Organically Templated Uranyl Sulfates and Selenates: Structural Complexity and Crystal Chemical Restrictions for Isotypic Compounds Formation
Abstract
:1. Introduction
2. Results and Discussion
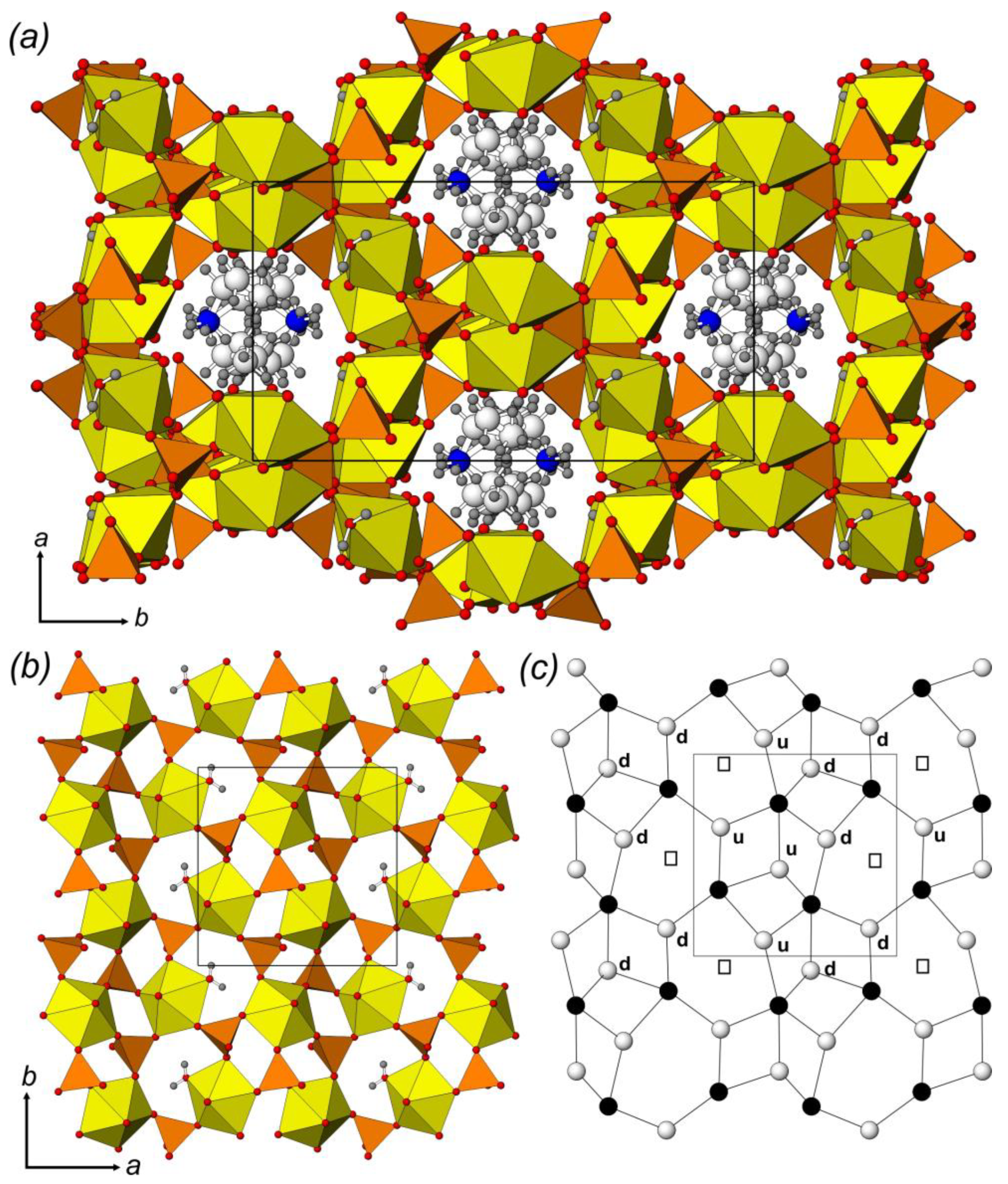
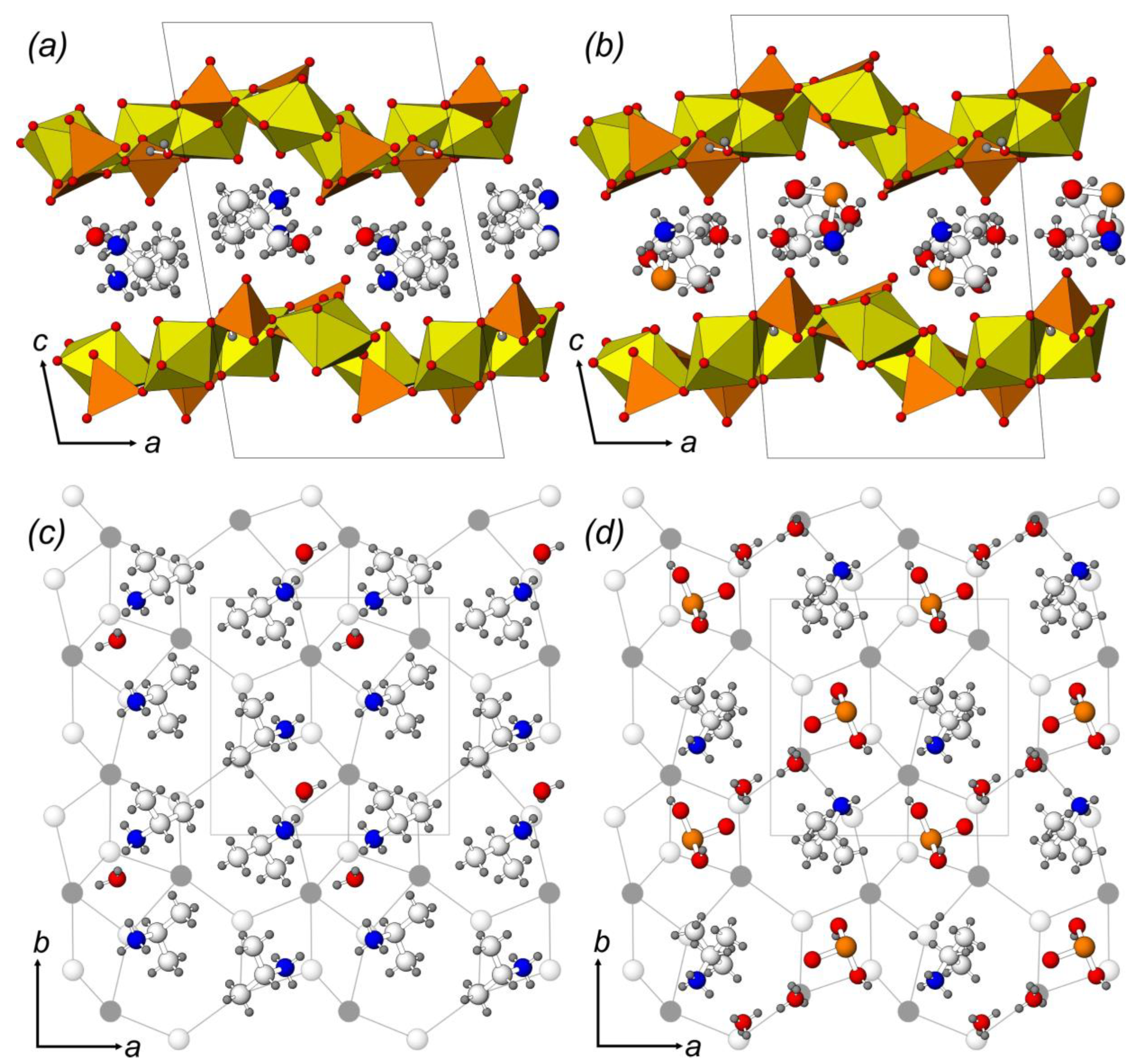
2.1. Crystal Structure Description
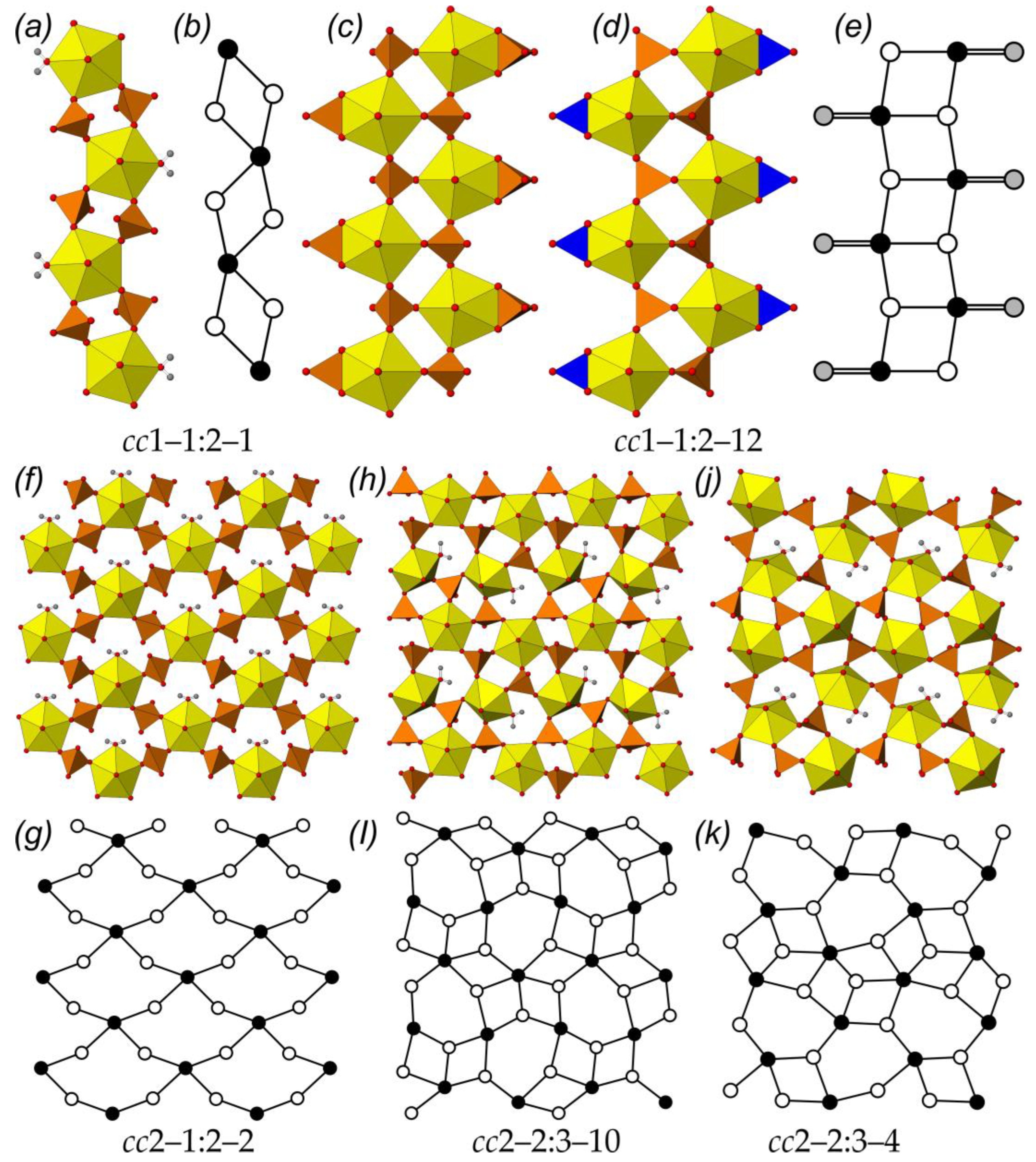
2.2. Structural Topology
3. Discussion
3.1. Isotypic Uranyl Sulfates and Selenates
3.2. Topology of U-Bearing Complexes
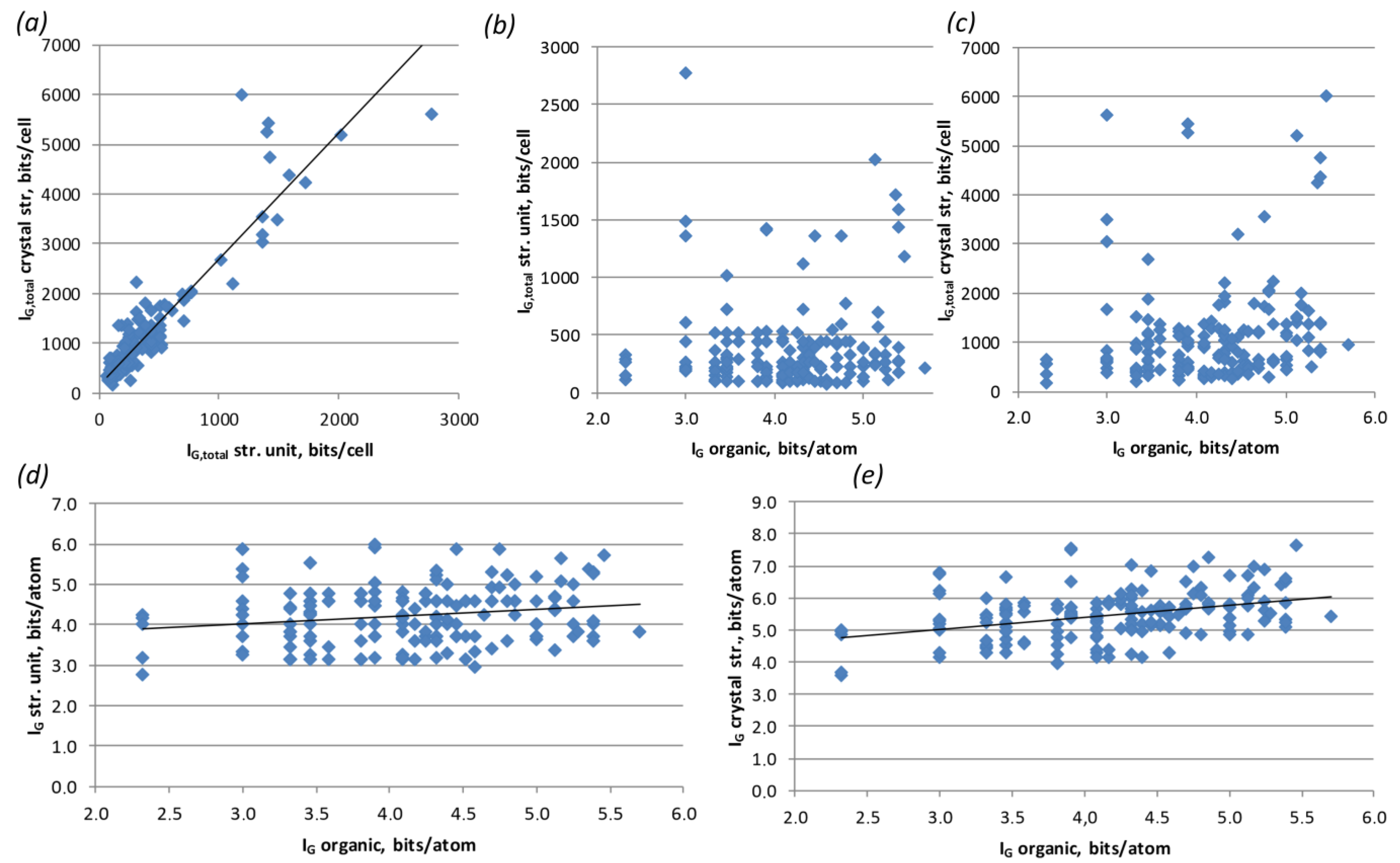
3.3. Structural Complexity
4. Materials and Methods
4.1. Synthesis
4.2. Chemical Analysis
4.3. Single-Crystal X-ray Diffraction
4.4. Structural Complexity Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serezhkin, V.N.; Soldatkina, M.A. Crystal Structure of the NH4[UO2SO4F]. Coord. Chem. 1985, 11, 103–105. [Google Scholar]
- Niinistö, L.; Toivonen, J.; Valkonen, J. Uranyl(VI) Compounds. I. The Crystal Structure of Ammonium Uranyl Sulfate Dihydrate, (NH4)2UO2(SO4)2·2H2O. Acta Chem. Scand. 1978, A32, 647–651. [Google Scholar] [CrossRef]
- Burns, P.C.; Deely, K.M.; Hayden, L.A. The crystal chemistry of the zippeite group. Can. Mineral. 2003, 41, 687–706. [Google Scholar] [CrossRef]
- Mikhailov, Y.N.; Gorbunova, Y.E.; Serezhkina, L.B.; Demchenko, E.A.; Serezhkin, V.N. Crystal Structure of the [(NH4)2][UO2(SeO4)2]·3H2O. Russ. J. Inorg. Chem. 1997, 42, 1413–1417. [Google Scholar]
- Gurzhiy, V.V.; Tyshchenko, D.V.; Krivovichev, S.V.; Tananaev, I.G. Symmetry Reduction in Uranyl Compounds with [(UO2)2(TO4)3]2− (T = Se, S, Mo) Layers: Crystal Structures of the New Guanidinium Uranyl Selenate and Methylammonium Uranyl Sulfate. Z. Krist.-Cryst. Mater. 2014, 229, 368–377. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Charkin, D.O.; Siidra, O.I.; Gurzhiy, V.V. Synthesis and Crystal Structures of New Layered Uranyl Compounds Containing Dimers [(UO2)2O8] of Edge-Linked Pentagonal Bipyramids. Radiochemistry 2018, 60, 498–506. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Gurzhiy, V.V.; Krivovichev, S.V. Structural Topology and Dimensional Reduction in Uranyl Oxysalts: Eight Novel Phases in the Methylamine–(UO2)(NO3)2–H2SeO4–H2O System. Struct. Chem. 2012, 23, 2003–2017. [Google Scholar] [CrossRef]
- Guo, H.X.; Weng, W.; Li, X.Z. Hydrothermal Synthesis, Crystal Structure and Luminescent Properties of an Organically Templated 2-D Uranyl Sulfate. Chin. J. Struct. Chem. 2008, 27, 1455–1458. [Google Scholar]
- Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G. Dehydration-Driven Evolution of Topological Complexity in Ethylamonium Uranyl Selenates. J. Solid State Chem. 2017, 247, 105–112. [Google Scholar] [CrossRef]
- Ling, J.; Sigmon, G.E.; Burns, P.C. Syntheses, Structures, Characterizations and Charge-Density Matching of Novel Amino-Templated Uranyl Selenates. J. Solid State Chem. 2009, 182, 402–408. [Google Scholar] [CrossRef]
- Bharara, M.S.; Gorden, A.E.V. Amine Templated Two- and Three-Dimensional Uranyl Sulfates. Dalton Trans. 2010, 39, 3557. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Gurzhii, V.V.; Tananaev, I.G.; Myasoedov, B.F. Topology of Inorganic Complexes as a Function of Amine Molecular Structure in Layered Uranyl Selenates. Dokl. Phys. Chem. 2006, 409, 228–232. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Gurzhiy, V.V.; Tananaev, I.G.; Myasoedov, B.F. Amine-Templated Uranyl Selenates with Chiral [(UO2)2(SeO4)3(H2O)]2– Layers: Topology, Isomerism, Structural Relationships. Z. Krist.-Cryst. Mater. 2009, 224, 316–324. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Mikhailenko, P.A.; Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Synthesis and Structure of a New Uranyl Selenate Complex with 1-Butylamine [CH3(CH2)3NH3](H5O2)[(UO2)2(SeO4)3(H2O)]. Russ. J. Gen. Chem. 2012, 82, 23–26. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V.; Tananaev, I.G.; Kaindl, R.; Mersdorf, E.; Myasoedov, B.F. Highly Porous Uranyl Selenate Nanotubules. J. Am. Chem. Soc. 2005, 127, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Tananaev, I.G.; Kahlenberg, V.; Myasoedov, B.F. Synthesis and Crystal Structure of a New Uranyl Selenite(IV)-Selenate(VI), [C5H14N]4[(UO2)3(SeO4)4(HSeO3)(H2O)](H2SeO3)(HSeO4). Radiochemistry 2006, 48, 217–222. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Geometric Isomerism of Layered Complexes of Uranyl Selenates: Synthesis and Structure of (H3O)[C5H14N]2[(UO2)3(SeO4)4(HSeO4)(H2O)] and (H3O)[C5H14N]2[(UO2)3(SeO4)4(HSeO4)(H2O)](H2O). Radiochemistry 2006, 48, 552–560. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Kalenberg, V.; Myasoedov, B.F. Synthesis and Crystal Structure of the First Uranyl Selenite (IV)-Selenate (VI), [C5H14N][(UO2)(SeO4)(SeO2OH)]. Dokl. Akad. Nauk. 2005, 403, 124–127. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Charge-Density Matching in Organic–Inorganic Uranyl Compounds. C. R. Chim. 2007, 10, 897–904. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V.; Tananaev, I.G.; Myasoedov, B.F. Amine-Templated Uranyl Selenates with Layered Structures. I Structural Diversity of Sheets with a U:Se Ratio of 1:2. Z. Anorg. Allg. Chem. 2005, 631, 2358–2364. [Google Scholar] [CrossRef]
- Liu Liu, D.-S.; Kuang, H.-M.; Chen, W.-T.; Luo, Q.-Y.; Sui, Y. Synthesis, Structure, and Photoluminescence Properties of an Organically-Templated Uranyl Selenite. Z. Anorg. Allg. Chem. 2015, 641, 2009–2013. [Google Scholar] [CrossRef]
- Norquist, A.J.; Doran, M.B.; O’Hare, D. The Effects of Linear Diamine Chain Length in Uranium Sulfates. Solid State Sci. 2003, 5, 1149–1158. [Google Scholar] [CrossRef]
- Thomas, P.M.; Norquist, A.J.; Doran, M.B.; O’Hare, D. Organically Templated Uranium(vi) Sulfates: Understanding Phase Stability Using Composition Space. J. Mater. Chem. 2003, 13, 88–92. [Google Scholar] [CrossRef]
- Doran, M.B.; Cockbain, B.E.; O’Hare, D. Structural Variation in Organically Templated Uranium Sulfate Fluorides. Dalton Trans. 2005, 10, 1774–1780. [Google Scholar] [CrossRef]
- Doran, M.B.; Cockbain, B.E.; Norquist, A.J.; O’Hare, D. The effects of hydrofluoric acid addition on the hydrothermal synthesis of templated uranium sulfates. Dalton Trans. 2004, 2004, 3810–3814. [Google Scholar] [CrossRef]
- Jouffret, L.J.; Wylie, E.M.; Burns, P.C. Amine Templating Effect Absent in Uranyl Sulfates Synthesized with 1,4-n-Butyldiamine. J. Solid State Chem. 2013, 197, 160–165. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Krivovichev, S.V.; Burns, P.C.; Tananaev, I.G.; Myasoedov, B.F. Supramolecular Templates for the Synthesis of New Nanostructured Uranyl Compounds: Crystal Structure of [NH3(CH2)9NH3][(UO2)(SeO4)(SeO2OH)](NO3). Radiochemistry 2010, 52, 1–6. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Gurzhiy, V.V.; Burns, P.C.; Tananaev, I.G.; Myasoedov, B.F. Partially Ordered Organic-Inorganic Nanocomposites in the System UO2SeO4–H2O–NH3(CH2)9NH3. Radiochemistry 2010, 52, 7–11. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V.; Avdontseva, E.Y.; Mersdorf, E.; Kaindl, R. Self-Assembly of Protonated 1,12-Dodecanediamine Molecules and Strongly Undulated Uranyl Selenate Sheets in the Structure of Amine-Templated Uranyl Selenate: (H3O)2[C12H30N2]3[(UO2)4(SeO4)8](H2O)5. Eur. J. Inorg. Chem. 2005, 2005, 1653–1656. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kovrugin, V.M.; Tyumentseva, O.S.; Mikhaylenko, P.A.; Krivovichev, S.V.; Tananaev, I.G. Topologically and Geometrically Flexible Structural Units in Seven New Organically Templated Uranyl Selenates and Selenite–Selenates. J. Solid State Chem. 2015, 229, 32–40. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Unprecedented layer topology in the crystal structure of a new organically templated uranyl selenite-selenate. Mendeleev Commun. 2012, 22, 11–12. [Google Scholar] [CrossRef]
- Serezhkina, L.N.; Trunov, V.K. Crystal structure of (N(CH3)4)[UO2SO4x2H2O]Cl. Zh. Neorg. Khim. 1989, 34, 968–970. [Google Scholar]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. catena-Poly-[tetra-methyl-ammonium-[[(nitrato-κ2O,O′)-dioxouranium]-μ3-sulfato]]. Acta Crystallogr. 2004, E59, m373–m375. [Google Scholar]
- Krivovichev, S.V.; Kahlenberg, V. Low-Dimensional Structural Units in Amine-Templated Uranyl Oxoselenates(VI): Synthesis and Crystal Structures of [C3H12N2][(UO2)(SeO4)2(H2O)2](H2O), [C5H16N2]2[(UO2)(SeO4)2(H2O)](NO3)2, [C4H12N][(UO2)(SeO4)(NO3)], and [C4H14N2][(UO2)(SeO4)2(H2O)]. Z. Anorg. Allg. Chem. 2005, 631, 2352–2357. [Google Scholar] [CrossRef]
- Doran, M.; Norquist, A.J.; O’Hare, D. [NC4H12]2[(UO2)6(H2O)2(SO4)7]: The First Organically Templated Actinide Sulfate with a Three-Dimensional Framework Structure. Chem. Commun. 2002, 2002, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Nazarchuk, E.V.; Charkin, D.O.; Kozlov, D.V.; Siidra, O.I.; Kalmykov, S.N. Topological Analysis of the Layered Uranyl Compounds Bearing Slabs with UO2:TO4 Ratio of 2:3. Radiochem. Acta 2020, 108, 249–260. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Charkin, D.O.; Siidra, O.I.; Gurzhiy, V.V. Crystal-Chemical Features of U(VI) Compounds with Inorganic Complexes Derived from [(UO2)(TO4)(H2O)n], T = S, Cr, Se: Synthesis and Crystal Structures of Two New Uranyl Sulfates. Radiochemistry 2018, 60, 345–351. [Google Scholar] [CrossRef]
- Baggio, R.F.; De Benyacar, M.A.R.; Perazzo, B.O.; De Perazzo, P.K. Crystal Structure of Ferroelectric Guanidinium Uranyl Sulphate Trihydrate. Acta Crystallogr. B 1977, 33, 3495–3499. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Belova, E.V.; Krivovichev, S.V. Chemically Induced Symmetry Breaking in the Crystal Structure of Guanidinium Uranyl Sulfate. Mendeleev Commun. 2019, 29, 408–410. [Google Scholar] [CrossRef]
- Medrish, I.V.; Vologzhanina, A.V.; Starikova, Z.A.; Antipin, M.Y. Synthesis and Crystal Structure of the Aminoguanidinium Uranyl Sulfate. Russ. J. Inorg. Chem. 2005, 50, 412–416. [Google Scholar]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. (C3H12N2)2[UO2(H2O)2(SO4)2]2·2H2O: An Organically Templated Uranium Sulfate with a Novel Dimer Type. Acta Crystallogr. E 2005, 61, m881–m884. [Google Scholar] [CrossRef]
- Norquist, A.J.; Doran, M.B.; Thomas, P.M.; O’Hare, D. Structural Diversity in Organically Templated Uranium Sulfates. Dalton Trans. 2003, 2003, 1168–1175. [Google Scholar] [CrossRef]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. Exploration of Composition Space in Templated Uranium Sulfates. Inorg. Chem. 2003, 42, 6989–6995. [Google Scholar] [CrossRef] [PubMed]
- Norquist, A.J.; Doran, M.B.; O’Hare, D. (C7H20N2)[(UO2)2(SO4)3(H2O)]: An Organically Templated Uranium Sulfate with a Novel Layer Topology. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m807–m810. [Google Scholar] [CrossRef]
- Kohlgruber, T.A.; Perry, S.N.; Sigmon, G.E.; Oliver, A.G.; Burns, P.C. Hydrogen Bond Network and Bond Valence Analysis on Uranyl Sulfate Compounds with Organic-Based Interstitial Cations. J. Solid State Chem. 2022, 307, 122871. [Google Scholar] [CrossRef]
- Ling, J.; Sigmon, G.E.; Ward, M.; Roback, N.; Carman Burns, P. Syntheses, Structures, and IR Spectroscopic Characterization of New Uranyl Sulfate/Selenate 1D-Chain, 2D-Sheet and 3D-Framework. Z. Kristallogr. Cryst. Mater. 2010, 225, 230–239. [Google Scholar] [CrossRef]
- Norquist, A.J.; Doran, M.B.; O’Hare, D. The Role of Amine Sulfates in Hydrothermal Uranium Chemistry. Inorg. Chem. 2005, 44, 3837–3843. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Actinide compounds containing hexavalent cations of the VI group elements (S, Se, Mo, Cr, W). In Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S.V., Burns, P.C., Tananaev, I.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 95–182. [Google Scholar]
- Doran, M.B.; Norquist, A.J.; Stuart, C.L.; O’Hare, D. (C8H26N4)0.5[(UO2)2(SO4)3(H2O)]·2H2O, an Organically Templated Uranyl Sulfate with a Novel Layer Type. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m996–m998. [Google Scholar] [CrossRef]
- Williams, J.M.; Pyrch, M.M.; Unruh, D.K.; Lightfoot, H.; Forbes, T.Z. Influence of Heterocyclic N-Donors on the Structural Topologies and Vibrational Spectra of Uranyl Selenate Phases. J. Solid State Chem. 2021, 304, 122619. [Google Scholar] [CrossRef]
- Jouffret, L.J.; Wylie, E.M.; Burns, P.C. Influence of the Organic Species and Oxoanion in the Synthesis of Two Uranyl Sulfate Hydrates, (H3O)2[(UO2)2(SO4)3(H2O)]·7H2O and (H3O)2[(UO2)2(SO4)3(H2O)]·4H2O, and a Uranyl Selenate-Selenite [C5H6N][(UO2)(SeO4)(HSeO3)]. Z. Anorg. Allg. Chem. 2012, 638, 1796–1803. [Google Scholar] [CrossRef]
- Wylie, E.M.; Smith, P.A.; Peruski, K.M.; Smith, J.S.; Dustin, M.K.; Burns, P.C. Effects of Ionic Liquid Media on the Cation Selectivity of Uranyl Structural Units in Five New Compounds Produced Using the Ionothermal Technique. Cryst. Eng. Commun. 2014, 16, 7236–7243. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Britvin, S.N.; Krivovichev, S.V.; Tananaev, I.G. Ring Opening of Azetidine Cycle: First Examples of 1-Azetidinepropanamine Molecules as a Template in Hybrid Organic-Inorganic Compounds. J. Mol. Struct. 2018, 1151, 88–96. [Google Scholar] [CrossRef]
- Norquist, A.J.; Thomas, P.M.; Doran, M.B.; O’Hare, D. Synthesis of Cyclical Diamine Templated Uranium Sulfates. Chem. Mater. 2002, 14, 5179–5184. [Google Scholar] [CrossRef]
- Almond, P.M.; Albrecht-Schmitt, T.E. Do Secondary and Tertiary Ammonium Cations Act as Structure-Directing Agents in the Formation of Layered Uranyl Selenites. Inorg. Chem. 2003, 42, 5693–5698. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.L.; Doran, M.B.; Norquist, A.J.; O’Hare, D. Catena-Poly[1-Methylpiperazinium [[Aquadioxouranium(VI)]-Di-μ-Sulfato-κ 4 O:O′]]. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m446–m448. [Google Scholar] [CrossRef]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. Catena-Poly[Cyclohexane-1,4-Diammonium [[Dioxo(Sulfato-κ2 O,O′)Uranium(VI)]-μ-Sulfato] Dihydrate]. Acta Crystallogr. E 2003, 59, m765–m767. [Google Scholar] [CrossRef]
- Wylie, E.M.; Dustin, M.K.; Smith, J.S.; Burns, P.C. Ionothermal Synthesis of Uranyl Compounds That Incorporate Imidazole Derivatives. J. Solid State Chem. 2013, 197, 266–272. [Google Scholar] [CrossRef]
- Kohlgruber, T.A.; Felton, D.E.; Perry, S.N.; Oliver, A.G.; Burns, P.C. Effect of Ionothermal Conditions on the Crystallization of Organically Templated Uranyl Sulfate Compounds. Cryst. Growth Des. 2021, 21, 861–868. [Google Scholar] [CrossRef]
- Norquist, A.J.; Doran, M.B.; Thomas, P.M.; O’Hare, D. Controlled Structural Variations in Templated Uranium Sulfates. Inorg. Chem. 2003, 42, 5949–5953. [Google Scholar] [CrossRef]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. Poly[[1,4-Bis-(3-Aminopropyl)Piperazinium] [[Dioxouranium(VI)]-Di-μ2,μ3 -Sulfato]]. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m762–m764. [Google Scholar] [CrossRef]
- Tyshchenko, D.V. Structural Study of Uranyl Sulfates with Inorganic and Organic Cations. Master’s Thesis, St. Petersburg State University, St. Petersburg, Russia, 2014. [Google Scholar]
- Kuporev, I.V.; Gurzhiy, V.V.; Krivovichev, S.V. Synthesis and Structural Study of the New Modular Uranyl selenite-Selenate with Melamine [(UO2)(SeO4)(H2SeO3)][(SeO4)(C3H8N6)]. In IV Conference and School for Young Scientists: Non-Ambient Diffraction and Nanomaterials; Book of Abstracts; St. Petersburg State University: St. Petersburg, Russia, 2020; p. 101. [Google Scholar]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Cyclic Polyamines as Templates for Novel Complex Topologies in Uranyl Sulfates and Selenates. Z. Kristallogr. 2018, 233, 233–245. [Google Scholar] [CrossRef]
- Rogers, R.D.; Bond, A.H.; Hipple, W.G.; Rollins, A.N.; Henry, R.F. Synthesis and Structural Elucidation of Novel Uranyl-Crown Ether Compounds Isolated from Nitric, Hydrochloric, Sulfuric, and Acetic Acids. Inorg. Chem. 1991, 30, 2671–2679. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Tyshchenko, D.V.; Krivovichev, S.V.; Tananaev, I.G. Crown-Ether-Templated Uranyl Selenates: Novel Family of Mixed Organic-Inorganic Actinide Compounds. Mendeleev Commun. 2016, 26, 309–311. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Gurzhiy, V.V.; Tananaev, I.G.; Myasoedov, B.F. Uranyl Selenates with Organic Templates: Principles of Structure and Characteristics of Self-Organization. Russ. J. Gen. Chem. 2009, 79, 2723–2730. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Hybrid One-Dimensional 15-Crown-5-Ether-Uranyl-Selenate Polymers in [K@(C10H20O5)][(UO2)(SeO4)(HSeO4)(H2O)]: Synthesis and Characterization. Z. Anorg. Allg. Chem. 2015, 641, 1110–1113. [Google Scholar] [CrossRef]
- Alekseev, E.V.; Krivovichev, S.V.; Depmeier, W. A Crown Ether as Template for Microporous and Nanostructured Uranium Compounds. Angew. Chem. Int. Ed. 2008, 47, 549–551. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Novel Type of Molecular Connectivity in One-Dimensional Uranyl Compounds: [K@(18-Crown-6)(H2O)][(UO2)(SeO4)(NO3)], a New Potassium Uranyl Selenate with 18-Crown-6 Ether. Inorg. Chem. Commun. 2014, 45, 93–96. [Google Scholar] [CrossRef]
- Charkin, D.O.; Bezzubov, S.I.; Siidra, O.I.; Borisov, A.S.; Kalmykov, S.N. Preparation and Crystal Structure of a New Uranyl Sulfate Templated by a Bis-Isothiouronium Cation. Z. Anorg. Allg. Chem. 2020, 646, 540–543. [Google Scholar] [CrossRef]
- Mikhajlov, Y.N.; Mistryukov, V.E.; Gorbunova, Y.E.; Serezhkina, L.B.; Demchenko, E.A.; Serezhkin, V.N. Crystal Structure of [UO2SO4x2H2O]XCH2ClCONH2. Zhurnal Neorg. Khimii 1995, 40, 1288–1290. [Google Scholar]
- Tyumentseva, O.S.; Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. First Organic–Inorganic Uranyl Chloroselenate: Synthesis, Crystal Structure and Spectroscopic Characteristics. J. Chem. Crystallogr. 2013, 43, 517–522. [Google Scholar] [CrossRef]
- Grechishnikova, E.V.; Serezhkina, L.B.; Virovets, A.V.; Peresypkina, E.V. Synthesis and Crystal Structure of (C2N4H7O)[UO2(SO4)(OH)] · 0.5H2O. Russ. J. Inorg. Chem. 2005, 50, 1800–1805. [Google Scholar]
- Nazarchuk, E.V.; Siidra, O.I.; Charkin, D.O. Specific Features of the Crystal Chemistry of Layered Uranyl Compounds with the Ratio UO2:TO4 = 5:8 (T = S6+, Cr6+, Se6+, Mo6+). Radiochemistry 2018, 60, 352–361. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Ikhalaynen, Y.A.; Charkin, D.O.; Siidra, O.I.; Petrov, V.G.; Kalmykov, S.N.; Borisov, A.S. Effect of Solution Acidity on the Structure of Amino Acid-Bearing Uranyl Compounds. Radiochem. Acta 2019, 107, 311–325. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Charkin, D.O.; Chukanov, N.V.; Zakharov, A.Y.; Kalmykov, S.N.; Ikhalaynen, Y.A.; Sharikov, M.I. Open-Framework Sodium Uranyl Selenate and Sodium Uranyl Sulfate with Protonated Morpholino-N-Acetic Acid. Z. Kristallogr. Cryst. Mater. 2019, 234, 109–118. [Google Scholar] [CrossRef]
- Smith, P.A.; Burns, P.C. Ligand Mediated Morphology of the Two-Dimensional Uranyl Aqua Sulfates [UO2(X)(SO4)(H2O)] [X = Cl− or (CH3)3NCH2COO]. Z. Anorg. Allg. Chem. 2019, 645, 504–508. [Google Scholar] [CrossRef]
- Siidra, O.; Nazarchuk, E.; Charkin, D.; Chukanov, N.; Depmeier, W.; Bocharov, S.; Sharikov, M. Uranyl Sulfate Nanotubules Templated by N-Phenylglycine. Nanomaterials 2018, 8, 216. [Google Scholar] [CrossRef]
- Smith, P.A.; Aksenov, S.M.; Jablonski, S.; Burns, P.C. Structural Unit Charge Density and Molecular Cation Templating Effects on Orientational Geometric Isomerism and Interlayer Spacing in 2-D Uranyl Sulfates. J. Solid State Chem. 2018, 266, 286–296. [Google Scholar] [CrossRef]
- Hu, K.; Zeng, L.; Kong, X.; Huang, Z.; Yu, J.; Mei, L.; Chai, Z.; Shi, W. Viologen-Based Uranyl Coordination Polymers: Anion-Induced Structural Diversity and the Potential as a Fluorescent Probe. Eur. J. Inorg. Chem. 2021, 2021, 5077–5084. [Google Scholar] [CrossRef]
- Tabachenko, V.V.; Kovba, L.M.; Serezhkin, V.N. Crystal structures of magnesium and strontium molybdateouranilates of Mg(UO2)6(MoO4)7·18H2O and Sr(UO2)6(MoO4)7·15H2O composition. Koord. Khim. 1984, 10, 558–562. [Google Scholar]
- Krivovichev, S.V.; Armbruster, T.; Chernyshov, D.Y.; Burns, P.C.; Nazarchuk, E.V.; Depmeier, W. Chiral open-framework uranyl molybdates. 2. Flexibility of the U:Mo = 6:7 frameworks: Syntheses and crystal structures of (UO2)0.82[C8H20N]0.36[(UO2)6(MoO4)7(H2O)2](H2O)n and [C6H14N2][(UO2)6(MoO4)7(H2O)2](H2O)m. Microporous Mesoporous Mater. 2005, 78, 217–224. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Armbruster, T.; Chernyshov, D.Y.; Burns, P.C.; Nazarchuk, E.V.; Depmeier, W. Chiral open-framework uranyl molybdates. 3. Synthesis, structure and the C2221–P212121 low-temperature phase transition of [C6H16N]2[(UO2)6(MoO4)7(H2O)2](H2O)2. Microporous Mesoporous Mater. 2005, 78, 225–234. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Bleiholder, C.; Gleiter, R.; Werz, D.B.; Köppel, H. Theoretical Investigations on Heteronuclear Chalcogen−Chalcogen Interactions: On the Nature of Weak Bonds between Chalcogen Centers. Inorg. Chem. 2007, 46, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.; Ling, K.; Cockroft, S. The Origin of Chalcogen-Bonding Interactions. J. Amer. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Santi, C.; Sancineto, L. Nonbonded Interaction: The Chalcogen Bond. In New Frontiers in Organoselenium Compounds; Springer: Cham, Switzerland, 2018; pp. 157–183. [Google Scholar]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendation). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Geometrical isomerism in uranyl chromates II. Crystal structures of Mg2[(UO2)3(CrO4)5](H2O)17 and Ca2[(UO2)3(CrO4)5](H2O)19. Z. Kristallogr. 2003, 218, 683–690. [Google Scholar] [CrossRef]
- Burns, P.C. The Crystal Chemistry of Uranium. In Uranium: Mineralogy, Geochemistry, and the Environment; Reviews in Mineralogy; Burns, P.C., Finch, R., Eds.; Walter de Gruyter GmbH & Co KG.: Berlin, Germany, 1999; Volume 38, pp. 23–90. [Google Scholar]
- Krivovichev, S.V. Structural Crystallography of Inorganic Oxysalts; Oxford University Press: Oxford, UK, 2008; 303p. [Google Scholar]
- Lussier, A.J.; Lopez, R.A.K.; Burns, P.C. A revised and expanded structure hierarchy of natural and synthetic hexavalent uranium compounds. Can. Mineral. 2016, 54, 177–283. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Hawthorne, F.C.; Williams, P.A. Structural complexity and crystallization: The Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Struct. Chem. 2017, 28, 153–159. [Google Scholar] [CrossRef]
- Plášil, J. Structural complexity of uranophane and uranophane-β: Implications for their formation and occurrence. Eur. J. Mineral. 2018, 30, 253–257. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskaya, O.V. Hydrated Calcium Oxalates: Crystal Structures, Thermal Stability and Phase Evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Krivovichev, S.V.; Charykova, M.V. Selenium Minerals: Structural and Chemical Diversity and Complexity. Minerals 2019, 9, 455. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Gurzhiy, V.V. Dimensional evolution in hydrated K+-bearing uranyl sulfates: From 2D-sheets to 3D frameworks. CrystEngComm 2020, 22, 4621–4629. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. 2019, B75, 39–48. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kuporev, I.V.; Kovrugin, V.M.; Murashko, M.N.; Kasatkin, A.V.; Plášil, J. Crystal chemistry and structural complexity of natural and synthetic uranyl selenites. Crystals 2019, 9, 639. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kalashnikova, S.A.; Kuporev, I.V.; Plášil, J. Crystal chemistry and structural complexity of the uranyl carbonate minerals and synthetic compounds. Crystals 2021, 11, 704. [Google Scholar] [CrossRef]
- Fraser, W. Diffractometers for modern X-ray crystallography: The XtaLAB Synergy X-ray diffractometer platform. Rigaku J. 2020, 36, 37–47. [Google Scholar]
- CrysAlisPro Software System, Version 1.171.41.94a; Rigaku Oxford Diffraction: Oxford, UK, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structural complexity of minerals and mineral parageneses: Information and its evolution in the mineral world. In Highlights in Mineralogical Crystallography; Danisi, R., Armbruster, T., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2015; pp. 31–73. [Google Scholar]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar]
- Krivovichev, S.V. Ladders of information: What contributes to the structural complexity in inorganic crystals. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth. Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Krivovichev, V.G.; Tananaev, I.G. Mixed uranyl sulfate-selenates: Variable composition and crystal structures. Cryst. Growth Des. 2016, 16, 4482–4492. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G.; Gurzhiy, V.V. Crystal chemistry of the M2+[(UO2)(T6+O4)2(H2O)](H2O)4 (M2+ = Mg, Mn, Fe, Co, Ni and Zn; T6+ = S, Se) compounds: The interplay between chemical composition, pH and structural architecture. CrystEngComm 2021, 23, 1140–1148. [Google Scholar] [CrossRef]
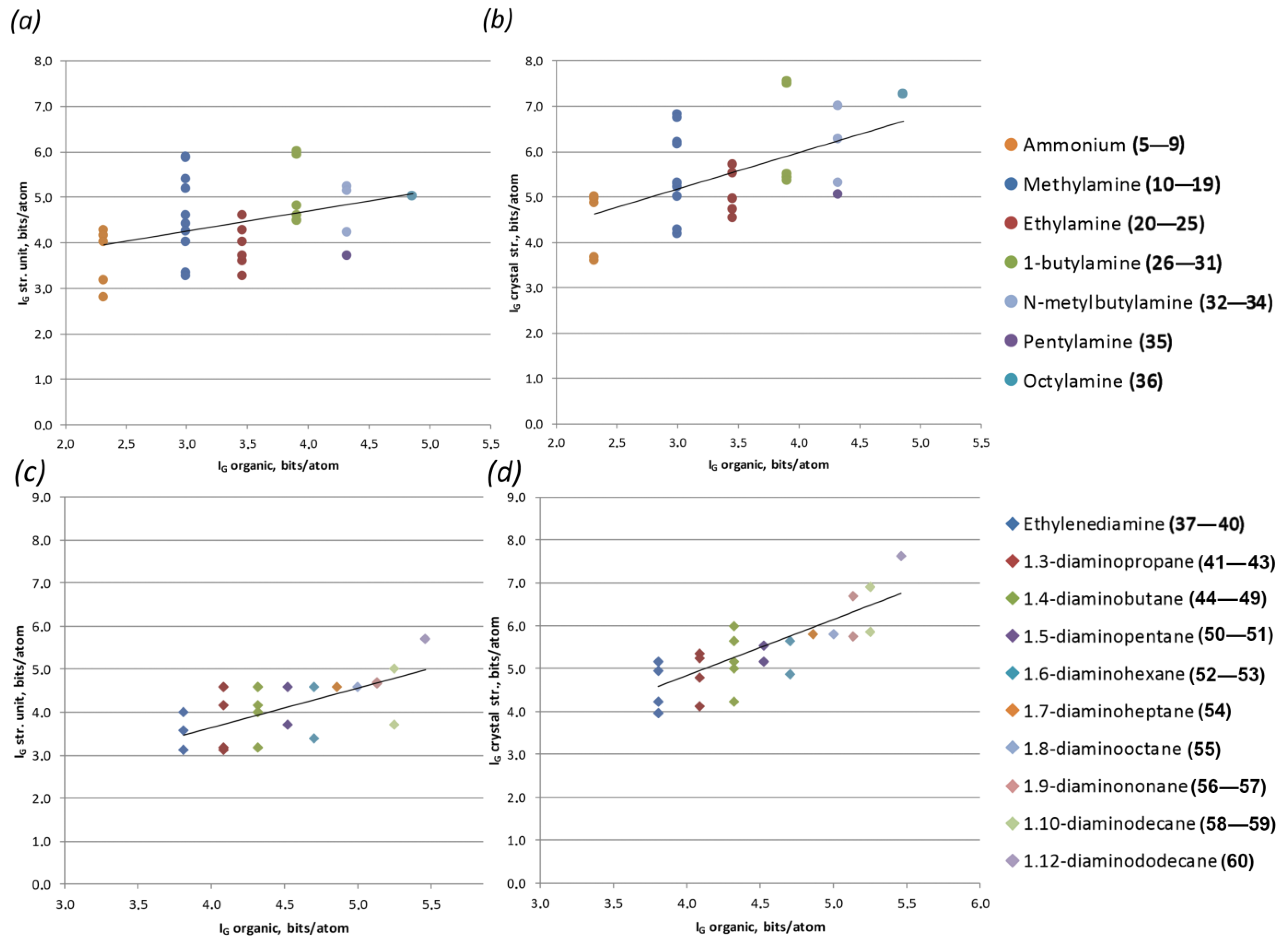
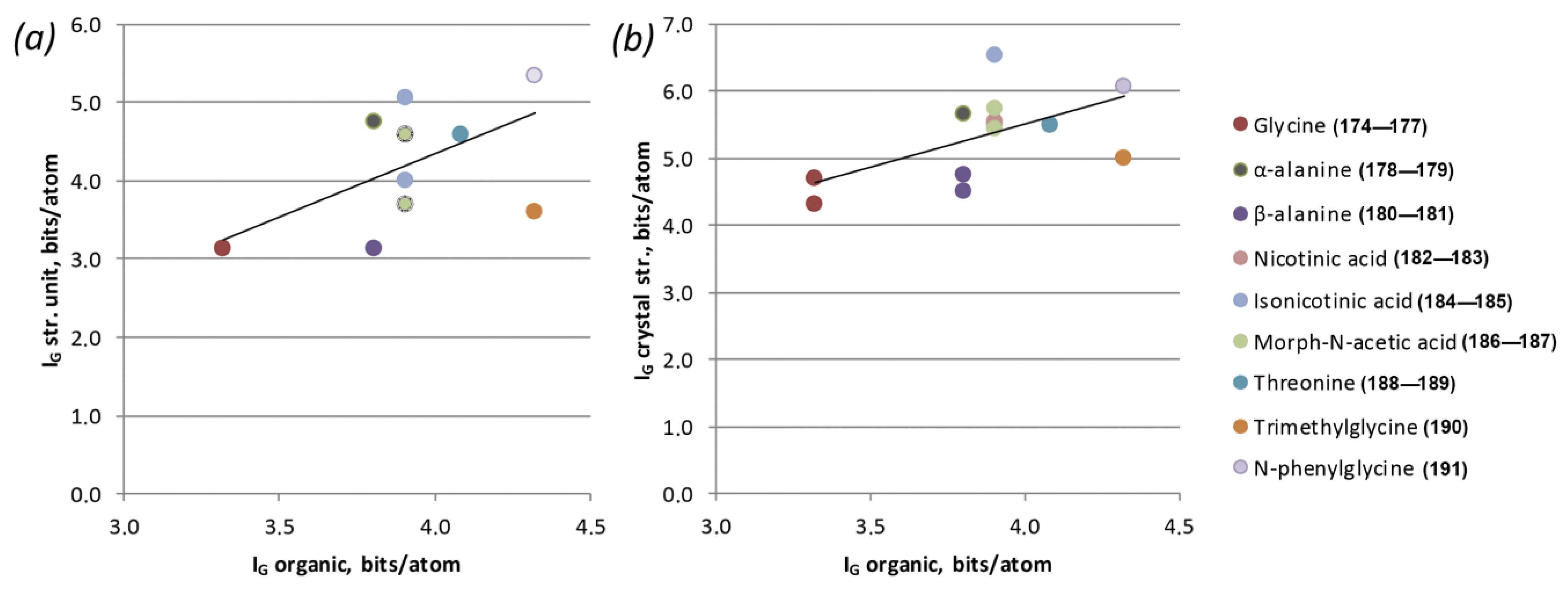

| No. | Chemical Formulae | Topology | Sp. Gr. | a, Å/α, ° | b, Å/β, ° | c, Å/γ, ° | Structural Complexity Parameters, Bits per Atom/Bits per Unit Cell | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Organic Molecule | U-Bearing Unit | Entire Structure | ||||||||
| Ammonium, NH4+ | 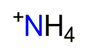 | 2.322/11.610 | ||||||||
| 5 | [NH4][(UO2)(SO4)F] | cc2–1:1–7 | Pb21a | 8.681(3)/90 | 11.319(8)/90 | 6.729(6)/90 | 3.170/114.117 | 3.585/172.078 | [1] | |
| 6 | [NH4]2[UO2(SO4)2(H2O)](H2O) | cc2–1:2–2 | P21/c | 7.783(5)/90 | 7.403(2)/102.25(4) | 20.918(9)/90 | 4.250/322.840 | 4.858/563.526 | [2] | |
| 7 | [NH4]4[(UO2)2(SO4)O2)2](H2O) | 524332 | C2/m | 8.6987(15)/90 | 14.166(2)/104.117(4) | 17.847(3)/90 | 4.150/281.950 | 4.956/564.949 | [3] | |
| 8 | [NH4]2[(UO2)2(SO4)O2)] | 524332 | Cmca | 14.2520(9)/90 | 8.7748(5)/90 | 17.1863(10)/90 | 2.780/144.420 | 3.654/336.168 | [3] | |
| 9 | [NH4]2[(UO2)(SeO4)2(H2O)](H2O)2 | cc2–1:2–3 | P212121 | 8.2036(9)/90 | 11.631(2)/90 | 14.028(2)/90 | 4.000/256.000 | 5.000/640.000 | [4] | |
| Methylamine, CH3NH3+ | 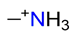 | 3.000/24.000 | ||||||||
| 10 | [CH6N]2[(UO2)2(SO4)3] | cc2–2:3–14 | P1 | 8.4784(6)/90.170(2) | 9.7873(8)/95.744(2) | 9.8121(7)/90.136(2) | 5.390/226.480 | 6.209/459.500 | [5] | |
| 11 | [CH6N][(UO2)(SO4)(OH)] | 61524232 | Pbca | 11.5951(8)/90 | 9.2848(6)/90 | 14.5565(9)/90 | 3.320/265.750 | 4.170/600.469 | [6] | |
| 12 | [CH6N]2[(UO2)(SeO4)2 (H2O)](H2O) | cc1–1:2–1 | Pnma | 7.5496(7)/90 | 12.0135(9)/90 | 15.8362(13)/90 | 3.250/208.000 | 4.272/598.100 | [7] | |
| 13 | [CH6N]2[(UO2)(SeO4)2 (H2O)] | cc2–1:2–3 | P21/c | 8.2366(10) /90 | 7.5888(6)/104.566(9) | 22.260(2)/90 | 4.000/256.000 | 5.000/640.000 | [7] | |
| 14 | [CH6N][H3O][(UO2)2 (SeO4)3(H2O)](H2O) | cc2–2:3–12 | P21/c | 8.4842(10)/90 | 10.2368(8)/102.803(9) | 24.228(2)/90 | 4.590/440.160 | 5.285/824.523 | [7] | |
| 15 | [CH6N]2[(UO2)2(SeO4)3] | cc2–2:3–14 | P21 | 8.5827(13)/90 | 10.0730(15)/95.980(12) | 10.0915(14)/90 | 4.390/184.480 | 5.209/385.500 | [7] | |
| 16 | [CH6N]4[(UO2)3(SeO4)5](H2O)4 | cc2–3:5–2 | Pnma | 16.4221(14)/90 | 18.4773(9)/90 | 10.3602(5)/90 | 4.230/608.470 | 5.311/1657.045 | [7] | |
| 17 | [CH6N][H5O2][H3O]2(UO2)3(SeO4)5] (H2O)4 | cc2–3:5–2 | Ibca | 20.956(2)/90 | 34.767(8)/90 | 18.663(2)/90 | 5.170/1488.940 | 6.150/3493.056 | [7] | |
| 18 | [CH6N]2[H3O]2[(UO2)5 (SeO4)8(H2O)](H2O)4 | cc2–5:8–2 | Pca21 | 31.505(2)/90 | 10.3688(6)/90 | 16.2424(11)/90 | 5.860/1359.050 | 6.807/3049.695 | [7] | |
| 19 | [CH6N]1.5[H5O2]1.5[H3O]3 [(UO2)5(SeO4)8(H2O)] (H2SeO4)2.6(H2O)3 | cc2–5:8–3 | Pnma | 30.9728(19)/90 | 37.022(2)/90 | 10.4171(5)/90 | 5.880/2776.610 | 6.749/5614.766 | [7] | |
| Ethylamine, C2H5NH3+ | 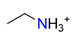 | 3.459/38.054 | ||||||||
| 20 | [C2H8N][(UO2)Cl(SO4)(H2O)] | cc2–1:1–1 | P21/c | 8.3545(17)/90 | 10.550(2)/102.64(3) | 12.370(3)/90 | 3.585/172.078 | 4.524/416.168 | [8] | |
| 21 | [C2H8N]2[(UO2)(SeO4)2(H2O)](H2O)2 | cc1–1:2–1 | Pnma | 7.6176(9)/90 | 12.1811(16)/90 | 19.258(2)/90 | 3.250/208.000 | 4.724/944.771 | [9] | |
| 22 | [C2H8N][H3O][(UO2)(SeO4)2(H2O)] | cc1–1:2–1 | P1 | 7.5635(15)/79.559(15) | 7.6188(15)/89.272(16) | 12.101(2)/82.356(16) | 4.000/128.000 | 4.954/307.160 | [9] | |
| 23 | [C2H8N]3[(UO2)(SeO4)2(HSeO4)] | cc1–1:3–2 | P21/c | 12.7463(11)/90 | 12.4261(7)/113.433(6) | 14.9928(11)/90 | 4.248/322.842 | 5.700/1185.691 | [9] | |
| 24 | [C2H8N][(UO2)(SeO4)(SeO2OH)] | cc2–1:2–4 | P21/n | 8.475(3)/90 | 12.264(2)/95.23(3) | 10.404(3)/90 | 3.700/192.423 | 4.954/614.320 | [9] | |
| 25 | [C2H8N]2[(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.2897(14)/90 | 12.349(2)/104.439(4) | 11.0379(18)/90 | 4.585/220.078 | 5.524/508.168 | [10] | |
| 1-butylamine, C4H7NH3+ |  | 3.907/58.603 | ||||||||
| 26 | [C4H10N]3[(UO2)2(SO4)3(OH)] (H2O)2 | cc2–2:3–10 | P21 | 8.439(5)/90 | 11.912(7)/102.79(10) | 10.636(6)/90 | 4.459/196.215 | 5.426/466.659 | [11] | |
| 27 | [C4H10N]8[(UO2)5(SO4)9](H2O) | framework | P212121 | 9.4586(8)/90 | 26.769(2)/90 | 32.377(3)/90 | 5.907/1417.654 | 7.500/5429.888 | [11] | |
| 28 | [C4H10N]2[(UO2)6(SO4)7(H2O)2] | framework | C2221 | 10.2776(12)/90 | 18.339(2)/90 | 22.788(3)/90 | 4.800/527.950 | 5.421/921.596 | [11] | |
| 29 | [C4H12N][H3O][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21/c | 10.7691(9)/90 | 12.5019(12)/98.172(7) | 15.4620(14)/90 | 4.585/440.156 | 5.492/988.534 | [12,13] | |
| 30 | [C4H12N][H5O2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.3908(11)/90 | 12.3602(11)/101.567(10) | 10.9150(13)/90 | 4.459/196.215 | 5.358/439.319 | [14] | |
| 31 | [C4H12N]14[(UO2)10(SeO4)17(H2O)] | cc2–3:5–2 nanotubules | I2mm | 10.8864(5)/90 | 29.532(2)/90 | 47.439(2)/90 | 5.999/1403.665 | 7.547/5268.064 | [15] | |
| N-methylbutylamine, C5H12NH2+ | 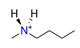 | 4.322/86.439 | ||||||||
| 32 | [C5H14N]4[(UO2)3(SeO4)4 (HSeO3)(H2O)](H2SeO3)(HSeO4) | cc2–3:5–3 | 11.7068(9)/73.899(6) | 14.8165(12)/76.221(7) | 16.9766(15)/89.861(6) | 5.209/385.500 | 7.011/1808.897 | [16] | ||
| 33 | [C5H14N]2[H3O][(UO2)3(SeO4)4(HSeO4) (H2O)] | cc2–3:5–3 | C2/c | 16.7572(13)/90 | 11.7239(12)/98.875(6) | 19.0490(13)/90 | 4.215/295.050 | 5.306/817.085 | [17] | |
| 34 | [C5H14N]2[H3O][(UO2)3 (SeO4)4(HSeO4)(H2O)](H2O) | cc2–3:5–3 | P21/n | 10.8252(10)/90 | 19.0007(10)/100.324(7) | 18.6463(15)/90 | 5.129/718.100 | 6.267/1930.170 | [17] | |
| Pentylamine, C5H11NH3+ |  | 4.322/86.439 | ||||||||
| 35 | [C5H14N][(UO2)(SeO4)(SeO2OH)] | cc2–1:2–4 | P21/n | 11.553(2)/90 | 10.6445(16)/108.045(15) | 12.138(2)/90 | 3.700/192.423 | 5.044/665.860 | [18] | |
| Octylamine, C8H17NH3+ |  | 4.858/140.881 | ||||||||
| 36 | [C8H20N]2[(UO2)(SeO4)2 (H2O)](H2O) | cc2–1:2–2 | 7.498(3)/89.69(3) | 11.897(4)/90.05(4) | 32.056(14)/88.80(3) | 5.000/320.000 | 7.267/2238.170 | [19] | ||
| Ethylenediamine, C2H4(NH3)22+ | 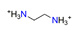 | 3.807/53.303 | ||||||||
| 37 | [C2H10N2][(UO2)(SeO4)2 (H2O)](H2O) | cc2–1:2–2 | C2/c | 11.787(2)/90 | 7.7007(10)/102.016(14) | 16.600(3)/90 | 3.125/100.000 | 4.225/304.235 | [20] | |
| 38 | [C2H10N2][(UO2)(SeO4)2 (H2O)](H2O)2 | cc2–1:2–2 | P21/c | 11.677(2)/90 | 7.908(1)/98.813(3) | 15.698(2)/90 | 4.000/256.000 | 5.170/744.469 | [10] | |
| 39 | [C2H10N2][(UO2)(SeO3) (HSeO3)](NO3)(H2O)0.5 | cc2–1:2–4 | Pbca | 13.170(3) /90 | 11.055(2)/90 | 18.009(4)/90 | 3.585/344.156 | 4.954/1228.641 | [21] | |
| 40 | [C2H4(NH3)2][UO2(SO4)2H2O] | cc1–1:2–1 | C2/c | 15.6163(4)/90 | 7.3018(2)/118.731(2) | 11.7114(3)/90 | 3.125/100.000 | 3.974/238.413 | [22] | |
| 1.3-diaminopropane, C3H6(NH3)22+ |  | 4.087/69.487 | ||||||||
| 41 | [C3H12N2][UO2(H2O)(SO4)2] | cc1–1:2–1 | P2/c | 7.2582(2)/90 | 7.3697(2)/99.4053(19) | 11.8514(3)/90 | 3.125/100.000 | 4.135/272.930 | [23] | |
| 42 | [C3H12N2][(UO2)2(H2O)(SO4)3] | cc2–2:3–4 | P21/n | 10.7391(3)/90 | 10.3791(3)/106.942(1) | 18.0265(7)/90 | 4.585/440.156 | 5.358/878.639 | [23] | |
| 43 | [N2C3H12][UO2F(SO4)]2(H2O) | cc2–1:1–10 | P21 | 6.7745(2)/90 | 8.1589(2)/94.556(1) | 14.3661(4)/90 | 4.170/150.117 | 5.248/398.842 | [24] | |
| 1.4-diaminobutane, C4H8(NH3)22+ |  | 4.322/86.439 | ||||||||
| 44 | [C4H14N2]2[UO2(SO4)3](H2O)2 | cc0–1:3–4 | 8.4584(1)/100.8158(5) | 10.2830(1)/96.3926(5) | 15.2943(2)/112.5170(5) | 4.170/150.117 | 6.000/768.000 | [22] | ||
| 45 | [C4H14N2][UO2(H2O)(SO4)2] | cc2–1:2–1 | 7.4199(2)/79.1237(9) | 7.8380(2)/79.9015(9) | 12.0319(3)/83.1098(9) | 4.000/128.000 | 5.170/372.235 | [25] | ||
| 46 | [C4H14N2][UO2F(SO4)]2 | cc2–1:1–10 | P21/c | 6.7754(5)/90 | 8.4094(8)/93.245(3) | 14.1492(14)/90 | 3.170/114.117 | 4.248/322.842 | [25] | |
| 47 | [C4H14N2][(UO2)2(SeO4)3(H2O)](H2O)2 | cc2–2:3–4 | P21/c | 11.068(3)/90 | 10.455(3)/114.555(19) | 20.266(3)/90 | 4.585/440.156 | 5.644/1128.771 | [12,13] | |
| 48 | (C4H14N2)[(UO2)2(SO4)3(H2O)]·2H2O | cc2–2:3–4 | P21/n | 10.9075(4)/90 | 10.4513(4)/97.908(2) | 17.7881(7)/90 | 4.585/440.156 | 5.644/1128.771 | [26] | |
| 49 | [C4H14N2][(UO2)(SO4)2(H2O)]·2H2O | cc2–1:2–3 | P21/n | 8.8570(4)/90 | 7.3299(3)/95.140(2) | 20.4260(9)/90 | 4.000/256.000 | 5.000/640.000 | [26] | |
| 1.5-diaminopentane, C5H10(NH3)22+ |  | 4.524/104.042 | ||||||||
| 50 | [C5H16N2][UO2(SO4)2] | cc2–1:2–21 | P21/c | 7.9825(1)/90 | 19.8458(4)/111.6563(9) | 9.7868(2)/90 | 3.700/192.423 | 5.170/744.469 | [22] | |
| 51 | [C5H16N2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.0491(11)/90 | 12.2633(16)/99.918(11) | 10.7239(16)/90 | 4.585/220.078 | 5.555/522.131 | [12,13] | |
| 1.6-diaminohexane, C6H12(NH3)22+ |  | 4.700/122.211 | ||||||||
| 52 | [C6H18N2][UO2(SO4)2]H2O | cc1–1:2–12 | P21/m | 10.1385(3)/90 | 6.9537(3)/99.287(2) | 11.7233(4)/90 | 3.393/88.211 | 4.880/478.242 | [22] | |
| 53 | [C6H18N2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.4020(18)/90 | 12.411(3)/102.951(17) | 10.923(2)/90 | 4.585/220.078 | 5.644/564.386 | [12,13] | |
| 1.7-diaminoheptane, C7H14(NH3)22+ |  | 4.858/140.881 | ||||||||
| 54 | [C7H20N2][(UO2)2(SeO4)3(H2O)](H2O) | cc2–2:3–10 | P21 | 8.7100(16)/90 | 12.4174(14)/101.348(14) | 10.8838(18)/90 | 4.585/220.078 | 5.807/650.424 | [12,13] | |
| 1.8-diaminooctane, C8H16(NH3)22+ |  | 5.000/160.000 | ||||||||
| 55 | [C8H22N2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.7793(16)/90 | 12.4874(15)/100.609(14) | 10.9331(18)/90 | 4.585/220.078 | 5.807/650.424 | [12,13] | |
| 1.9-diaminononane, C9H18(NH3)22+ |  | 5.129/179.525 | ||||||||
| 56 | [C9H24N2][(UO2)(SeO4)(SeO2OH)] (NO3) | cc2–1:2–4 | 10.7480(7) /109.960(1) | 13.8847(9)/103.212(2) | 14.6363(10)/90.409(1) | 4.700/244.423 | 6.700/1393.691 | [27] | ||
| 57 | [C9H24N2]2[(UO2)3(SeO4)5(H2O)2](H2O)x | cc2–3:5–4 | P63/mmc | 19.5572(5)/90 | 19.5572(5)/90 | 47.878(2)/120 | 4.670/2017.408 | 5.755/5190.982 | [28] | |
| 1.10-diaminodecane, C10H20(NH3)22+ | 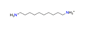 | 5.248/199.421 | ||||||||
| 58 | [C10H26N2][(UO2)(SeO4)2(H2O)] (H2SeO4)0.85(H2O)2 | cc1–1:2–1 | 7.5461(6)/77.678(6) | 14.9910(12)/85.463(6) | 22.3789(17)/82.717(6) | 5.000/320.000 | 6.895/1640.967 | [19] | ||
| 59 | [C10H26N2][(UO2)(SeO4)2] (H2SeO4)0.5(H2O) | cc2–1:2–4 | C2/c | 29.280(2)/90 | 13.3013(10)/93.295(5) | 11.4513(7)/90 | 3.700/192.423 | 5.879/1375.665 | [19] | |
| 1.12-diaminododecane, C12H24(NH3)22+ |  | 5.459/240.215 | ||||||||
| 60 | [C12H30N2]3[H3O]2[(UO2)4(SeO4)8] (H2O)5 | cc2–1:2–13 | P21/n | 11.3437(7)/90 | 24.8042(12)/96.701(5) | 29.2496(19)/90 | 5.700/1185.691 | 7.622/6006.177 | [29] | |
| Dimethylamine, C2H6NH2+ | 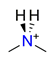 | 3.459/38.054 | ||||||||
| 61 | [C2H8N]2[(UO2)(SeO4)2(H2O)] | cc1–1:2–1 | P212121 | 7.5363(7)/90 | 12.2021(11)/90 | 16.7601(16)/90 | 4.000/256.000 | 5.248/797.685 | [30] | |
| 62 | [C2H8N]2[(UO2)2(SeO4)3(H2O)] | cc2–2:3–4 | P212121 | 11.2154(5)/90 | 11.2263(5)/90 | 16.9138(8)/90 | 4.585/440.156 | 5.524/1016.335 | [30] | |
| 63 | [C2H8N]3[H5O2][(UO2)2(SeO4)3(H2O)2]2 (H2O)5 | cc2–2:3–5 | P21/c | 12.451(5)/90 | 31.126(5)/120.39(2) | 14.197(4)/90 | 5.524/1016.335 | 6.658/2689.917 | [30] | |
| 64 | [C2H8N]2[H3O][(UO2)3 (SeO4)4(HSeO3)(H2O)](H2SeO3)0.2 | cc2–3:5–3 | P21/m | 8.3116(4)/90 | 18.6363(8)/97.582(1) | 11.5623(5)/90 | 4.264/289.947 | 5.078/619.550 | [30] | |
| 65 | [C2H8N][(H5O2)(H2O)] [(UO2)2(SeO4)3(H2SeO3)](H2O) | cc2–1:2–14 | P21/n | 14.7979(8)/90 | 10.0238(6)/111.628(1) | 16.4176(9)/90 | 4.755/513.528 | 5.672/1157.175 | [31] | |
| 66 | [C2H8N]3[C2H7N][(UO2)3(SeO4)4 (HSeO3)(H2O)] | cc2–3:5–3 | Pnma | 11.6591(11)/90 | 14.9556(17)/90 | 22.194(2)/90 | 4.472/715.508 | 5.607/1883.819 | [30] | |
| 67 | [C2H8N]3[H3O][(UO2)3(SeO4)4(SeO3) (H2O)](H2O) | cc2–3:5–3 | P21/m | 8.941(2)/90 | 19.300(4)/97.510(4) | 11.377(3)/90 | 4.329/303.050 | 5.599/996.681 | [30] | |
| Isopropylamine, C3H7NH3+ | 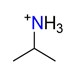 | 3.807/53.303 | ||||||||
| 1 | [C3H10N]2[(UO2)6(SO4)7(H2O)2] | framework | C2221 | 10.2560(2)/90 | 18.4062(4)/90 | 22.8900(4)/90 | 4.900/578.152 | 5.454/949.072 | This work | |
| 2 | [C3H10N]2[(UO2)2(SeO4)3(H2O)](H2O) | cc2–2:3–4 | P21/c | 11.4644(2)/90 | 11.2426(2)/99.421(2) | 18.7555(4)/90 | 4.585/440.156 | 5.781/1271.899 | This work,[12,13] | |
| 3 | [C3H10N]2[(UO2)2(SO4)3(H2O)](H2O) | cc2–2:3–4 | P21/c | 11.0470(1)/90 | 10.8926(1)/100.180(1) | 18.5397(2)/90 | 4.585/440.156 | 5.781/1271.899 | This work | |
| 4 | [C3H10N](H3O)[(UO2)2 (SeO4)3(H2O)](H2SeO3) | cc2–2:3–4 | P21/c | 11.2894(4)/90 | 11.1012(3)/94.717(3) | 18.1368(6)/90 | 4.585/440.156 | 5.585/1072.313 | This work | |
| Tert-butylamine, C4H9NH3+ | 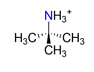 | 4.087/69.487 | ||||||||
| 68 | [C4H12N]2[(UO2)(SeO4)2(H2O)] | cc2–1:2–3 | C2/c | 27.212(10)/90 | 7.372(3)/117.75(2) | 23.113(7)/90 | 4.000/256.000 | 5.644/1128.771 | [20] | |
| 69 | [C4H12N]2[(UO2)2(SeO4)3(H2O)] | cc2–2:3–4 | P21/c | 11.3478(14)/90 | 11.3850(9)/91.865(11) | 18.959(3)/90 | 4.585/440.156 | 5.858/1359.052 | [12,13] | |
| Tetramethylammonium, C4H12N+ |  | 4.087/69.487 | ||||||||
| 70 | [C4H12N][(UO2)(SO4)(H2O)2]Cl | cc1–1:1–2 | P21 | 8.989(6)/90 | 6.877(4)/109.77(4) | 10.981(8)/90 | 3.807/106.606 | 5.000/320.000 | [32] | |
| 71 | [C4H12N][(UO2)(SO4)(NO3)] | cc1–1:2–12 | C2/m | 21.106(1)/90 | 6.9350(3)/97.5468(18) | 8.4284(5)/90 | 3.252/78.039 | 4.306/249.763 | [33] | |
| 72 | [C4H12N][(UO2)(SeO4)(NO3)] | cc1–1:2–12 | C2/m | 21.244(5)/90 | 7.1092(11)/97.693(17) | 8.6581(18)/90 | 3.252/78.039 | 4.375/280.000 | [34] | |
| 73 | [C4H12N]2[(UO2)6(SO4)7(H2O)2] | framework | C2221 | 10.3466(2)/90 | 18.5415(3)/90 | 22.7001(4)/90 | 4.800/527.950 | 5.487/976.681 | [35] | |
| Triethylamine, C6H15NH+ | 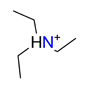 | 4.524/104.042 | ||||||||
| 74 | [C6H16N][H3O][(UO2)2(SeO4)3(H2O)] (H2O) | cc2–2:3–4 | P21 | 8.8162(16)/90 | 12.4459(15)/103.695(14) | 10.8212(19)/90 | 4.585/220.078 | 5.755/621.528 | [12,13] | |
| 75 | [C6H16N][H5O2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21 | 8.8477(3)/90 | 12.4835(5)/103.382(1) | 10.8373(4)/90 | 4.585/220.078 | 5.755/621.528 | [36] | |
| 76 | (H5O2)[C6H16N][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21/c | 10.753(1)/90 | 12.3221(8)/91.050(9) | 18.142(2)/90 | 4.585/440.156 | 5.755/1243.056 | [13] | |
| Guanidine, CH6N3+ |  | 3.322/33.219 | ||||||||
| 77 | [CH6N3]2[(UO2)(SO4)(H2O)2](NO3)2 (H2O) | cc1–1:1–2 | P21/n | 12.3824(7)/90 | 7.0329(4)/99.598(2) | 21.5362(12)/90 | 3.807/213.212 | 5.492/988.534 | [37] | |
| 78 | [CH6N3]2[(UO2)(SO4)2(H2O)](H2O)2 | cc2–1:2–2 | C2/c | 11.220(8)/90 | 8.027(4)/101.00(7) | 18.681(8)/90 | 3.125/100.000 | 4.440/372.955 | [38] | |
| 79 | [CH6N3]2[(UO2)2(SO4)3] | cc2–2:3–14 | P21212 | 9.907(3)/90 | 9.597(3)/90 | 9.762(3)/90 | 3.440/144.477 | 4.480/367.319 | [39] | |
| 80 | [CH6N3]2[(UO2)(SeO4)2(H2O)](H2O)1.5 | cc2–1:2–2 | C2/c | 37.314(4)/90 | 7.1771(6)/109.267(8) | 13.2054(14)/90 | 4.000/256.000 | 5.352/867.056 | [20] | |
| 81 | [CH6N3]3[(UO2)2(SeO4)3(HSeO4)](H2O)2 | cc2–1:2–4 | P212121 | 10.7261(9)/90 | 13.9178(16)/90 | 18.3213(17)/90 | 4.755/513.528 | 5.977/1506.275 | [20] | |
| 82 | [CH6N3]2[(UO2)2(SeO4)3] | cc2–2:3–14 | P2 | 9.9448(15)/90 | 9.727(2)/90.213(12) | 10.1508(15)/90 | 4.440/186.477 | 5.480/449.319 | [5] | |
| Aminoguanidine, CH7N4+ | 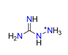 | 3.585/43.020 | ||||||||
| 83 | [CH7N4]2[(UO2)(SO4)2(H2O)] | cc2–1:2–2 | C2/c | 11.297(2)/90 | 7.8336(16)/100.18(3) | 17.984(4)/90 | 3.125/100.000 | 4.627/444.156 | [40] | |
| 1,2-diaminopropane, C3H12N22+ | 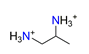 | 4.087/69.487 | ||||||||
| 84 | [C3H12N2]2[(UO2)2(SO4)4(H2O)4](H2O)2 | cc1–1:2–1 | 7.3983(2)/95.1761(12) | 7.6333(2)/94.6412(13) | 12.5946(5)/96.578(2) | 4.248/161.421 | 5.285/412.261 | [41] | ||
| 85 | [C3H12N2][UO2(H2O)(SO4)2] | cc1–1:1–1 | 7.3296(2)/92.0309(13) | 7.3702(2)/106.041(1) | 11.6822(2)/93.6783(9) | 4.000/128.000 | 5.044/332.930 | [42] | ||
| 86 | [C3H12N2][UO2F(SO4)]2·H2O | cc2–1:1–9 | Pnma | 13.5775(3)/90 | 14.6180(4)/90 | 8.1168(2)/90 | 3.170/228.235 | 4.752/912.313 | [24] | |
| 87 | [C3H12N2][(UO2)(SeO4)2(H2O)2](H2O) | cc0–1:2–3 | 7.5611(16)/94.604(18) | 7.7650(17)/94.405(17) | 12.925(3)/96.470(17) | 4.248/161.421 | 5.285/412.261 | [34] | ||
| N.N-dimethylethylene diamine, C4H14N22+ | 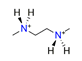 | 4.322/86.439 | ||||||||
| 88 | [C4H14N2][UO2(SO4)2] | cc2–1:2–20 | P212121 | 9.3322(1)/90 | 9.7743(2)/90 | 13.8897(3)/90 | 3.700/192.423 | 5.044/665.860 | [43] | |
| 89 | [C4H14N2][(UO2)2(H2O)(SO4)3](H2O) | cc2–2:3–4 | P21/c | 11.2460(2)/90 | 10.5387(2)/92.9884(6) | 17.0432(3)/90 | 4.585/440.156 | 5.555/1044.263 | [43] | |
| 90 | [C4H14N2][(UO2)(SeO4)2(H2O)] | cc2–1:2–8 | 6.853(2)/99.62(3) | 10.537(3)/94.45(3) | 10.574(3)/100.52(3) | 4.000/128.000 | 5.170/372.235 | [34] | ||
| 91 | [C4H14N2][(UO2)2(SeO4)3(H2O)](H2O) | cc2–2:3–4 | P21/c | 11.568(4)/90 | 10.857(4)/95.545(11) | 17.229(7)/90 | 4.585/440.156 | 5.555/1044.263 | [36] | |
| Diethylenetriamine, C4H15N33+ | 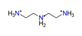 | 4.459/98.107 | ||||||||
| 92 | [C4H15N3][H3O]0.5[(UO2)2(SeO4)3 (H2O)](NO3)0.5 | cc2–2:3–4 | P21/c | 11.1679(4)/98.019(1) | 10.9040(4)/90 | 17.9913(6)/90 | 4.459/392.430 | 5.615/1100.483 | [30] | |
| 1.3-diaminopentane, C5H16N22+ | 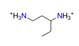 | 4.524/104.042 | ||||||||
| 93 | [C5H16N2]2[(UO2)(SeO4)2(H2O)](NO3)2 | cc1–1:2–1 | C2/c | 28.916(5)/90 | 8.0836(10)/110.909(11) | 11.9856(16)/90 | 3.125/100.000 | 5.158/722.100 | [34] | |
| N,N-Diethylethylenediamine, C6H18N22+ | 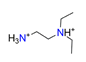 | 4.700/122.211 | ||||||||
| 94 | [C3H8N]2[(UO2)2(SeO4)3(H2O)](H2O) | cc2–2:3–4 | P21/c | 12.0301(15)/90 | 10.7845(9)/91.865(10) | 17.490(2)/90 | 4.585/440.156 | 5.728/1214.319 | [13] | |
| Tetramethylethylenediamine, C6H18N22+ | 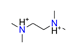 | 4.700/122.211 | ||||||||
| 95 | [C6H18N2][(UO2)2(SO4)3(H2O)] | cc2–2:3–4 | P21 | 8.4460(7)/90 | 11.966(1)/104.043(2) | 10.6635(9)/90 | 4.585/220.078 | 5.644/564.386 | [36] | |
| 1.2-ethylamino ethane, C6H18N22+ | 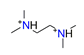 | 4.700/122.211 | ||||||||
| 96 | [C6H18N2][(UO2)2(H2O)3(SO4)3] | cc1–1:1–2 cc1–1:2–8 | 6.8234(1)/101.3691(6) | 8.7384(1)/98.1340(6) | 19.2381(4)/90.0480(11) | 4.907/294.413 | 5.807/650.424 | [42] | ||
| N,N-diethylethane-1,2-diamine, C6H18N22+ | 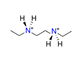 | 4.700/122.211 | ||||||||
| 97 | [C6H18N2]2[UO2F(SO4)]4·H2O | cc2–1:1–6 | 10.8832(2)/75.6604(8) | 10.9386(2)/73.6101(7) | 16.5325(3)/89.7726(7) | 5.285/412.261 | 6.508/1184.419 | [24] | ||
| N,N,N′,N′-tetramethyl-1,3-propanediamine, C7H20N22+ |  | 4.858/140.881 | ||||||||
| 98 | [C7H20N2][(UO2)2(SO4)3(H2O)] | cc2–2:3–17 | 6.7861(1)/88.6230(9) | 8.5143(1)/81.6364(8) | 19.0442(3)/84.8577(6) | 4.585/220.078 | 5.728/607.160 | [44] | ||
| N-(3-aminopropyl)-1,3-propanediamine, N3C6H203+ | 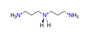 | 4.858/140.881 | ||||||||
| 99 | (N3C6H20)(H5O2)[(UO2)4(SO4)6(H2O)2]· 4H2O | cc2–2:3–4 | P21/n | 10.8576(1)/90 | 10.4120(1)/97.518(1) | 17.8726(3)/90 | 4.585/440.156 | 5.858/1359.052 | [45] | |
| 100 | (N3C6H20)[(UO2)(SO4)2(SO3OH)]·H2O | cc1–1:3–2 | 7.9164(1)/92.892(1) | 11.0632(1)/97.938(1) | 11.3354(1)/107.497(1) | 4.248/161.421 | 5.672/578.587 | [45] | ||
| Triethylenetetramine, C6H22N44+ | 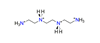 | 5.000/160.000 | ||||||||
| 101 | [C6H22N4][UO2(H2O)(SO4)2]2(H2O)6 | cc1–1:2–8 | 6.7186(5)/72.337(2) | 9.2625(7)/89.198(2) | 13.1078(9)/70.037(1) | 4.000/128.000 | 5.358/439.319 | [46] | ||
| 102 | [C6H22N4][UO2(SO4)2]2 | cc2–1:2–20 | Pbca | 9.3771(2)/90 | 12.9523(3)/90 | 18.9065(6)/90 | 3.700/384.846 | 4.858/1127.052 | [47] | |
| 103 | [C6H22N4][(UO2)(SeO4)2(H2O)](H2O) | cc2–1:2–3 | P21/n | 13.002(2)/90 | 7.962(1)/114.077(2) | 14.754(2)/90 | 4.000/256.000 | 5.129/718.100 | [10] | |
| 104 | [N4C6H22][UO2(H2O)(SO4)2]2(H2O)6 | cc1–1:2–8 | 6.7318(1)/72.3395(6) | 9.2975(1)/89.1401(7) | 13.1457(3)/70.0267(12) | 4.000/128.000 | 5.358/439.319 | [47] | ||
| Tris(2-aminoethyl)-amine, C6H21N44+ | 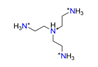 | 5.000/160.000 | ||||||||
| 105 | [C6H21N4][(UO2)(SeO4)2(HSeO4)] | cc1–1:3–2 | P21/m | 9.2218(6)/90 | 12.2768(9)/116.165(1) | 9.4464(7)/90 | 3.616/137.421 | 4.931/512.846 | [10] | |
| 106 | (N4C6H22)[(UO2)2(SO4)4(H2O)2]·3H2O | cc2–1:2–2 | P21/n | 7.4982(1)/90 | 16.9531(5)/90.729(2) | 11.4496(2)/90 | 4.000/256.000 | 5.700/1185.691 | [45] | |
| 107 | [C6H22N4]2[(UO2)2(SO4)6](H2O) | cc0–1:3–4 | 11.2315(1)/88.4073(5) | 13.2136(1)/74.5896(5) | 14.3521(2)/66.5370(6) | 5.170/372.235 | 6.687/1377.419 | [22] | ||
| 1,5,8,12-tetraazadodecane, C8H26N44+ |  | 5.248/199.421 | ||||||||
| 108 | [C8H26N4][(UO2)(SeO4)2(H2O)](H2O) | cc2–1:2–2 | P21/n | 7.8198(11)/90 | 16.516(3)/90.662(11) | 11.6831(16)/90 | 4.000/256.000 | 5.285/824.523 | [48] | |
| 109 | [C8H26N4]0.5[(UO2)2(SO4)3(H2O)](H2O)2 | cc2–2:3–12 | P21/n | 11.8400(2)/90 | 10.3190(2)/107.7718(9) | 16.5919(4)/90 | 4.585/440.156 | 5.615/1100.483 | [49] | |
| Tetraethylenepentamine, C8H28N55+ |  | 5.358/219.660 | ||||||||
| 110 | [C8H28N5]2[(UO2)5(H2O)5(SO4)10]H2O | cc2–1:2–2 | Pbnm | 7.7638(5)/90 | 14.16890(5)/90 | 56.46930(5)/90 | 5.372/1719.017 | 6.409/4229.773 | [47] | |
| Imidazole, C3H5N2+ | 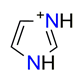 | 3.322/33.219 | ||||||||
| 111 | [C3H5N2]2[(UO2)2(SO4)3] | cc2–2:3–14 | P212121 | 9.7683(3)/90 | 10.0252(3)/90 | 19.9136(7)/90 | 4.392/368.955 | 5.358/878.639 | [42] | |
| Pyrazine, C4H5N22+ |  | 3.459/38.054 | ||||||||
| 112 | (C4H5N2)2[(UO2)(SeO4)2(H2O)] | cc2–1:2–1 | C2/c | 18.2026(8)/90 | 7.9997(3)/106.947(2) | 11.6866(5)/90 | 3.125/100.000 | 4.301/326.842 | [50] | |
| 113 | (C4H5N2)2[(UO2)2(SeO4)3(H2O)]·3H2O | cc2–2:3–11 | 8.8130(5)/108.286(2) | 11.5642(6)/94.279(2) | 13.1308(7)/105.157(2) | 4.755/256.764 | 5.781/635.950 | [50] | ||
| 114 | (H3O)(C4H5N2)2[(UO2)3(SeO4)5(H2O)]· H2O | cc2–3:5–3 | Pbcm | 11.573(3)/90 | 19.220(6)/90 | 14.465(5)/90 | 4.472/715.508 | 5.469/1465.712 | [50] | |
| Pyridine, C5H6N+ | 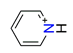 | 3.585/43.020 | ||||||||
| 115 1 | [C5H6N][(UO2)(SeO4)(HSeO3)] | cc2–1:2–4 | P21/n | 8.993(3)/90 | 13.399(5)/108.230(4) | 10.640(4)/90 | - | - | [51] | |
| 116 | [C5H6N]2[(UO2)2(SeO4)3] | cc2–2:3–14 | Pccn | 9.987(7)/90 | 10.251(7)/90 | 20.957(14)/90 | 3.440/288.955 | 4.589/789.318 | [52] | |
| 117 | (C5H6N)2[(UO2)2(SeO4)3(H2O)]∙3H2O | cc2–2:3–10 | P21/n | 10.6354(4)/90 | 12.3334(5)/103.182(1) | 18.8810(8)/90 | 4.585/440.156 | 5.755/1243.056 | [50] | |
| Azetidine, C3H8N+ | 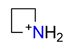 | 3.585/43.020 | ||||||||
| 118 | [C3H8N]2[(UO2)2(SeO4)3(H2O)] | cc2–2:3–4 | P212121 | 10.8620(5)/90 | 11.1105(5)/90 | 17.8815(8)/90 | 4.585/440.156 | 5.585/1072.313 | [53] | |
| 4-aminopyridine, C5H7N2+ |  | 3.907/58.603 | ||||||||
| 119 | [C5H7N2]2[(UO2)(SO4)2] | cc1–1:2–12 | 7.0126(9)/68.187(5) | 10.3352(13)/78.940(5) | 13.8027(19)/71.339(3) | 3.700/96.211 | 5.426/466.659 | [37] | ||
| Benzylamine, NC7H10+ | 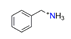 | 4.170/75.059 | ||||||||
| 120 | [NC7H10]2[(UO2)2(SO4)3]·H2O | cc2–2:3–14 | P21/n | 10.3238(2)/90 | 9.1710(2)/91.414(2) | 27.1113(7)/90 | 4.392/368.955 | 5.907/1417.654 | [45] | |
| 121 | [C7H10N]2[(UO2)(SeO4)2(H2O)](H2O) | cc2–1:2–2 | Pna21 | 24.221(2)/90 | 11.9169(11)/90 | 7.4528(7)/90 | 4.000/256.000 | 5.781/1271.899 | [10] | |
| Piperazine, C4H12N22+ | 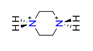 | 4.170/75.059 | ||||||||
| 122 | [C4H12N2][UO2(H2O)(SO4)2] | cc1–1:2–1 | C2/c | 14.7676(3)/90 | 7.6585(2)/104.837(2) | 11.6807(2)/90 | 3.125/100.000 | 4.146/281.947 | [54] | |
| 123 | [C4H12N2][(UO2)(SeO4)2 (H2O)] | cc1–1:2–1 | C2/c | 15.7651(10)/90 | 7.4093(5)/101.121(2) | 11.9639(8)/90 | 3.125/100.000 | 4.146/281.947 | [50] | |
| 124 | [C4H12N2]0.5[(UO2)(HSeO3)(SeO3)] | cc2–1:2–20 | P21/c | 10.9378(5)/90 | 8.6903(4)/90.3040(8) | 9.9913(5)/90 | 3.585/172.078 | 4.392/368.955 | [55] | |
| 1-methylpiperazine, C5H14N22+ | 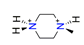 | 4.392/92.239 | ||||||||
| 125 | [C5H14N2][UO2(H2O)(SO4)2] | cc1–1:2–1 | 8.0031(2)/72.704(1) | 8.1873(2)/81.7766(11) | 10.8911(3)/78.7917(9) | 4.000/128.000 | 5.209/385.500 | [56] | ||
| 2-methylpiperazine, C5H14N22+ |  | 4.392/92.239 | ||||||||
| 126 | [C5H14N2][UO2(H2O)(SO4)2] | cc1–1:2–4 | 10.7537(2)/87.998(1) | 11.4297(2)/79.660(1) | 11.5797(2)/80.6313(6) | 5.000/320.000 | 6.209/918.999 | [54] | ||
| 127 | [C5H14N2][UO2F(H2O)(SO4)]2 | cc2–1:1–7 | P21/n | 8.4354(2)/90 | 15.5581(4)/96.666(1) | 14.8442(6)/90 | 4.585/440.156 | 5.585/1072.313 | [24] | |
| Homopiperazine, C5H14N22+ |  | 4.392/92.239 | ||||||||
| 128 | [C5H14N2]2[UO2(SO4)3] | cc0–1:3–2 | C2/c | 14.4975(3)/90 | 11.9109(3)/110.475(1) | 13.0157(3)/90 | 3.281/118.117 | 4.940/592.827 | [43] | |
| 129 | [C5H14N2][UO2(H2O)(SO4)2] | cc1–1:1–2 | P22121 | 7.6955(2)/90 | 11.7717(3)/90 | 14.7038(4)/90 | 4.125/264.000 | 4.125/264.000 | [43] | |
| 1.4-diaminocyclohexane, C6H16N22+ | 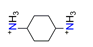 | 4.585/110.039 | ||||||||
| 130 | [N2C6H16][UO2F2(SO4)] | cc1–1:1–13 | 6.9105(2)/72.659(1) | 9.6605(2)/87.068(1) | 10.1033(2)/77.957(1) | 3.322/66.439 | 5.087/345.947 | [24] | ||
| 131 | [C6H16N2][UO2F2(SO4)] | cc2–1:1–14 | Pmmn | 6.9503(1)/90 | 17.2147(4)/90 | 7.0867(1)/90 | 2.948/106.117 | 4.309/534.320 | [24] | |
| 132 | [C6H16N2][UO2(SO4)2]·2H2O | cc1–1:2–12 | 6.7813(1)/76.7537(7) | 10.0636(2)/75.6074(7) | 12.9753(3)/74.3971(13) | 3.700/96.211 | 5.426/466.659 | [57] | ||
| Azetidinopropaneamine, C6H16N2+ | 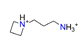 | 4.585/110.039 | ||||||||
| 133 | [C6H16N2][(UO2)2(SeO4)3(H2O)](H2O) | cc2–2:3–4 | P21/c | 11.3575(5)/90 | 11.021(5)/90.608(1) | 17.8038(8)/90 | 4.585/440.156 | 5.728/1214.319 | [53] | |
| 134 | [C3H8N]2(H5O2)[(UO2)2(SO4)3(HSO4)] | cc2–1:2–13 | P21/n | 8.677(3)/90 | 10.294(3)/97.521(7) | 26.474(8)/90 | 4.755/513.528 | 5.858/1359.052 | [53] | |
| 1-ethyl-3-methyl imidazolium, C6H11N2+ | 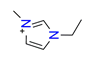 | 4.248/80.711 | ||||||||
| 135 1 | [C6H11N2]2[(UO2)(SO4)2] | cc1–1:2–12 | C2/c | 31.90(1)/90 | 9.383(5)/93.999(7) | 13.770(7)/90 | - | - | [58] | |
| 136 | [C6N2H11](Na)[(UO2)4(SO4)2(OH)2(O)2]· 3(H2O) | 5 2 4 3 3 2 | P21/c | 17.182(5)/90 | 8.852(3)/100.693(4) | 17.162(5)/90 | 4.755/513.528 | 5.803/1288.360 | [59] | |
| 137 | [C6N2H11](H9O4)[(UO2)(SO4)2] | cc1–1:2–12 | 6.9504(11)/95.993(2) | 9.9247(15)/95.024(2) | 14.966(2)/103.323(2) | 3.700/96.211 | 5.931/723.550 | [59] | ||
| 138 | [C6N2H11]2[(UO2)2(SO4)3(H2O)] | cc2–2:3–22 | 9.5715(11)/81.803(1) | 10.4399(12)/81.394(1) | 13.7023(16)/86.480(1) | 4.585/220.078 | 5.954/738.320 | [59] | ||
| 139 | [C6N2H11]2[(UO2)2(SO4)3(H2O)2]·2(H2O) | cc1–2:3–3 | P21/n | 12.952(2)/90 | 19.302(3)/116.891(2) | 13.224(2)/90 | 4.755/513.528 | 6.150/1746.528 | [59] | |
| 140 | [C6N2H11][(UO2)2(SO4)(OH)(O)] | 5 2 4 3 3 2 | 8.859(2)/107.671(3) | 8.926(2)/97.350(3) | 9.893(3)/104.502(3) | 3.807/106.606 | 5.044/332.930 | [59] | ||
| 1-(3-aminopropyl) imidazole, N3C6H13+ | 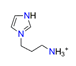 | 4.459/98.107 | ||||||||
| 141 | [N3C6H13][(UO2)(SO4)2] | cc1–1:2–12 | 6.8164(1)/76.749(1) | 7.6357(1)/88.091(1) | 14.1979(2)/86.533(1) | 3.700/96.211 | 5.129/359.050 | [45] | ||
| 1-butyl-3-methylimidazole, C8H15N2+ | 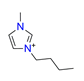 | 4.644/116.096 | ||||||||
| 142 | [C8H15N2]2[(UO2)4(SeO3)5] | 6 1 5 2 4 2 3 2 | Pnma | 18.860(2)/90 | 18.010(2)/90 | 11.140(1)/90 | 4.250/544.000 | 5.455/1789.277 | [52] | |
| 2-piperazinoethylamine, C6H18N33+ | 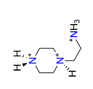 | 4.755/128.382 | ||||||||
| 143 | [C6H18N3][(UO2)2(H2O)(SO4)3(HSO4)] (H2O)4.5 | cc2–1:2–12 | P21/a | 15.7673(4)/90 | 10.5813(3)/99.9216(9) | 16.7710(5)/90 | 4.907/588.827 | 6.129/1716.199 | [60] | |
| 144 | [C6H18N3]2[(UO2)5(H2O)(SO4)8](H2O)5 | cc2–5:8–2 | P21/n | 21.5597(3)/90 | 10.2901(2)/96.7436(7) | 22.8403(3)/90 | 5.858/1359.052 | 6.989/3550.252 | [60] | |
| 1,4-bis(3-aminopropyl)piperazine, C10H28N44+ | 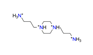 | 5.392/226.477 | ||||||||
| 145 | (N4C10H28)0.5[(UO2)(SO4)2(H2O)]·H2O | cc2–1:2–2 | P21/n | 7.5484(2)/90 | 16.9859(4)/90.580(2) | 11.4581(3)/90 | 4.000/256.000 | 5.322/851.508 | [45] | |
| 146 | [C10H28N4][(UO2)2(SO4)4] | cc2–1:2–20 | Pbca | 9.5831(2)/90 | 15.6060(3)/90 | 18.1212(3)/90 | 3.700/384.846 | 5.087/1383.790 | [61] | |
| 1,2,3-benzotriazole, C6H6N3+ |  | 3.907/58.603 | ||||||||
| 147 | [C6H6N3][H5O2][(UO2)2(SeO4)3(H2O)] | cc2–2:3–10 | P21/c | 12.167(3)/90 | 12.316(3)/108.270(4) | 14.909(3)/90 | 4.585/440.156 | 5.392/905.909 | [36] | |
| 148 | [C6H6N3][H7O3][(UO2)2(SO4)3(H2O)] (H2O) | cc2–2:3–10 | C2 | 19.678(7)/90 | 10.600(4)/95.979(7) | 10.925(4)/90 | 4.585/220.078 | 5.720/594.846 | [36] | |
| Melamine, C3H8N62+ |  | 4.087/69.487 | ||||||||
| 149 | [C3H8N6][(UO2)2(SO4)3(H2O)](H2O) | cc2–2:3–4 | P21/n | 11.1194(4)/90 | 10.5921(3)/101.405(2) | 17.0143(6)/90 | 4.585/440.156 | 5.459/960.860 | [62] | |
| 150 | [(C3H8N6)(SeO4)] [(UO2)(SeO4) (H2SeO3)2] | cc2–1:3–6 | P21/c | 16.247(4)/90 | 8.680(2)/90.615(5) | 13.347(3)/90 | 4.644/464.386 | 5.392/905.909 | [63] | |
| 4.4′-Bipyridine, C10H10N22+ | 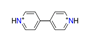 | 4.459/98.107 | ||||||||
| 151 | [C10H10N2][UO2(SO4)2]H2O | cc1–1:2–12 | 6.9507(1)/79.1992(7) | 7.7097(1)/80.1403(8) | 15.9200(4)/80.9717(14) | 3.700/96.211 | 5.248/398.842 | [42] | ||
| Terpyridine, C15H14N33+ | 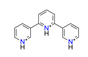 | 5.000/160.000 | ||||||||
| 152 | [C15H14N3][(UO2)(SO4)2](NO3)(H2O)2 | cc1–1:2–12 | 6.9732(7)/111.809(2) | 13.569(1)/102.386(2) | 13.641(1)/93.833(2) | 3.700/96.211 | 5.781/635.950 | [46] | ||
| 1.4-diazabicyclo(2.2.2)octane, C6H14N22+ | 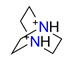 | 4.459/98.107 | ||||||||
| 153 | [C6H14N2][UO2(H2O)(SO4)2] | cc2–1:2–3 | P21/n | 8.6480(1)/90 | 7.7135(1)/90.7254(9) | 21.2554(3)/90 | 4.000/256.000 | 5.248/797.685 | [54] | |
| 3-Aminotropane, C8H18N22+ | 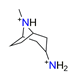 | 4.807/134.606 | ||||||||
| 154 | [C8H18N2](H5O2)2[(UO2)3(SeO4)5(H2O)] (H2O) | cc2–3:5–5 | P21/n | 10.210(2)/90 | 19.151(4)/98.959(3) | 17.819(3)/90 | 5.209/770.999 | 6.340/2054.111 | [64] | |
| 155 | [C8H18N2](H5O2)2[(UO2)3(SO4)5(H2O)] (H2O) | cc2–3:5–5 | P21/n | 10.147(3)/90 | 18.726(6)/99.043(7) | 17.076(5)/90 | 5.209/770.999 | 6.322/2023.017 | [64] | |
| Cyclen, C8H24N44+ | 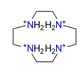 | 5.170/186.117 | ||||||||
| 156 | [C8H24N4][(UO2)3(SO4)5] (H2O)3 | cc2–3:5–2 | Pna21 | 16.8623(10)/90 | 18.0113(11)/90 | 10.1928(6)/90 | 5.087/691.895 | 6.304/1991.995 | [64] | |
| 157 | (C8H24N4)(H3O)2[(UO2)4(SeO4)7(H2O)] (H2O)6.75 | cc2–4:7–3 | 8.7587(14)/73.807(3) | 13.067(2)/88.980(4) | 23.009(4)/86.129(3) | 5.644/564.386 | 6.977/1758.275 | [64] | ||
| 12-crown-4 ether, C8H16O4 | 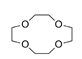 | 4.807/134.606 | ||||||||
| 158 | [C8H16O4]0.5[UO2(SO4)(H2O)](H2O) | cc1–1:1–2 | 7.007(1)/91.31(1) | 8.0408(6)/93.60(2) | 10.776(2)/100.18(1) | 3.585/86.039 | 4.858/281.763 | [65] | ||
| 159 | [C8H16O4]2[(H5O2)3(H9O4)] [(UO2)2(SeO4)3(H2O)]2 | cc2–2:3–10 | P21/c | 10.7328(6)/90 | 12.2828(5)/110.102(5) | 22.7085(17)/90 | 4.585/440.156 | 6.087/1655.790 | [66,67] | |
| 15-crown-5-ether, C10H20O5 | 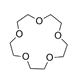 | 5.129/179.525 | ||||||||
| 160 | [K@(C10H20O5)][(UO2)(SeO4)(HSeO4) (H2O)] | cc1–1:2–1 | Pnma | 15.386(3)/90 | 10.771(2)/90 | 13.239(3)/90 | 3.382/229.947 | 4.860/1030.319 | [68] | |
| 161 | [(H5O2)(H3O)3](C10H20O5)[(UO2)3 (SeO4)5(H2O)] | cc2–3:5–3 | P21/m | 11.6754(5)/90 | 18.9887(10)/112.282(3) | 12.2047(5)/90 | 4.399/325.500 | 6.064/1491.859 | [66,67] | |
| 162 | [(H5O2)x(H3O)4-x](C10H20O5) [(UO2)3(SeO4)5(H2O)](H2O)y | cc2–3:5–3 | C2/c | 24.2575(15)/90 | 11.7501(7)/101.996(1) | 18.9243(12)/90 | 4.362/340.261 | 6.012/1527.126 | [66,67] | |
| 18-crown-6 ether, C12H24O6 |  | 5.392/226.477 | ||||||||
| 163 | [C12H24O6]0.5[(UO2)(SO4)(H2O)3] | cc1–1:1–1 | P21/n | 9.314(5)/90 | 9.339(3)/103.62(3) | 16.734(3)/90 | 4.087/277.947 | 5.248/797.685 | [65] | |
| 164 | [(H3O)@(C12H24O6)]2(H3O)8 [(UO2)14(SO4)19(H2O)4](H2O)20.5 | framework | I4/m | 28.023(1)/90 | 28.023(1)/90 | 19.6840(7)/90 | 5.313/1583.312 | 6.531/4375.972 | [69] | |
| 165 | [K@(C12H24O6)][(UO2)(SeO4)(NO3)] (H2O) | cc1–1:2–12 | P21/c | 7.2402(2)/90 | 21.2024(7)/91.581(1) | 15.7322(5)/90 | 3.585/172.078 | 5.858/1359.052 | [70] | |
| 166 | [(H3O)@(C12H24O6)]K[H3O]2 [(UO2)3(SeO4)5](H2O)4 | cc2–3:5–2 nanotubules | Ccmm | 11.292(1)/90 | 37.158(1)/90 | 38.504(1)/90 | 5.264/1431.790 | 6.622/4754.269 | [69] | |
| Benzo-15-crown-5 ether, C14H20O5 | 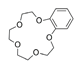 | 5.285/206.131 | ||||||||
| 167 | [C14H20O5]0.5[(UO2)(SO4)(H2O)2](H2O) | cc1–1:1–2 | 6.908(2)/79.46(2) | 8.717(4)/75.28(2) | 13.578(2)/89.98(3) | 3.807/106.606 | 5.524/508.168 | [65] | ||
| Thiourea, CN2H4S | 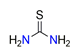 | 3.000/24.000 | ||||||||
| 168 | [CN2H4S]2[UO2(SO4)2]·0.3H2O | cc1–1:2–12 | P212121 | 6.9283(1)/90 | 13.3983(3)/90 | 15.2250(3)/90 | 3.700/192.423 | 5.044/665.860 | [71] | |
| Chloroacetamide, ClCH2CONH2 | 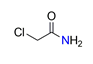 | 3.322/33.219 | ||||||||
| 169 | (C2H4NCOCl)[UO2(SO4)(H2O)2] | cc1–1:1–2 | 6.892(3)/104.40(3) | 8.786(6)/109.71(3) | 9.494(6)/90.33(3) | 3.807/106.606 | 4.524/208.084 | [72] | ||
| Choline, C5H12NO+ | 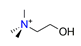 | 4.248/80.711 | ||||||||
| 170 | [C5H12NO][(UO2)(SeO4)Cl(H2O)] | cc2–1:1–1 | P21/n | 10.745(4)/90 | 11.236(4)/114.580(5) | 12.477(4)/90 | 3.585/172.078 | 5.044/665.860 | [73] | |
| 3-hydroxypiperidine, C5H7NO+ | 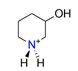 | 3.807/53.303 | ||||||||
| 171 | [(C5H7NO)2(H2O)][(UO2)2(SeO4)3 (H2O)2](H2O) | cc2–2:3–11 | 9.4248(7)/85.456(1) | 11.2711(8)/79.571(1) | 13.1059(10)/73.439(1) | 4.585/220.078 | 5.781/635.950 | [36] | ||
| Carbamoylguanidine, C2N4H7O22+ | 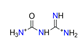 | 3.907/58.603 | ||||||||
| 172 | [C2N4H7O][(UO2)(SO4)(OH)](H2O)0.5 | 6 1 5 2 4 2 3 2 | P21/c | 10.5135(7)/90 | 11.3744(7)/110.880(2) | 9.2731(5)/90 | 3.170/114.117 | 4.747/503.160 | [74] | |
| 1-(hydroxyethyl)-5-nitroimidazole (Metronidazole), C6H10N3O3+ | 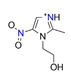 | 4.459/98.107 | ||||||||
| 173 | [(C6H10N3O3)(H5O2)2(H2O)][(H5O2)3 (H2O)][(UO2)5(SO4)8(H2O)] | cc2–5:8–2 | P2/c | 18.1693(17)/90 | 10.0732(10)/103.427(2) | 30.098(3)/90 | 5.858/1359.052 | 6.858/3182.103 | [75] | |
| Glycine, C₂H₅NO₂+ |  | 3.322/33.219 | ||||||||
| 174 | [(glyH2+)(H2O)]2[(UO2)(SO4)2(H2O)] | cc2–1:2–2 | C2/c | 11.5914(5)/90 | 7.3412(3)/103.993(2) | 23.5958(9)/90 | 3.125/100.000 | 4.684/468.386 | [76] | |
| 175 2 | [(glyH+)(H2O)]2[(UO2)(SeO4)2(H2O)] | cc2–1:2–2 | C2/c | 11.5854(5)/90 | 7.3322(3)/103.623(2) | 23.5768(9)/90 | 3.125/100.000 | 4.684/468.386 | [76] | |
| 176 | (glyH+)2[(UO2)(SeO4)2(H2O) | cc2–1:2–2 | P2/c | 7.646(2)/90 | 9.496(3)/104.832(6) | 11.477(3)/90 | 3.125/100.000 | 4.301/326.842 | [76] | |
| 177 3 | (glyH+)2[(UO2)(SO4)2(H2O)] | cc2–1:2–2 | P2/c | 7.690(2)/90 | 9.505(3)/104.805(6) | 11.433(3)/90 | 3.125/100.000 | 4.301/326.842 | [76] | |
| α-alanine, C3H8NO2+ | 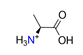 | 3.807/53.303 | ||||||||
| 178 | (α-AlaH+)(H5O2)(H2O)3[(UO2)2(SO4)3 (H2O)2] | cc2–2:3–5 | P21/c | 11.000(2)/90 | 15.402(3)/91.320(6) | 13.688(3)/90 | 4.755/513.528 | 5.644/1128.771 | [76] | |
| 179 4 | (α-AlaH+)(H5O2)(H2O)3[(UO2)2(SeO4)3 (H2O)2] | cc2–2:3–5 | P21/c | 11.150(3)/90 | 15.510(2)/92.00(2) | 13.500(5)/90 | 4.755/513.528 | 5.644/1128.771 | [76] | |
| β-alanine, C3H8NO2+ | 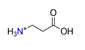 | 3.807/53.303 | ||||||||
| 180 | (β-AlaH+)2[(UO2)(SO4)2(H2O)] | cc1–1:2–1 | C2/c | 20.660(3)/90 | 7.3138(11)/91.934(5) | 11.8449(17)/90 | 3.125/100.000 | 4.739/492.846 | [76] | |
| 181 | (β-AlaH+)2[(UO2)(SeO4)2(H2O)] | cc1–1:2–1 | C2/c | 20.909(2)/90 | 7.4754(8)/92.589(2) | 12.1693(13)/90 | 3.125/100.000 | 4.505/396.430 | [76] | |
| Nicotinic acid, C6H6NO2+ | 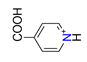 | 3.907/58.603 | ||||||||
| 182 | [(nicH+)(H5O2)(H2O)][(UO2)(SO4)2 (H2O)] | cc2–2:3–10 | P21/n | 12.4322(9)/90 | 11.9693(9)/106.574(2) | 14.5768(11)/90 | 4.585/440.156 | 5.487/976.681 | [76] | |
| 183 | [(nicH+)(H5O2)(H2O)][(UO2)(SeO4)2 (H2O)] | cc2–2:3–10 | P21/n | 12.616(2)/90 | 12.329(3)/107.221(5) | 14.819(3)/90 | 4.585/440.156 | 5.550/1032.284 | [76] | |
| Isonicotinic acid, C6H6NO2+ | 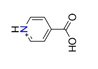 | 3.907/58.603 | ||||||||
| 184 | (IsonicH+)2[(UO2)(SO4)2(H2O)] | cc1–1:2–1 | 8.5774(9)/97.034(2) | 11.2800(12)/105.214(2) | 11.4608(12)/106.737(2) | 4.000/128.000 | 5.524/508.168 | [76] | ||
| 185 | (IsonicH+)2[(UO2)(SeO4)2(H2O)] | cc1–1:2–1 | 8.629(2)/98.22(5) | 11.588(3)/105.180(4) | 11.588(3)/105.180(4) | 5.044/166.465 | 6.524/600.168 | [76] | ||
| Protonated morpholino-N-acetic acid, C6H6O3+ | 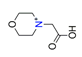 | 3.907/58.603 | ||||||||
| 186 | Na(C6H6O3)[(UO2)2(SeO4)3(H2O)](H2O)2 | cc2–2:3–10 | P21/c | 10.7767(5)/90 | 12.2679(5)/92.126(1) | 17.9043(8)/90 | 4.585/440.156 | 5.728/1214.319 | [77] | |
| 187 | Na2(SO3OH)(C6H6O3)[(UO2)(SO4)2] | cc1–1:2–12 | 6.860(3)/85.186(6) | 10.546(4)/88.017(5) | 13.047(5)/79.752(5) | 3.700/96.211 | 5.426/466.659 | [77] | ||
| Threonine, C4H9NO3+ | 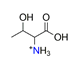 | 4.087/69.487 | ||||||||
| 188 | [(TrhH+)(H2O)]2[(UO2)2(SO4)3(H2O)] | cc2–2:3–4 | P212121 | 10.5155(6)/90 | 10.516(1)/90 | 17.3804(12)/90 | 4.585/440.156 | 5.492/988.534 | [76] | |
| 189 5 | [(TrhH+)(H2O)]2[(UO2)2(SeO4)3(H2O)] | cc2–2:3–4 | P212121 | 10.5602(6)/90 | 10.485(5)/90 | 17.5804(2)/90 | 4.585/440.156 | 5.492/988.534 | [76] | |
| Trimethylglycine, C5H12NO2+ | 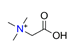 | 4.322/86.439 | ||||||||
| 190 | [C5H12NO2][UO2(Cl)(SO4)(H2O)] | cc2–1:1–1 | P21/n | 9.0486(7)/90 | 12.5735(9)/111.4560(7) | 12.3064(9)/90 | 3.585/172.078 | 5.000/640.000 | [78] | |
| Protonated N-phenylglycine, C8H9NO2+ | 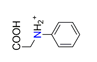 | 4.322/86.439 | ||||||||
| 191 | Na(C6H5CH(NH2)CO2)7[(UO2)6(SO4)10] (H2O)3.5 | cc2–3:5–2 nanotubules | R3m | 44.001(10)/90 | 44.001(10)/90 | 10.367(2)/90 | 5.329/1119.149 | 6.062/2218.650 | [79] | |
| 1-methyl-3-carboxy methylimidazolium, C6H10N2O2+ |  | 4.322/86.439 | ||||||||
| 192 | (C7H15N2O2)(H3O)[(UO2)2(SO4)3(H2O)]· 1.5H2O | cc2–2:3–4 | P21/n | 10.7858(6)/90 | 10.7092(6)/98.493(1) | 19.776(1)/90 | 4.585/440.156 | 5.755/1243.056 | [80] | |
| N-(3-aminopropyl)-2-pyrrolidinone, C7H14N2O+ | 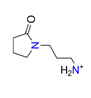 | 4.585/110.039 | ||||||||
| 193 | (N2C6H17COOH)[(UO2)2(SO4)3(H2O)] | cc2–2:3–4 | P21/c | 11.4656(3)/90 | 10.6562(2)/99.604(3) | 17.7267(5)/90 | 4.585/440.156 | 5.728/1214.319 | [45] | |
| N,N′-bis(3,5-dicarboxylatophenyl)-4,4′-bipyridinium dihydrate, C26H16N2O82+ | 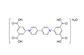 | 5.700/296.423 | ||||||||
| 194 | (C26H16N2O8)0.5[(UO2)(SO4)(H2O)2]·H2O | cc1–1:1–2 | C2/c | 6.8993(14)/90 | 18.396(4)/93.191(7) | 27.847(5)/90 | 3.807/213.212 | 5.426/933.318 | [81] | |
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Crystallographic Data | ||||
| Space Group | C2221 | P21/c | P21/c | P21/c |
| a [Å] | 10.2560(2) | 11.4644(2) | 11.0470(1) | 11.2894(4) |
| b [Å] | 18.4062(4) | 11.24259(17) | 10.8926(1) | 11.1012(3) |
| c [Å] | 22.8900(4) | 18.7555(4) | 18.5397(2) | 18.1368(6) |
| β [°] | 90 | 99.421(2) | 100.180(1) | 94.717(3) |
| V [Å3] | 4321.03(15) | 2384.77(8) | 2195.77(4) | 2265.30(12) |
| Z | 4 | 4 | 4 | 4 |
| Data Collection Parameters | ||||
| Angle range 2θ [o] | 6.94–55.00 | 7.12–52.00 | 6.49–55.00 | 6.65–55.00 |
| Total reflections | 21,967 | 28,650 | 71,790 | 18,562 |
| Unique reflections | 4968 | 4656 | 5027 | 5195 |
| Reflections with F2 > 2σ(F2) | 4715 | 4326 | 4773 | 4616 |
| Rint, Rσ [%] | 4.19, 3.63 | 4.14, 2.93 | 7.86, 2.72 | 2.77, 2.93 |
| Refinement Parameters | ||||
| R1 (F2 > 2σ(F2)), wR2 (F2 > 2σ(F2)) [%] | 2.88, 6.61 | 2.29, 4.99 | 1.86, 4.48 | 2.44, 4.69 |
| R1 and wR2 (all data) [%] | 3.12, 6.69 | 2.61, 5.09 | 2.02, 4.53 | 3.11, 4.86 |
| S | 1.052 | 1.068 | 1.048 | 1.024 |
| ρmax, ρmin [e− Å−3] | 2.008/−1.932 | 1.940/−1.026 | 1.477/−1.733 | 1.453/−0.883 |
| CCDC | 2,285,071 | 2,285,072 | 2,285,073 | 2,285,074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durova, E.V.; Kuporev, I.V.; Gurzhiy, V.V. Organically Templated Uranyl Sulfates and Selenates: Structural Complexity and Crystal Chemical Restrictions for Isotypic Compounds Formation. Int. J. Mol. Sci. 2023, 24, 13020. https://doi.org/10.3390/ijms241613020
Durova EV, Kuporev IV, Gurzhiy VV. Organically Templated Uranyl Sulfates and Selenates: Structural Complexity and Crystal Chemical Restrictions for Isotypic Compounds Formation. International Journal of Molecular Sciences. 2023; 24(16):13020. https://doi.org/10.3390/ijms241613020
Chicago/Turabian StyleDurova, Elizaveta V., Ivan V. Kuporev, and Vladislav V. Gurzhiy. 2023. "Organically Templated Uranyl Sulfates and Selenates: Structural Complexity and Crystal Chemical Restrictions for Isotypic Compounds Formation" International Journal of Molecular Sciences 24, no. 16: 13020. https://doi.org/10.3390/ijms241613020
APA StyleDurova, E. V., Kuporev, I. V., & Gurzhiy, V. V. (2023). Organically Templated Uranyl Sulfates and Selenates: Structural Complexity and Crystal Chemical Restrictions for Isotypic Compounds Formation. International Journal of Molecular Sciences, 24(16), 13020. https://doi.org/10.3390/ijms241613020







