Huntingtin Interacting Proteins and Pathological Implications
Abstract
1. Introduction
2. Structure of HTT and Expression of Mutant HTT
3. HTT Interacting Proteins
3.1. Proteins Binding to HTT Enhanced by Poly Q Expansion
3.1.1. Huntingtin-Associated Protein 1 (HAP1)
3.1.2. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
3.1.3. Transcription Factor Sp1 (Sp1)
3.1.4. Homocysteine-Induced Endoplasmic Reticulum Protein (Herp)
3.2. Proteins Binding to HTT Inhibited by PolyQ Expansion
3.2.1. Huntingtin-Interacting Protein 1 (HIP1)
3.2.2. Kynurenine 3-Monooxygenase (KMO)
4. Interacting Protein that Can Enhance the mHTT Toxicity
5. HTT Interacting Proteins that Can Alter mHtt Aggregates
6. Proteins with Impaired Function When Interacting with mHtt
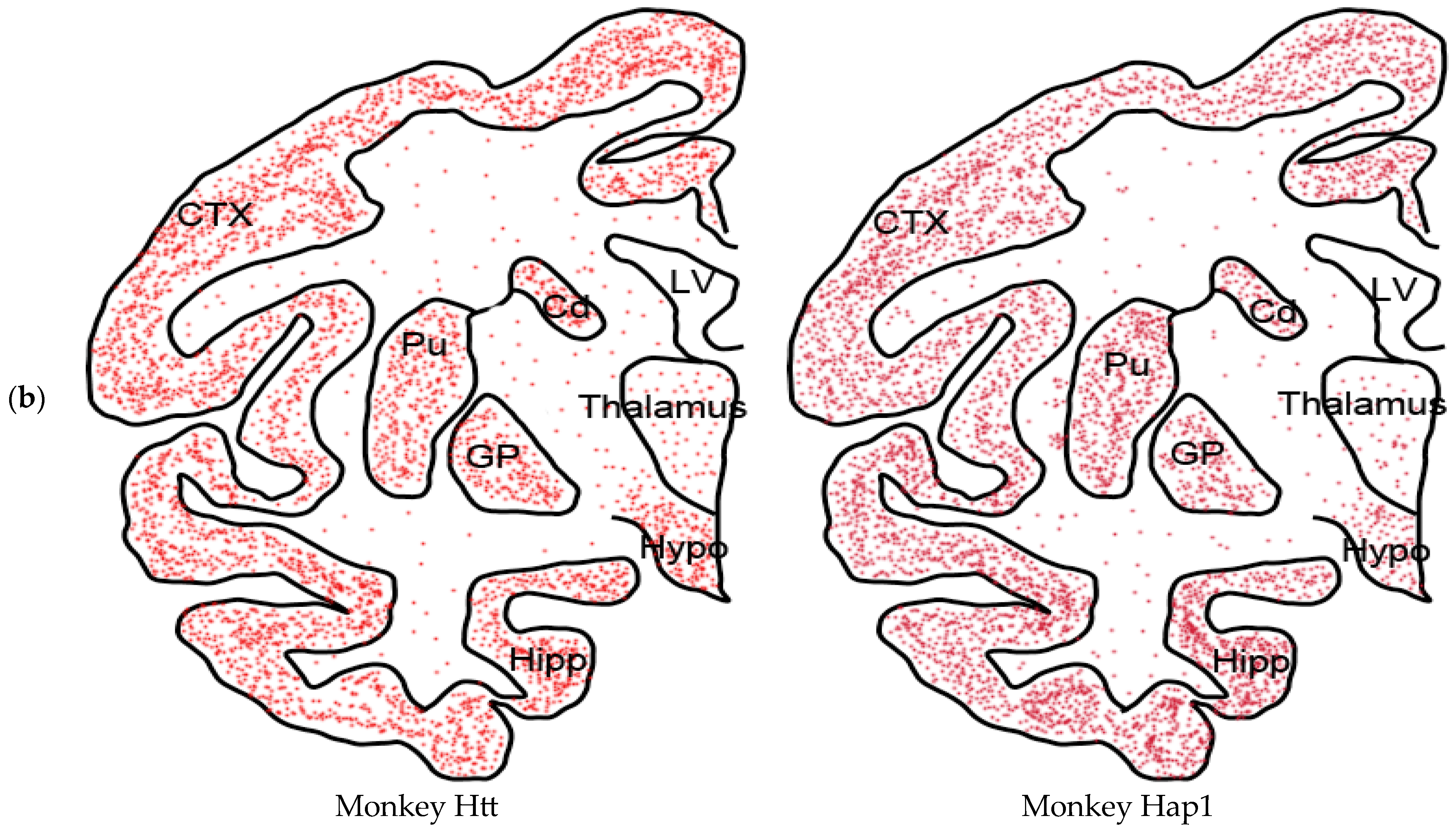
7. New Insights in HAP1
- (1)
- HAP1 in the primate brains
- (2)
- HAP1 is neuroprotective in monkey tissue
- (3)
- Other functions of HAP1 in humans
8. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Tabrizi, S.J. Clinical Features of Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Chiarugi, S.; Margheriti, F.; Garau, G. Mapping, Structure and Modulation of PPI. Front. Chem. 2021, 9, 718405. [Google Scholar] [CrossRef] [PubMed]
- Wanker, E.E.; Ast, A.; Schindler, F.; Trepte, P.; Schnoegl, S. The pathobiology of perturbed mutant huntingtin protein–protein interactions in Huntington’s disease. J. Neurochem. 2019, 151, 507–519. [Google Scholar] [CrossRef]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.P.; Raymond, L. Huntington Disease. In Neurobiology of Brain Disorders; Elsevier: Amsterdam, The Netherlands, 2015; pp. 303–320. [Google Scholar] [CrossRef]
- Ochaba, J.; Lukacsovich, T.; Csikos, G.; Zheng, S.; Margulis, J.; Salazar, L.; Mao, K.; Lau, A.L.; Yeung, S.Y.; Humbert, S.; et al. Potential function for the Huntingtin protein as a scaffold for selective autophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 16889–16894. [Google Scholar] [CrossRef]
- Pandey, N.K.; Isas, J.M.; Rawat, A.; Lee, R.V.; Langen, J.; Pandey, P.; Langen, R. The 17-residue-long N terminus in huntingtin controls stepwise aggregation in solution and on membranes via different mechanisms. J. Biol. Chem. 2018, 293, 2597–2605. [Google Scholar] [CrossRef]
- Pigazzini, M.L.; Lawrenz, M.; Margineanu, A.; Schierle, G.S.K.; Kirstein, J. An Expanded Polyproline Domain Maintains Mutant Huntingtin Soluble in vivo and During Aging. Front. Mol. Neurosci. 2021, 14, 721749. [Google Scholar] [CrossRef]
- Xia, J.; Lee, D.H.; Taylor, J.; Vandelft, M.; Truant, R. Huntingtin contains a highly conserved nuclear export signal. Hum. Mol. Genet. 2003, 12, 1393–1403. [Google Scholar] [CrossRef]
- Desmond, C.R.; Atwal, R.S.; Xia, J.; Truant, R. Identification of a karyopherin β1/β2 proline-tyrosine nuclear localization signal in huntingtin protein. J. Biol. Chem. 2012, 287, 39626–39633. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, A.; Holmes, B.B.; Marasa, J.C.; Diamond, M.I. An N-terminal nuclear export signal regulates trafficking and aggregation of Huntingtin (Htt) protein exon 1. J. Biol. Chem. 2013, 288, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, T.; Woloshansky, T.; Xia, J.; Truant, R. The huntingtin N17 domain is a multifunctional CRM1 and Ran-dependent nuclear and cilial export signal. Hum. Mol. Genet. 2013, 22, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-G.; Yan, X.-Z.; Song, A.-X.; Chang, Y.-G.; Gao, X.-C.; Jiang, N.; Zhang, Q.; Hu, H.-Y. Structural Insights into the Specific Binding of Huntingtin Proline-Rich Region with the SH3 and WW Domains. Structure 2006, 14, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Arbez, N.; Ratovitski, T.; Roby, E.; Chighladze, E.; Stewart, J.C.; Ren, M.; Wang, X.; Lavery, D.J.; Ross, C.A. Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity. J. Biol. Chem. 2017, 292, 19238–19249. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Jing, L.; Huang, L.; Chen, L.; Zhao, X.; Yang, W.; Pan, Y.; Yin, P.; Qin, Z.S.; et al. Truncation of mutant huntingtin in knock-in mice demonstrates exon1 huntingtin is a key pathogenic form. Nat. Commun. 2020, 11, 2582. [Google Scholar] [CrossRef]
- Sathasivam, K.; Neueder, A.; Gipson, T.A.; Landles, C.; Benjamin, A.C.; Bondulich, M.K.; Smith, D.L.; Faull, R.L.; Roos, R.A.; Howland, D.; et al. Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease. Proc. Natl. Acad. Sci. USA 2013, 110, 2366–2370. [Google Scholar] [CrossRef]
- Labadorf, A.T.; Myers, R.H. Evidence of Extensive Alternative Splicing in Post Mortem Human Brain HTT Transcription by mRNA Sequencing. PLoS ONE 2015, 10, e0141298. [Google Scholar] [CrossRef]
- Neueder, A.; Landles, C.; Ghosh, R.; Howland, D.; Myers, R.H.; Faull, R.L.M.; Tabrizi, S.J.; Bates, G.P. The pathogenic exon 1 HTT protein is produced by incomplete splicing in Huntington’s disease patients. Sci. Rep. 2017, 7, 1307. [Google Scholar] [CrossRef]
- Franich, N.R.; Hickey, M.A.; Zhu, C.; Osborne, G.F.; Ali, N.; Chu, T.; Bove, N.H.; Lemesre, V.; Lerner, R.P.; Zeitlin, S.O.; et al. Phenotype onset in Huntington’s disease knock-in mice is correlated with the incomplete splicing of the mutant huntingtin gene. J. Neurosci. Res. 2019, 7, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Gafni, J.; Ellerby, L.M. Calpain activation in Huntington’s disease. J. Neurosci. 2002, 22, 4842–4849. [Google Scholar] [CrossRef]
- Lunkes, A.; Lindenberg, K.S.; Ben-Haïem, L.; Weber, C.; Devys, D.; Landwehrmeyer, G.B.; Mandel, J.L.; Trottier, Y. Proteases acting on mutant huntingtin generate cleaved products that differentially build up cytoplasmic and nuclear inclusions. Mol. Cell 2002, 10, 259–269. [Google Scholar] [CrossRef]
- Landles, C.; Sathasivam, K.; Weiss, A.; Woodman, B.; Moffitt, H.; Finkbeiner, S.; Sun, B.; Gafni, J.; Ellerby, L.M.; Trottier, Y.; et al. Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease. J. Biol. Chem. 2010, 285, 8808–8823. [Google Scholar] [CrossRef] [PubMed]
- Juenemann, K.; Weisse, C.; Reichmann, D.; Kaether, C.; Calkhoven, C.F.; Schilling, G. Modulation of mutant huntingtin N-terminal cleavage and its effect on aggregation and cell death. Neurotox. Res. 2011, 20, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Ratovitski, T.; Chighladze, E.; Waldron, E.; Hirschhorn, R.R.; Ross, C.A. Cysteine proteases bleomycin hydrolase and cathepsin Z mediate N-terminal proteolysis and toxicity of mutant huntingtin. J. Biol. Chem. 2011, 286, 12578–12589. [Google Scholar] [CrossRef] [PubMed]
- Tebbenkamp, A.T.; Crosby, K.W.; Siemienski, Z.B.; Brown, H.H.; Golde, T.E.; Borchelt, D.R. Analysis of proteolytic processes and enzymatic activities in the generation of huntingtin n-terminal fragments in an HEK293 cell model. PLoS ONE 2012, 7, e50750. [Google Scholar] [CrossRef]
- Schulte, J.; Littleton, J.T. The biological function of the Huntingtin protein and its relevance to Huntington’s Disease pathology. Curr. Trends Neurol. 2011, 5, 65–78. [Google Scholar]
- Li, S.-H.; Li, X.-J. Huntington and its Role in Neuronal Degeneration. Neuroscientist 2004, 10, 467–475. [Google Scholar] [CrossRef]
- Caron, N.S.; Banos, R.; Yanick, C.; Aly, A.E.; Byrne, L.M.; Smith, E.D.; Xie, Y.; Smith, S.E.P.; Potluri, N.; Findlay Black, H.; et al. Mutant Huntingtin is cleared from the Brain via Active Mechanisms in Huntington Disease. J. Neurosci. 2021, 41, 780–796. [Google Scholar] [CrossRef]
- Li, X.-J.; Li, S.-H.; Sharp, A.H.; Nucifora, F.C.; Schilling, G.; Lanahan, A.; Worley, P.; Snyder, S.H.; Ross, C.A. A huntingtin-associated protein enriched in brain with implications for pathology. Nature 1995, 378, 398–402. [Google Scholar] [CrossRef]
- Wu, L.L.; Zhou, X.F. Huntingtin associated protein 1 and its functions. Cell Adhes. Migr. 2009, 3, 71–76. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, S.; Yang, H.; Zhu, L.; Pan, Y.; Jing, L.; Tang, B.; Li, S.; Li, X.-J. Loss of Hap1 selectively promotes striatal degeneration in Huntington disease mice. Proc. Natl. Acad. Sci. USA 2020, 117, 20265–20273. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Chen, L.; Chen, X.-S.; Pan, M.; Zhang, Y.; Wang, Q.; Yang, W.; Yin, P.; He, D.; et al. Differential expression and roles of Huntingtin and Huntingtin-associated protein 1 in the mouse and primate brains. Cell Mol. Life Sci. 2022, 79, 554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Li, S.-H. HAP1 and intracellular Trafficking. Trends Pharmacol. 2005, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kang, S.; Wang, X.; Rosales, J.L.; Gao, X.; Byun, H.-G.; Jin, Y.; Fu, S.; Wang, J.; Lee, K.-Y. HAP1 loss confers l-asparaginase resistance in ALL by downregulating the calpain-1-Bid-caspase-3/12 pathway. Blood 2019, 133, 2222–2232. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, A.; Wang, Z.; Xu, X.-H.; Tao, Y. Biological functions and potential therapeutic applications of huntingtin-associated protein 1: Progress and prospects. Clin. Transl. 2022, 24, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Wang, J.; Mao, L.; Tang, B.; Vanderklish, P.W.; Liao, X.; Xiong, Z.Q.; Liao, L. HAP1 is an in vivo UBE3A target that augments autophagy in a mouse model of Angelman syndrome. Neurobiol. Dis. 2019, 132, 104585. [Google Scholar] [CrossRef]
- Shinoda, K.; Nagano, M.; Osawa, Y. An aromatase-associated cytoplasmic inclusion, the “stigmoid body”, in the rat brain: II. Ultrastructure (with a review of its history and nomenclature). J. Comp. Neurol. 1993, 329, 1–19. [Google Scholar] [CrossRef]
- Li, S.H.; Gutekunst, C.A.; Hersch, S.M.; Li, X.J. Association of HAP1 isoforms with a unique cytoplasmic structure. J. Neurochem. 1998, 71, 2178–2185. [Google Scholar] [CrossRef]
- Sheng, G.; Xu, X.; Lin, Y.-F.; Wang, C.-E.; Rong, J.; Cheng, D.; Peng, J.; Jiang, X.; Li, S.-H.; Li, X.-J. Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J. Clin. Investig. 2008, 118, 2785–2795. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, S.; Xin, N.; Gaertig, M.A.; Reeves, R.H.; Li, S.; Li, X.-J. DYRK1A regulates Hap1-Dcaf7/WDR68 binding with implication for delayed growth in Down syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E1224–E1233. [Google Scholar] [CrossRef]
- Fujinaga, R.; Yanai, A.; Nakatsuka, H.; Yoshida, K.; Takeshita, Y.; Uozumi, K.; Zhao, C.; Hirata, K.; Kokubu, K.; Nagano, M.; et al. Anti-human placental antigen complex X-P2 (hPAX-P2) anti-serum recognizes C-terminus of huntingtin-associated protein 1A common to 1B as a determinant marker for the stigmoid body. Histochem. Cell Biol. 2007, 128, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, E.J. Glyceraldehyde-3-phosphate Dehydrogenase Is Phosphorylated by Protein Kinase Cι/λ and Plays a Role in Microtubule Dynamics in the Early Secretory Pathway. J. Biol. Chem. 2002, 277, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.R.; Enghild, J.J.; Martin, M.E.; Jou, Y.-S.; Myers, R.M.; Roses, A.D.; Vance, J.M.; Strittmatter, W.J. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nat. Med. 1996, 2, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.-I.; Hara, M.R.; Cascio, M.B.; Wellington, C.L.; Hayden, M.R.; Ross, C.A.; Ha, H.C.; Li, X.-J.; Snyder, S.H.; Sawa, A. Mutant Huntingtin: Nuclear translocation and cytotoxicity mediated by GAPDH. Proc. Natl. Acad. Sci. USA 2006, 103, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Disatnik, M.H.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef]
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Li, S.-H.; Cheng, A.L.; Zhou, H.; Lam, S.; Rao, M.; Li, H.; Li, X.-J. Interaction of Huntington Disease Protein with Transcriptional Activator Sp1. Mol. Cell Biol. 2002, 22, 1277–1287. [Google Scholar] [CrossRef]
- Dunah, A.W.; Jeong, H.; Griffin, A.; Kim, Y.-M.; Standaert, D.G.; Hersch, S.M.; Mouradian, M.M.; Young, A.B.; Tanese, N.; Krainc, D. Sp1 and TAFII130 Transcriptional Activity Disrupted in Early Huntington’s Disease. Science 2002, 296, 2238–2243. [Google Scholar] [CrossRef]
- Bradford, J.; Shin, J.-Y.; Roberts, M.; Wang, C.-E.; Li, X.-J.; Li, S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. USA 2009, 106, 22480–22485. [Google Scholar] [CrossRef]
- Wang, R.; Luo, Y.; Ly, P.T.; Cai, F.; Zhou, W.; Zou, H.; Song, W. Sp1 regulates human huntingtin gene expression. J. Mol. Neurosci. 2012, 47, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Norflus, F.; Singh, B.; Swindell, M.K.; Buzescu, R.; Bejarano, M.; Chopra, R.; Zucker, B.; Benn, C.L.; Dirocco, D.P.; et al. Sp1 Is Up-regulated in Cellular and Transgenic Models of Huntington Disease, and Its Reduction Is Neuroprotective. J. Biol. Chem. 2006, 281, 16672–16680. [Google Scholar] [CrossRef] [PubMed]
- Kokame, K.; Agarwala, K.L.; Kato, H.; Miyata, T. Herp, a New Ubiquitin-like Membrane Protein Induced by Endoplasmic Reticulum Stress. J. Biol. Chem. 2000, 275, 32846–32853. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cao, L.; Liang, X.; Du, A.; Peng, T.; Li, H. Herp Promotes Degradation of Mutant Huntingtin: Involvement of the Proteasome and Molecular Chaperones. Mol. Neurobiol. 2018, 55, 7652–7668. [Google Scholar] [CrossRef]
- Kalchman, M.; Koide, H.; McCutcheon, K.; Graham, R.; Nichol, K.; Nishiyama, K.; Kazemi-Esfarjani, P.; Lynn, F.; Wellington, C.; Metzler, M.; et al. HIP1, a human homologue of S-cerevisiae Sla2p, interacts with membrane-associated huntingtin in the brain. Nat. Genet. 1997, 16, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wanker, E.E.; Rovira, C.; Scherzinger, E.; Hasenbank, R.; Wälter, S.; Tait, D.; Colicelli, J.; Lehrach, H. HIP-I: A huntingtin interacting protein isolated by the yeast two-hybrid system. Hum. Mol. Genet. 1997, 6, 487–495. [Google Scholar] [CrossRef]
- Waelter, S.; Scherzinger, E.; Hasenbank, R.; Nordhoff, E.; Lurz, R.; Goehler, H.; Gauss, C.; Sathasivam, K.; Bates, G.P.; Lehrach, H.; et al. The huntingtin interacting protein HIP1 is a clathrin and alpha-adaptin-binding protein involved in receptor-mediated endocytosis. Hum. Mol. Genet. 2001, 10, 1807–1817. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, S.; Fang, X.; Jiang, Y.; Fang, T.; Liu, J.; Lu, K. Therapeutic Advances of Rare ALK Fusions in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 7816–7831. [Google Scholar] [CrossRef]
- Teresina, L.; Max, B.; Amit, L.; Erjing, G.; Carolyn, H.; Percio, S.G. Huntingtin-interacting protein 1 (HIP1) regulates arthritis severity and synovial fibroblast invasiveness by altering PDGFR and Rac1 signalling. Ann. Rheum. Dis. 2018, 77, 1627. [Google Scholar] [CrossRef]
- Nicita, F.; Garone, G.; Spalice, A.; Savasta, S.; Striano, P.; Pantaleoni, C.; Spartà, M.V.; Kluger, G.; Capovilla, G.; Pruna, D.; et al. Epilepsy is a possible feature in Williams-Beuren syndrome patients harboring typical deletions of the 7q11.23 critical region. Am. J. Med. Genet. Part A 2016, 170, 148–155. [Google Scholar] [CrossRef]
- Swaih, A.M.; Breda, C.; Sathyasaikumar, K.V.; Allcock, N.; Collier, M.E.W.; Mason, R.P.; Feasby, A.; Herrera, F.; Outeiro, T.F.; Schwarcz, R.; et al. Kynurenine 3-Monooxygenase Interacts with Huntingtin at the Outer Mitochondrial Membrane. Biomedicines 2022, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Pérez De La Cruz, V.; Pineda, B.; Vázquez Cervantes, G.I.; Ramírez Ortega, D.; Donley, D.W.; Severson, P.L.; West, B.L.; Giorgini, F.; Fox, J.H.; et al. Cellular Localization of Kynurenine 3-Monooxygenase in the Brain: Challenging the Dogma. Antioxidants 2022, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Hajibeygi., R.; Shool., S.; Sodeifian., F.; Klegeris, A.; McElhinney, A.; et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front. Immunol. 2022, 13, 997240. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.-Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.-Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef]
- Bondulich, M.K.; Fan, Y.; Song, Y.; Giorgini, F.; Bates, G.P. Ablation of kynurenine 3-monooxygenase rescues plasma inflammatory cytokine levels in the R6/2 mouse model of Huntington’s disease. Sci. Rep. 2021, 11, 5484. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sixt, K.M.; Barrow, R.; Snyder, S.H. Rhes, a Striatal Specific Protein, Mediates Mutant-Huntingtin Cytotoxicity. Science 2009, 324, 1327–1330. [Google Scholar] [CrossRef]
- Ramirrez-Jarquin, U.N.; Manish Sharmaa, M.; Zhou, W.; Shahani, N.; Subramaniam, S. Deletion of SUMO1 attenuates behavioral and anatomical deficits by regulating autophagic activities in Huntington disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2107187119. [Google Scholar] [CrossRef]
- Mealer, R.G.; Subramaniam, S.; Snyder, S.H. Rhes Deletion Is Neuroprotective in the 3-Nitropropionic Acid Model of Huntington’s Disease. J. Neurosci. 2013, 33, 4206–4210. [Google Scholar] [CrossRef]
- Mealer, R.G.; Murray, A.J.; Shahani, N.; Subramaniam, S.; Snyder, S.H. Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J. Biol. Chem. 2014, 289, 3547–3554. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Sinha, M.; Mukhopadhyay, D.; Bhattacharyya, N.P. HYPK, a Huntingtin interacting protein, reduces aggregates and apoptosis induced by N-terminal Huntingtin with 40 glutamines in Neuro2a cells and exhibits chaperone-like activity. Hum. Mol. Genet. 2008, 17, 240–255. [Google Scholar] [CrossRef]
- Choudhury, K.R.; Bhattacharyya, N.P. Chaperone protein HYPK interacts with the first 17 amino acid region of Huntingtin and modulates mutant HTT-mediated aggregation and cytotoxicity. Biochem. Biophys. Res. Commun. 2015, 456, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K.; Roy, A.; Ranjan, A. The ATPase VCP/p97 functions as a disaggregase against toxic Huntingtin-exon1 aggregates. FEBS Lett. 2018, 592, 2680–2692. [Google Scholar] [CrossRef] [PubMed]
- Topolska-Woś, A.M.; Chazin, W.J.; Filipek, A. CacyBP/SIP—Structure and variety of functions. Biochim. Biophys. Acta BBA—Gen. Subj. 2016, 1860, 79–85. [Google Scholar] [CrossRef]
- Latoszek, E.; Wiweger, M.; Ludwiczak, J.; Dunin-Horkawicz, S.; Kuznicki, J.; Czeredys, M. Siah-1-interacting protein regulates mutated huntingtin protein aggregation in Huntington’s disease models. Cell Biosci. 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bensalel, J.; Raju, S.; Capobianco, E.; Lu, M.L.; Wei, J. Characterization of huntingtin interactomes and their dynamic responses in living cells by proximity proteomics. J. Neurochem. 2023, 164, 512–528. [Google Scholar] [CrossRef]
- Morrow, C.S.; Porter, T.J.; Xu, N.; Arndt, Z.P.; Ako-Asare, K.; Heo, H.J.; Thompson, E.A.N.; Moore, D.L. Vimentin Coordinates Protein Turnover at the Aggresome during Neural Stem Cell Quiescence Exit. Cell Stem Cell 2020, 26, 558–568.e559. [Google Scholar] [CrossRef]
- Bauer, P.O.; Hudec, R.; Goswami, A.; Kurosawa, M.; Matsumoto, G.; Mikoshiba, K.; Nukina, N. ROCK-phosphorylated vimentin modifies mutant huntingtin aggregation via sequestration of IRBIT. Mol. Neurodeger. 2012, 7, 43. [Google Scholar] [CrossRef]
- Tousley, A.; Iuliano, M.; Weisman, E.; Sapp, E.; Zhang, N.; Vodicka, P.; Alexander, J.; Aviolat, H.; Gatune, L.; Reeves, P.; et al. Rac1 Activity Is Modulated by Huntingtin and Dysregulated in Models of Huntington’s Disease. J. Huntingt. Dis. 2019, 8, 53–69. [Google Scholar] [CrossRef]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.-L. Roles of Rho GTPases in Intracellular Transport and Cellular Transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Brakebusch, C. Rho GTPase Signaling in Health and Disease: A Complex Signaling Network. Cells 2021, 10, 401. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Bento, C.F.; Ricketts, T.; Vicinanza, M.; Siddiqi, F.; Pavel, M.; Squitieri, F.; Hardenberg, M.C.; Imarisio, S.; Menzies, F.M.; et al. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature 2017, 545, 108–111. [Google Scholar] [CrossRef]
- Zeitlin, S.; Liu, J.P.; Chapman, D.L.; Papaioannou, V.E.; Efstratiadis, A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat. Genet. 1995, 11, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lesinskienė, S.; Rojaka, D.; Praninskienė, R.; Morkūnienė, A.; Matulevičienė, A.; Utkus, A. Juvenile Huntington’s disease: Two case reports and a review of the literature. J. Med. Case Rep. 2020, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.F.; Ross, C.A. Isolation of a 40-kDa Huntingtin-associated Protein. J. Biol. Chem. 2001, 276, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Santelli, E.; Leone, M.; Li, C.; Fukushima, T.; Preece, N.E.; Olson, A.J.; Ely, K.R.; Reed, J.C.; Pellecchia, M.; Liddington, R.C.; et al. Structural Analysis of Siah1-Siah-interacting Protein Interactions and Insights into the Assembly of an E3 Ligase Multiprotein Complex. J. Biol. Chem. 2005, 280, 34278–34287. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Lu, T.; Furuya, T.; Degterev, A.; Mizushima, N.; Yoshimori, T.; Macdonald, M.; Yankner, B.; Yuan, J. Regulation of Intracellular Accumulation of Mutant Huntingtin by Beclin 1. J. Biol. Chem. 2006, 281, 14474–14485. [Google Scholar] [CrossRef]
- Pircs, K.; Drouin-Ouellet, J.; Horváth, V.; Gil, J.; Rezeli, M.; Garza, R.; Grassi, D.A.; Sharma, Y.; St-Amour, I.; Harris, K.; et al. Distinct subcellular autophagy impairments in induced neurons from patients with Huntington’s disease. Brain 2022, 145, 3035–3057. [Google Scholar] [CrossRef]
- Croce, K.R.; Yamamoto, A. A role for autophagy in Huntington’s disease. Neurobiol. Dis. 2019, 122, 16–22. [Google Scholar] [CrossRef]
- Valionyte, E.; Yang, Y.; Roberts, S.L.; Kelly, J.; Lu, B.; Luo, S. Lowering Mutant Huntingtin Levels and Toxicity: Autophagy-Endolysosome Pathways in Huntington’s Disease. J. Mol. Biol. 2020, 432, 2673–2691. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, S.; Li, X.-J. Genetically modified large animal models for investigating neurodegenerative diseases. Cell Biosci. 2021, 11, 218. [Google Scholar] [CrossRef]
- Yin, P.; Li, S.; Li, X.-J.; Yang, W. New pathogenic insights from large animal models of neurodegenerative diseases. Protein Cell 2022, 13, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Caijuan, L.; Jun, L.; Liangxue, L.; Shihua, L.; Sen, Y. Genetically engineered pig models of neurological diseases. Ageing Neurodegener. Dis. 2022, 2, 13. [Google Scholar] [CrossRef]
- Li, S.-H.; Hosseini, S.H.; Gutekunst, C.-A.; Hersch, S.M.; Ferrante, R.J.; Li, X.-J. A Human HAP1 Homologue. J. Biol. Chem. 1998, 273, 19220–19227. [Google Scholar] [CrossRef]
- Bertaux, F.; Sharp, A.H.; Ross, C.A.; Lehrach, H.; Bates, G.P.; Wanker, E. HAP1-huntingtin interactions do not contribute to the molecular pathology in Huntington’s disease transgenic mice. FEBS Lett. 1998, 426, 229–232. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Harper, P.S.; Shaw, D.; Datson, N.; Snell, R.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Schilling, G.; Young, W.S.; Li, X.; Margolis, R.L.; Stine, O.C.; Wagster, M.V.; Abbott, M.H.; Franz, M.L.; Ranen, N.G.; et al. Huntington’s disease gene (IT15) is widely expressed in human and rat tissues. Neuron 1993, 11, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Strong, T.V.; Tagle, D.A.; Valdes, J.M.; Elmer, L.W.; Boehm, K.; Swaroop, M.; Kaatz, K.W.; Collins, F.S.; Albin, R.L. Widespread expression of the human and rat Huntington’s disease gene in brain and nonneural tissues. Nat. Genet. 1993, 5, 259–265. [Google Scholar] [CrossRef]
- Sharp, A.H.; Loev, S.J.; Schilling, G.; Li, S.-H.; Li, X.-J.; Bao, J.; Wagster, M.V.; Kotzuk, J.A.; Steiner, J.P.; Lo, A.; et al. Widespread expression of Huntington’s disease gene (IT15) protein product. Neuron 1995, 14, 1065–1074. [Google Scholar] [CrossRef]
- Landwehrmeyer, G.B.; McNeil, S.M.; Dure, L.S.; Ge, P.; Aizawa, H.; Huang, Q.; Ambrose, C.M.; Duyao, M.P.; Bird, E.D.; Bonilla, E.; et al. Huntington’s disease gene: Regional and cellular expression in brain of normal and affected individuals. Ann. Neurol. 1995, 37, 218–230. [Google Scholar] [CrossRef]
- Harjes, P.; Wanker, E. The hunt for huntingtin function: Interaction partners tell many different stories. Trends Biochem. 2003, 28, 425–433. [Google Scholar] [CrossRef]
- Kaltenbach, L.S.; Romero, E.; Becklin, R.R.; Chettier, R.; Bell, R.; Phansalkar, A.; Strand, A.; Torcassi, C.; Savage, J.; Hurlburt, A.; et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007, 3, e82. [Google Scholar] [CrossRef]
- Miller, J.; Hughes, R.E. Protein Interactions and Target Discovery in Huntington’s Disease. In Neurobiology of Huntington Disease (Applications to Drug Discovery) Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2011; ISBN 978-0-8493-9000. [Google Scholar]
- Vonsattel, J.-P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P., Jr. Neuropathological Classification of Huntington’s Disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Vonsattel, J.P.G.; Keller, C.; Pilar Amaya, M.d. Neuropathology of Huntington’s Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 89, pp. 599–618. [Google Scholar]
- Yan, S.; Tu, Z.; Liu, Z.; Fan, N.; Yang, H.; Yang, S.; Yang, W.; Zhao, Y.; Ouyang, Z.; Lai, C.; et al. A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neurodegeneration in Huntington’s Disease. Cell 2018, 173, 989–1002.e1013. [Google Scholar] [CrossRef]
- Caviston, J.P.; Holzbaur, E.L.F. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009, 19, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Page, K.-J.; Potter, L.; Aronni, S.; Everitt, B.J.; Dunnett, S.B. The expression of Huntingtin-associated protein (HAP1) mRNA in developing, adult and ageing rat CNS: Implications for Huntington’s disease neuropathology. Eur. J. Neurosci. 1998, 10, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, S.-H.; Gao, X.; Cai, Q.; Li, X.-J. Expression and Localization of Huntingtin-Associated Protein 1 (HAP1) in the Human Digestive System. Dig. Dis. Sci. 2019, 64, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-M.; Chen, A.; Zhao, X.; Wang, Z.; Guo, D.; Shao, S.-L.; Tao, Y.-Y.; Li, Q.-J.; Wang, M.-Y.; Ma, W.-S. Huntingtin-associated protein 1 is a potential tumor suppressor for gastric cancer. Mol. Biol. Rep. 2023, 50, 1517–1531. [Google Scholar] [CrossRef]
- Zhu, L.; Song, X.; Tang, J.; Wu, J.; Ma, R.; Cao, H.; Ji, M.; Jing, C.; Wang, Z. Huntingtin-associated protein 1: A potential biomarker of breast cancer. Oncol. Rep. 2013, 29, 1881–1887. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.-Y.; Yin, L.; Wu, J.-Z.; Guo, W.-J.; Wu, J.-F.; Chen, M.; Xia, Y.-Y.; Tang, J.-H.; Ma, Y.-C.; et al. HAP1 gene expression is associated with radiosensitivity in breast cancer cells. Biochem. Biophys. Res. 2015, 456, 162–166. [Google Scholar] [CrossRef]
- Wang, W.; Yan, H.; Zhang, Q.; Song, W.; Li, H.; Xu, J. Evaluating the association of polymorphisms in the HAP1 gene with lung cancer risk: A meta-analysis. Tumor Biol. 2014, 35, 10825–10831. [Google Scholar] [CrossRef] [PubMed]
- Duyao, M.P.; Auerbach, A.B.; Ryan, A.; Persichetti, F.; Barnes, G.T.; McNeil, S.M.; Ge, P.; Vonsattel, J.P.; Gusella, J.F.; Joyner, A.L.; et al. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science 1995, 269, 407–410. [Google Scholar] [CrossRef]
- Nasir, J.; Floresco, S.B.; O’Kusky, J.R.; Diewert, V.M.; Richman, J.M.; Zeisler, J.; Borowski, A.; Marth, J.D.; Phillips, A.G.; Hayden, M.R. Targeted disruption of the Huntington’s disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell 1995, 81, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bin Huang Cheng, J.; Seefelder, M.; Engler, T.; Pfeifer, G.; Oeckl, P.; Otto, M.; Moser, F.; Maurer, M.; Pautsch, A.; et al. The cryo-electron microscopy structure of huntingtin. Nature 2018, 555, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.J.; Deme, J.C.; Hevler, J.F.; Tamara, S.; Lemak, A.; Cantle, J.P.; Szewczyk, M.M.; Begeja, N.; Goss, S.; Zuo, X.; et al. Huntingtin structure is orchestrated by HAP40 and shows a polyglutamine expansion-specific interaction with exon 1. Commun. Biol. 2021, 4, 1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Seefelder, M.; Buck, E.; Engler, T.; Lindenberg, K.S.; Klein, F.; Landwehrmeyer, G.B.; Kochanek, S. HAP40 protein levels are huntingtin-dependent and decrease in Huntington disease. Neurobiol. Dis. 2021, 158, 105476. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, G.; Ye, X.; Chen, D.; Chen, Z.; Xu, Z.; Daniele, M.; Tambone, S.; Ceccacci, A.; Tomei, L.; et al. HAP40 is a conserved central regulator of Huntingtin and a potential modulator of Huntington’s disease pathogenesis. PLoS Genet. 2022, 18, e1010302. [Google Scholar] [CrossRef]
- Metzger, S.; Rong, J.; Nguyen, H.-P.; Cape, A.; Tomiuk, J.; Soehn, A.S.; Propping, P.; Freudenberg-Hua, Y.; Freudenberg, J.; Tong, L.; et al. Huntingtin-associated protein-1 is a modifier of the age-at-onset of Huntington’s disease. Hum. Mol. Genet. 2008, 17, 1137–1146. [Google Scholar] [CrossRef]
- Lee, J.-M.; Huang, Y.; Orth, M.; Gillis, T.; Siciliano, J.; Hong, F.; Mysore, J.S.; Lucente, D.; Vanessa, C.; Wheeler, V.C.; et al. Genetic modifiers of Huntington disease differentially influence motor and cognitive domains. Am. J. Hum. Genet. 2022, 109, 885–899. [Google Scholar] [CrossRef]
- Bhide, P.G.; Day, M.; Sapp, E.; Schwarz, C.; Sheth, A.; Kim, J.; Young, A.B.; Penney, J.; Golden, J.; Aronin, N.; et al. Expression of Normal and Mutant Huntingtin in the Developing Brain. J. Neurosci. 1996, 16, 5523–5535. [Google Scholar] [CrossRef]
- Barnat, M.; Capizzi, M.; Aparicio, E.; Boluda, S.; Wennagel, D.; Kacher, R.; Kassem, R.; Lenoir, S.; Agasse, F.; Braz, B.Y.; et al. Huntington’s disease alters human neurodevelopment. Science 2020, 369, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Molero, A.E.; Arteaga-Bracho, E.E.; Chen, C.H.; Gulinello, M.; Winchester, M.L.; Pichamoorthy, N.; Gokhan, S.; Khodakhah, K.; Mehler, M.F. Selective expression of mutant huntingtin during development recapitulates characteristic features of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2016, 113, 5736–5741. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Li, S. Influence of Species Differences on the Neuropathology of Transgenic Huntington’s Disease Animal Models. J. Genet. Genom. 2012, 39, 239–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eaton, S.L.; Wishart, T.M. Bridging the gap: Large animal models in neurodegenerative research. Mamm. Genome 2017, 28, 324–337. [Google Scholar] [CrossRef] [PubMed]


| Name | Function | Region in Htt for Binding | PolyQ-Length Influence | Identification Method | References |
|---|---|---|---|---|---|
| Huntingtin associated protein 1 (HAP1) | multiple functions | N-terminal | Enhances | Y2-H | [30,31,32,33,34,35,36,37,38,39,40] |
| GAPDH | Glycolitic enzyme | Polyproline | Enhances | Affinity chromatography | [41,42,43,44,45] |
| Sp1 | Transcription activator | Amino acid 1–171 | Enhances | CO-IP, Y2-H | [46,47,48,49,50,51] |
| Homocysteine-induced endoplasmic reticulum protein (Herp) | Ubiquitin ligase | N-terminal | Enhances | Co-IP | [52,53] |
| Huntingtin-interacting protein 1 (Hip1) | Clathrin-mediated endocytosis and trafficking | Amino acid 1–540 | Decreases | Y2-H | [54,55,56,57,58,59] |
| Kynurenine 3-Monooxygenase (KMO) | Flavin monooxygenase | Unknown | Decreases | BiFC, Co-IP | [60,61,62,63,64] |
| Rhes | Small gtpase/E3 ligase | N-terminal | Increases | Co-IP | [65,66,67,68] |
| HYPK | Chaperone protein | N-terminal 17 amino acid domain | Unknown | Co-IP | [69,70] |
| ATPase valosin-containing protein (VCP/p97) | Facilitate the process of protein polyubiquitination | N-terminal 17 amino acid domain | Unknown | IP | [71] |
| Siah1-interacting protein(SIP) | Ubiquitin ligase | Unknown | Unknown | Co-IP, Autophagy test | [72,73,74] |
| Vimentin | Stabilize intracellular structure | Unknown | Unknown | Co-IP, Proximity ligation assay (PLA) | [75,76] |
| RAC1 (Rac family small GTPase 1) | Plasma membrane-associated small GTPase | Unknown | Unknown | IP | [77,78,79] |
| Beclin 1 | A key initiator of autophagy | Unknown | Unknown | IP | [80,81,82] |
| HAP40 | Stabilize HTT protein | C-terminal | N/A | Co-purification | [83,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Tong, H.; Sun, Y.; Chen, X.; Yang, T.; Zhou, G.; Li, X.-J.; Li, S. Huntingtin Interacting Proteins and Pathological Implications. Int. J. Mol. Sci. 2023, 24, 13060. https://doi.org/10.3390/ijms241713060
Liu L, Tong H, Sun Y, Chen X, Yang T, Zhou G, Li X-J, Li S. Huntingtin Interacting Proteins and Pathological Implications. International Journal of Molecular Sciences. 2023; 24(17):13060. https://doi.org/10.3390/ijms241713060
Chicago/Turabian StyleLiu, Li, Huichun Tong, Yize Sun, Xingxing Chen, Tianqi Yang, Gongke Zhou, Xiao-Jiang Li, and Shihua Li. 2023. "Huntingtin Interacting Proteins and Pathological Implications" International Journal of Molecular Sciences 24, no. 17: 13060. https://doi.org/10.3390/ijms241713060
APA StyleLiu, L., Tong, H., Sun, Y., Chen, X., Yang, T., Zhou, G., Li, X.-J., & Li, S. (2023). Huntingtin Interacting Proteins and Pathological Implications. International Journal of Molecular Sciences, 24(17), 13060. https://doi.org/10.3390/ijms241713060






