Reticular Chemistry for Optical Sensing of Anions
Abstract
:1. Introduction
- (1)
- Sensitivity—the presence of sustainable pores enhances guest-host interactions and pre-accumulates the anions, which increases detection sensitivity;
- (2)
- Electronic tunability—the electronic properties can be finely tuned by varying the degree of conjugation of the organic linkers, which changes the HOMO-LUMO band gap and thereby tunes the absorption and emission properties;
- (3)
- Selectivity—functional groups within the framework, such as Lewis acidic or hydrogen bonding sites in the ligands or varying the nature of the metals in the clusters (in the case of MOFs and ZIFs), can further promote binding of the preferred anion and thereby improve the selectivity of detection;
- (4)
- Structural recognition—structure-property correlations and anion-sensor interactions may be thoroughly investigated due to the highly ordered crystalline nature, which makes it easier to characterize and identify structures precisely;
- (5)
- Strong emissions—incorporating organic linkers into a rigid framework can minimize the non-radiative relaxation caused by the free rotation and vibration of the linkers, thereby enhancing the emission strength, e.g., aggregation-induced emission (AIE);
- (6)
- Thermal and chemical stability—the thermal and chemical stability of these materials is relatively high, allowing them to retain their crystalline structure at elevated temperatures. Thus, they can preserve their optical properties (absorbance and fluorescence) at relatively high temperatures, allowing them to be utilized when binding to a particular anion that necessitates an elevated temperature.
2. MOFs for Optical Sensing of Anions
2.1. Pre-Functionalized MOFs
2.2. Post-Functionalized MOFs
2.3. Non-Functionalized MOFs
3. ZIFs for Optical Sensing of Anions
4. COFs for Optical Sensing of Anions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe MYaghi, O.M. Reticular Chemistry: Occurrence and Taxonomy of Nets and Grammar for the Design of Frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular Chemistry—Construction, Properties, and Precision Reactions of Frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Chen, Z.; Jiang, H.; Belmabkhout, Y.; Mouchaham, G.; Aggarwal, H.; Adil, K.; Abou-Hamad, E.; Czaban-Jóźwiak, J.; Tchalala, M.R.; et al. Extremely hydrophobic POPs to access highly porous storage media and capturing agent for organic vapors. Chem 2019, 5, 180–191. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, 585–593. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yaghi, O.M. Reticular Chemistry in All Dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic imidazolate frameworks: Synthesis, functionalization, and catalytic/adsorption applications. Catal. Surv. Jpn. 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Chen, B.L.; Yang, Z.X.; Zhu, Y.Q.; Xia, Y.D. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–organic frameworks for separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Fan, L.; Sun, D. Recent advances and challenges of metal–organic framework membranes for gas separation. J. Mater. Chem. A 2017, 5, 10073–10091. [Google Scholar] [CrossRef]
- Denny, M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal–organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1, 16078. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Helal, A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. Multivariate metal-organic frameworks. Natl. Sci. Rev. 2017, 4, 296–298. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–organic frameworks for water harvesting from air. Adv. Mater. 2018, 30, 1704304. [Google Scholar] [CrossRef]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Cordova, K.E.; Arafat, M.E.; Usman, M.; Yamani, Z.H. Defect-engineering a metal-organic framework for CO2 fixation in the synthesis of bioactive oxazolidinones. Inorg. Chem. Front. 2020, 7, 3571–3577. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Sun, L.; Campbell, M.G.; Dinca, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Zheng, W.; Tsang, C.-S.; Lee, L.Y.S.; Wong, K.-Y. Two-dimensional metal-organic framework and covalent-organic framework: Synthesis and their energy-related applications. Mater. Today Chem. 2019, 12, 34–60. [Google Scholar] [CrossRef]
- Helal, A.; Shah, S.S.; Usman, M.; Khan, M.Y.; Aziz, M.A.; Rahman, M.M. Potential Applications of Nickel-Based Metal-Organic Frameworks and their Derivatives. Chem. Rec. 2022, 22, e202200055. [Google Scholar] [CrossRef]
- Diercks, C.S.; Liu, Y.; Cordova, K.E.; Yaghi, O.M. The role of reticular chemistry in the design of CO2 reduction catalysts. Nat. Mater. 2018, 17, 301–307. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Doonan, C.J.; Sumby, C.J. Metal–organic framework catalysis. CrystEngComm 2017, 19, 4044–4048. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Cohen, S.M. Photocatalytic metal–organic frameworks for organic transformations. CrystEngComm 2017, 19, 4126–4136. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme encapsulation in metal–organic frameworks for applications in catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Helal, A.; Sanhoob, M.A.; Hoque, B.; Usman, M.; Zahir, M.H. Bimetallic Metal-Organic Framework Derived Nanocatalyst for CO2 Fixation through Benzimidazole Formation and Methanation of CO2. Catalysts 2023, 13, 357–369. [Google Scholar] [CrossRef]
- Peller, M.; Böll, K.; Zimpel, A.; Wuttke, S. Metal–organic framework nanoparticles for magnetic resonance imaging. Inorg. Chem. Front. 2018, 5, 1760–1779. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal–organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Sophie, E.M.; Teplensky, H.; Michelle, Z.; Peyman, M.; Fairen-Jimenez, D. Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 2016, 6, 20160027. [Google Scholar]
- Helal, A.; Nguyen, H.L.; Al-Ahmed, A.; Cordova, K.E.; Yamani, Z.H. An Ultrasensitive and Selective Metal−Organic Framework Chemosensor for Palladium Detection in Water. Inorg. Chem. 2019, 58, 1738–1741. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–organic framework nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, S.; Lismont, M.; Escudero, A.; Rungtaweevoranit, B.; Parak, W.J. Positioning metal-organic framework nanoparticles within the context of drug delivery–a comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 2017, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: Nanoscale Metal–Organic Frameworks and Nanoscale Coordination Polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Chidambaram, A.; Stylianou, K.C. Electronic metal–organic framework sensors. Inorg. Chem. Front. 2018, 5, 979–998. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Maity, R.; Chakraborty, D.; Nandi, S.; Yadav, A.K.; Mullangi, D.; Vinod, C.P.; Vaidhyanathan, R. Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework. ACS Appl. Nano Mater. 2019, 2, 5169–5178. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mullangi, D.; Chandran, C.; Vaidhyanathan, R. Nanopores of a Covalent Organic Framework: A Customizable Vessel for Organocatalysis. ACS Omega 2022, 7, 15275–15295. [Google Scholar] [CrossRef] [PubMed]
- Mullangi, D.; Evans, H.A.; Yildirim, T.; Wang, Y.; Deng, Z.; Zhang, Z.; Mai, T.T.; Wei, F.; Wang, J.; Hight Walker, A.R.; et al. Noncryogenic Air Separation Using Aluminum Formate Al(HCOO)3 (ALF). J. Am. Chem. Soc. 2023, 145, 9850–9856. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Naeem, M.; Arafat, M.E.; Rahman, M.M. Europium doped Ni (BTC) metal-organic framework for detection of heteroaromatic compounds in mixed aqueous media. Mater. Res. Bull. 2022, 146, 111604–111609. [Google Scholar] [CrossRef]
- Anderson, M.P.; Gregory, R.J.; Thompson, S.; Souza, D.W.; Paul, S.; Mulligan, R.C.; Smith, A.E.; Welsh, M.J. Demonstration That CFTR Is a Chloride Channel by Alteration of Its Anion Selectivity. Science 1991, 253, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.H.P.; Ellwood, D.C. The removal of the pertechnetate ion and actinides from radioactive waste streams at Hanford, Washington, USA and Sellafield, Cumbria, UK: The role of iron-sulfide-containing adsorbent materials. Nucl. Eng. Des. 2003, 226, 375–385. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Dyke, J.V.; Kirk, A.B.; Jackson, W.A. Perchlorate in the United States.Analysis of relative source contributions to the food chain. Environ. Sci. Technol. 2006, 40, 6608–6614. [Google Scholar] [CrossRef]
- Zhitkovich, A. Importance of chromium—DNA adducts in mutagenicity and toxicity of chromium (VI). Chem. Res. Toxicol. 2005, 18, 3–11. [Google Scholar] [CrossRef]
- Moradi, E.; Rahimi, R.; Farahani, Y.D.; Safarifard, V. Porphyrinic zirconium-based MOF with exposed pyrrole Lewis base site as a luminescent sensor for highly selective sensing of Cd2+ and Br− ions and THF small molecule. J. Solid State Chem. 2020, 282, 121103. [Google Scholar] [CrossRef]
- Asha, K.S.; Bhattacharjee, R.; Mandal, S. Complete transmetalation in a metal–organic framework by metal ion metathesis in a single crystal for selective sensing of phosphate ions in aqueous media. Angew. Chem. Int. Ed. 2016, 55, 11528–11532. [Google Scholar] [CrossRef]
- Goswami, R.; Seal, N.; Dash, S.; Tyagi, R.A.; Neogi, S. Devising Chemically Robust and Cationic Ni(II)–MOF with Nitrogen-Rich Micropores for Moisture-Tolerant CO2 Capture: Highly Regenerative and Ultrafast Colorimetric Sensor for TNP and Multiple Oxo–Anions in Water with Theoretical Revelation. ACS Appl. Mater. Interfaces 2019, 11, 40134–40150. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sikdar, N.; Maji, T.K. An excited-state intramolecular proton-transfer responsive nanoscale MOF for dual sensing of water and chromate ions. J. Mater. Chem. C 2022, 10, 7558–7566. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Biswas, S. Post-synthetic modification of a metal–organic framework with a chemodosimeter for the rapid detection of lethal cyanide via dual emission. Dalton Trans. 2020, 49, 8684–8692. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-N.; Zhou, L.-J.; Li, P.; Sui, Q.; Gao, E.-Q. Space-confined indicator displacement assay inside a metal–organic framework for fluorescence turn-on sensing. Chem. Sci. 2019, 10, 3307–3321. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Du, P.-Y.; Gu, W.; Liu, X. Highly selective luminescence sensing of nitrite and benzaldehyde based on 3d–4f heterometallic metal–organic frameworks. Dalton Trans. 2016, 45, 8700–8704. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, J.; Gao, X.; Ji, G.; Wang, D.; Liu, Z. A multi-chemosensor based on Zn-MOF: Ratio-dependent color transition detection of Hg (II) and highly sensitive sensor of Cr (VI). Sens. Actuators B 2018, 269, 164–172. [Google Scholar] [CrossRef]
- Singh, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. A luminescent cadmium based MOF as selective and sensitive iodide sensor in aqueous medium. J. Photochem. Photobiol. A 2018, 356, 389–396. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, J.; Zhou, Q.; Lv, Z.; Li, C.; Hu, G. Enhanced luminescence of NH2-UiO-66 for selectively sensing fluoride anion in water medium. J. Lumin. 2019, 208, 67–74. [Google Scholar] [CrossRef]
- Li, P.-C.; Zhang, L.; Yang, M.; Zhang, K.-L. A novel luminescent 1D→2D polyrotaxane Zn(II)-organic framework showing dual responsive fluorescence sensing for Fe3+ cation and Cr(VI) anions in aqueous medium. J. Lumin. 2019, 207, 351–360. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, N.; Zhang, X.; Wang, Y.; Zhao, L.; Yang, Q. A stable Cu-MOF as a dual function sensor with high selectivity and sensitivity detection of picric acid and CrO42-in aqueous solution. Microchem. J. 2020, 153, 104498. [Google Scholar] [CrossRef]
- Safaei, S.; Wang, J.; Junk, P.C. Incorporation of thiazolothiazole fluorophores into a MOF structure: A highly luminescent Zn(II)-based MOF as a selective and reversible sensor for Cr2O72− and MnO4− anions. J. Solid State Chem. 2021, 294, 121762. [Google Scholar] [CrossRef]
- Helal, A.; Shaikh, M.N.; Aziz, M.A. Dual sensing of copper ion and chromium (VI) oxyanions by benzotriazole functionalized UiO-66 metal-organic framework in aqueous media. J. Photochem. Photobiol. A 2020, 389, 112238. [Google Scholar] [CrossRef]
- Gogoi, C.; Nagarjun, N.; Roy, S.; Mostakim, S.K.; Volkmer, D.; Dhakshinamoorthy, A.; Biswas, S. A Zr-Based Metal–Organic Framework with a DUT-52 Structure Containing a Trifluoroacetamido-Functionalized Linker for Aqueous Phase Fluorescence Sensing of the Cyanide Ion and Aerobic Oxidation of Cyclohexane. Inorg. Chem. 2021, 60, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Moorthy, J.N. Zwitterionic Luminescent 2D Metal–Organic Framework Nanosheets (LMONs): Selective Turn-On Fluorescence Sensing of Dihydrogen Phosphate. Inorg. Chem. 2022, 61, 3942–3950. [Google Scholar] [CrossRef]
- Zhu, B.; Jin, Y.; Chu, J.; Zuo, M.; Cui, S. Metal–organic framework bearing new viologen ligand for ammonia and Cr2O72− sensing. RSC Adv. 2022, 12, 6951–6957. [Google Scholar] [CrossRef]
- Yan, Y.T.; Wu, Y.L.; Zheng, L.N.; Cai, W.; Tang, P.F.; Wu, W.P.; Zhang, W.Y.; Wang, Y.Y. Two porous three-dimensional (3D) metal–organic frameworks based on diverse metal clusters selective sensing of Fe3+ and Cr2O72−. New J. Chem. 2022, 46, 4292–4299. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Eu (III)-functionalized MIL-124 as fluorescent probe for highly selectively sensing ions and organic small molecules especially for Fe (III) and Fe (II). ACS Appl. Mater. Interfaces 2014, 7, 721–729. [Google Scholar] [CrossRef]
- Hao, J.-N.; Yan, B. Ln3+ post-functionalized metal–organic frameworks for color tunable emission and highly sensitive sensing of toxic anions and small molecules. New J. Chem. 2016, 40, 4654–4661. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. Post-synthetic modification of a metal-organic framework with fluorescent-tag for dual naked-eye sensing in aqueous medium. Sens. Actuators B 2017, 239, 759–767. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Yan, B. A novel sensitive fluorescent probe of S2O82− and Fe3+ based on covalent post-functionalization of a zirconium (iv) metal–organic framework. Dalton Trans. 2018, 47, 11586–11592. [Google Scholar] [CrossRef]
- Sen, A.; Desai, A.V.; Samanta, P.; Dutta, S.; Let, S.; Ghosh, S.K. Post-synthetically modified metal–organic framework as a scaffold for selective bisulphite recognition in water. Polyhedron 2018, 156, 1–5. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.-S.; Zhao, B. A water-stable lanthanide-organic framework as a recyclable luminescent probe for detecting pollutant phosphorus anions. Chem. Commun. 2015, 51, 10280–10283. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.-W.; Yan, B.; Weng, H. Europium activated yttrium hybrid microporous system for luminescent sensing toxic anion of Cr (VI) species. Microporous Mesoporous Mater. 2015, 217, 196–202. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. Hydrolytically stable luminescent cationic metal organic framework for highly sensitive and selective sensing of chromate anions in natural water systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Zheng, H.-Q.; Zheng, H.-Y.; Lin, L.-P.; Xin, Q.; Cao, R. Efficient Capture and Effective Sensing of Cr2O72– from Water Using a Zirconium Metal–Organic Framework. Inorg. Chem. 2017, 56, 14178–14188. [Google Scholar] [CrossRef]
- Yu, L.; Wang, C.; Hu, C.-J.; Dong, W.-W.; Wu, Y.-P.; Li, D.-S.; Zhao, J. A bifunctional luminescent Tb (III)-metal-organic framework by a tetracarboxylate ligand for highly selective detection of Fe3+ cation and Cr2O72−anion. J. Solid State Chem. 2018, 262, 282–286. [Google Scholar] [CrossRef]
- Li, S.; Lu, L.; Zhu, M.; Yuan, C.; Feng, S. A bifunctional chemosensor for detection of volatile ketone or hexavalent chromate anions in aqueous solution based on a Cd (II) metal–organic framework. Sens. Actuators B 2018, 258, 970–980. [Google Scholar] [CrossRef]
- Chen, B.C.; Xiao, C.Q.; Hu, J.J.; Peng, Y.; Wen, H.R.; Liu, S.J. Synthesis and Structure of an Aqueous Stable Europium-Based Metal–Organic Framework with Ratiometric Fluorescence Sensing for Phosphate and Luminescence Quenching for Salicylaldehyde. Inorg. Chem. 2023, 62, 6255–6262. [Google Scholar] [CrossRef]
- Liu, C.; Yan, B. Luminescent zinc metal—Organic framework (ZIF-90) for sensing metal ions, anions and small molecules. Photochem. Photobiol. Sci. 2015, 14, 1644–1650. [Google Scholar] [CrossRef]
- Karmakar, A.; Kumar, N.; Samanta, P.; Desai, A.V.; Ghosh, S.K. A post-synthetically modified MOF for selective and sensitive aqueous-phase detection of highly toxic cyanide ions. Chem. A Eur. J. 2016, 22, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hai, J.; Li, S.; Wang, B.; Yang, Z. Luminescent magnetic nanoparticles encapsulated in MOFs for highly selective and sensitive detection of ClO−/SCN− and anti-counterfeiting. Nanoscale 2018, 10, 8667–8676. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhan, K.; Wu, Y.; Zhu, Y.; Yan, J.; Liu, B.; Chen, Y. A novel fluorescence probe based MAPbBr3@ ZIF-8 for detecting hypochlorite in water samples. Microchem. J. 2022, 172, 106924. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-linked conjugated microporous polymers: Sensing and removal of fluoride ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef]
- Li, Z.; Huang, N.; Lee, K.H.; Feng, Y.; Tao, S.; Jiang, Q.; Nagao, Y.; Irle, S.; Jiang, D. Light-emitting covalent organic frameworks: Fluorescence improving via pinpoint surgery and selective switch-on sensing of anions. J. Am. Chem. Soc. 2018, 140, 12374–12377. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Z.; Xiong, Y. Water dispersed two-dimensional ultrathin Fe(III)- modified covalent triazine framework nanosheets: Peroxidase like activity and colorimetric biosensing applications. Nanoscale 2018, 10, 20120–20128. [Google Scholar] [CrossRef]
- Li, M.; Cui, Z.; Pang, S.; Meng, L.; Ma, D.; Li, Y.; Shi, Z.; Feng, S. Luminescent covalent organic framework as a recyclable turn-off fluorescent sensor for cations and anions in aqueous solution. J. Mater. Chem. C 2019, 7, 11919–11927. [Google Scholar] [CrossRef]
- Singh, H.; Devi, M.; Jena, N.; Iqbal, M.M.; Nailwal, Y.; Sarkar, A.D.; Pal, S.K. Proton-triggered fluorescence switching in self-exfoliated ionic covalent organic nanosheets for applications in selective detection of anions. ACS Appl. Mater. Interfaces 2020, 12, 13248–13255. [Google Scholar] [CrossRef]
- Huang, M.; Chong, J.; Hu, C.; Yang, Y. Ratiometric fluorescent detection of temperature and MnO4− using a modified covalent organic framework. Inorg. Chem. Commun. 2020, 119, 108094–108105. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yin, H.Q.; Yin, X.B. MOF@ COFs with strong multiemission for differentiation and ratiometric fluorescence detection. ACS Appl. Mater. Interfaces 2020, 12, 20973–20981. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M.; Farrokhpour, H.; Zamora, F. Imine-Linked Covalent Organic Framework with a Naphthalene Moiety as a Sensitive Phosphate Ion Sensing. ACS Appl. Mater. Interfaces 2022, 14, 22398–22406. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Shi, W.; Li, Y.; Yu, Y.; Wu, X.; Li, Z.; Lee, S.Y.; Lee, K.H. Excellent Crystallinity and Stability Covalent–Organic Frameworks with High Emission and Anions Sensing. Macromol. Rapid Commun. 2022, 43, 2200393–2200399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Wu, S.; Li, X.M.; Sun, Q. Excited-state intramolecular proton transfer-based covalent organic framework for fluorescence anion sensing. New J. Chem. 2022, 46, 14122–14126. [Google Scholar] [CrossRef]

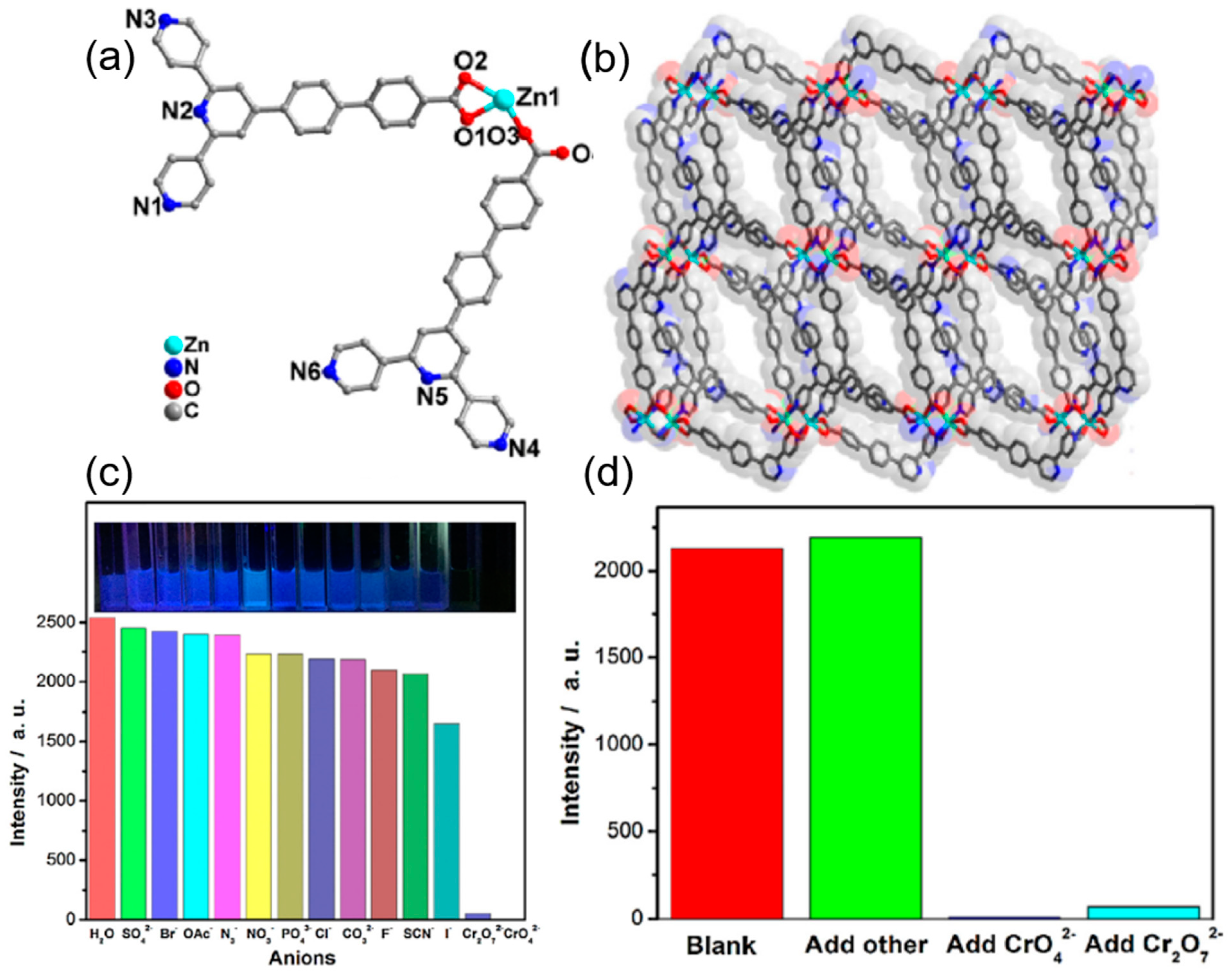
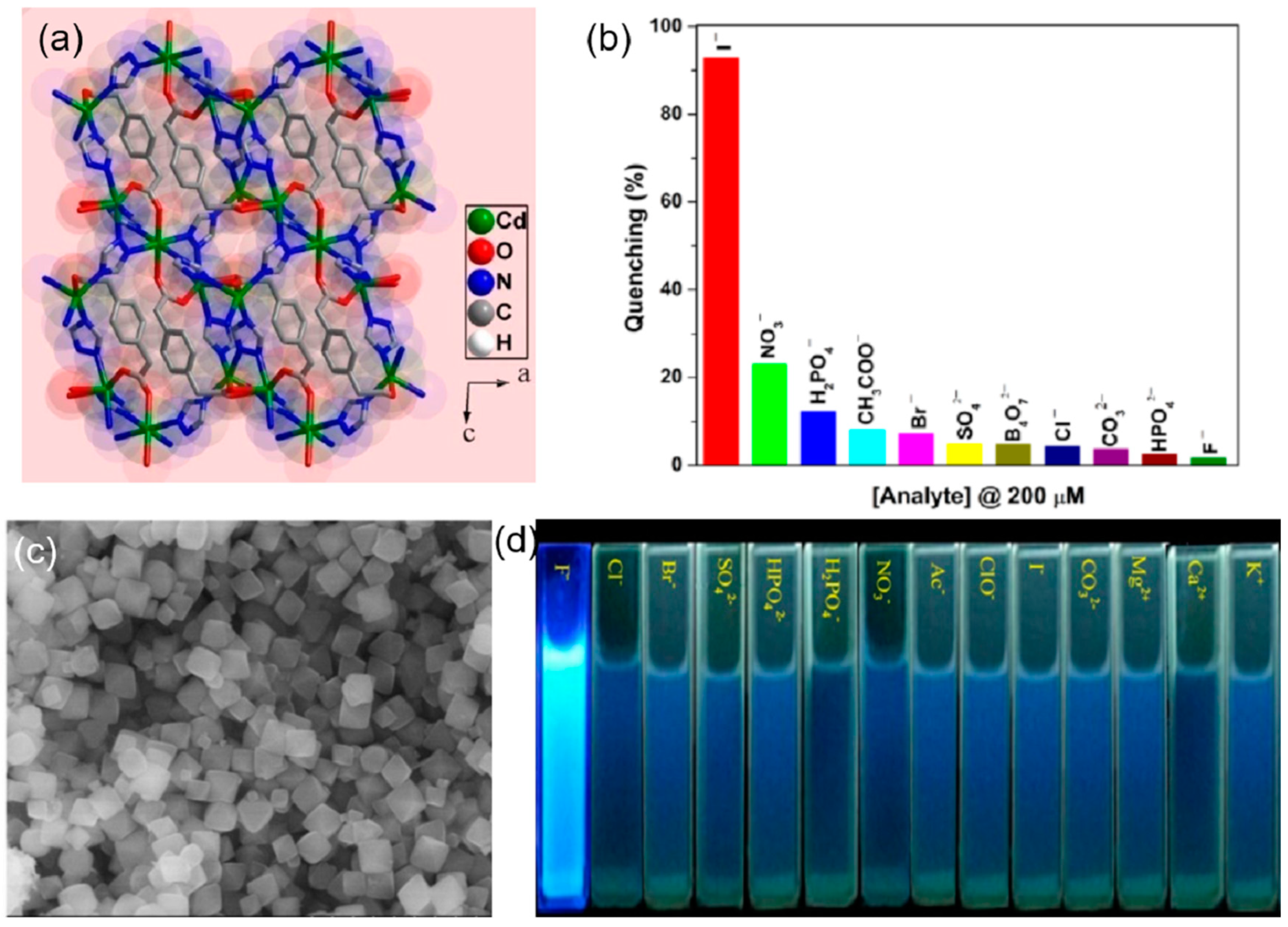
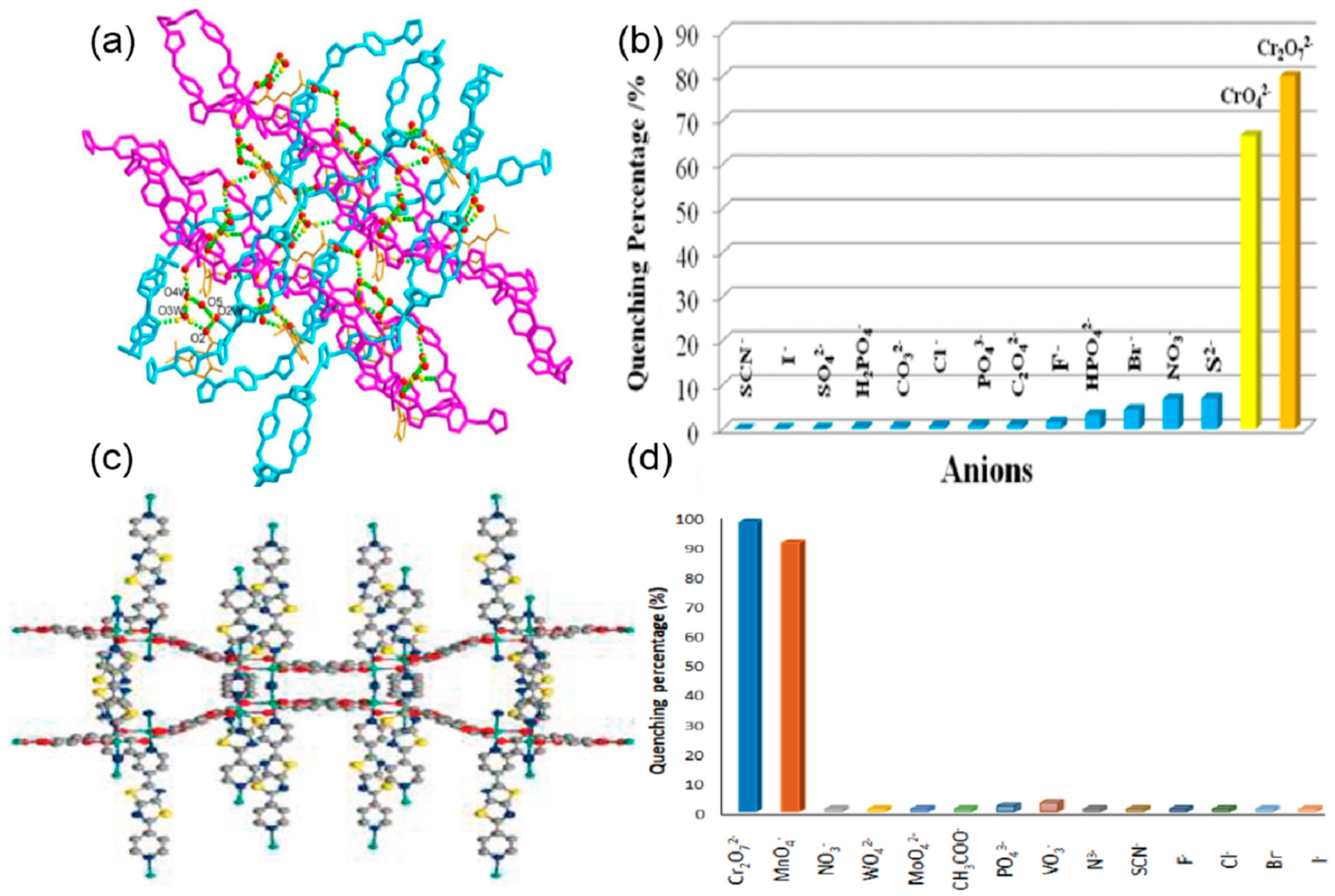
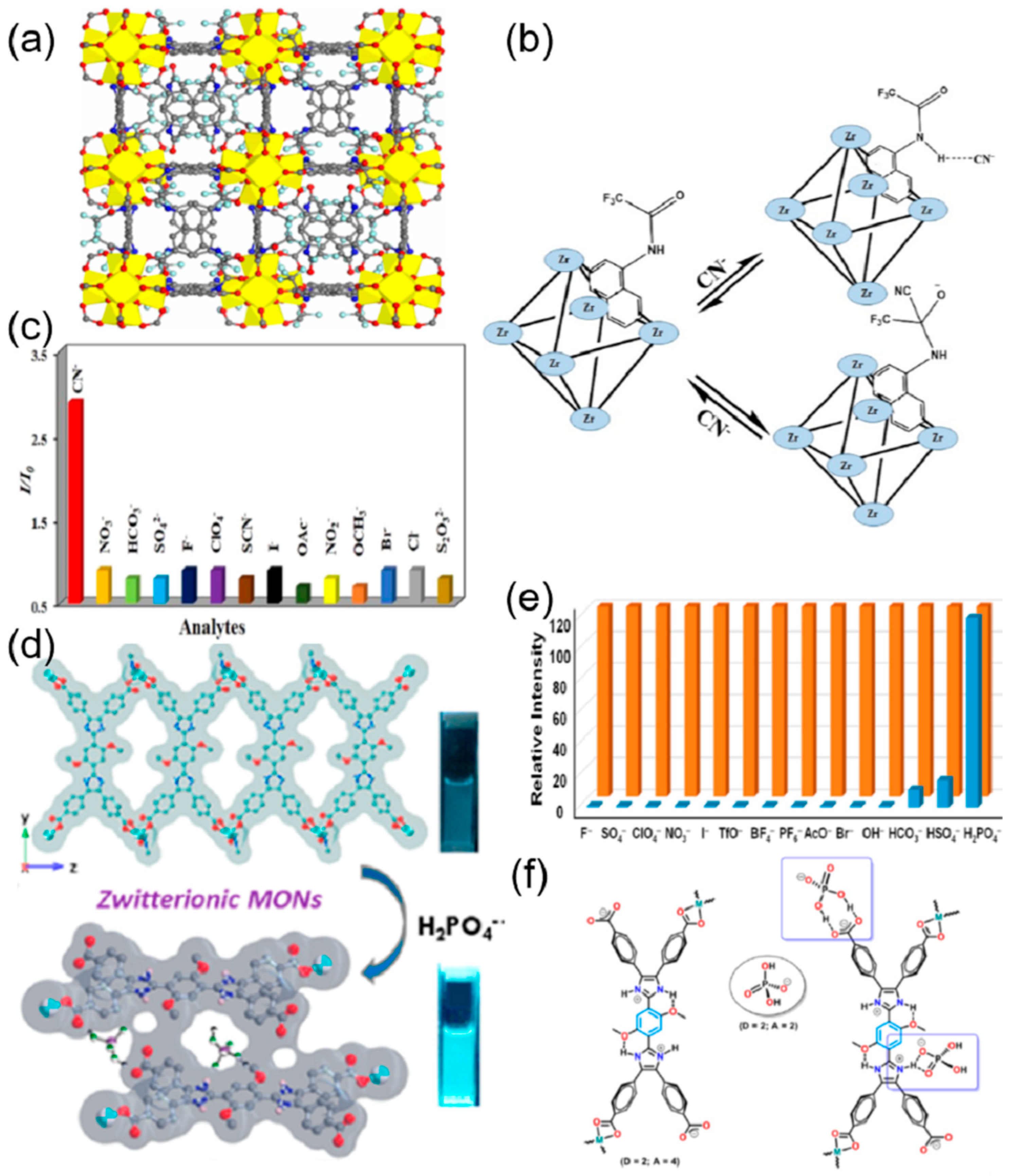
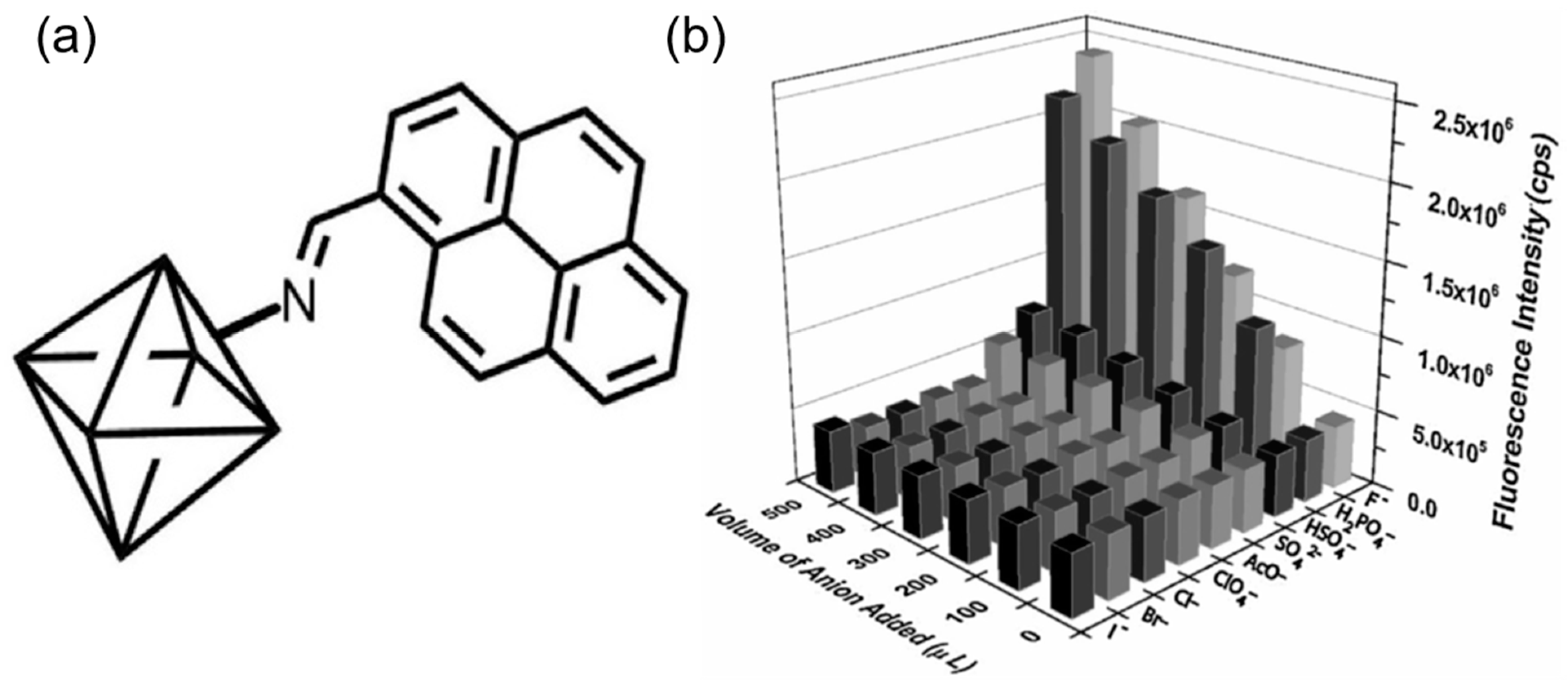
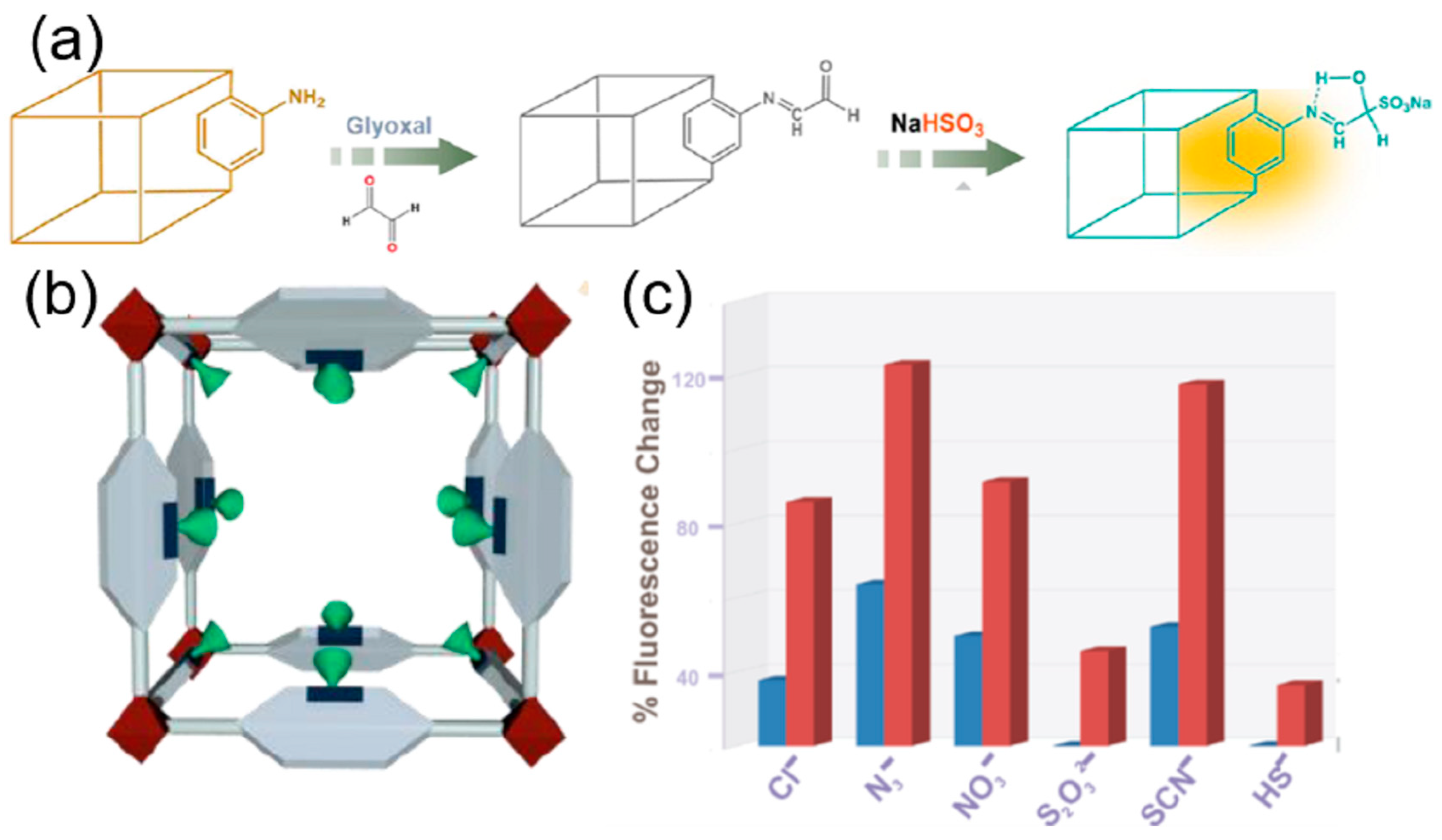
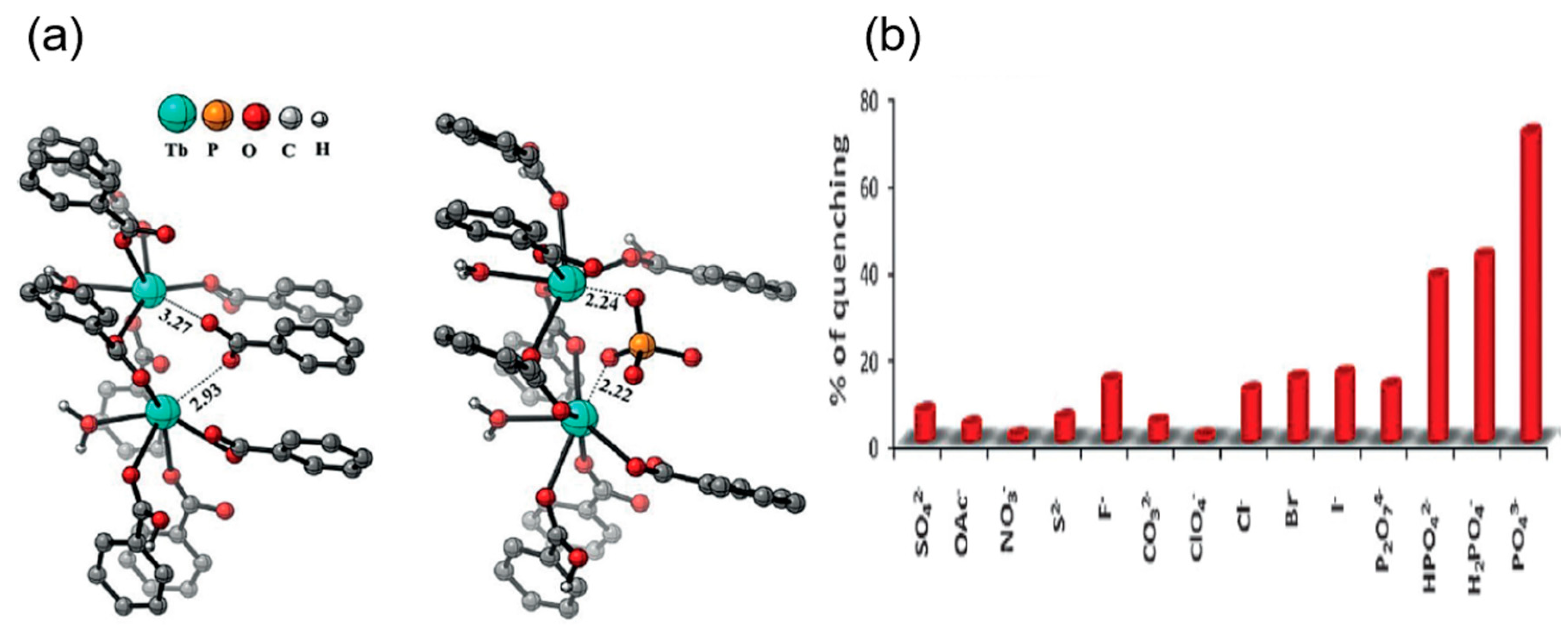
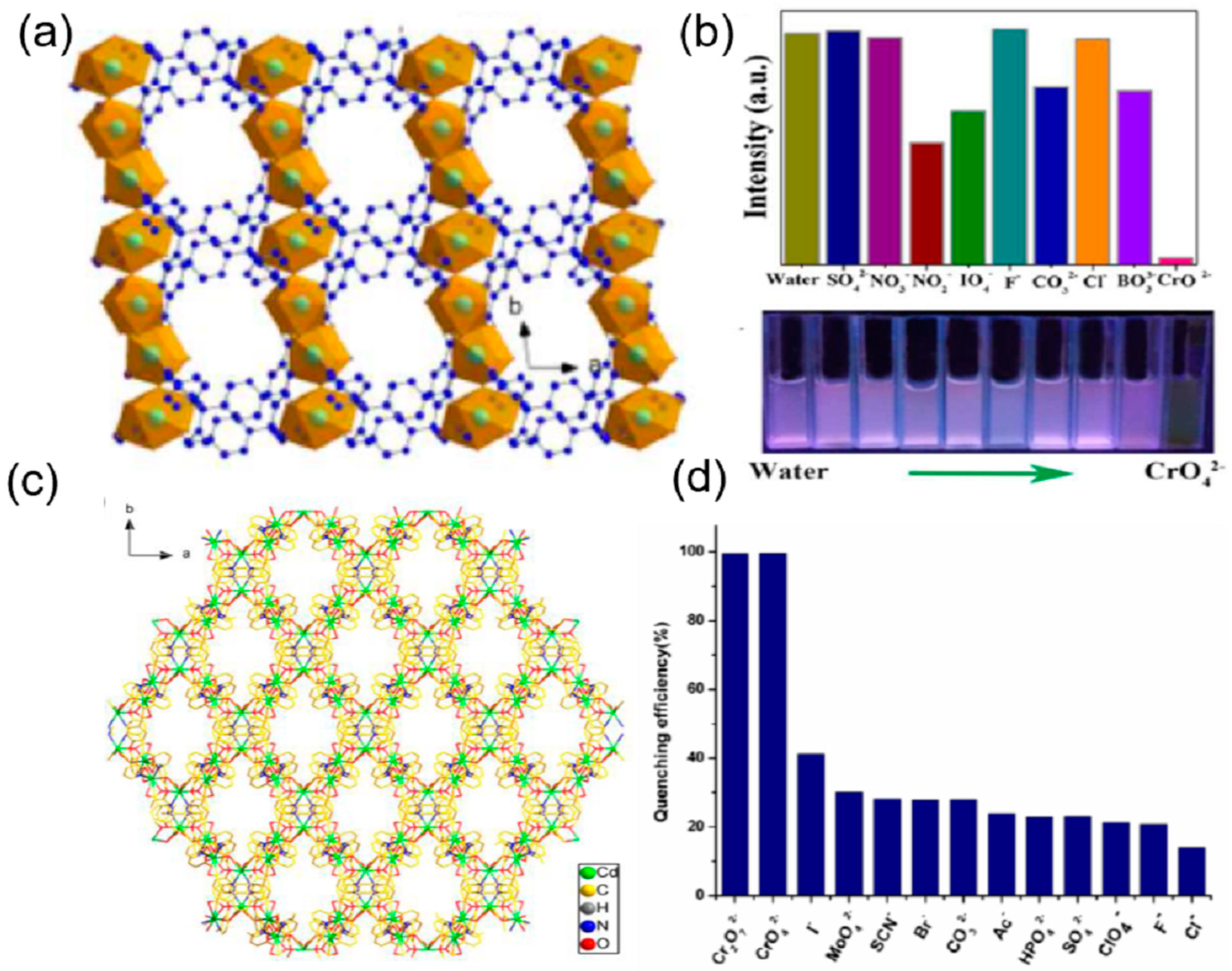
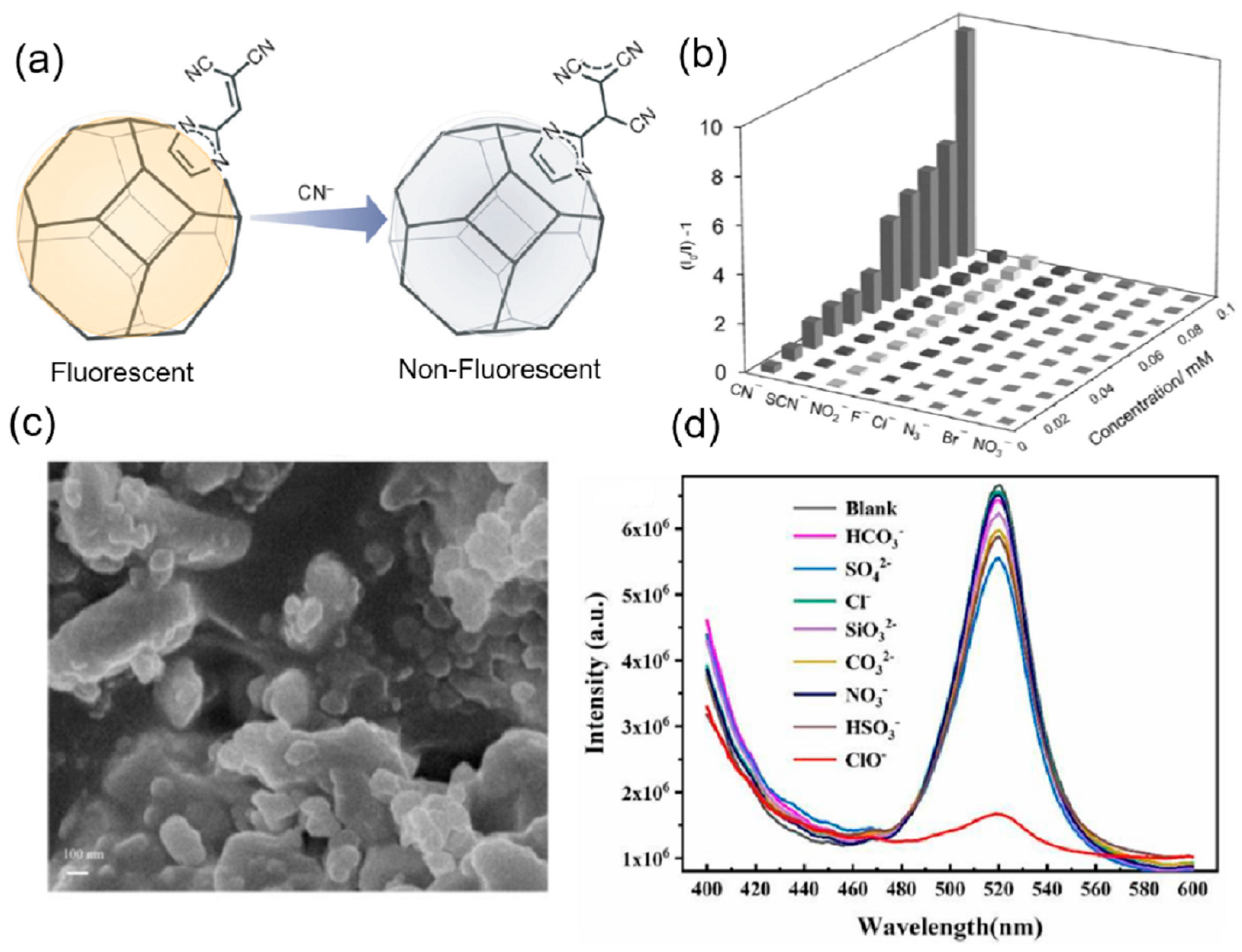
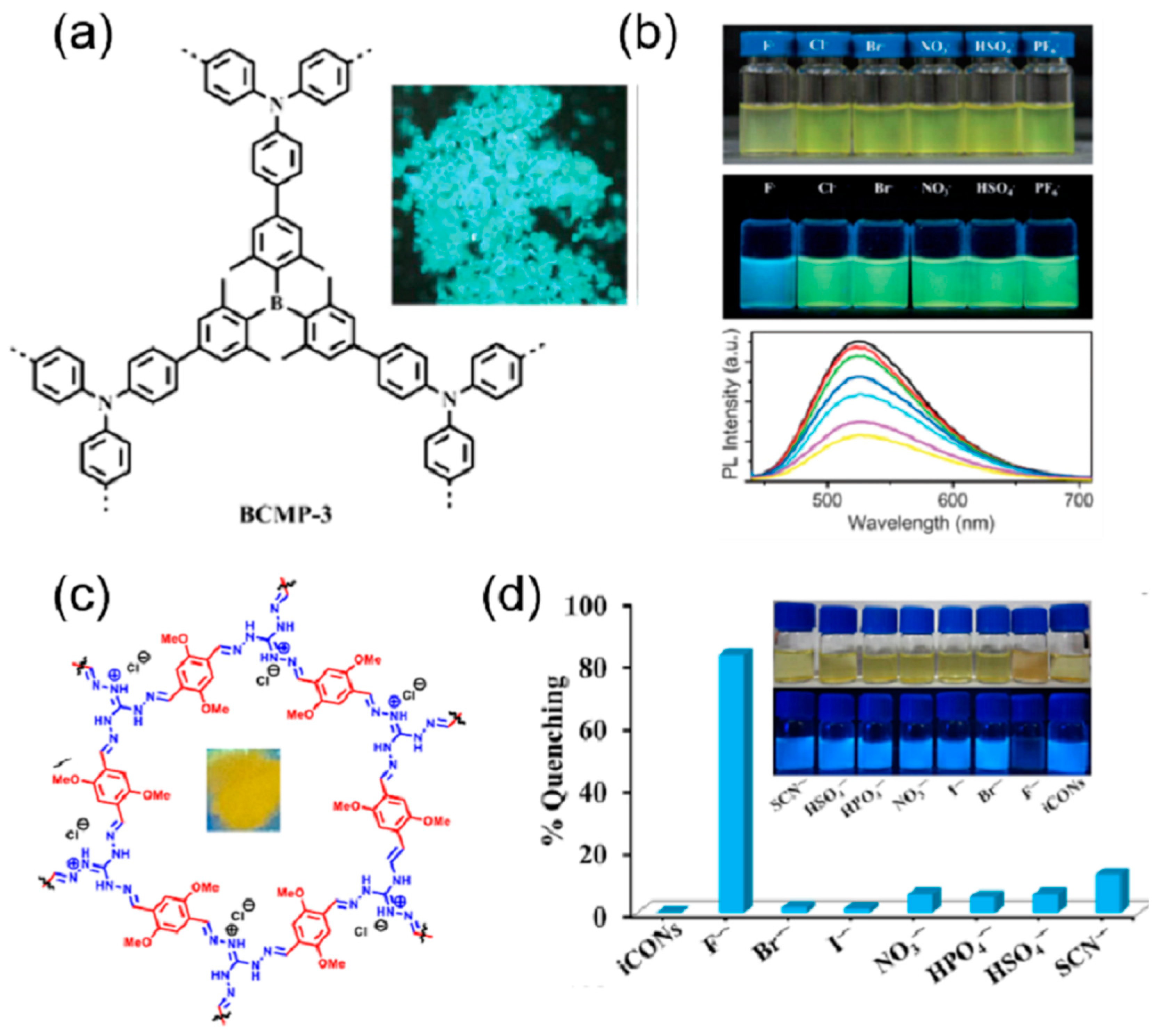

| S. N. | Material | Analyte | Binding Constant (M−1) | LOD | Surface Area (m2g−1) | Recyclability (Cycles) | Media | Ref. |
|---|---|---|---|---|---|---|---|---|
| Metal-Organic Frameworks (MOFs) | ||||||||
| 1 | UiO-66-NH2 | PO43− | 1.25 μM | 671 | water | [66] | ||
| 2 | TbZn(abtc) | NO2− | water | [67] | ||||
| 3 | [Zn(tpbpc)2] | CrO42− and Cr2O72− | 1.65 × 105 and 1.13 × 104 | 4.66 × 10−8 M and 6.76 × 10−7 M | 6 | water | [68] | |
| 4 | Cd-MOF | I− | 1.8 × 104 | 0.63 µM | water | [69] | ||
| 5 | NH2-UiO-66 | F− | 0.229 mg L−1 | 1200 | water | [70] | ||
| 6 | [Zn(afsba)(bbtz)1.5(H2O)2]·2H2O | CrO42− and Cr2O72− | 2.06 × 104 and 4.42 × 104 | 0.22 ppm and 0.26 ppm | water | [71] | ||
| 7 | [Cu2(tpt)2(tda)2].H2O | CrO42− | 2.1 × 104 | 1.64 × 10−5 M | water | [72] | ||
| 8 | UiO-66-NH-BT | CrO42− and Cr2O72− | 6.7 × 103 and 3.9 × 103 | 47.7 ppb and 280 ppb | 384 | 5 | water | [74] |
| 9 | [Zn2(TzTz)2(BDC)2]·2DMF | Cr2O72− and MnO4− | 9 × 107 and 4.8 × 103 | 4.0 μM | 4 | water | [73] | |
| 10 | DUT-52 | CN− | 0.23 μM | 1105 | 2 | water | [75] | |
| 11 | Zn-DMBI | H2PO4− | 5.1 × 104 | 0.13 ppm | 72.9 | water | [76] | |
| 12 | Compound 1 | Cr2O72− | 9.12 × 103 | 3.28 μM | water | [77] | ||
| 13 | Compound 2 | Cr2O72− | 1.56 × 104 | 7.69 μM | water | [77] | ||
| 14 | Compound 3 | Cr2O72− | 8.60 × 103 | 10.40 μM | water | [77] | ||
| 15 | {[H2N(Me)2]2[Zn5(L)2(OH)2]·3DMF·4H2O}n | Cr2O72− | 1.455 × 104 | 1.86 × 10−4 μM | 5 | water | [78] | |
| 11 | Eu3+@MIL-124 | Cr2O72− | 6.034 × 104 | 0.15 µM | water | [79] | ||
| 12 | Eu3+@MIL-121 | F− and Cr2O72− | 2.07 × 103 and 4.34 × 103 | 0.063 μM and 0.054 μM | 165 | water | [80] | |
| 13 | Pyrene taggedUiO-66-NH2 | F− and H2PO4− | 8.2 × 10−7 M and 7.3 × 10−7 M | water | [81] | |||
| 14 | UiO-66-NH2-IM | S2O82− | 2.883 × 103 | 8.63 µM | 352 | water | [82] | |
| 15 | NH2-MIL-68(In)@CHO | HSO3− | 0.047 ppm | water | [83] | |||
| 16 | Eu-BTB | PO43− | 7.97 × 103 | 10−5 mol/L | water | [84] | ||
| 17 | Y(BTC)(H2O)6]n:0.1Eu | CrO42− and Cr2O72− | 1.18 × 103 and 4.52 × 103 | 0.03 μM and 0.04 μM | 166.04 | water | [85] | |
| 18 | Tb(H2O)(BTB) | PO43− | 35 μM | water | [61] | |||
| 19 | [Eu7(mtb)5(H2O)16].NO3 | Cr2O72− | 3.3 × 104 | 0.56 ppb | 634.5 | water | [86] | |
| 20 | NU-1000B | Cr2O72− | 1.3 × 104 | 1.8 μM | 2288 | 3 | water | [87] |
| 21 | [Tb2(ptptc)1.5(H2O)2]n | Cr2O72− | water | [88] | ||||
| 22 | [Cd3(cpota)2(phen)3]n·5nH2O | CrO42− and Cr2O72− | 6.9 × 103 and 1.21 × 104 | 4.18 × 10−7 M and 3.70 × 10−7 M | water | [89] | ||
| 23 | {[(CH3)2NH2][Eu(TCPB)(H2O)2]·DMF}n | PO43−, HPO42− | 0.139 mM | water | [90] | |||
| Zeolitic Imidazolate Frameworks (ZIFs) | ||||||||
| 24 | ZIF-90a | CrO42− | 5.03 × 103 | 829.2341 | water | [91] | ||
| 25 | M-ZIF-90 | CN− | 3.3 × 105 | 2 μM | water | [92] | ||
| 26 | nano-ZIF-8 | ClO− and SCN− | 0.133 nM 0.204 nM | 5 | water | [93] | ||
| 27 | MAPbBr3@ZIF-8 | ClO− | 0.141 × 106 | 31.9 nM | 207.9 | water | [94] | |
| Covalent-Organic Frameworks (COFs) | ||||||||
| 28 | BCMP-3 | F− | 950 | 5 | THF | [95] | ||
| 29 | TFPPy-DETHz-COF | F− | 50.5 ppb | 1090 | water | [96] | ||
| 30 | Fe-CTF | F− | 5 nM | water | [97] | |||
| 31 | COF-TT | CrO42−, Cr2O72−, and MnO4− | 1.4 × 104, 1.4 × 104, and 1.5 × 104 | 3.43 × 10−4 M, 3.43 × 10−4 M, and 3.20 × 10−4 M | 446 | water | [98] | |
| 32 | PP-TzDa | MnO4− | 3.279 × 103 | 0.01 mM | 583 | water | [99] | |
| 33 | DATGCl-iCONs | F− | 2.25 × 103 | 5 ppb | 155 | 5 | water | [100] |
| 34 | UiO@COF1 | PO43− | 0.067 μM | 504 | water | [101] | ||
| 35 | IC-COF | PO43− and CO32− | 3.5 × 103 and 3.1 × 103 | 0.61 μM 1.2 μM | 647 | 5 | water | [102] |
| 36 | TFHPB-TAPB-COF | F− | 3.2 × 104 | 1592 × 10−9 M | 751 | water | [103] | |
| 37 | TFHPB-TTA-COF | F− | 3.4 × 104 | 1125 × 10−9 M | 1472 | water | [103] | |
| 38 | ACOF | F− | 1.2 × 104 | 2.5 μM | 674 | 3 | water | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, A.; Khan, M.Y.; Khan, A.; Usman, M.; Zahir, M.H. Reticular Chemistry for Optical Sensing of Anions. Int. J. Mol. Sci. 2023, 24, 13045. https://doi.org/10.3390/ijms241713045
Helal A, Khan MY, Khan A, Usman M, Zahir MH. Reticular Chemistry for Optical Sensing of Anions. International Journal of Molecular Sciences. 2023; 24(17):13045. https://doi.org/10.3390/ijms241713045
Chicago/Turabian StyleHelal, Aasif, Mohd Yusuf Khan, Abuzar Khan, Muhammad Usman, and Md. Hasan Zahir. 2023. "Reticular Chemistry for Optical Sensing of Anions" International Journal of Molecular Sciences 24, no. 17: 13045. https://doi.org/10.3390/ijms241713045
APA StyleHelal, A., Khan, M. Y., Khan, A., Usman, M., & Zahir, M. H. (2023). Reticular Chemistry for Optical Sensing of Anions. International Journal of Molecular Sciences, 24(17), 13045. https://doi.org/10.3390/ijms241713045







