Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease, with a complex genetic background. Apart from rare, familial cases, a combination of multiple risk loci contributes to the susceptibility of the disease. Genome-wide association studies (GWAS) have identified numerous AD risk loci. Changes in cerebrospinal fluid (CSF) biomarkers and imaging techniques can detect AD-related brain changes before the onset of clinical symptoms, even in the presence of preclinical mild cognitive impairment. In this study, we aimed to assess the associations between SNPs in well-established GWAS AD risk loci and CSF biomarker levels or cognitive test results in Slovenian patients with cognitive decline. The study included 82 AD patients, 28 MCI patients with pathological CSF biomarker levels and 35 MCI patients with normal CSF biomarker levels. Carriers of at least one polymorphic TOMM40 rs157581 C allele had lower Aβ42 (p = 0.033) and higher total tau (p = 0.032) and p-tau181 levels (p = 0.034). Carriers of at least one polymorphic T allele in SORCS1 rs1358030 had lower total tau (p = 0.019), while polymorphic SORCS1 rs1416406 allele was associated with lower total tau (p = 0.013) and p-tau181 (p = 0.036). In addition, carriers of at least one polymorphic T allele in BCHE rs1803274 had lower cognitive test scores (p = 0.029). The study findings may contribute to the identification of genetic markers associated with AD and MCI and provide insights into early disease diagnostics.
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease, affecting a significant part of the worldwide population. It is a leading cause of dementia, that typically occurs in the elderly population, with the vast majority of cases in people aged 65 or older [1]. As the elderly population at increased risk for developing late-onset AD is expected to continue to grow in the future, the disease prevalence will continue to increase, making AD one of the most important health and societal issues. Multiple risk factors contribute to the development of the disease, with age being one of the most important [2]. The appearance of symptoms in the younger population is unusual and is considered early-onset AD [3].
Pathophysiologically, AD manifests with two major disease hallmarks–deposition of amyloid β (Aβ) in neuritic plaques and accumulation of tau protein neurofibrillary tangles [4]. The induced brain changes can be measured with cerebrospinal fluid (CSF) biomarkers (Aβ42, Aβ40, p-tau181) and modern imaging approaches (PET scan). Although AD generally manifests in the elderly population, initial changes in CSF biomarker levels can be observed years before the onset of clinical symptoms [5,6]. The protective mechanisms of the brain are still sufficient at this preclinical stage, to prevent further impairment and memory loss [1]. Mild cognitive impairment (MCI) is the main feature of the pre-dementia stage of the disease, with changes in AD biomarkers and subtle cognitive impairments [7]. These cognitive problems are noticeable but do not affect daily activities. MCI may eventually progress to AD. It is estimated that up to one-third of MCI patients develop dementia due to AD within five years [8]. Still, some individuals with MCI do not progress to AD [1]. It is clinically relevant to identify individuals at higher risk for developing AD; thus, the search for predictive AD biomarkers is of great importance in current research.
Similar to other common chronic diseases, a combination of multiple factors contributes to the development of AD. A small proportion of AD cases show a familial, highly heritable form of AD [4]. Mutations in three genes—amyloid precursor protein (APP), presenilin-1 (PSEN1), and presenilin-2 (PSEN2)—were found in familial AD with earlier onset of symptoms [3,9]. However, there are no common causative genes for the sporadic form of the disease. Numerous genome-wide association studies (GWAS) have identified different AD risk loci [10,11,12,13]. Of the many genes that increase AD risk, APOE has the strongest impact on late-onset AD. Two common APOE polymorphisms, rs429358 (p.Cys112 Arg) and rs7412 (p.Arg158 Cys), define a combination of alleles (ε2, ε3, and ε4) that affect AD risk [14]. The risk for AD is 2-3-fold higher in carriers of one APOE ε4 allele and about 12-fold higher in those with two APOE ε4 alleles [15,16]. On the other hand, the APOE ε2 allele is protective [17]. In addition to APOE, many other genes involved in different molecular pathways contribute to susceptibility to develop AD.
In a previously published systematic review, we used GWAS data to identify key pathways of AD pathogenesis: cellular processes, metabolic processes, biological regulation, localization, transport, regulation of cellular processes, and neurological system processes [18]. Among genes involved in localization pathways, TOMM40 and SORCS1 were frequently reported as potential AD biomarkers. TOMM40 encodes the subunit of outer mitochondrial membrane translocase, a channel-forming pore for protein uptake in mitochondria [19]. SORCS1 is a member of the Vps10 p family of sorting receptors, important in APP processing [20]. Serine esterase BCHE is important in neurotransmitter activation and has been found to be enriched in senile plaques of AD brains [21,22]. Angiotensin-converting enzyme, encoded by the ACE gene is primarily known as a vasoconstrictor; however, its role in Aβ degradation has also been reported [23]. IL6 R is the receptor for IL6, a cytokine important for neuronal cell growth and differentiation. The genetic variability of IL6 R was previously studied in association with elevated IL6 activity in AD brain [24].
The search for robust and non-invasive biomarkers of early AD detection is still ongoing. Therefore, the aim of our study was to investigate the association of genetic variability in genes, previously identified in GWAS studies with AD biomarker levels and cognitive decline in Slovenian patients with AD and MCI.
2. Results
2.1. Patients’ Characteristic
Our study included 145 patients with cognitive impairment: 82 AD patients, 28 MCI (AD), and 35 MCI (NOT AD). Clinical characteristics of all patients with cognitive impairment and of each separate group (AD, MCI (AD) and MCI (NOT AD)) are summarized in Table 1. AD patients were significantly older compared to patients with MCI (AD) and MCI (NOT AD) (p = 0.039). More female subjects were included in both AD and MCI (AD) groups (p = 0.008). Significant differences in all CSF biomarker levels (Aβ42, Aβ42/40, total tau and p-tau181) were observed between groups (all p < 0.001). AD patients also achieved significantly lower MMSE scores (p < 0.001).

Table 1.
Clinical characteristics of all patients with cognitive impairment (N = 145) and of patients with AD (N = 82), MCI (N = 28) and MCI (NOT AD) diagnosis (N = 35).
Genotype frequencies of all nine investigated SNPs in SORCS1, BCHE, TOMM40, ACE and IL6 R genes in the entire cohort are presented in Table S1.
2.2. Association of Investigated SNPs with Cognitive Impairment and AD Susceptibility
The genotype frequency distribution of investigated polymorphisms in different patient groups with cognitive impairment is presented in Table 2. APOE rs429358 polymorphic C allele was more frequent in AD and MCI (AD) (p = 0.004). Normal TOMM40 rs157581 T allele was more frequent in MCI (not AD) group and the difference was statistically significant in both additive and dominant models (p = 0.005 and p = 0.001, respectively). Furthermore, at least one polymorphic TOMM40 rs2075650 G allele was more frequent in the AD and MCI (AD) group, compared to MCI (not AD); however, the nominal difference was only observed in the dominant model (p = 0.025).

Table 2.
Comparison of genotype frequencies among patients with different types of cognitive impairment.
2.3. Association of Investigated SNPs with CSF Biomarker Levels and MMSE
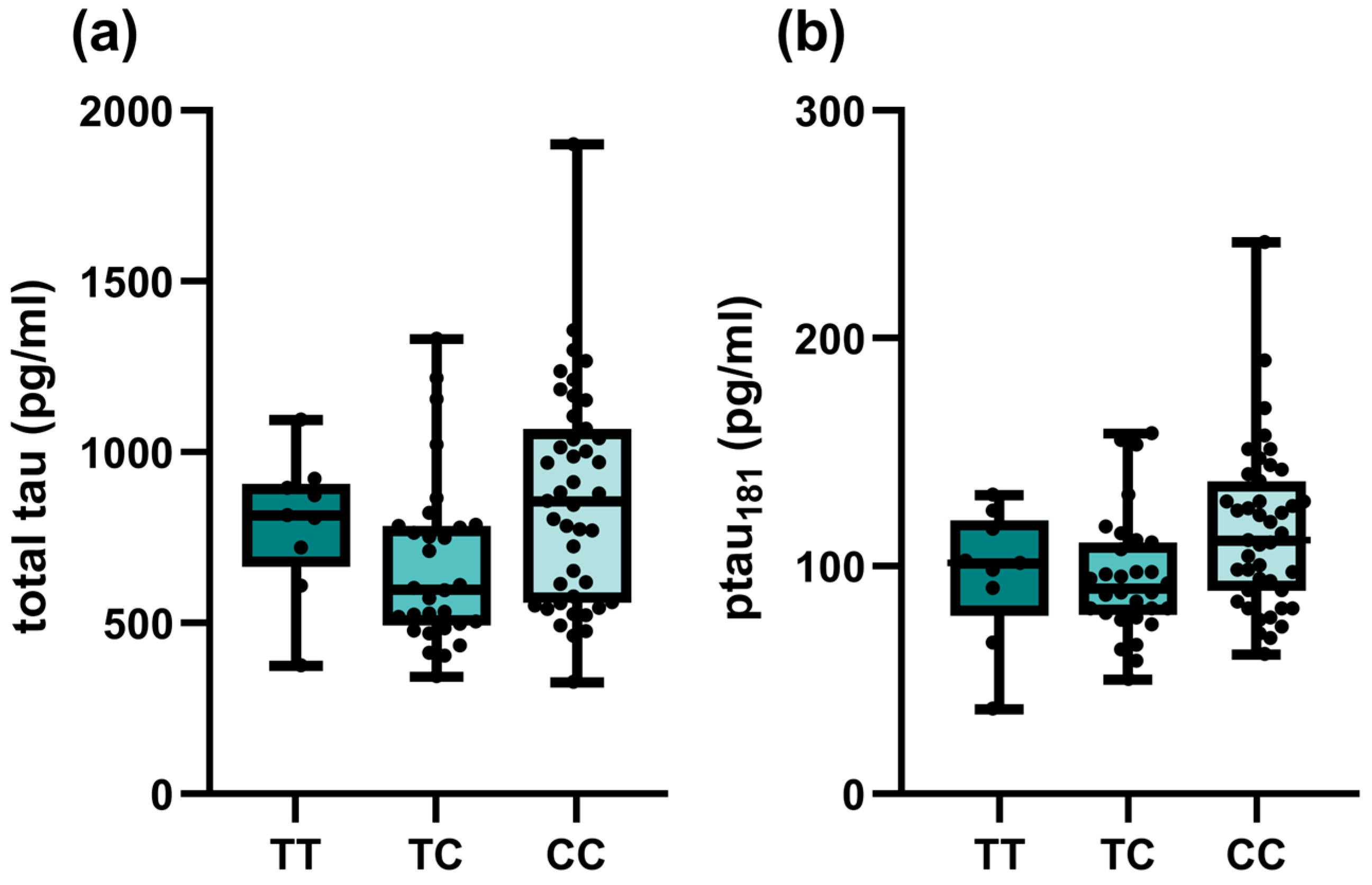
Among investigated SNPs, associations of TOMM40 rs157581 with different CSF biomarkers were observed among all patients with cognitive impairment (Table 3). Carriers of at least one polymorphic C allele had lower Aβ42 (p = 0.033) and higher total tau (p = 0.032) and p-tau (p = 0.034) levels, respectively. However, the associations did not remain significant when only AD patients were included in the analysis (Table S2). On the other hand, significant associations of SORCS1 polymorphisms with tau biomarkers were observed in the AD group. Carriers of at least one polymorphic SORCS1 rs1358030 T allele had lower total tau (p = 0.019). Carriers of one polymorphic C allele in SORCS1 rs1416406 had lower total tau (p = 0.013) and p-tau (p = 0.036) levels (Figure 1). The association did not remain significant in the dominant model.

Table 3.
Association of investigated polymorphisms with cerebrospinal fluid biomarkers among all patients with cognitive impairment.
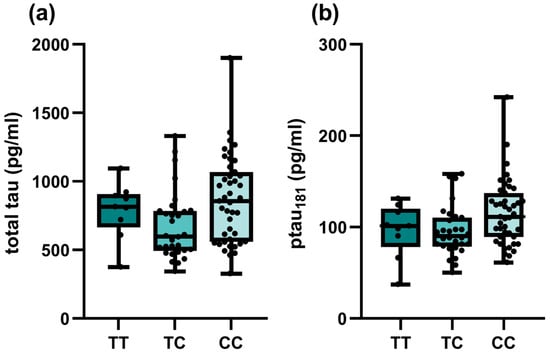
Figure 1.
Associations of SORCS1 rs1416406 genotypes of AD patients and CSF biomarker levels: (a) total tau; (b) p-tau181.
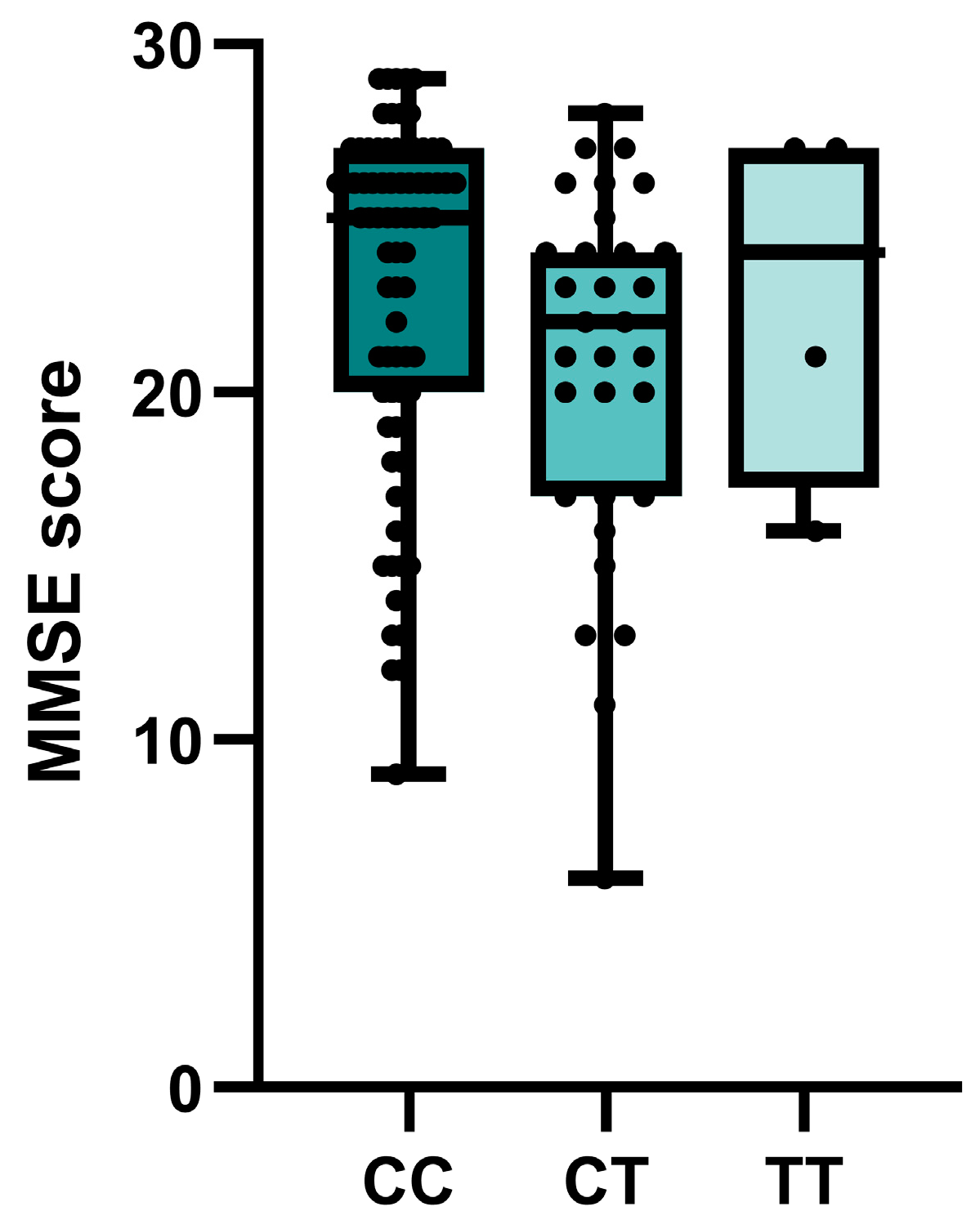
Nominally significant associations of BCHE rs1803274 with the MMSE cognitive test scores were observed in the entire group of patients with cognitive impairment. Carriers of at least one polymorphic T allele had lower test scores (p = 0.029) (Figure 2). No significant or nominally significant associations with MMSE were observed for other investigated SNPs (Table S3).
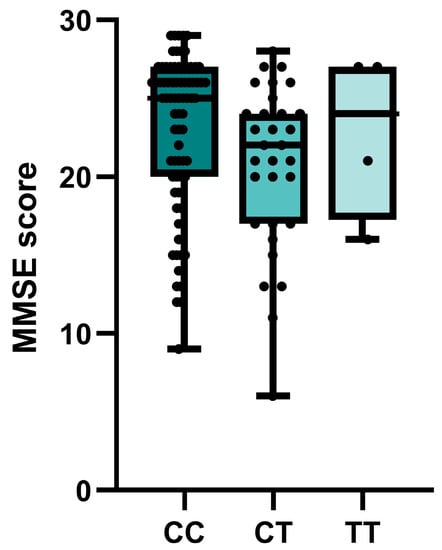
Figure 2.
Associations of BCHE rs1803274 genotypes of patients with cognitive impairment and MMSE score.
3. Discussion
We investigated the association of selected SNPs from GWAS-identified AD risk genes with CSF biomarker levels and cognitive test results in patients with cognitive impairment. TOMM40 and SORCS1 were associated with both amyloid and tau CSF biomarkers, while BCHE was associated with cognitive decline.
Numerous GWAS and case–control studies identified these genes as potential AD risk loci [25,26,28,29,30,31]. Among investigated SNPs, both TOMM40 rs157581 and rs2075650 were previously associated with increased AD susceptibility in European [32,33] and Asian [27,34,35] populations. Similarly, increased AD risk was observed for BCHE rs1803274 in European [36] and ACE rs1800764 in the Asian population [37]. Furthermore, the genetic variance within these genes was also associated with Aβ and tau levels in CSF [38,39,40,41] and blood plasma [42,43]; therefore, our study focused on common functional polymorphisms in established AD risk loci. In our study, lower CSF Aβ42 and higher total tau and p-tau levels have been observed in carriers of the polymorphic C allele in TOMM40 rs157581. As previously shown, TOMM40 genetic variability is associated with CSF Aβ and tau levels [38,44]. Polymorphic alleles in both SORCS1 SNPs have been associated with lower total tau, while carriers of SORCS1 rs1416406 C allele additionally had lower p-tau. In concordance with the literature, the APOE effect on amyloid and tau pathology was also confirmed.
Among investigated genes, the TOMM40 was the only one associated with both Aβ and tau CSF biomarkers. On a pathophysiological level, TOMM40 was associated with APP accumulation in the mitochondrion. APP is the precursor molecule for processing of Aβ proteins of various lengths [45] and APP mutations result in overproduction and aggregation of Aβ42 [4,46]. By entering and obstructing the TOMM40 pore, APP induces mechanisms for mitochondrial dysfunction [47]. Furthermore, TOMM40 impacts AD-vulnerable brain areas by downstream apoptotic processes that forego extracellular Aβ aggregation. Considering those pieces of evidence, TOMM40 is an important gene presumably contributing to AD-related mitochondria dysfunction [48]. Its genomic location, adjacent to the APOE region gene on chromosome 19, raised interest in the assessment of TOMM40 genetic variability on AD susceptibility. Both TOMM40 rs157581 and rs2075650 were associated with AD risk in GWAS and meta-analyses [25,28,30,49,50]. Our present study shows the effect of TOMM40 rs157581 on CSF biomarkers, thus further supporting the importance of this gene in the development of AD. However, CSF Aβ42, p-tau181/Aβ42 and total-tau/Aβ42 as quantitative traits in GWAS were previously associated with rs2075650 [38]. A similar effect was observed in another study [39], where TOMM40 rs2075650 showed a genome-wide association with CSF tau and Aβ42. In concordance with our results, the genome-wide association of TOMM40 rs2075650 with lower Aβ42 levels in CSF of AD patients was also reported [44]. Since the close proximity to APOE, the potentially combined effect of TOMM40-APOE on AD pathology was also addressed. The polygenic profile of APOE-TOMM40-APOC1 was associated with increased CSF tau levels, suggesting the important role of TOMM40 in the modulation of the APOE ε4 effect in AD [51]. As previously reported, our study confirmed both decreased Aβ42 and increased tau CSF levels.
We have also observed the association between both SORCS1 polymorphisms and tau CSF levels. SORCS1 is another gene important in APP processing. Since SorCS1 is prominently expressed in the nervous system [52], it might affect AD pathophysiology. Overexpression of SorCS1 might lead to lower Aβ levels through reduced γ-secretase activity [53]. Aβ is derived by the proteolytic cleavage of APP by a successive β-secretase cleavage at the N-terminus, followed by γ-secretase cleavage of the membrane-bound C-terminal site [4,54]. SORCS1 was proposed as an AD risk locus in several GWAS [53,55,56]. To the best of our knowledge, no association studies on CSF biomarkers were conducted on AD patients. The observed associations of genetic variability in SORCS1 with tau pathology in our study are, thus, novel findings that can further contribute to the understanding of the effect of APP processing on tau aggregation.
Apart from CSF biomarkers, biomarkers related to cognitive decline can also serve as a marker of neurodegeneration. The observed lower MMSE scores associated with BCHE rs1803274 highlight the potential of serine esterase in AD. Butyrylcholinesterase (BCHE) as a serine esterase is involved in organophosphate ester hydrolysis [21]. It is important in neurotransmitter activation and elevated BCHE activity was observed in the brain affected by amyloid plagues [22,57]. Furthermore, a link between BCHE and the accumulation of tau protein in neurofibrillary tangles was found in AD brain [58]. In terms of genetic variability, a significant association of BCHE rs509208 with cortical Aβ in AD subjects was found in GWAS [40]. The effect of the BCHE rs1803274 genotype on impaired BCHE activity in brain tissue and CSF was found [36,59]. What is more, the effect of BCHE rs1803274 on cognitive decline, measured with MMSE in combination with donepezil treatment in MCI treatment was evaluated [60]. Similar to our findings, a faster MMSE decline was associated with a polymorphic allele. Our results thus confirm the previously found negative effect of BCHE on cognitive decline. Although they were previously associated with AD risk in GWAS, polymorphisms in ACE and IL6 R did not reach significant or nominally significant associations with CSF biomarker levels and MMSE scores.
Our study has some limitations. The sample size of different pathologies was relatively small and some clinical parameters, especially cognitive test scores, were not available for all patients. We are aware, that our study cannot compare with larger consortium-based approaches in GWAS studies, for the assessment of disease risk loci. However, the detailed patient-related data enabled us to focus assessment of the genetic variability of selected genes in relation to CSF biomarkers and cognitive tests in addition to evaluating these SNPs as potential risk factors. The smaller sample size is therefore partly due to the fact that the patients were included during their lumbar punction appointment to assess CSF biomarkers. Although it is difficult to detect the contributions of many factors in a smaller sample size study, a similar effect can occur due to phenotypic heterogeneity in larger studies as well. We also accounted for multiple comparisons in the statistical analysis. For a more thorough evaluation of observed associations, an independent study with a larger sample could be conducted. On the other hand, our study had several strengths. All the patients were recruited from the same department and evaluated according to the same protocol. We comprehensively assessed the simultaneous influence of several clinical and genetic parameters on AD risk and pathology. We were the first to assess the genetic variability in some of the GWAS-identified AD risk loci in Slovenian patients. Furthermore, only a few studies focused on the association of selected genes with CSF biomarkers. We believe our work might serve as a valuable addition to the field of translation of AD genetic risk to biomarker levels in patients.
4. Materials and Methods
4.1. Subjects
Our study included patients with cognitive impairment as they had appointments for clinical evaluation and lumbar puncture at the Department of Neurology, University Medical Centre Ljubljana, Slovenia, between June 2019 and December 2022. Inclusion criteria were age above 55 and diagnosis of AD or MCI. Patients with comorbidities significantly affecting cognitive performance and dementia due to diseases other than AD were excluded from the study. A structured interview with patients and their caregivers was performed to obtain demographic and clinical data. Additional information was obtained from medical records.
The study protocol was approved by the National Medical Ethics Committee of the Republic of Slovenia (0120–523/2017–4) and all the subjects provided written informed consent in accordance with the Declaration of Helsinki.
4.2. Assessment
Cognitive impairment was diagnosed through a standardized clinical evaluation and an assessment of patients’ cognitive decline history. The Mini-mental State Examination (MMSE) was administered to screen for cognitive deficit [61]. A thorough diagnostic work-up was conducted, combining structural brain imaging, blood laboratory tests, neuropsychological assessment and CSF AD biomarker testing. Following a consensus meeting with clinicians and neuropsychologists, patients received a cognitive impairment diagnosis based on the DSM V criteria [62].
The patients were categorized into three groups, AD, MCI (AD) and MCI (NOT AD), based on CSF biomarker levels, dementia criteria and Winblad & Peterson MCI diagnostic criteria [63], as previously described [64]. Locally validated biomarker cut-off levels were applied for Aβ42 (>570 pg/mL), Aβ42/40 (>0.07), p-tau181 (<60 pg/mL) and total tau (<400 pg/mL), respectively. Patients with elevated total, p-tau181 and reduced Aβ42 and/or Aβ42/40 levels and with impaired daily activities were defined as the AD group. Patients with MCI and AD CSF biomarker profiles and normal daily functioning were included in MCI (AD) group. Patients with normal biomarker levels and MCI, having preserved daily functioning were assigned to the MCI (NOT AD) group.
4.3. Cerebrospinal Fluid Analysis
CSF was obtained via lumbar puncture between the L3/L4 and L4/L5 intervertebral space using a 25 gauge needle and collected in polypropylene tubes (Sarstedt AG & Co., Nümbrecht, Germany) (Figure 3). Biomarker analysis was performed at the Laboratory for CSF Diagnostics, Department of Neurology, University Medical Centre Ljubljana, Slovenia. The levels of Aβ42, Aβ40, p-tau181 and total tau were measured as previously described [64]. CSF biomarker analyses were performed according to manufacturers’ instructions using Innotest (Fujirebio Europe, Gent, Belgium) immunoassays with intra-assay variability < 5%. Between-assay coefficients and locally validated biomarker cut-off levels are listed elsewhere [65].
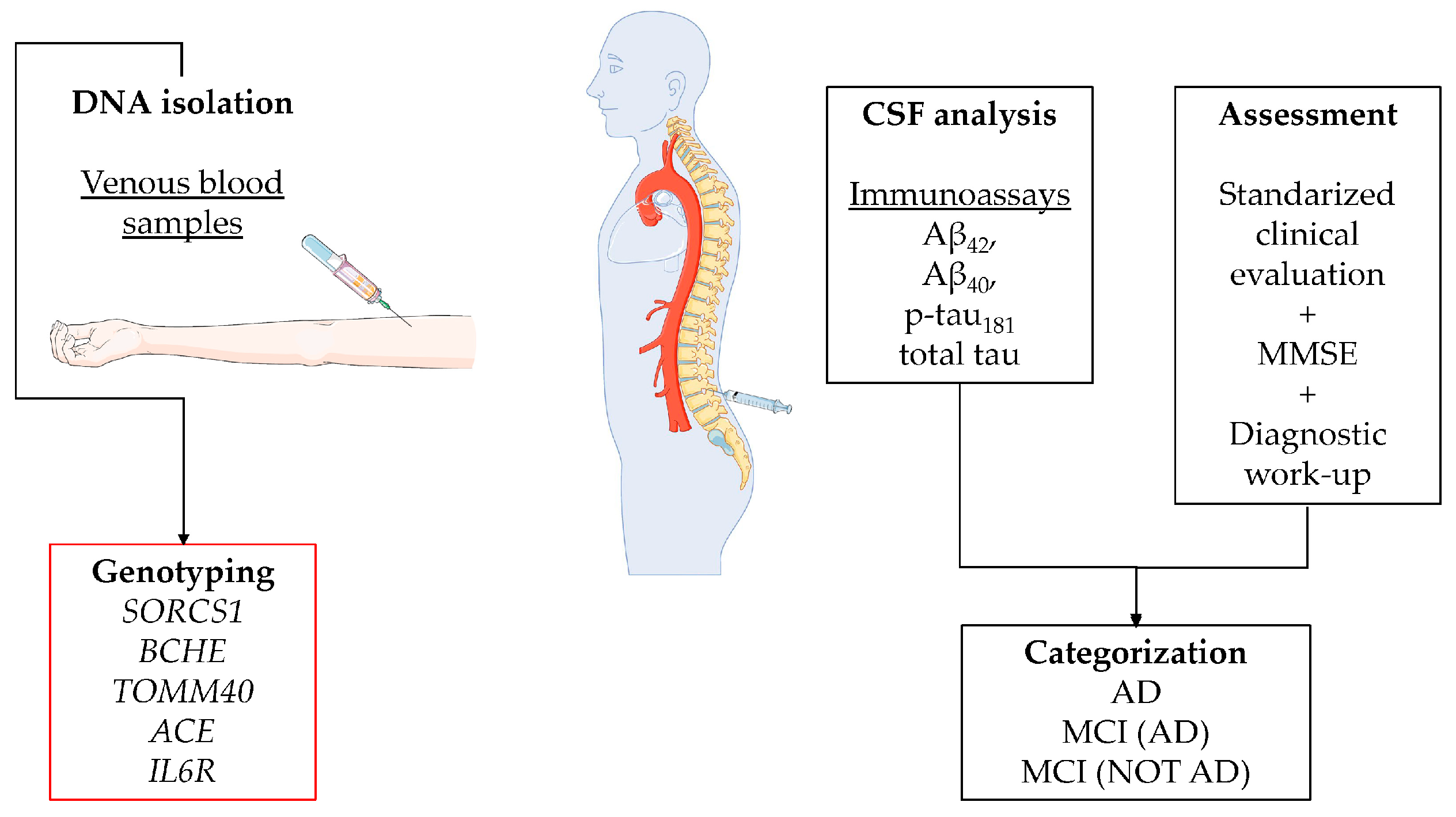
Figure 3.
Workflow of methods: DNA isolation and genotyping, CSF analysis and assessment. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://creativecommons.org/licenses/by/3.0/ 20 July 2023).
4.4. Genotyping
Genomic DNA was isolated using the E.Z.N.A.® SQ Blood DNA Kit II (Omega Bio-tek, Inc., Norcross, GA, USA) from peripheral venous blood samples following the manufacturer’s protocol. Genotyping was performed for single-nucleotide polymorphisms (SNPs) in 5 genes previously associated with AD in different GWAS studies: SORCS1, BCHE, TOMM40, ACE, and IL6 R (Figure 3). Polymorphism was selected among the previously identified GWAS genes, based on the data available from the published literature and their function prediction. Only potentially functional SNPs with a minor allele frequency of at least 0.05 were selected. For SORCS1 and BCHE, we choose different SNPs than those that were identified in the GWAS approach, due to the lack of potential functionality. In total, nine SNPs were included in the analysis (Table S1).
All of the selected SNPs were genotyped with competitive allele-specific PCR (KASP assays, LGC Biosearch Technologies, Hoddesdon, UK), according to the manufacturer’s instructions.
Additionally, APOE rs7412 and rs429358 were genotyped for the assessment of APOE4 status using real-time PCR-based Taqman assay (Applied Biosystems, Foster City, CA, USA). Ten percent of samples were genotyped in duplicate as quality control and all the results were concordant.
4.5. Statistical Analysis
We used median and interquartile range (25–75%) to describe continuous variables, and frequencies to describe categorical variables. The interquartile range was determined using weighted averages if more than two samples were included in the group and using Tukey’s hinges if only two samples were included in the group. Fisher’s exact test or Kruskal–Wallis test was used to compare patients’ characteristics and genotype frequencies between groups. The agreement of genotype frequencies with Hardy–Weinberg equilibrium (HWE) was assessed using the Chi-squared test. Both dominant and additive genetic models were used in the analysis. Mann–Whitney test or Kruskal–Wallis test with post hoc Bonferroni corrections for pairwise comparisons were used to evaluate the association of SNPs with MMSE and CSF biomarker levels. Bonferroni correction was used to account for multiple comparisons. As nine SNPs were included in the final analysis, the significance threshold was set to 0.0056, and p-values below 0.0056 were considered statistically significant, while p-values between 0.0056 and 0.050 were considered nominally significant. IBM SPSS Statistics version 27.0 (IBM Corporation, Armonk, NY, USA) was used for all analyses. All tests were two-sided and the level of significance was set at 0.05. Figures were prepared using GraphPad Prism version 9 (GraphPad Software, LLC., San Diego, CA, USA).
Based on the sample size, this study had 80% power to detect differences in CSF Aβ42 levels between 167 and 205 pg/ml for polymorphisms with minor allele frequencies between 0.20 and 0.40. Power calculation was performed using the PS power and sample size calculations, version 3.1.6 [66].
5. Conclusions
Analysis of genetic variability in GWAS identified AD risk and showed associations with CSF AD biomarker levels and cognitive decline in Slovenian patients. We associated TOMM40 and SORCS1 with both amyloid and tau pathologies, whereas BCHE was associated with cognitive decline. Our findings support previously published results and also propose some of the new associations with common polymorphisms of AD risk loci involved in various aspects of cellular signaling and localization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241612966/s1.
Author Contributions
Conceptualization, D.V., K.G. and V.D.; methodology D.V., M.G.K., A.E., S.Č., K.G. and V.D.; validation, D.V. and K.G.; formal analysis, D.V. and K.G.; investigation, D.V., M.G.K., A.E., S.Č., K.G. and V.D.; resources, M.G.K., A.E., S.Č. and V.D.; writing—original draft preparation, D.V.; writing—review and editing, M.G.K., A.E., S.Č., K.G. and V.D.; visualization, D.V.; supervision, K.G. and V.D.; project administration, K.G. and V.D.; funding acquisition, K.G. and V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Javna Agencija za Raziskovalno Dejavnost RS (Eng. Slovenian Research Agency) (ARRS) research grants P1–0170.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Republic of Slovenia National Medical Ethics Committee (0120–523/2017–4, 24 October 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the data are presented within the article and in the Supplementary Materials. Any additional information is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alzheimer’s Association 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Hebert, L.E.; Bienias, J.L.; Aggarwal, N.T.; Wilson, R.S.; Bennett, D.A.; Shah, R.C.; Evans, D.A. Change in risk of Alzheimer disease over time. Neurology 2010, 75, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Efthymiou, A.G.; Goate, A.M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H.M. Rate of Conversion from Prodromal Alzheimer’s Disease to Alzheimer’s Dementia: A Systematic Review of the Literature. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 320–332. [Google Scholar] [CrossRef]
- Naj, A.C.; Schellenberg, G.D. Genomic variants, genes, and pathways of Alzheimer’s disease: An overview. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 5–26. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.P.; Jansen, I.E.; Savage, J.E.; Shadrin, A.A.; Bahrami, S.; Holland, D.; Rongve, A.; Børte, S.; Winsvold, B.S.; Drange, O.K.; et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 2021, 53, 1276–1282. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef]
- Zannis, V.I.; Breslow, J.L.; Utermann, G.; Mahley, R.W.; Weisgraber, K.H.; Havel, R.J.; Goldstein, J.L.; Brown, M.S.; Schonfeld, G.; Hazzard, W.R.; et al. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J. Lipid Res. 1982, 23, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.W. APOE Genotype Effects on Alzheimer’s Disease Definition of AD Epidemiology of AD. J. Mol. Neurosci. 2004, 23, 157–165. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, D.M. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer’s disease. 2014, 10, 861–868. Alzheimers Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Vogrinc, D.; Goričar, K.; Dolžan, V. Genetic Variability in Molecular Pathways Implicated in Alzheimer’s Disease: A Comprehensive Review. Front. Aging Neurosci. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.D.; Streimann, I.C.; Stojanovski, D.; Johnston, A.J.; Yano, M.; Hoogenraad, N.J.; Ryan, M.T. Dissection of the mitochondrial import and assembly pathway for human Tom40. J. Biol. Chem. 2005, 280, 11535–11543. [Google Scholar] [CrossRef]
- Olgiati, P.; Politis, A.M.; Papadimitriou, G.N.; De Ronchi, D.; Serretti, A. Genetics of late-onset Alzheimer’s disease: Update from the Alzgene database and analysis of shared pathways. Int. J. Alzheimers. Dis. 2011, 11, 832379. [Google Scholar] [CrossRef] [PubMed]
- Amitay, M.; Shurki, A. The structure of G117H mutant of butyrylcholinesterase: Nerve agents scavenger. Proteins Struct. Funct. Bioinform. 2009, 77, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Kehoe, P.G. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: Progress toward disease prevention and treatment? J. Alzheimer’s Dis. 2018, 62, 1443–1466. [Google Scholar] [CrossRef]
- Haddick, P.C.G.; Larson, J.L.; Rathore, N.; Bhangale, T.R.; Phung, Q.T.; Srinivasan, K.; Hansen, D.V.; Lill, J.R.; Pericak-Vance, M.A.; Haines, J.; et al. A Common Variant of IL-6R is Associated with Elevated IL-6 Pathway Activity in Alzheimer’s Disease Brains. J. Alzheimer’s Dis. 2017, 56, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Grupe, A.; Abraham, R.; Li, Y.; Rowland, C.; Hollingworth, P.; Morgan, A.; Jehu, L.; Segurado, R.; Stone, D.; Schadt, E.; et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Hum. Mol. Genet. 2007, 16, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Hamshere, M.; Pahwa, J.S.; Moskvina, V.; Williams, A.; Jones, N.; Thomas, C.; et al. Genome-Wide Association Study Identifies Variants at CLU and PICALM Associated with Alzheimer’s Disease, and Shows Evidence for Additional Susceptibility Genes. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, J.H.; Kim, S.Y.; You, S.; Kim, M.J.; Lee, J.Y.; Koh, J. Association of GWAS top hits with late-onset alzheimer disease in korean population. Alzheimer Dis. Assoc. Disord. 2013, 27, 250–257. [Google Scholar] [CrossRef]
- Seshadri, S.; Fitzpatrick, A.L.; Ikram, M.A.; DeStefano, A.L.; Gudnason, V.; Boada, M.; Bis, J.C.; Smith, A.V.; Carassquillo, M.M.; Lambert, J.C.; et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA-J. Am. Med. Assoc. 2010, 303, 1832–1840. [Google Scholar] [CrossRef]
- Feulner, T.M.; Laws, S.M.; Friedrich, P.; Wagenpfeil, S.; Wurst, S.H.R.; Riehle, C.; Kuhn, K.A.; Krawczak, M.; Schreiber, S.; Nikolaus, S.; et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol. Psychiatry 2010, 15, 756–766. [Google Scholar] [CrossRef]
- Wijsman, E.M.; Pankratz, N.D.; Choi, Y.; Rothstein, J.H.; Faber, K.M.; Cheng, R.; Lee, J.H.; Bird, T.D.; Bennett, D.A.; Diaz-Arrastia, R.; et al. Genome-wide association of familial late-onset alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011, 7, e1001308. [Google Scholar] [CrossRef]
- Laumet, G.; Chouraki, V.; Grenier-Boley, B.; Legry, V.; Heath, S.; Zelenika, D.; Fievet, N.; Hannequin, D.; Delepine, M.; Pasquier, F.; et al. Systematic analysis of candidate genes for Alzheimer’s disease in a French, genome-wide association study. J. Alzheimer’s Dis. 2010, 20, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Blue, E.E.; Cheng, A.; Chen, S.; Yu, C.E. Association of Uncommon, Noncoding Variants in the APOE Region with Risk of Alzheimer Disease in Adults of European Ancestry. JAMA Netw. Open 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, S.; Piaceri, I.; Tedde, A.; Bessi, V.; Bracco, L.; Sorbi, S.; Nacmias, B. Tomm40 polymorphisms in Italian Alzheimer’s disease and frontotemporal dementia patients. Neurol. Sci. 2013, 34, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, S.C.; Lee, Y.S.; Ho, W.M.; Huang, Y.H.; Wu, Y.Y.; Chu, Y.C.; Wu, K.H.; Wei, L.S.; Wang, H.L.; et al. TOMM40 Genetic Variants Cause Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4085. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, C.; Liu, K.; Cong, L.; Wang, Y.; Liu, R.; Fa, W.; Tian, N.; Cheng, Y.; Wang, N.; et al. Association and interaction of TOMM40 and PVRL2 with plasma amyloid-β and Alzheimer’s disease among Chinese older adults: A population-based study. Neurobiol. Aging 2022, 113, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gok, M.; Madrer, N.; Zorbaz, T.; Bennett, E.R.; Greenberg, D.; Bennett, D.A.; Soreq, H. Altered levels of variant cholinesterase transcripts contribute to the imbalanced cholinergic signaling in Alzheimer’s and Parkinson’s disease. Front. Mol. Neurosci. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Cui, Y.; Zheng, L.; Wei, Y. Association between ACE gene polymorphisms and Alzheimer’s disease in Han population in Hebei Peninsula. Int. J. Clin. Exp. Pathol. 2017, 10, 10134–10139. [Google Scholar]
- Kim, S.; Swaminathan, S.; Shen, L.; Risacher, S.L.; Nho, K.; Foroud, T.; Shaw, L.M.; Trojanowski, J.Q.; Potkin, S.G.; Huentelman, M.J.; et al. Genome-wide association study of CSF biomarkers Aβ1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology 2011, 76, 69–79. [Google Scholar] [CrossRef]
- Cruchaga, C.; Kauwe, J.S.K.; Harari, O.; Jin, S.C.; Cai, Y.; Karch, C.M.; Benitez, B.A.; Jeng, A.T.; Skorupa, T.; Carrell, D.; et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron 2013, 78, 256–268. [Google Scholar] [CrossRef]
- Ramanan, V.K.; Risacher, S.L.; Nho, K.; Kim, S.; Swaminathan, S.; Shen, L.; Foroud, T.M.; Hakonarson, H.; Huentelman, M.J.; Aisen, P.S.; et al. APOE and BCHE as modulators of cerebral amyloid deposition: A florbetapir PET genome-wide association study. Mol. Psychiatry 2014, 19, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Bush, W.S.; Gifford, K.A.; Chibnik, L.B.; Mukherjee, S.; De Jager, P.L.; et al. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain 2019, 142, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Oatman, S.R.; Reddy, J.S.; Quicksall, Z.; Carrasquillo, M.M.; Wang, X.; Liu, C.C.; Yamazaki, Y.; Nguyen, T.T.; Malphrus, K.; Heckman, M.; et al. Genome-wide association study of brain biochemical phenotypes reveals distinct genetic architecture of Alzheimer’s disease related proteins. Mol. Neurodegener. 2023, 18, 1–23. [Google Scholar] [CrossRef]
- Damotte, V.; van der Lee, S.J.; Chouraki, V.; Grenier-Boley, B.; Simino, J.; Adams, H.; Tosto, G.; White, C.; Terzikhan, N.; Cruchaga, C.; et al. Plasma amyloid β levels are driven by genetic variants near APOE, BACE1, APP, PSEN2: A genome-wide association study in over 12,000 non-demented participants. Alzheimer’s Dement. 2021, 17, 1663–1674. [Google Scholar] [CrossRef]
- Souza, M.B.R.; Araújo, G.S.; Costa, I.G.; Oliveira, J.R.M. Combined Genome-Wide CSF Aβ-42’s Associations and Simple Network Properties Highlight New Risk Factors for Alzheimer’s Disease. J. Mol. Neurosci. 2016, 58, 120–128. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Golde, T.E.; Eckman, C.B.; Younkin, S.G. Biochemical detection of Aβ isoforms: Implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2000, 1502, 172–187. [Google Scholar] [CrossRef]
- Devi, L.; Prabhu, B.M.; Galati, D.F.; Avadhani, N.G.; Anandatheerthavarada, H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006, 26, 9057–9068. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, W.K.; Lutz, M.W.; He, Y.T.; Saunders, A.M.; Daniel, K.; Roses, A.D.; Chiba-falek, O.; Hill, C. The Broad Impact of TOM40 on Neurodegenerative Diseases in Aging. J. Park. Dis. Alzheimer’s Dis. 2014, 1, 12. [Google Scholar] [CrossRef]
- He, Y.; Li, C.; Yang, Y.; Li, Y.; Wang, Y.; Yang, H.; Jin, T.; Chen, S. Meta-analysis of the rs2075650 polymorphism and risk of Alzheimer disease. Aging Clin. Exp. Res. 2016, 28, 805–811. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Xu, B.; Ma, X.; Dai, Q.; Li, T.; Xue, F.; Chen, B. The TOMM40 gene rs2075650 polymorphism contributes to Alzheimer’s disease in Caucasian, and Asian populations. Neurosci. Lett. 2016, 628, 142–146. [Google Scholar] [CrossRef]
- Kulminski, A.M.; Jain-Washburn, E.; Loiko, E.; Loika, Y.; Feng, F.; Culminskaya, I. Associations of the APOE ε2 and ε4 alleles and polygenic profiles comprising APOE-TOMM40-APOC1 variants with Alzheimer’s disease biomarkers. Aging 2022, 14, 9782–9804. [Google Scholar] [CrossRef]
- Hermey, G.; Riedel, I.B.; Rezgaoui, M.; Westergaard, U.B.; Schaller, C.; Hermans-Borgmeyer, I. SorCS1, a member of the novel sorting receptor family, is localized in somata and dendrites of neurons throughout the murine brain. Neurosci. Lett. 2001, 313, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, J.; Wang, Y.; Tang, H.; Deng, Y.; Ren, R.; Wang, G.; Niu, W.; Ma, J.; Wu, Y.; et al. The Genetic Variation of SORCS1 Is Associated with Late-Onset Alzheimer’s Disease in Chinese Han Population. PLoS ONE 2013, 8, e63621. [Google Scholar] [CrossRef] [PubMed]
- Edbauer, D.; Winkler, E.; Regula, J.T.; Pesold, B.; Steiner, H.; Haass, C. Reconstitution of γ-secretase activity. Nat. Cell Biol. 2003, 5, 486–488. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, I.; Youm, E.M.; Lee, S.; Park, J.-H.; Lee, J.; Lee, D.Y.; Byun, M.S.; Lee, J.H.; Yi, D.; et al. Novel Alzheimer’s disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers. Transl. Psychiatry 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Yu, J.T.; Zhang, W.; Wang, W.; Liu, Q.Y.; Ma, X.Y.; Ding, H.M.; Tan, L. SORCS1 and APOE polymorphisms interact to confer risk for late-onset Alzheimer’s disease in a Northern Han Chinese population. Brain Res. 2012, 1448, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Brückner, M.K.; Lange, M.; Bigl, V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development—A study of molecular forms. Neurochem. Int. 1992, 21, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.A.; Geula, C.; Mesulam, M.-M. Electron microscopic localization of cholinesterase activity in Alzheimer brain tissue. Brain Res. 1991, 540, 204–208. [Google Scholar] [CrossRef]
- Darreh-Shori, T.; Siawesh, M.; Mousavi, M.; Andreasen, N.; Nordberg, A. Apolipoprotein ε4 Modulates Phenotype of Butyrylcholinesterase in CSF of Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 28, 443–458. [Google Scholar] [CrossRef]
- Sokolow, S.; Li, X.; Chen, L.; Taylor, K.D.; Rotter, J.I.; Rissman, R.A.; Aisen, P.S.; Apostolova, L.G. Deleterious Effect of Butyrylcholinesterase K-Variant in Donepezil Treatment of Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 56, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.; De Leon, M.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Backman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment - beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vogrinc, D.; Gregorič Kramberger, M.; Emeršič, A.; Čučnik, S.; Goričar, K.; Dolžan, V. Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia. Antioxidants 2023, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Perovnik, M.; Tomše, P.; Jamšek, J.; Emeršič, A.; Tang, C.; Eidelberg, D.; Trošt, M. Identification and validation of Alzheimer’s disease-related metabolic brain pattern in biomarker confirmed Alzheimer’s dementia patients. Sci. Rep. 2022, 12, 11752 . [Google Scholar] [CrossRef]
- Dupont, W.D.; Plummer, W.D.J. Power and sample size calculations: A review and computer program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).