Complete Chloroplast Genomes and Phylogenetic Relationships of Bougainvillea spectabilis and Bougainvillea glabra (Nyctaginaceae)
Abstract
:1. Introduction
2. Results
2.1. Basic Characteristics and Comparative Analysis of the cp Genomes
2.2. Gene Composition of the cp Genomes
2.3. Codon Usage
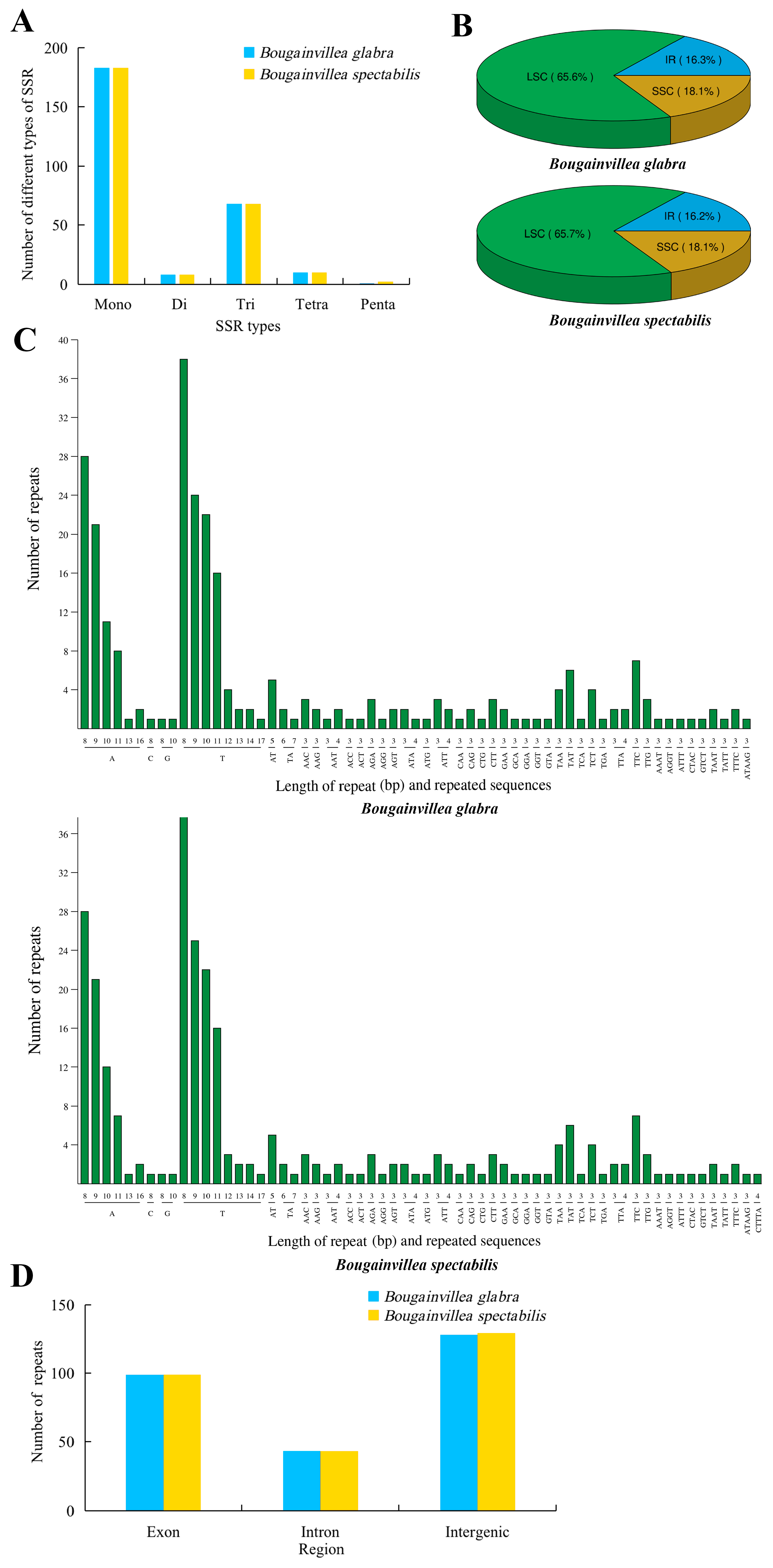
2.4. Simple Sequence Repeat Analysis
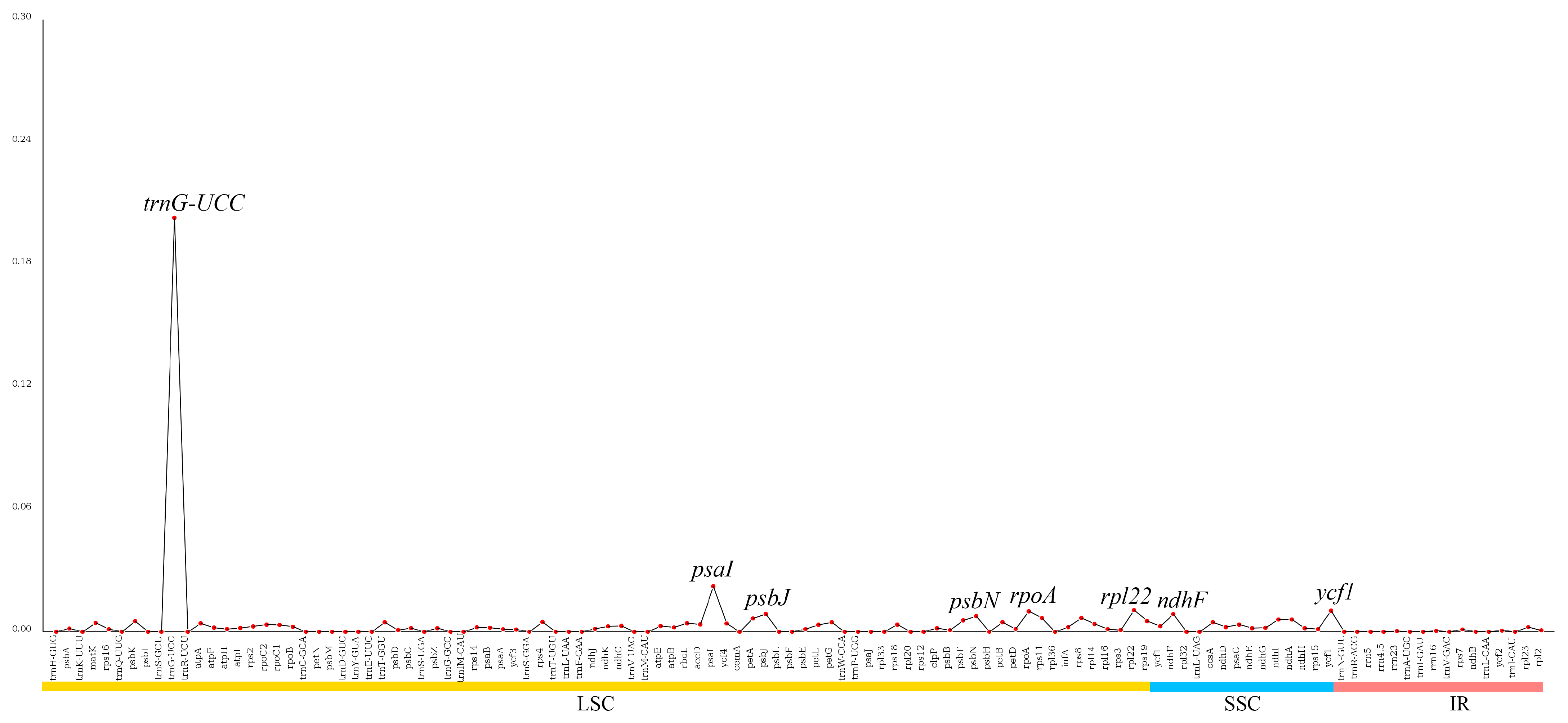
2.5. Nucleotide Diversity of Genes
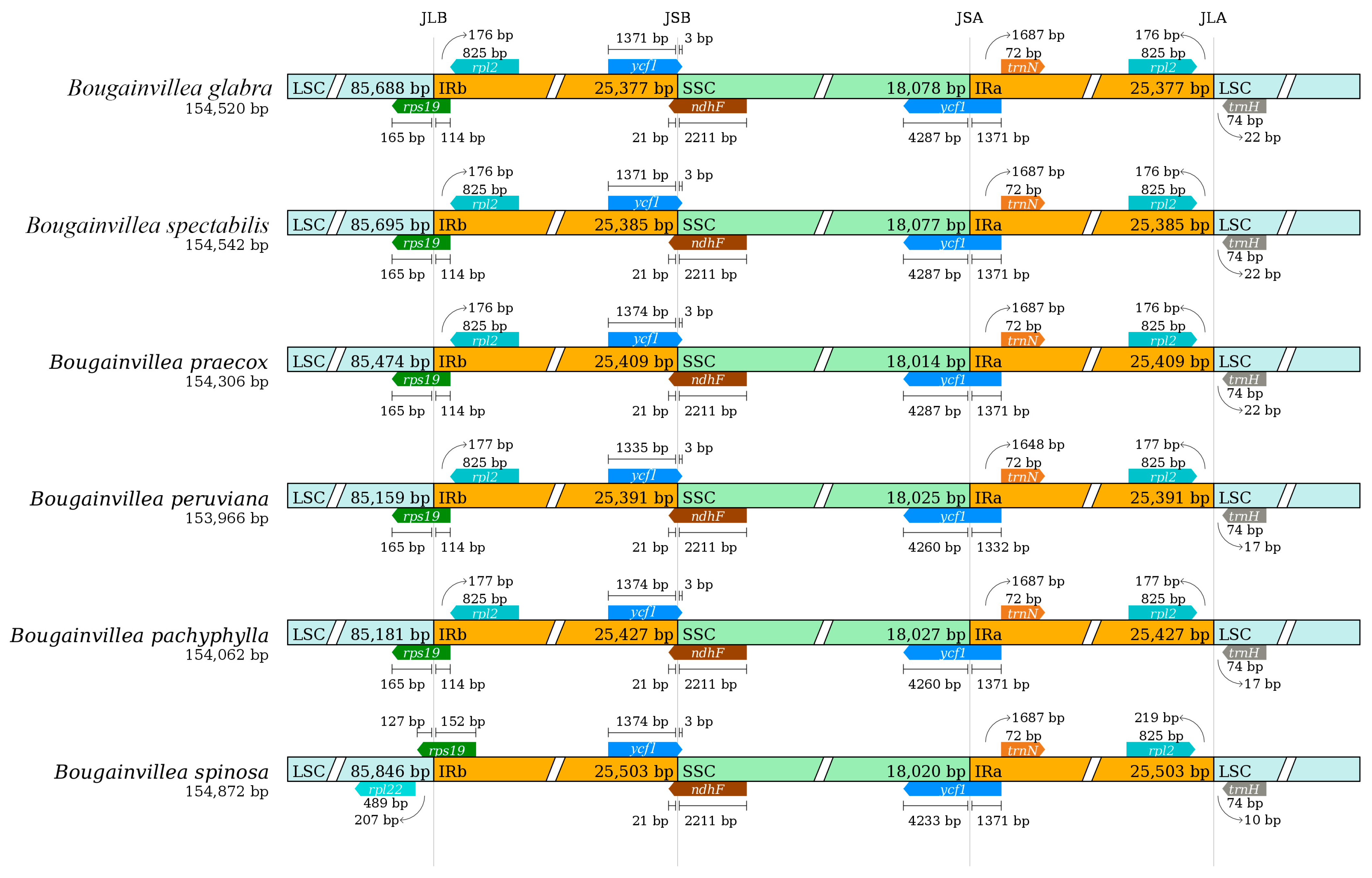
2.6. Analysis of IR Boundary Changes
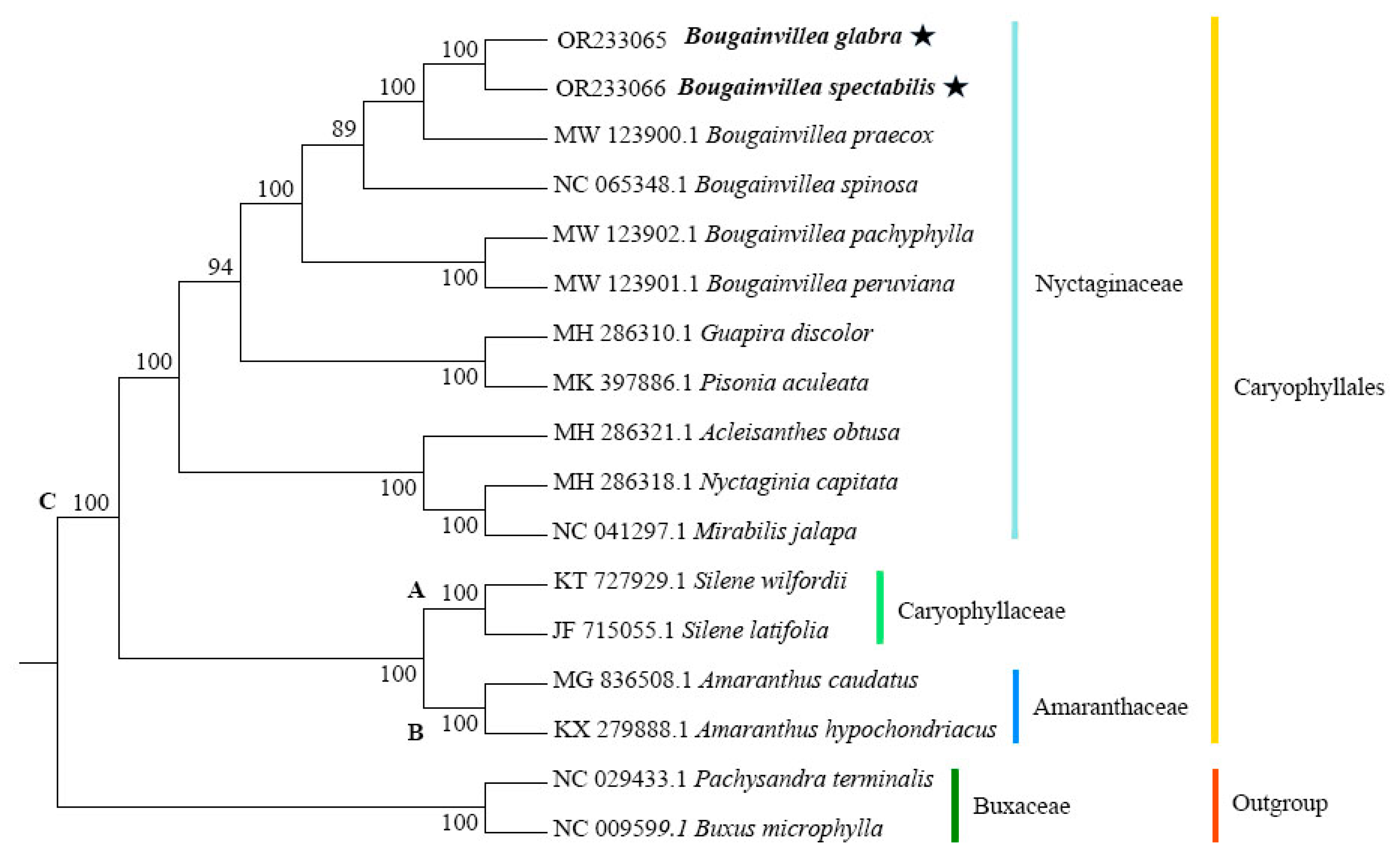
2.7. Phylogenetic Relationships
3. Discussion
4. Materials and Methods
4.1. Sampling, DNA Extraction, and Sequencing
4.2. Chloroplast Genome Assembly and Annotation
4.3. Analysis of SSRs, Nucleotide Polymorphisms, and IR Boundary Changes
4.4. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Wang, X.H.; Zhuang, Y.T.; Jin, X. The complete chloroplast genome of Bougainvillea glabra. Mitochondrial DNA B Resour. 2020, 5, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kulshrestha, A.; Shrivastava, S.; Sharma, B.; Goswamy, H.M.; Prasad, G.B.K.S. Bougainvillea spectabilis exhibits antihyperglycemic and antioxidant activities in experimental diabetes. J. Evid. Based Complement. Altern. Med. 2016, 21, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea genus: A review on phytochemistry, pharmacology, and toxicology. J. Evid. Based Complement. Altern. Med. 2018, 2018, 9070927. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Avoseh, O.N.; Olasunkanmi, K.N.; Lawal, O.A.; Ascrizzi, R.; Guido, F. Chemical composition, anti-nociceptive and anti-inflammatory activities of essential oil of Bougainvillea glabra. J. Ethnopharmacol. 2019, 232, 188–192. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Petricevich, V.L. Extract from Bougainvillea × buttiana (variety Orange) inhibits production of LPS-induced inflammatory mediators in macrophages and exerts a protective effect in vivo. Biomed. Res. Int. 2019, 2019, 2034247. [Google Scholar] [CrossRef]
- Rauf, M.A.; Oves, M.; Rehman, F.U.; Khan, A.R.; Husain, N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed. Pharmacother. 2019, 116, 108983. [Google Scholar] [CrossRef]
- Bautista, M.A.C.; Zheng, Y.; Hu, Z.; Deng, Y.; Chen, T. Comparative analysis of complete chloroplast genome sequences of wild and cultivated Bougainvillea (Nyctaginaceae). Plants 2020, 9, 1671. [Google Scholar] [CrossRef]
- Saleem, H.; Usman, A.; Mahomoodally, M.F.; Ahemad, N. Bougainvillea glabra (choisy): A comprehensive review on botany, traditional uses, phytochemistry, pharmacology and toxicity. J. Ethnopharmacol. 2021, 266, 113356. [Google Scholar] [CrossRef]
- Zhou, Q.; Bao, Y.H.; Liao, L.H.; Luo, Q. Current situation analysis and transformation of the Bougainvillea garden in Xiamen. J. Sichuan Forest. Sci. Technol. 2013, 34, 72–75. [Google Scholar]
- Zhao, T.; Chang, S.X.; Leng, Q.Y.; Xu, S.S.; Yin, J.M.; Niu, J.H. Development of SSR molecular markers based on transcriptome sequencing of Bougainvillea. Mol. Plant Breed. 2019, 17, 4331–4341. [Google Scholar]
- Ding, Y.N.; Bi, G.Y.; Hu, S.W.; Zhou, Y.; Li, H.M.; Xia, Z. Characterization and phylogenetic analysis of complete chloroplast genome of different color medicinal plant Carthamus tinctorius. Chin. Tradit. Herb. Drugs 2023, 54, 262–271. [Google Scholar]
- Yu, X.Y.; Zuo, L.H.; Lu, D.D.; Lu, B.; Yang, M.; Wang, J. Comparative analysis of chloroplast genomes of five Robinia species: Genome comparative and evolution analysis. Gene 2019, 689, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Comparative organization of chloroplast genomes. Annu. Rev. Genet. 1985, 19, 325–354. [Google Scholar] [CrossRef]
- Bock, R.; Knoop, V. Genomics of chloroplasts and mitochondria. In Advances in Photosynthesis and Respiration; Govindjee, Sharkey, T.D., Eds.; Springer: New York, NY, USA; London, UK, 2012; Volume 35, p. 377. [Google Scholar]
- Binh, N.V.; Ngoc, L.V.; Espinosa, W.N.; Hyun-Seung, P.; Nam-Hoon, K.; Woojong, J.; Junki, L.; Yang, T.J. Comprehensive comparative analysis of chloroplast genomes from seven Panax species and development of an authentication system based on species-unique single nucleotide polymorphism markers. J. Ginseng Res. 2020, 44, 135–144. [Google Scholar]
- Bautista, M.A.C.; Zheng, Y.; Boufford, D.E.; Hu, Z.; Deng, Y.; Chen, T. Phylogeny and taxonomic synopsis of the genus Bougainvillea (Nyctaginaceae). Plants 2022, 11, 1700. [Google Scholar] [CrossRef]
- Yang, L.Y.; Zhao, W.Z.; Xin, J.; Dong, Z.H.; Ma, L.Y.; Li, W.Y.; Wang, F.; Xin, P.Y. Comparative analysis of complete chloroplast genome sequences of Bougainvillea glabra and Nyctaginaceae. J. West China For. Sci. 2022, 51, 66–73+79. [Google Scholar]
- Ni, J.Z.; Lee, S.Y.; Hu, X.; Wang, W.; Zhang, J.F.; Ruan, L.; Dai, S.P.; Liu, G.F. The complete chloroplast genome of a commercially exploited ornamental plant, Bougainvillea glabra (Caryophyllales: Nyctaginaceae). Mitochondrial DNA B Resour. 2019, 4, 3390–3391. [Google Scholar] [CrossRef]
- Mabberley, D.J. The Plant Book; Cambridge University Press: Cambridge, UK, 1987; pp. 1–706. [Google Scholar]
- Standley, P.C. The Nyctaginaceae and Chenopodiaceae of Northwestern South America; Field Museum of Natural History-Botanical Series: Chicago, IL, USA, 1931; Volume 11, pp. 73–114. [Google Scholar]
- Standley, P.C. Nyctaginaceae. In Flora of Peru; Macbride, J.F., Ed.; Field Museum of Natural History-Botanical Series: Chicago, IL, USA, 1937; Volume 13, pp. 518–546. [Google Scholar]
- Datta, S.K.; Jayanthi, R.; Janakiram, T. Bougainvillea. In Floriculture and Ornamental Plants. Handbooks of Crop Diversity: Conservation and Use of Plant Genetic Resources; Datta, S.K., Gupta, Y.C., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Li, W.; Meng, Y.P.; Huang, Y.G.; Liu, X.W. Study on ecological adaptability of five Bougainvillea species in Hengyang. Hunan Agric. Sci. 2018, 7, 19–22. [Google Scholar]
- Chen, X.B.; Zhou, Q.; Zhang, D.M.; Luo, Y.L.; Chen, Q.; Yin, L.J.; Wang, L.G. Study and practice on cold-tolerance adaptability of Bougainvillea spp. applied in urban greening of Shanghai. Landscape 2023, 40, 120–126. [Google Scholar]
- Li, Z.B.; Ren, T.; Deng, J.; Zhou, S.; Zeng, X.; Ma, J.; Li, F. Comparative analysis of the chloroplast genomes of three cultivars of Hibiscus mutabilis and its related species. Guihaia 2022, 42, 2007–2020. [Google Scholar]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon usage bias. Mol. Biol. Rep. 2022, 49, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, A.; Miyashita, N.T. Patterns of codon usage bias in three dicot and four monocot plant species. Genes Genet. Syst. 2003, 78, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Sugiura, M. Fine structural features of the chloroplast genome: Comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991, 19, 983–995. [Google Scholar] [CrossRef]
- Wang, H.K.; Lou, X.M.; Zhang, Z. Application in germplasm resource research using chloroplast simple sequence repeat. Mol. Plant Breed. 2006, 4, 92–98. [Google Scholar]
- Gichira, A.; Li, Z.Z.; Saina, J.K.; Long, Z.C.; Hu, G.W.; Gituru, R.W.; Wang, Q.F.; Chen, J.M. The complete chloroplast genome sequence of an endemic monotypic genus Hagenia (Rosaceae): Structural comparative analysis, gene content and microsatellite detection. PeerJ 2017, 5, e2846. [Google Scholar] [CrossRef]
- Yi, X.; Gao, L.; Wang, B.; Su, Y.-J.; Wang, T. The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): Evolutionary comparison of cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol. Evol. 2013, 5, 688–698. [Google Scholar] [CrossRef]
- Chen, J.; Hao, Z.; Xu, H.; Yang, L.; Liu, G.; Shen, Y.; Zhen, C.; Zhen, W.; Cheng, T.; Shi, J. The complete chloroplast genome sequence of the relict woody plant Metasequoia glyptostroboides Hu et Cheng. Front. Plant Sci. 2015, 6, 447. [Google Scholar] [CrossRef]
- Dong, W.P.; Xu, C.; Li, C.H.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. Ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, T.; Duan, D.; Yang, J.; Feng, L.; Zhao, G. Comparative analysis of the complete chloroplast genomes of five Quercus species. Front. Plant Sci. 2016, 7, 959. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.; Resende-Moreira, L.C.; Buzatti, R.; Nazareno, A.G.; Carlsen, M.; Lobo, F.P. Chloroplast genomes of Byrsonima species (Malpighiaceae): Comparative analysis and screening of high divergence sequences. Sci. Rep. 2018, 8, 2210. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Shi, T.; Luo, W.; Ni, X.; Iqbal, S.; Ni, Z.; Huang, X.; Yao, D.; Shen, Z.; Gao, Z. Comparative analysis of the complete chloroplast genome among Prunus mume, P. armeniaca, and P. salicina. Hortic. Res. 2019, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Mehmood, F.; Shahzadi, I.; Waseem, S.; Mirza, B.; Ahmed, I.; Waheed, M.T. Chloroplast genome of Hibiscus rosa-sinensis (Malvaceae): Comparative analyses and identification of mutational hotspots. Genomics 2020, 112, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, S.; Ding, Y.; Xu, J.; Li, M.F.; Zhu, S.; Chen, N. Chloroplast genomic resource of Paris for species discrimination. Sci. Rep. 2017, 7, 3427. [Google Scholar] [CrossRef]
- Liu, L.X.; Wang, Y.W.; He, P.Z.; Li, P.; Lee, J.K.; Soltis, D.E.; Fu, C.X. Chloroplast genome analyses and genomic resource development for epilithic sister genera Oresitrophe and Mukdenia (Saxifragaceae), using genome skimming data. BMC Genom. 2018, 19, 1–17. [Google Scholar] [CrossRef]
- Pahlich, E.; Gerlitz, C. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1980, 19, 11–13. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Boetzer, M.; Henkel, C.V.; Jansen, H.J.; Butler, D.; Pirovano, W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 2011, 27, 578–579. [Google Scholar] [CrossRef]
- Boetzer, M.; Pirovano, W. Toward almost closed genomes with GapFiller. Genome Biol. 2012, 13, R56. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; Chen, F.; Teng, N.J.; Xu, Y.C.; Dai, Z.L. Comparative analysis of the complete chloroplast genome of seven Nymphaea species. Aquat. Bot. 2021, 170, 103353. [Google Scholar] [CrossRef]
- Yang, J.; Yue, M.; Niu, C.; Ma, X.F.; Li, Z.H. Comparative analysis of the complete chloroplast genome of four endangered herbals of Notopterygium. Genes 2017, 8, 124. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Peden, J.F. Analysis of Codon Usage. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2000. [Google Scholar]
- Zhou, X.J.; Song, L.L.; Peng, Z.F.; Sun, S.S.; Ya, H.Y.; Cheng, Y.W.; Zhang, Y. The complete chloroplast genome sequence of Paeonia jishanensis (Paeoniaceae), a rare wild tree peony. Mitochondrial DNA B Resour. 2019, 4, 503–504. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Cuirao-Rico, S.; Librado, P.; Ramons-Onsins, S.E.; Sánchez-Garcia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]




| Genome Feature | Bougainvillea glabra | Bougainvillea spectabilis | Bougainvillea peruviana | Bougainvillea pachyphylla | Bougainvillea praecox | Bougainvillea spinosa |
|---|---|---|---|---|---|---|
| Genome size (bp) | 154,520 | 154,542 | 153,966 | 154,062 | 154,306 | 154,872 |
| LSC length (bp) | 85,688 | 85,695 | 85,159 | 85,181 | 85,474 | 85,846 |
| SSC length (bp) | 18,078 | 18,077 | 18,025 | 18,027 | 18,014 | 18,020 |
| IR length (bp) | 25,377 | 25,385 | 25,391 | 25,427 | 25,409 | 25,503 |
| Number of genes | 131 | 131 | 131 | 131 | 131 | 131 |
| Number of protein-coding genes | 86 | 86 | 86 | 86 | 86 | 86 |
| Number of rRNA genes | 8 | 8 | 8 | 8 | 8 | 8 |
| Number of tRNA genes | 37 | 37 | 37 | 37 | 37 | 37 |
| GC content (%) | 36.5 | 36.5 | 36.6 | 36.5 | 36.5 | 36.4 |
| GC content in LSC (%) | 34.2 | 34.2 | 34.3 | 34.3 | 34.3 | 34.1 |
| GC content in SSC (%) | 29.5 | 29.5 | 29.6 | 29.6 | 29.5 | 29.4 |
| GC content in IR (%) | 42.8 | 42.8 | 42.8 | 42.8 | 42.8 | 42.9 |
| Category | Group | Genes |
|---|---|---|
| Photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of NADH dehydrogenase | ndhA *, ndhB * (2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB *, petD *, petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI | |
| Large subunit of rubisco | rbcL | |
| Subunits protochlorophyllide reductase | - | |
| Self-replication | Proteins of large ribosomal subunit | rpl14, rpl16 *, rpl2 * (2), rpl20, rpl22, rpl23 (2), rpl32, rpl33, rpl36 |
| Proteins of small ribosomal subunit | rps11, rps12 ** (2), rps14, rps15, rps16 *, rps18, rps19, rps2, rps3, rps4, rps7 (2), rps8 | |
| Subunits of RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 | |
| Ribosomal RNAs | rrn16 (2), rrn23 (2), rrn4.5 (2), rrn5 (2) | |
| Transfer RNAs | trnA-UGC * (2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC, trnG-UCC *, trnH-GUG, trnI-CAU (2), trnI-GAU * (2), trnK-UUU *, trnL-CAA (2), trnL-UAA *, trnL-UAG, trnM-CAU, trnN-GUU (2), trnP-UGG, trnQ-UUG, trnR-ACG (2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC (2), trnV-UAC *, trnW-CCA, trnY-GUA, trnfM-CAU | |
| Other genes | Maturase | matK |
| Protease | clpP ** | |
| Envelope membrane protein | cemA | |
| Acetyl-CoA carboxylase | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Translation initiation factor | infA | |
| Other | - | |
| Genes of unknown function | Conserved hypothetical chloroplast open reading frame | ycf1 (2), ycf2 (2), ycf3 **, ycf4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Huang, T.; Zhou, Q.; Sheng, Q.; Zhu, Z. Complete Chloroplast Genomes and Phylogenetic Relationships of Bougainvillea spectabilis and Bougainvillea glabra (Nyctaginaceae). Int. J. Mol. Sci. 2023, 24, 13044. https://doi.org/10.3390/ijms241713044
Zhang H, Huang T, Zhou Q, Sheng Q, Zhu Z. Complete Chloroplast Genomes and Phylogenetic Relationships of Bougainvillea spectabilis and Bougainvillea glabra (Nyctaginaceae). International Journal of Molecular Sciences. 2023; 24(17):13044. https://doi.org/10.3390/ijms241713044
Chicago/Turabian StyleZhang, Huihui, Tao Huang, Qi Zhou, Qianqian Sheng, and Zunling Zhu. 2023. "Complete Chloroplast Genomes and Phylogenetic Relationships of Bougainvillea spectabilis and Bougainvillea glabra (Nyctaginaceae)" International Journal of Molecular Sciences 24, no. 17: 13044. https://doi.org/10.3390/ijms241713044





