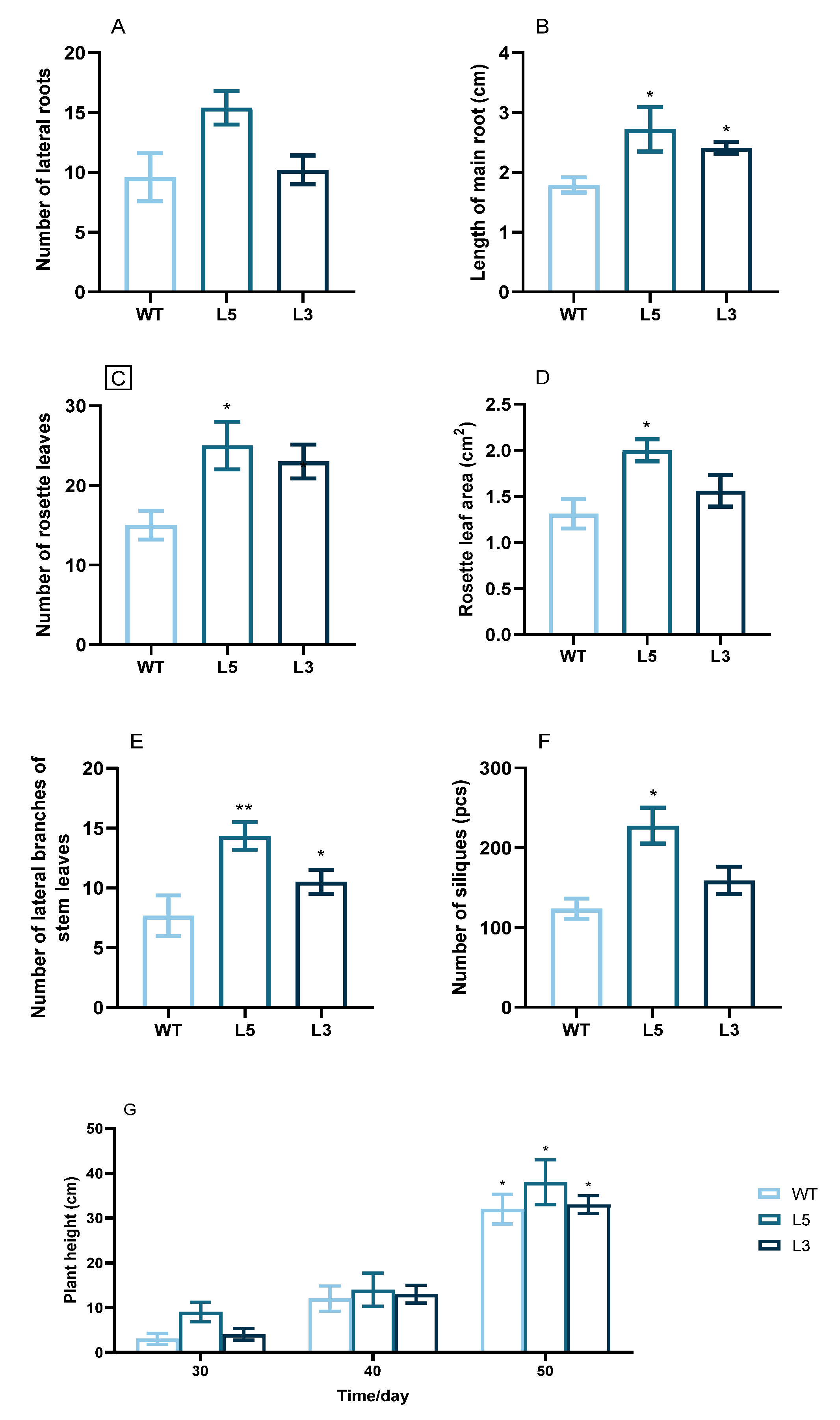
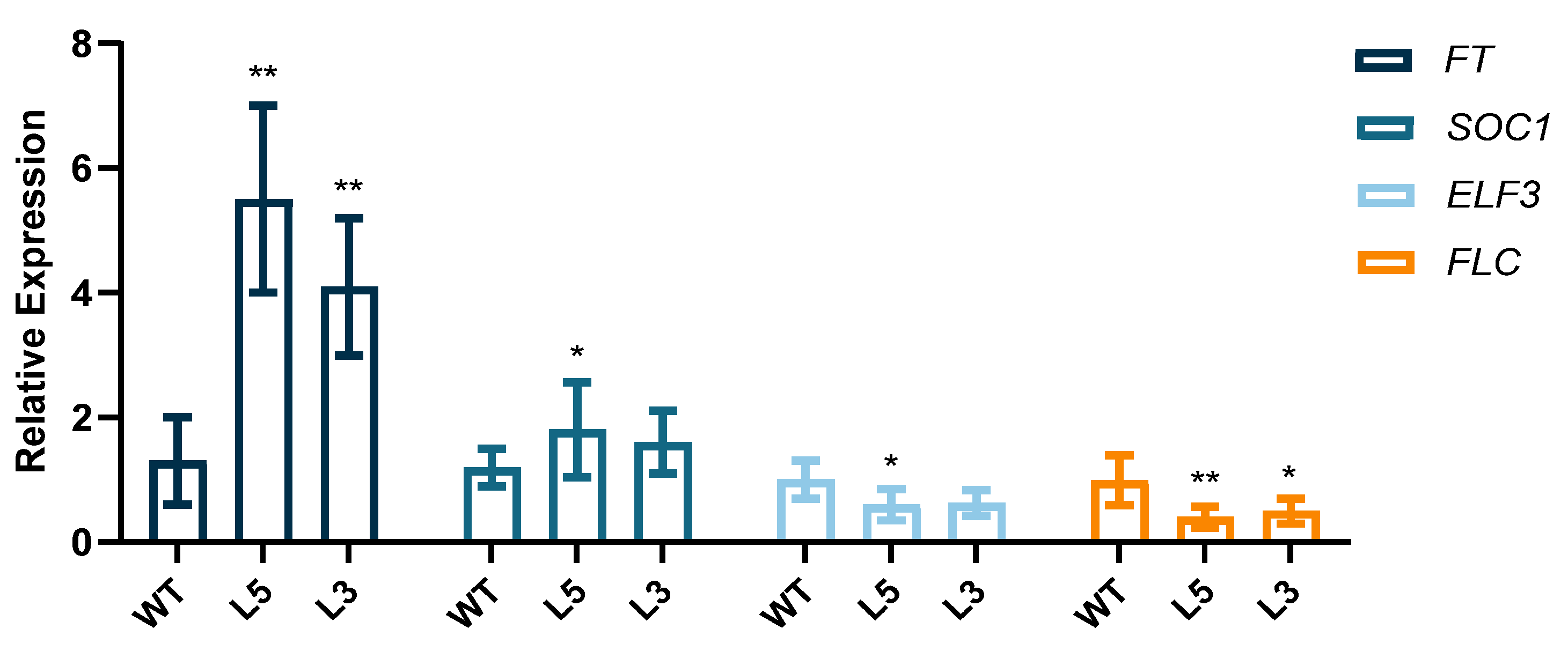

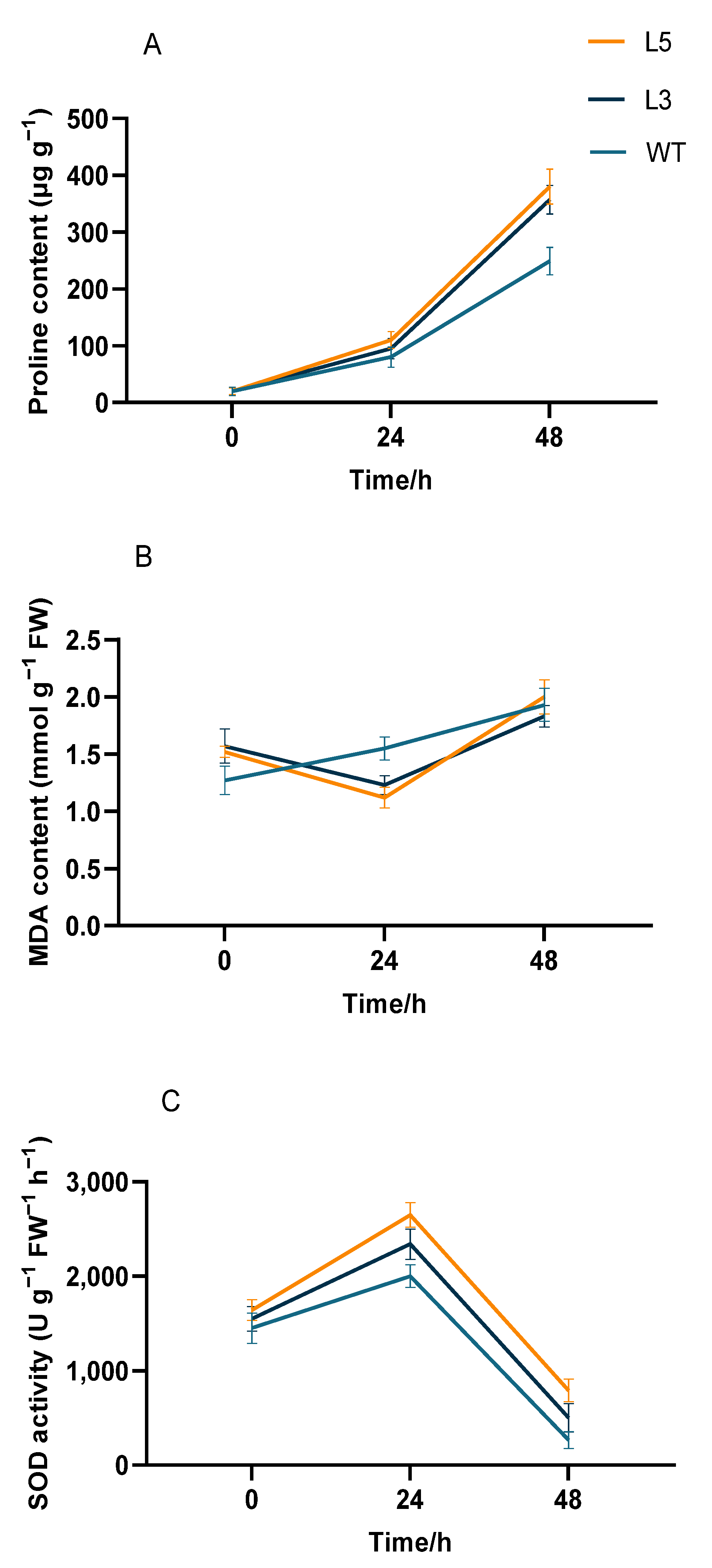
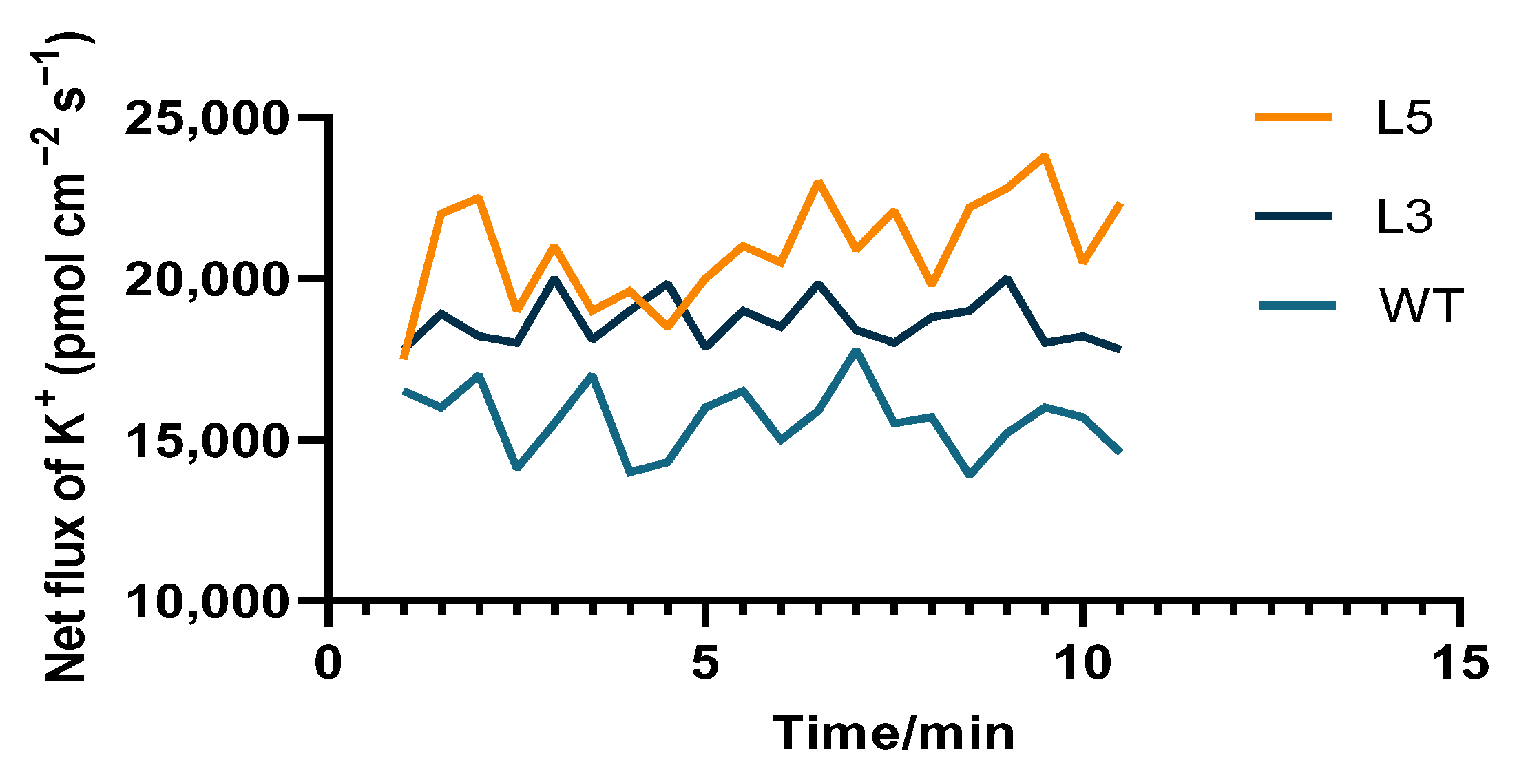

| Phenotypic Trait | Wild Type | L5 | L3 |
|---|---|---|---|
| First rosette leaf collateral branch appeared (d) | 36.33 ± 0.58 a | 33.00 ± 1.00 c | 34.33 ± 0.53 b |
| First lateral branch of the stem appeared (d) | 32.67 ± 1.53 ab | 28.33 ± 1.53 c | 31.00 ± 1.00 b |
| First inflorescence appeared (d) | 29.00 ± 1.00 a | 26.00 ± 0.07 d | 27.00 ± 1.73 bc |
| First flower opened (d) | 33.67 ± 1.53 a | 28.33 ± 0.58 d | 30.67 ± 0.58 b |
| First silique appeared (d) | 36.33 ± 1.15 a | 30.67 ± 0.58 c | 33.00 ± 1.73 b |
| First yellow leaf appeared (d) | 39.33 ± 0.58 a | 36.33 ± 1.53 cd | 37.00 ± 1.00 bc |
| No. | Regulatory Element | Site | Sequence | Function of Site |
|---|---|---|---|---|
| 1 | ABRE | −986, −374 | TACGTG | Cis-acting elements involved in abscisic acid response |
| 2 | ARE | −1741 | AAACCA | Cis-acting regulatory element essential for anaerobic induction |
| 3 | AT1-motif | −538 | ATTAATTTTACA | Part of a light-responsive module |
| 4 | AuxRR-core | −1329 | GGTCCAT | Cis-acting regulatory element involved in auxin responsiveness |
| 5 | Box 4 | −667, −491 | ATTAAT | Part of a conserved DNA module involved in light responsiveness |
| 6 | W-Box | +124, −368 | TTGACC | WRKY transcription factor binding site involved in defense responses |
| 7 | DRE | −456 | TTCGACC | Induced by both drought and low temperature |
| 9 | ERE | −1623, −1058 | ATTTCAAA | Ethylene-responsive element |
| 10 | G-Box | −987, −374 | CACGTT | Cis-acting regulatory element involved in light responsiveness |
| 11 | GT1-motif | −1628 | GGAGATG | Light-responsive element |
| 12 | L-box | −58 | ATCCCACCTAC | Part of a light-responsive element |
| 13 | LTR | −249 | CCGAAA | Cis-acting element involved in low-temperature responsiveness |
| 14 | MYB | +151, −186, −1231 | TAACCA | Response to drought and salinity stress tolerance |
| 15 | MYC | −1107, −1159, −995 | CATTTG | Response to drought and abscisic acid signals |
| 16 | SARE | −1050 | TTCGACCATCTT | Salicylic-acid-responsive element |
| 17 | STRE | +143, −173, −207, −342 | AGGGG | Stress-responsive elements |
| 18 | Sp1 | −66 | GGGCGG | Light-responsive element |
| 19 | TCA | −46 | GAGAAGAATA | Cis-acting element involved in salicylic acid responsiveness |
| 20 | TCT-motif | −1464 | TCTTAC | Part of a light-responsive element |
| 21 | WUN-motif | −1830 | CCATTTCAA | Injury-related element |
| 22 | Telo-box | +172 | AAACCCTAACCCTAA | Motifs associated with MYB binding sites |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Xie, M.; Zhou, S.; Wen, B.; Sui, S.; Li, M.; Ma, J. CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 12945. https://doi.org/10.3390/ijms241612945
Lv Y, Xie M, Zhou S, Wen B, Sui S, Li M, Ma J. CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(16):12945. https://doi.org/10.3390/ijms241612945
Chicago/Turabian StyleLv, Yimeng, Mingfang Xie, Shiqing Zhou, Bixia Wen, Shunzhao Sui, Mingyang Li, and Jing Ma. 2023. "CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 16: 12945. https://doi.org/10.3390/ijms241612945
APA StyleLv, Y., Xie, M., Zhou, S., Wen, B., Sui, S., Li, M., & Ma, J. (2023). CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana. International Journal of Molecular Sciences, 24(16), 12945. https://doi.org/10.3390/ijms241612945






