The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies
Abstract
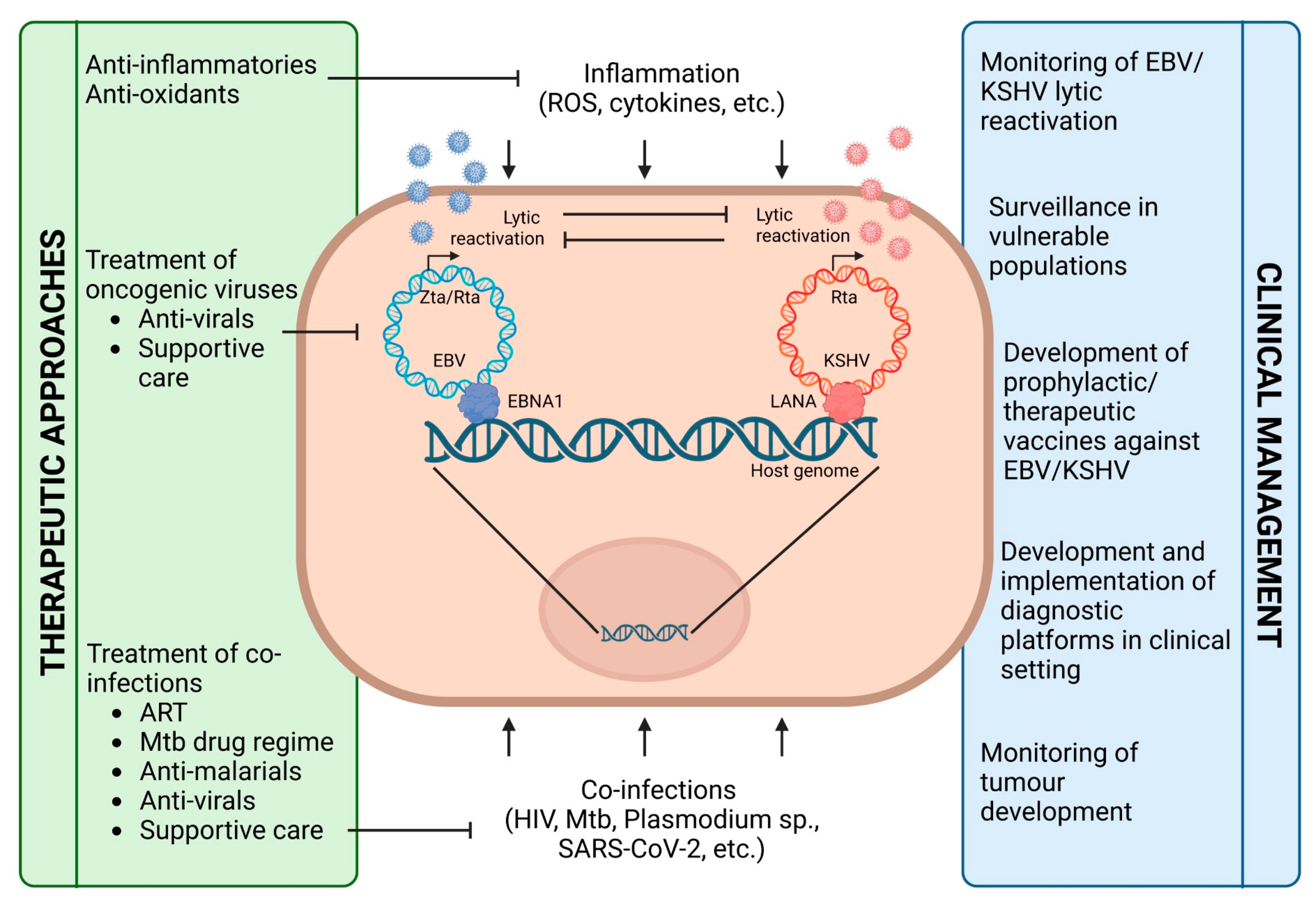
:1. Introduction
2. Epidemiology of the Oncogenic Gammaherpesviruses EBV and KSHV
2.1. EBV
2.2. KSHV
3. The Life Cycle of Oncogenic Gammaherpesviruses
3.1. The EBV Life Cycle and the Contribution of Key Latent and Lytic Gene Products
3.2. The KSHV Life Cycle and the Contribution of Key Latent and Lytic Gene Products
4. Co-Infection and/or Inflammation Triggers Lytic Reactivation of Oncogenic Gammaherpesviruses
4.1. Lytic Reactivation of EBV
4.1.1. HIV
4.1.2. Mycobacterium Tuberculosis
4.1.3. Plasmodium sp.
4.1.4. SARS-CoV-2
4.1.5. Other Co-Infections
4.2. Lytic Reactivation of KSHV
4.2.1. HIV
4.2.2. Mycobacterium Tuberculosis
4.2.3. Plasmodium sp.
4.2.4. SARS-CoV-2
4.2.5. Other Co-Infections
4.3. The Interplay between EBV and KSHV
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boehmer, P.E.; Nimonkar, A.V. Herpes virus replication. IUBMB Life 2003, 55, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Grinde, B. Herpesviruses: Latency and reactivation–viral strategies and host response. J. Oral Microbiol. 2013, 5, 22766. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Mohr, I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012, 20, 604–611. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wilson, A.C.; Chao, M.V.; Mohr, I. Control of viral latency in neurons by axonal mTOR signaling and the 4E-BP translation repressor. Genes Dev. 2012, 26, 1527–1532. [Google Scholar] [CrossRef]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef]
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H., Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 2011, 24, 193–209. [Google Scholar] [CrossRef]
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef]
- Wang, M.; Gu, B.; Chen, X.; Wang, Y.; Li, P.; Wang, K. The function and therapeutic potential of Epstein-Barr virus-encoded microRNAs in cancer. Mol. Ther.-Nucleic Acids 2019, 17, 657–668. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein–Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front. Oncol. 2018, 8, 211. [Google Scholar] [CrossRef]
- Palser, A.L.; Grayson, N.E.; White, R.E.; Corton, C.; Correia, S.; Ba Abdullah, M.M.; Watson, S.J.; Cotten, M.; Arrand, J.R.; Murray, P.G.; et al. Genome diversity of Epstein-Barr virus from multiple tumor types and normal infection. J. Virol. 2015, 89, 5222–5237. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Bridges, R.; Wegner, F.; Venturini, C.; Palser, A.; Middeldorp, J.M.; Cohen, J.I.; Lorenzetti, M.A.; Bassano, I.; White, R.E.; et al. Sequence Variation of Epstein-Barr Virus: Viral Types, Geography, Codon Usage, and Diseases. J. Virol. 2018, 92, e01132-01118. [Google Scholar] [CrossRef] [PubMed]
- Rickinson, A.B.; Young, L.S.; Rowe, M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J. Virol. 1987, 61, 1310–1317. [Google Scholar] [CrossRef]
- Tzellos, S.; Correia, P.B.; Karstegl, C.E.; Cancian, L.; Cano-Flanagan, J.; McClellan, M.J.; West, M.J.; Farrell, P.J. A single amino acid in EBNA-2 determines superior B lymphoblastoid cell line growth maintenance by Epstein-Barr virus type 1 EBNA-2. J. Virol. 2014, 88, 8743–8753. [Google Scholar] [CrossRef]
- Kuri, A.; Jacobs, B.M.; Vickaryous, N.; Pakpoor, J.; Middeldorp, J.; Giovannoni, G.; Dobson, R. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health 2020, 20, 912. [Google Scholar] [CrossRef]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, G.C.; Krajewski, A.S.; Crawford, D.H. The ins and outs of EBV infection. Trends Microbiol. 2000, 8, 185–189. [Google Scholar] [CrossRef]
- Alfieri, C.; Tanner, J.; Carpentier, L.; Perpete, C.; Savoie, A.; Paradis, K.; Delage, G.; Joncas, J. Epstein-Barr virus transmission from a blood donor to an organ transplant recipient with recovery of the same virus strain from the recipient’s blood and oropharynx. Blood 1996, 87, 812–817. [Google Scholar] [CrossRef]
- Niederman, J.; Evans, A.; Subrahmanyan, L.; McCollum, R. Prevalence, incidence and persistence of EB virus antibody in young adults. N. Engl. J. Med. 1970, 282, 361–365. [Google Scholar] [CrossRef]
- Papesch, M.; Watkins, R. Epstein-Barr virus infectious mononucleosis. Clin. Otolaryngol. Allied Sci. 2001, 26, 3–8. [Google Scholar] [CrossRef]
- Trottier, H.; Buteau, C.; Robitaille, N.; Duval, M.; Tucci, M.; Lacroix, J.; Alfieri, C. Transfusion-related Epstein-Barr virus infection among stem cell transplant recipients: A retrospective cohort study in children. Transfusion 2012, 52, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, G.; Henle, W.; Henle, G.; Lennette, E.T.; Biggar, R.J. Primary infection with Epstein-Barr virus in infants in the United States: Clinical and serologic observations. J. Infect. Dis. 1979, 139, 553–558. [Google Scholar] [CrossRef]
- Horwitz, C.A.; Henle, W.; Henle, G.; Goldfarb, M.; Kubic, P.; Gehrz, R.C.; Balfour, H.H.; Fleisher, G.R.; Krivit, W. Clinical and Laboratory Evaluation of Infants and Children With Epstein-Barr Virus-Induced Infectious Mononucleosis: Report of 32 Patients (Aged 10–48 Months). Blood 1981, 57, 933–938. [Google Scholar] [CrossRef]
- Biggar, R.J.; Henle, G.; Böcker, J.; Lennette, E.T.; Fleisher, G.; Henle, W. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int. J. Cancer 1978, 22, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Richardo, T.; Prattapong, P.; Ngernsombat, C.; Wisetyaningsih, N.; Iizasa, H.; Yoshiyama, H.; Janvilisri, T. Epstein-Barr Virus Mediated Signaling in Nasopharyngeal Carcinoma Carcinogenesis. Cancers 2020, 12, 2441. [Google Scholar] [CrossRef]
- Kaymaz, Y.; Oduor, C.I.; Aydemir, O.; Luftig, M.A.; Otieno, J.A.; Ong’echa, J.M.; Bailey, J.A.; Moormann, A.M. Epstein-Barr Virus Genomes Reveal Population Structure and Type 1 Association with Endemic Burkitt Lymphoma. J. Virol. 2020, 94, e02007-19. [Google Scholar] [CrossRef]
- Mpunga, T.; Clifford, G.M.; Morgan, E.A.; Milner, D.A., Jr.; de Martel, C.; Munyanshongore, C.; Muvugabigwi, G.; Combes, J.D. Epstein-Barr virus prevalence among subtypes of malignant lymphoma in Rwanda, 2012 to 2018. Int. J. Cancer 2022, 150, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Means, R.E.; Lang, S.M.; Jung, J.U. Human gammaherpesvirus immune evasion strategies. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Gulley, M.L.; Tang, W. Laboratory assays for Epstein-Barr virus-related disease. J. Mol. Diagn. 2008, 10, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Chetaille, B.; Bertucci, F.; Finetti, P.; Esterni, B.; Stamatoullas, A.; Picquenot, J.M.; Copin, M.C.; Morschhauser, F.; Casasnovas, O.; Petrella, T. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood J. Am. Soc. Hematol. 2009, 113, 2765–3775. [Google Scholar] [CrossRef]
- Neri, A.; Barriga, F.; Inghirami, G.; Knowles, D.M.; Neequaye, J.; Magrath, I.T.; Dalla-Favera, R. Epstein-Barr Virus Infection Precedes Clonal Expansion in Burkitt’s and Acquired Immunodeficiency Syndrome-Associated Lymphoma. Blood 1991, 77, 1092–1095. [Google Scholar] [CrossRef]
- Rasul, A.E.; Nagy, N.; Sohlberg, E.; Ádori, M.; Claesson, H.-E.; Klein, G.; Klein, E. Simultaneous detection of the two main proliferation driving EBV encoded proteins, EBNA-2 and LMP-1 in single B cells. J. Immunol. Methods 2012, 385, 60–70. [Google Scholar] [CrossRef]
- Bigi, R.; Landis, J.T.; An, H.; Caro-Vegas, C.; Raab-Traub, N.; Dittmer, D.P. Epstein–Barr virus enhances genome maintenance of Kaposi sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, E11379–E11387. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.M.; Valderrama, S.; Gualtero, S.; Hernández, C.; López, M.; Herrera, M.V.; Solano, J.; Fiorentino, S.; Quijano, S. Loss of T-cell multifunctionality and TCR-Vβ repertoire against Epstein-Barr virus is associated with worse prognosis and clinical parameters in HIV+ patients. Front. Immunol. 2018, 9, 2291. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.P.; Azzi, T.; Hui, K.F.; Wong, A.M.G.; McHugh, D.; Caduff, N.; Chan, K.H.; Münz, C.; Chiang, A.K.S. Co-infection of Cytomegalovirus and Epstein-Barr Virus Diminishes the Frequency of CD56(dim)NKG2A(+)KIR(-) NK Cells and Contributes to Suboptimal Control of EBV in Immunosuppressed Children With Post-transplant Lymphoproliferative Disorder. Front. Immunol. 2020, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Caduff, N.; Barros, M.H.M.; Rämer, P.C.; Raykova, A.; Murer, A.; Landtwing, V.; Quast, I.; Styles, C.T.; Spohn, M.; et al. Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression. Cell Host Microbe 2017, 22, 61–73.e67. [Google Scholar] [CrossRef]
- Moormann, A.M.; Chelimo, K.; Sumba, O.P.; Lutzke, M.L.; Ploutz-Snyder, R.; Newton, D.; Kazura, J.; Rochford, R. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J. Infect. Dis. 2005, 191, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, T.; Zeng, M.; Peng, T. Herpes simplex virus type 1 infection activates the Epstein-Barr virus replicative cycle via a CREB-dependent mechanism. Cell Microbiol. 2012, 14, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Diakite, M.; Shaw-Saliba, K.; Lau, C.-Y. Malignancy and viral infections in Sub-Saharan Africa: A review. Front. Virol. 2023, 3. [Google Scholar] [CrossRef]
- Sinfield, R.; Molyneux, E.; Banda, K.; Borgstein, E.; Broadhead, R.; Hesseling, P.; Newton, R.; Casabonne, D.; Mkandawire, N.; Nkume, H. Spectrum and presentation of pediatric malignancies in the HIV era: Experience from Blantyre, Malawi, 1998–2003. Pediatr. Blood Cancer 2007, 48, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Uldrick, T.S.; Whitby, D. Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer Lett. 2011, 305, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Dedicoat, M.; Newton, R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br. J. Cancer 2003, 88, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Ziegler, J.; Bourboulia, D.; Casabonne, D.; Beral, V.; Mbidde, E.; Carpenter, L.; Reeves, G.; Parkin, D.M.; Wabinga, H. The sero-epidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in adults with cancer in Uganda. Int. J. Cancer 2003, 103, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.J.; Schutz, C.; Barr, D.; Locketz, M.; Marshall, V.; Whitby, D.; Katz, A.A.; Uldrick, T.; Meintjes, G.; Schäfer, G. The Contribution of Kaposi’s Sarcoma–Associated Herpesvirus to Mortality in Hospitalized Human Immunodeficiency Virus–Infected Patients Being Investigated for Tuberculosis in South Africa. J. Infect. Dis. 2019, 220, 841–851. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Sanders, M.K.; Brulois, K.F.; Dittmer, D.P.; Damania, B. The K1 Protein of Kaposi’s Sarcoma-Associated Herpesvirus Augments Viral Lytic Replication. J. Virol. 2016, 90, 7657–7666. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Biryahwaho, B.; Downing, R.; Hatzakis, A.; Alessi, E.; Cusini, M.; Ruocco, V.; Katongole-Mbidde, E.; Loquercio, G.; Buonaguro, L.; et al. Human herpesvirus type 8 variants circulating in Europe, Africa and North America in classic, endemic and epidemic Kaposi’s sarcoma lesions during pre-AIDS and AIDS era. Virology 2010, 398, 280–289. [Google Scholar] [CrossRef]
- Hladik, W.; Dollard, S.C.; Mermin, J.; Fowlkes, A.L.; Downing, R.; Amin, M.M.; Banage, F.; Nzaro, E.; Kataaha, P.; Dondero, T.J. Transmission of human herpesvirus 8 by blood transfusion. N. Engl. J. Med. 2006, 355, 1331–1338. [Google Scholar] [CrossRef]
- de Sanjose, S.; Mbisa, G.; Perez-Alvarez, S.; Benavente, Y.; Sukvirach, S.; Hieu, N.T.; Shin, H.-R.; Anh, P.T.H.; Thomas, J.; Lazcano, E. Geographic variation in the prevalence of Kaposi sarcoma–associated herpesvirus and risk factors for transmission. J. Infect. Dis. 2009, 199, 1449–1456. [Google Scholar] [CrossRef]
- de França, T.R.T.; de Araújo, R.A.; Ribeiro, C.M.B.; Leao, J.C. Salivary shedding of HHV-8 in people infected or not by human immunodeficiency virus 1. J. Oral Pathol. Med. 2011, 40, 97–102. [Google Scholar] [CrossRef]
- Giffin, L.; Damania, B. KSHV: Pathways to tumorigenesis and persistent infection. Adv. Virus Res. 2014, 88, 111–159. [Google Scholar]
- Minhas, V.; Wood, C. Epidemiology and transmission of Kaposi’s sarcoma-associated herpesvirus. Viruses 2014, 6, 4178–4194. [Google Scholar] [CrossRef]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-sssociated kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Polizzotto, M.N.; Uldrick, T.S.; Hu, D.; Yarchoan, R. Clinical manifestations of Kaposi sarcoma herpesvirus lytic activation: Multicentric Castleman disease (KSHV–MCD) and the KSHV inflammatory cytokine syndrome. Front. Microbiol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Uldrick, T.S.; Wyvill, K.M.; Aleman, K.; Marshall, V.; Wang, V.; Whitby, D.; Pittaluga, S.; Jaffe, E.S.; Millo, C. Clinical features and outcomes of patients with symptomatic Kaposi sarcoma herpesvirus (KSHV)-associated inflammation: Prospective characterization of KSHV inflammatory cytokine syndrome (KICS). Clin. Infect. Dis. 2016, 62, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.H.; Uldrick, T.S.; Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017, 31, 1903. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Thakker, S.; Verma, S.C. Co-infections and pathogenesis of KSHV-associated malignancies. Front. Microbiol. 2016, 7, 151. [Google Scholar] [CrossRef]
- Powles, T.; Stebbing, J.; Bazeos, A.; Hatzimichael, E.; Mandalia, S.; Nelson, M.; Gazzard, B.; Bower, M. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann. Oncol. 2009, 20, 775–779. [Google Scholar] [CrossRef]
- Staskus, K.A.; Sun, R.; Miller, G.; Racz, P.; Jaslowski, A.; Metroka, C.; Brett-Smith, H.; Haase, A.T. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J. Virol. 1999, 73, 4181–4187. [Google Scholar] [CrossRef]
- Parravicini, C.; Chandran, B.; Corbellino, M.; Berti, E.; Paulli, M.; Moore, P.S.; Chang, Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am. J. Pathol. 2000, 156, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Uldrick, T.S.; Wang, V.; O’Mahony, D.; Aleman, K.; Wyvill, K.M.; Marshall, V.; Steinberg, S.M.; Pittaluga, S.; Maric, I.; Whitby, D. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin. Infect. Dis. 2010, 51, 350–358. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A. EBV persistence—Introducing the virus. Epstein Barr Virus Volume 1: One Herpes Virus: Many Diseases. Curr. Top. Microbiol. Immunol. 2015, 390 Pt 1, 151–209. [Google Scholar] [PubMed]
- Yap, L.F.; Wong, A.K.C.; Paterson, I.C.; Young, L.S. Functional Implications of Epstein-Barr Virus Lytic Genes in Carcinogenesis. Cancers 2022, 14, 5780. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M. Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium. Biomedicines 2023, 11, 1444. [Google Scholar] [CrossRef]
- Wang, X.; Kenyon, W.J.; Li, Q.; Müllberg, J.; Hutt-Fletcher, L.M. Epstein-Barr Virus Uses Different Complexes of Glycoproteins gH and gL To Infect B Lymphocytes and Epithelial Cells. J. Virol. 1998, 72, 5552–5558. [Google Scholar] [CrossRef]
- Borza, C.M.; Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat. Med. 2002, 8, 594–599. [Google Scholar] [CrossRef]
- Burton, E.M.; Voyer, J.; Gewurz, B.E. Epstein–Barr virus latency programs dynamically sensitize B cells to ferroptosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2118300119. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Yin, H.; Qu, J.; Peng, Q.; Gan, R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med. Microbiol. Immunol. 2019, 208, 573–583. [Google Scholar] [CrossRef]
- Deakyne, J.S.; Malecka, K.A.; Messick, T.E.; Lieberman, P.M. Structural and Functional Basis for an EBNA1 Hexameric Ring in Epstein-Barr Virus Episome Maintenance. J. Virol. 2017, 91, e01046-17. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.; Rowe, D.T.; Gregory, C.D.; Young, L.S.; Farrell, P.J.; Rupani, H.; Rickinson, A.B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987, 6, 2743–2751. [Google Scholar] [CrossRef]
- Hatzubai, A.; Anafi, M.; Masucci, M.G.; Dillner, J.; Lerner, R.A.; Klein, G.; Sulitzeanu, D. Down-regulation of the EBV-encoded membrane protein (LMP) in Burkitt lymphomas. Int. J. Cancer 1987, 40, 358–364. [Google Scholar] [CrossRef]
- Gregory, C.D.; Rowe, M.; Rickinson, A.B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt’s lymphoma cell line. J. Gen. Virol. 1990, 71, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Babcock, G.J.; Thorley-Lawson, D.A. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc. Natl. Acad. Sci. USA 2000, 97, 12250–12255. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wang, Y.; Wang, X.-F.; Liang, H.; Yan, L.-P.; Huang, B.-H.; Zhao, P. Expression of Epstein-Barr virus genes in EBV-associated gastric carcinomas. World J. Gastroenterol. WJG 2005, 11, 629. [Google Scholar] [CrossRef]
- Hochberg, D.; Middeldorp, J.M.; Catalina, M.; Sullivan, J.L.; Luzuriaga, K.; Thorley-Lawson, D.A. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 239–244. [Google Scholar] [CrossRef]
- Desbien, A.L.; Kappler, J.W.; Marrack, P. The Epstein–Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA 2009, 106, 5663–5668. [Google Scholar] [CrossRef]
- Bentz, G.; Liu, R.; Hahn, A.; Shackelford, J.; Pagano, J. Epstein-Barr virus immediate-early protein RTA negatively regulates interferon regulatory factors. Virology 2010, 402, 121. [Google Scholar] [CrossRef]
- Geiger, T.R.; Martin, J.M. The Epstein-Barr virus-encoded LMP-1 oncoprotein negatively affects Tyk2 phosphorylation and interferon signaling in human B cells. J. Virol. 2006, 80, 11638–11650. [Google Scholar] [CrossRef]
- Amon, W.; Farrell, P.J. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 2005, 15, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.M.; Tanizawa, H.; Caruso, L.B.; Hulse, M.; Kossenkov, A.; Madzo, J.; Keith, K.; Tan, Y.; Boyle, S.; Lieberman, P.M.; et al. The three-dimensional structure of Epstein-Barr virus genome varies by latency type and is regulated by PARP1 enzymatic activity. Nat. Commun. 2022, 13, 187. [Google Scholar] [CrossRef]
- Kim, K.-D.; Tanizawa, H.; De Leo, A.; Vladimirova, O.; Kossenkov, A.; Lu, F.; Showe, L.C.; Noma, K.-I.; Lieberman, P.M. Epigenetic specifications of host chromosome docking sites for latent Epstein-Barr virus. Nat. Commun. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Ning, S. Innate immune modulation in EBV infection. Herpesviridae 2011, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wikramasinghe, P.; Norseen, J.; Tsai, K.; Wang, P.; Showe, L.; Davuluri, R.V.; Lieberman, P.M. Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1). Virol. J. 2010, 7, 262. [Google Scholar] [CrossRef]
- Verhoeven, R.J.A.; Tong, S.; Zong, J.; Chen, Y.; Tsao, S.W.; Pan, J.; Chen, H. NF-κB Signaling Regulates Epstein-Barr Virus BamHI-Q-Driven EBNA1 Expression. Cancers 2018, 10, 119. [Google Scholar] [CrossRef]
- Countryman, J.; Jenson, H.; Seibl, R.; Wolf, H.; Miller, G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J. Virol. 1987, 61, 3672–3679. [Google Scholar] [CrossRef]
- Ragoczy, T.; Heston, L.; Miller, G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 1998, 72, 7978–7984. [Google Scholar] [CrossRef]
- Leong, M.M.L.; Cheung, A.K.L.; Dai, W.; Tsao, S.W.; Tsang, C.M.; Dawson, C.W.; Mun Yee Ko, J.; Lung, M.L. EBV infection is associated with histone bivalent switch modifications in squamous epithelial cells. Proc. Natl. Acad. Sci. USA 2019, 116, 14144–14153. [Google Scholar] [CrossRef]
- Gruffat, H.; Manet, E.; Sergeant, A. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 2002, 3, 141–146. [Google Scholar] [CrossRef]
- Gruhne, B.; Sompallae, R.; Marescotti, D.; Kamranvar, S.A.; Gastaldello, S.; Masucci, M.G. The Epstein–Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2009, 106, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kamranvar, S.A.; Masucci, M. Oxidative stress enables Epstein–Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors. Oncogene 2016, 35, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Arakawa, F.; Kiyasu, J.; Sato, K.; Miyoshi, H.; Niino, D.; Kimura, Y.; Takeuchi, M.; Yoshida, M.; Ishibashi, Y. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: Histology, E pstein–B arr virus, and clonality are important predictors of disease progression and regression. Eur. J. Haematol. 2013, 91, 20–28. [Google Scholar] [CrossRef]
- Sausen, D.G.; Bhutta, M.S.; Gallo, E.S.; Dahari, H.; Borenstein, R. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 2021, 11, 1380. [Google Scholar] [CrossRef]
- Jiang, L.; Lan, R.; Huang, T.; Chan, C.-F.; Li, H.; Lear, S.; Zong, J.; Wong, W.-Y.; Muk-Lan Lee, M.; Dow Chan, B.; et al. EBNA1-targeted probe for the imaging and growth inhibition of tumours associated with the Epstein–Barr virus. Nat. Biomed. Eng. 2017, 1, 0042. [Google Scholar] [CrossRef]
- Hjalgrim, H.F.J.; Melbye, M. The epidemiology of EBV and its association with malignant disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kenney, S.C.; Mertz, J.E. Regulation of the latent-lytic switch in Epstein–Barr virus. Semin. Cancer Biol. 2014, 26, 60–68. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Wang, C.; Gan, R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Kim, Y.S.; Lee, H.-K.; Song, H.; Cho, D.-H.; Lee, W.J.; Hur, D.Y. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J. Immunol. 2010, 185, 7274–7284. [Google Scholar] [CrossRef]
- Murata, T.; Sugimoto, A.; Inagaki, T.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H. Molecular Basis of Epstein-Barr Virus Latency Establishment and Lytic Reactivation. Viruses 2021, 13, 2344. [Google Scholar] [CrossRef]
- Yan, L.; Majerciak, V.; Zheng, Z.-M.; Lan, K. Towards better understanding of KSHV life cycle: From transcription and posttranscriptional regulations to pathogenesis. Virol. Sin. 2019, 34, 135–161. [Google Scholar] [CrossRef]
- Ganem, D. KSHV-induced oncogenesis. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lagos, D.; Boshoff, C. Immunobiology and host response to KSHV infection. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Dollery, S.J. Towards understanding KSHV fusion and entry. Viruses 2019, 11, 1073. [Google Scholar] [CrossRef]
- Della Bella, S.; Taddeo, A.; Calabro, M.L.; Brambilla, L.; Bellinvia, M.; Bergamo, E.; Clerici, M.; Villa, M.L. Peripheral blood endothelial progenitors as potential reservoirs of Kaposi’s sarcoma-associated herpesvirus. PLoS ONE 2008, 3, e1520. [Google Scholar] [CrossRef] [PubMed]
- Monini, P.; Colombini, S.; Sturzl, M.; Goletti, D.; Cafaro, A.; Sgadari, C.; Butto, S.; Franco, M.; Leone, P.; Fais, S. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi’s sarcoma. Blood J. Am. Soc. Hematol. 1999, 93, 4044–4058. [Google Scholar]
- Ye, F.; Lei, X.; Gao, S.-J. Mechanisms of Kaposi’s sarcoma-associated herpesvirus latency and reactivation. Adv. Virol. 2011, 2011, 3468–3478. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Issa, A.T.; Ching, K.H.; Wyvill, K.M.; Little, R.F.; Iadarola, M.J.; Kovacs, J.A.; Yarchoan, R. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J. Infect. Dis. 2010, 201, 1919–1922. [Google Scholar] [CrossRef]
- Gaglia, M.M. Kaposi’s sarcoma-associated herpesvirus at 27. Tumour Virus Res. 2021, 12, 200223. [Google Scholar] [CrossRef]
- Boshoff, C.; Schulz, T.F.; Kennedy, M.M.; Graham, A.K.; Fisher, C.; Thomas, A.; McGee, J.O.D.; Weiss, R.A.; O’Leary, J.J. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1995, 1, 1274–1278. [Google Scholar] [CrossRef]
- Chandran, B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. J. Virol. 2010, 84, 2188–2199. [Google Scholar] [CrossRef]
- Kedes, D.H.; Lagunoff, M.; Renne, R.; Ganem, D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J. Clin. Investig. 1997, 100, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, L.; Platt, G.M.; Simpson, G.R.; Sarid, R.; Gao, S.-J.; Stoiber, H.; Herrington, C.S.; Moore, P.S.; Schulz, T.F. The 222-to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 1997, 71, 5915–5921. [Google Scholar] [CrossRef]
- Sadler, R.; Wu, L.; Forghani, B.; Renne, R.; Zhong, W.; Herndier, B.; Ganem, D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999, 73, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Uppal, T.; Banerjee, S.; Sun, Z.; Verma, S.C.; Robertson, E.S. KSHV LANA—The master regulator of KSHV latency. Viruses 2014, 6, 4961–4998. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Grundhoff, A. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 2010, 6, e1000935. [Google Scholar] [CrossRef] [PubMed]
- Journo, G.; Tushinsky, C.; Shterngas, A.; Avital, N.; Eran, Y.; Karpuj, M.V.; Frenkel-Morgenstern, M.; Shamay, M. Modulation of cellular CpG DNA methylation by Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2018, 92, e00008-18. [Google Scholar] [CrossRef]
- Campbell, M.; Yang, W.-S.; Yeh, W.W.; Kao, C.-H.; Chang, P.-C. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus latency. Front. Microbiol. 2020, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Toth, Z.; Brulois, K.; Jung, J.U. The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses 2013, 5, 1346–1373. [Google Scholar] [CrossRef]
- Chen, H.-S.; Lu, F.; Lieberman, P.M. Epigenetic regulation of EBV and KSHV latency. Curr. Opin. Virol. 2013, 3, 251–259. [Google Scholar] [CrossRef]
- Shamay, M.; Krithivas, A.; Zhang, J.; Hayward, S.D. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi’s sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. USA 2006, 103, 14554–14559. [Google Scholar] [CrossRef]
- West, J.T.; Wood, C. The role of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene 2003, 22, 5150–5163. [Google Scholar] [CrossRef]
- Di Bartolo, D.L.; Cannon, M.; Liu, Y.-F.; Renne, R.; Chadburn, A.; Boshoff, C.; Cesarman, E. KSHV LANA inhibits TGF-β signaling through epigenetic silencing of the TGF-β type II receptor. Blood J. Am. Soc. Hematol. 2008, 111, 4731–4740. [Google Scholar] [CrossRef]
- Purushothaman, P.; Uppal, T.; Verma, S.C. Molecular biology of KSHV lytic reactivation. Viruses 2015, 7, 116–153. [Google Scholar] [CrossRef]
- Friborg, J., Jr.; Kong, W.-P.; Hottiger, M.O.; Nabel, G.J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999, 402, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Santag, S.; Jäger, W.; Karsten, C.; Kati, S.; Pietrek, M.; Steinemann, D.; Sarek, G.; Ojala, P.; Schulz, T. Recruitment of the tumour suppressor protein p73 by Kaposi’s Sarcoma Herpesvirus latent nuclear antigen contributes to the survival of primary effusion lymphoma cells. Oncogene 2013, 32, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.X.; Cusano, T.; Yuan, Y. Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999, 73, 5556–5567. [Google Scholar] [CrossRef]
- Saveliev, A.K.; Zhu, F.X.; Yuan, Y. Transcription mapping and expression patterns of genes in the major immediate-early region of Kaposi’s sarcoma-associated herpesvirus. Virology 2002, 299, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, V.; de La Fuente, C.; Kashanchi, F.; Pumfery, A. Kaposi’s sarcoma-associated herpesvirus immediate early gene activity. Front. Biosci.-Landmark 2004, 9, 2245–2272. [Google Scholar] [CrossRef]
- Lan, K.; Murakami, M.; Choudhuri, T.; Kuppers, D.A.; Robertson, E.S. Intracellular-activated Notch1 can reactivate Kaposi’s sarcoma-associated herpesvirus from latency. Virology 2006, 351, 393–403. [Google Scholar] [CrossRef]
- Chang, H.; Dittmer, D.P.; Chul, S.-Y.; Hong, Y.; Jung, J.U. Role of Notch signal transduction in Kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 2005, 79, 14371–14382. [Google Scholar] [CrossRef]
- Carroll, K.D.; Bu, W.; Palmeri, D.; Spadavecchia, S.; Lynch, S.J.; Marras, S.A.; Tyagi, S.; Lukac, D.M. Kaposi’s Sarcoma-associated herpesvirus lytic switch protein stimulates DNA binding of RBP-Jk/CSL to activate the Notch pathway. J. Virol. 2006, 80, 9697–9709. [Google Scholar] [CrossRef]
- Toth, Z.; Maglinte, D.T.; Lee, S.H.; Lee, H.-R.; Wong, L.-Y.; Brulois, K.F.; Lee, S.; Buckley, J.D.; Laird, P.W.; Marquez, V.E. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog. 2010, 6, e1001013. [Google Scholar] [CrossRef]
- Fröhlich, J.; Grundhoff, A. Epigenetic control in Kaposi sarcoma-associated herpesvirus infection and associated disease. Proc. Semin. Immunopathol. 2020, 42, 143–157. [Google Scholar] [CrossRef]
- Vieira, J.; O’Hearn, P.; Kimball, L.; Chandran, B.; Corey, L. Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 2001, 75, 1378–1386. [Google Scholar] [CrossRef]
- Tang, Q.; Qin, D.; Lv, Z.; Zhu, X.; Ma, X.; Yan, Q.; Zeng, Y.; Guo, Y.; Feng, N.; Lu, C. Herpes simplex virus type 2 triggers reactivation of Kaposi’s sarcoma-associated herpesvirus from latency and collaborates with HIV-1 Tat. PLoS ONE 2012, 7, e31652. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zeng, Y.; Huang, Z.; Huang, L.; Qian, C.; Tang, G.; Qin, D. Human herpesvirus 6 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus. Am. J. Pathol. 2005, 166, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Anders, P.M.; Montgomery, N.D.; Montgomery, S.A.; Bhatt, A.P.; Dittmer, D.P.; Damania, B. Human herpesvirus–encoded kinase induces B cell lymphomas in vivo. J. Clin. Investig. 2018, 128, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Wong, J.P.; Weinberg, M.S.; Host, K.M.; Giffin, L.C.; Buijnink, J.; Van Dijk, E.; Izumiya, Y.; Kung, H.-J.; Temple, B.R. A viral kinase mimics S6 kinase to enhance cell proliferation. Proc. Natl. Acad. Sci. USA 2016, 113, 7876–7881. [Google Scholar] [CrossRef]
- Montaner, S.; Sodhi, A.; Molinolo, A.; Bugge, T.H.; Sawai, E.T.; He, Y.; Li, Y.; Ray, P.E.; Gutkind, J.S. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 2003, 3, 23–36. [Google Scholar] [CrossRef]
- Ma, Z.; Jacobs, S.R.; West, J.A.; Stopford, C.; Zhang, Z.; Davis, Z.; Barber, G.N.; Glaunsinger, B.A.; Dittmer, D.P.; Damania, B. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl. Acad. Sci. USA 2015, 112, E4306–E4315. [Google Scholar] [CrossRef]
- Abere, B.; Schulz, T.F. KSHV non-structural membrane proteins involved in the activation of intracellular signaling pathways and the pathogenesis of Kaposi’s sarcoma. Curr. Opin. Virol. 2016, 20, 11–19. [Google Scholar] [CrossRef]
- Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Spender, L.C.; Cornish, G.H.; Rowland, B.; Kempkes, B.; Farrell, P.J. Direct and indirect regulation of cytokine and cell cycle proteins by EBNA-2 during Epstein-Barr virus infection. J. Virol. 2001, 75, 3537–3546. [Google Scholar] [CrossRef]
- Mrad, M.F.; Saba, E.S.; Nakib, L.; Khoury, S.J. Exosomes from subjects with multiple sclerosis express EBV-derived proteins and activate monocyte-derived macrophages. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1004. [Google Scholar] [CrossRef]
- Bernal, K.D.E.; Whitehurst, C.B. Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus Res. 2023, 334, 199157. [Google Scholar] [CrossRef]
- Chinen, J.; Shearer, W.T. Molecular virology and immunology of HIV infection. J. Allergy Clin. Immunol. 2002, 110, 189–198. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS Global HIV & AIDS statistics—Fact sheet 2022. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 6 July 2023).
- Satoh, S.; Boyer, E. HIV in South Africa. Lancet 2019, 394, 467. [Google Scholar] [CrossRef]
- Cassim, S.; Antel, K.; Chetty, D.R.; Oosthuizen, J.; Opie, J.; Mohamed, Z.; Verburgh, E. Diffuse large B-cell lymphoma in a South African cohort with a high HIV prevalence: An analysis by cell-of-origin, Epstein–Barr virus infection and survival. Pathology 2020, 52, 453–459. [Google Scholar] [CrossRef] [PubMed]
- WHO HIV Data and Statistics. Available online: https://www.who.int/data/gho/data/indicators (accessed on 15 May 2023).
- Heath, K.; Levi, J.; Hill, A. The Joint United Nations Programme on HIV/AIDS 95–95–95 targets: Worldwide clinical and cost benefits of generic manufacture. AIDS 2021, 35, S197–S203. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, L.; Vella, S. A brief history of antiretroviral therapy of HIV infection: Success and challenges. Ann. Dell’istituto Super. Di Sanitã 2011, 47, 44–48. [Google Scholar]
- Grulich, A.E.; Van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Biggar, R.J.; Engels, E.A.; Goedert, J.J.; AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. Jama 2001, 285, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Coghill, A.E.; Newcomb, P.A.; Madeleine, M.M.; Richardson, B.A.; Mutyaba, I.; Okuku, F.; Phipps, W.; Wabinga, H.; Orem, J.; Casper, C. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 2013, 27, 2933. [Google Scholar] [CrossRef]
- Carbone, A.; Cesarman, E.; Spina, M.; Gloghini, A.; Schulz, T.F. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Pather, S.; Wainwright, R.D.; Sahid, F.; Mashele, T.; van den Berg, E.J.; Mohanlal, R.D. Human immunodeficiency virus-related Epstein-Barr virus-associated smooth muscle tumours: South African experience from Chris Hani Baragwanath Academic Hospital. South. Afr. J. Infect. Dis. 2017, 32, 115–118. [Google Scholar]
- Rahman, M.A.; Kingsley, L.A.; Atchison, R.W.; Belle, S.; Breinig, M.C.; Ho, M.; Rinaldo, C.R. Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. J. Clin. Microbiol. 1991, 29, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, C.B.; Rizk, M.; Teklezghi, A.; Spagnuolo, R.A.; Pagano, J.S.; Wahl, A. HIV Co-infection Augments EBV-Induced Tumorigenesis in vivo. Front. Virol. 2022, 2, 861628. [Google Scholar] [CrossRef]
- Nazim, F.; Kayani, H.A.; Ali Nathwani, A.; Mir, F.; Abidi, S.H. CMV and EBV Co-Infection in HIV-Infected Children: Infection Rates and Analysis of Differential Expression of Cytokines in HIV Mono- and HIV-CMV-EBV Co-Infected Groups. Viruses 2022, 14, 1823. [Google Scholar] [CrossRef]
- Mujtaba, S.; Varma, S.; Sehgal, S. Coinfection with epstein barr virus in north Indian patients with HIV/AIDS. Indian J. Pathol. Microbiol. 2005, 48, 349–353. [Google Scholar]
- Stevens, S.J.; Blank, B.S.; Smits, P.H.; Meenhorst, P.L.; Middeldorp, J.M. High Epstein–Barr virus (EBV) DNA loads in HIV-infected patients: Correlation with antiretroviral therapy and quantitative EBV serology. AIDS 2002, 16, 993–1001. [Google Scholar] [CrossRef]
- Slyker, J.A.; Casper, C.; Tapia, K.; Richardson, B.; Bunts, L.; Huang, M.-L.; Maleche-Obimbo, E.; Nduati, R.; John-Stewart, G. Clinical and virologic manifestations of primary Epstein-Barr virus (EBV) infection in Kenyan infants born to HIV-infected women. J. Infect. Dis. 2013, 207, 1798–1806. [Google Scholar] [CrossRef]
- Ferbas, J.; Rahman, M.A.; Kingsley, L.A.; Armstrong, J.A.; Ho, M.; Zhou, S.Y.J.; Rinaldo, C.R., Jr. Frequent oropharyngeal shedding of Epstein-Barr virus in homosexual men during early HIV infection. AIDS 1992, 6, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mitoma, F.; Ruiz, A.; Flowerdew, G.; Houston, S.; Romanowski, B.; Kovithavongs, T.; Preiksaitis, J.; Tyrrell, D.L. High levels of Epstein-Barr virus in the oropharynx: A predictor of disease progression in human immunodeficiency virus infection. J. Med. Virol. 1990, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ren, Y.; Chen, R.; Hu, J.; Ji, Y.; Yang, J.; Shen, J.; Hu, L.; Pei, H.; Wang, J.; et al. Evaluation of Epstein-Barr Virus Salivary Shedding in HIV/AIDS Patients and HAART Use: A Retrospective Cohort Study. Virol. Sin. 2018, 33, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Modification of EBV-Associated Pathologies and Immune Control by Coinfections. Front. Oncol. 2021, 11, 756480. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Myburgh, R.; Caduff, N.; Spohn, M.; Kok, Y.L.; Keller, C.W.; Murer, A.; Chatterjee, B.; Rühl, J.; Engelmann, C.; et al. EBV renders B cells susceptible to HIV-1 in humanized mice. Life Sci. Alliance 2020, 3, e202000640. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Mizrahi, V. Mycobacterium tuberculosis. Trends Microbiol. 2018, 26, 555–556. [Google Scholar] [CrossRef] [PubMed]
- van de Water, B.J.; Fulcher, I.; Cilliers, S.; Meyer, N.; Wilson, M.; Young, C.; Gaunt, B.; le Roux, K. Association of HIV infection and antiretroviral therapy with the occurrence of an unfavorable TB treatment outcome in a rural district hospital in Eastern Cape, South Africa: A retrospective cohort study. PLoS ONE 2022, 17, e0266082. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, E.; López-Varela, E.; Broderick, C.; Seddon, J.A. Examining the Complex Relationship Between Tuberculosis and Other Infectious Diseases in Children. Front. Pediatr. 2019, 7, 233. [Google Scholar] [CrossRef]
- Gledhill, R.; Greyling, M. Epstein-Barr virus infection in a patient with active pulmonary tuberculosis-a case report. South Afr. Med. J. 1986, 70, 761–762. [Google Scholar]
- Kakoullis, L.; Hentschel, C.; Colgrove, R. Headache, Fever, and Myalgias in an HIV-Positive Male with a History of Tuberculosis: Epstein–Barr Virus Aseptic Meningitis. Trop. Med. Infect. Dis. 2023, 8, 191. [Google Scholar]
- Shibanov, A.; Vasilèva, I.; Karazhas, N.; Abramchenko, A.; Pul’nova, N.Y.; Ribalkina, T.y.; Bosh’yan, R. Lung tuberculosis characteristics in association with herpesvirus infection. ERJ Open Res. 2022, 8, 168. [Google Scholar] [CrossRef]
- Miller, H.E.; Johnson, K.E.; Tarakanova, V.L.; Robinson, R.T. γ-herpesvirus latency attenuates Mycobacterium tuberculosis infection in mice. Tuberculosis 2019, 116, 56–60. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Schofield, L.; Grau, G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735. [Google Scholar] [CrossRef]
- Chêne, A.; Donati, D.; Guerreiro-Cacais, A.O.; Levitsky, V.; Chen, Q.; Falk, K.I.; Orem, J.; Kironde, F.; Wahlgren, M.; Bejarano, M.T. A Molecular Link between Malaria and Epstein–Barr Virus Reactivation. PLOS Pathog. 2007, 3, e80. [Google Scholar] [CrossRef]
- Matar, C.G.; Anthony, N.R.; O’Flaherty, B.M.; Jacobs, N.T.; Priyamvada, L.; Engwerda, C.R.; Speck, S.H.; Lamb, T.J. Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity. PLOS Pathog. 2015, 11, e1004858. [Google Scholar] [CrossRef]
- Oluoch, P.O.; Forconi, C.S.; Oduor, C.I.; Ritacco, D.A.; Akala, H.M.; Bailey, J.A.; Juliano, J.J.; Ong’echa, J.M.; Münz, C.; Moormann, A.M. Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes. Int. J. Mol. Sci. 2023, 24, 6711. [Google Scholar] [CrossRef]
- Simone, O.; Bejarano, M.T.; Pierce, S.K.; Antonaci, S.; Wahlgren, M.; Troye-Blomberg, M.; Donati, D. TLRs innate immunereceptors and Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) CIDR1α-driven human polyclonal B-cell activation. Acta Trop. 2011, 119, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Snider, C.J.; Chelimo, K. The company malaria keeps: How co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr. Opin. Infect. Dis. 2011, 24, 435–441. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Happi, A.N.; Ugwu, C.A.; Happi, C.T. Tracking the emergence of new SARS-CoV-2 variants in South Africa. Nat. Med. 2021, 27, 372–373. [Google Scholar] [CrossRef]
- WHO COVID-19 Dashboard. World Health Organization (WHO): Geneva, Switzerland. Available online: https://covid19.who.int/ (accessed on 4 May 2023).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 15 May 2023).
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Hafezi, B.; Chan, L.; Knapp, J.P.; Karimi, N.; Alizadeh, K.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells 2021, 10, 1761. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Paolucci, S.; Cassaniti, I.; Novazzi, F.; Fiorina, L.; Piralla, A.; Comolli, G.; Bruno, R.; Maserati, R.; Gulminetti, R.; Novati, S.; et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. 2021, 104, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Engelmann, I.; Moreau, A.-S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein–Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cao, S.; Dong, H.; Lv, H.; Teng, X.; Zhang, J.; Wang, T.; Zhang, X.; Qin, Y.; Chai, Y.; et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect. Dis. 2021, 21, 955. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Church, T.; Swaminathan, S. Epstein-Barr Virus Lytic Replication Induces ACE2 Expression and Enhances SARS-CoV-2 Pseudotyped Virus Entry in Epithelial Cells. J. Virol. 2021, 95, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef]
- Nunn, A.V.; Guy, G.W.; Botchway, S.W.; Bell, J.D. SARS-CoV-2 and EBV; the cost of a second mitochondrial “whammy”? Immun. Ageing 2021, 18, 1–4. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Wei, C.; Huang, Y.; Zhao, D. Coinfection with EBV/CMV and other respiratory agents in children with suspected infectious mononucleosis. Virol. J. 2010, 7, 247. [Google Scholar] [CrossRef]
- Ito, Y.; Shibata-Watanabe, Y.; Kawada, J.; Maruyama, K.; Yagasaki, H.; Kojima, S.; Kimura, H. Cytomegalovirus and Epstein-Barr virus coinfection in three toddlers with prolonged illnesses. J. Med. Virol. 2009, 81, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Mehraein, Y.; Lennerz, C.; Ehlhardt, S.; Zang, K.D.; Madry, H. Replicative multivirus infection with cytomegalovirus, herpes simplex virus 1, and parvovirus B19, and latent Epstein-Barr virus infection in the synovial tissue of a psoriatic arthritis patient. J. Clin. Virol. 2004, 31, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Zhang, N.; Jia, M.; Su, M. Association Between Cytomegalovirus and Epstein-Barr Virus Co-Reactivation and Hematopoietic Stem Cell Transplantation. Front. Cell. Infect. Microbiol. 2022, 12, 818167. [Google Scholar] [CrossRef] [PubMed]
- Mosca de Cerqueira, A.M.; de Cerqueira, F.M.; Campos Moreira, L.F.; Oliveira, S.D.S. Coinfection by herpes simplex virus and Epstein-Barr virus causing erythema multiforme. Proc. J. Am. Acad. Dermatol. 2010, 62, AB36. [Google Scholar]
- Delaney, A.S.; Thomas, W.; Balfour, H.H., Jr. Coprevalence of Epstein-Barr Virus, Cytomegalovirus, and Herpes Simplex Virus Type-1 Antibodies Among United States Children and Factors Associated With Their Acquisition. J. Pediatr. Infect. Dis. Soc. 2014, 4, 323–329. [Google Scholar] [CrossRef]
- Okoye, J.O.; Ngokere, A.A.; Onyenekwe, C.C.; Omotuyi, O.; Dada, D.I. Epstein-Barr virus, human papillomavirus and herpes simplex virus 2 co-presence severely dysregulates miRNA expression. Afr. J. Lab. Med. 2021, 10, 975. [Google Scholar] [CrossRef]
- Blanco, R.; Carrillo-Beltrán, D.; Osorio, J.C.; Calaf, G.M.; Aguayo, F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives. Pathogens 2020, 9, 685. [Google Scholar] [CrossRef]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.-F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Wal, M.; Kuch, A.; Pieta, P. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) cervical infections in women with normal and abnormal cytology. Pol. J. Microbiol. 2004, 53, 95–99. [Google Scholar]
- Khenchouche, A.; Sadouki, N.; Boudriche, A.; Houali, K.; Graba, A.; Ooka, T.; Bouguermouh, A. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol. J. 2013, 10, 340. [Google Scholar] [CrossRef]
- Santos, N.B.M.; Villanova, F.E.; Andrade, P.M.; Ribalta, J.; Focchi, J.; Otsuka, A.Y.; Dale Silva, I. Epstein-Barr virus detection in invasive and pre-invasive lesions of the uterine cervix. Oncol. Rep. 2009, 21, 403–405. [Google Scholar] [PubMed]
- Sosse, S.A.; Tadlaoui, K.A.; Benhassou, M.; Elkarroumi, M.; Elmzibri, M.; Ennaji, M.M. Viral co-infection of oncogenic Human papillomavirus with Epstein–Barr Virus, Human herpesvirus 8 and Herpes Simplex Virus type 2 in malignant cervical cancer. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Sauvageot, J.; Fedor, H.L.; Dick, J.D.; De Marzo, A.M.; Isaacs, W.B. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate 2008, 68, 306–320. [Google Scholar] [CrossRef]
- Grinstein, S.; Preciado, M.V.; Gattuso, P.; Chabay, P.A.; Warren, W.H.; De Matteo, E.; Gould, V.E. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res. 2002, 62, 4876–4878. [Google Scholar]
- Nahand, J.S.; Khanaliha, K.; Mirzaei, H.; Moghoofei, M.; Baghi, H.B.; Esghaei, M.; Khatami, A.R.; Fatemipour, M.; Bokharaei-Salim, F. Possible role of HPV/EBV coinfection in anoikis resistance and development in prostate cancer. BMC Cancer 2021, 21, 926. [Google Scholar] [CrossRef] [PubMed]
- Glenn, W.K.; Heng, B.; Delprado, W.; Iacopetta, B.; Whitaker, N.J.; Lawson, J.S. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS ONE 2012, 7, e48788. [Google Scholar] [CrossRef]
- Fang, L.-Z.; Dong, Y.-H.; Yan, Z.-J.; Zhou, C.-M.; Yu, X.-J.; Qin, X.-R. Reactivation of Epstein-Barr virus in SFTSV infected patients. Infect. Med. 2023. [Google Scholar] [CrossRef]
- Chen, J.; Ueda, K.; Sakakibara, S.; Okuno, T.; Parravicini, C.; Corbellino, M.; Yamanishi, K. Activation of latent Kaposi’s sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc. Natl. Acad. Sci. USA 2001, 98, 4119–4124. [Google Scholar] [CrossRef]
- Yan, Q.; Li, W.; Tang, Q.; Yao, S.; Lv, Z.; Feng, N.; Ma, X.; Bai, Z.; Zeng, Y.; Qin, D. Cellular microRNAs 498 and 320d regulate herpes simplex virus 1 induction of Kaposi’s sarcoma-associated herpesvirus lytic replication by targeting RTA. PLoS ONE 2013, 8, e55832. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Barrett, L.; James, J.; Plaisance-Bonstaff, K.; Post, S.R.; Qin, Z. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun. Biol. 2021, 4, 682. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Sun, R. Oxidative stress induces reactivation of Kaposi’s sarcoma-associated herpesvirus and death of primary effusion lymphoma cells. J. Virol. 2011, 85, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhou, F.; Bedolla, R.G.; Jones, T.; Lei, X.; Kang, T.; Guadalupe, M.; Gao, S.J. Reactive oxygen species hydrogen peroxide mediates Kaposi’s sarcoma-associated herpesvirus reactivation from latency. PLoS Pathog. 2011, 7, e1002054. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lamplugh, Z.L.; Lang, F.; Yuan, Y.; Lieberman, P.; You, J.; Robertson, E.S. KSHV-encoded LANA protects the cellular replication machinery from hypoxia induced degradation. PLoS Pathog. 2019, 15, e1008025. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Rinderknecht, A.S.; Zoeteweij, J.P.; Aoki, Y.; Read-Connole, E.L.; Tosato, G.; Blauvelt, A.; Yarchoan, R. Hypoxia induces lytic replication of Kaposi sarcoma–associated herpesvirus. Blood J. Am. Soc. Hematol. 2001, 97, 3244–3250. [Google Scholar] [CrossRef]
- Iftode, N.; Rădulescu, M.A.; Aramă, Ș.S.; Aramă, V. Update on Kaposi sarcoma-associated herpesvirus (KSHV or HHV8)–review. Rom. J. Intern. Med. 2020, 58, 199–208. [Google Scholar] [CrossRef]
- Mbisa, G.L.; Miley, W.; Gamache, C.J.; Gillette, W.K.; Esposito, D.; Hopkins, R.; Busch, M.P.; Schreiber, G.B.; Little, R.F.; Yarchoan, R.; et al. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: A new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J. Immunol. Methods 2010, 356, 39–46. [Google Scholar] [CrossRef]
- Labo, N.; Miley, W.; Benson, C.A.; Campbell, T.B.; Whitby, D. Epidemiology of Kaposi’s sarcoma-associated herpesvirus in HIV-1-infected US persons in the era of combination antiretroviral therapy. Aids 2015, 29, 1217–1225. [Google Scholar] [CrossRef]
- Varthakavi, V.; Smith, R.M.; Deng, H.; Sun, R.; Spearman, P. Human immunodeficiency virus type-1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus through induction of KSHV Rta. Virology 2002, 297, 270–280. [Google Scholar] [CrossRef]
- Albini, A.; Soldi, R.; Giunciuclio, D.; Giraudo, E.; Benelli, R.; Primo, L.; Noonan, D.; Salio, M.; Camussi, G.; Rock, W. The angiogenesis induced by HIV–1 Tat protein is mediated by the Flk–1/KDR receptor on vascular endothelial cells. Nat. Med. 1996, 2, 1371–1375. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Huang, Z.; Cheng, L.; Yao, S.; Qin, D.; Chen, X.; Tang, Q.; Lv, Z.; Zhang, L. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus: Role of JAK/STAT signaling. J. Virol. 2007, 81, 2401–2417. [Google Scholar] [CrossRef]
- Mercader, M.; Taddeo, B.; Panella, J.R.; Chandran, B.; Nickoloff, B.J.; Foreman, K.E. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am. J. Pathol. 2000, 156, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Cavallin, L.E.; Goldschmidt-Clermont, P.; Mesri, E.A. Molecular and cellular mechanisms of KSHV oncogenesis of Kaposi’s sarcoma associated with HIV/AIDS. PLoS Pathog. 2014, 10, e1004154. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Lai, C.-L.; Tsao, S.-M.; Lin, M.-N.; Hsieh, T.-C.; Lu, J.-J.; Chu, T.-Y. High prevalence of human herpesvirus type 8 infection in patients with pulmonary tuberculosis in Taiwan. Clin. Microbiol. Infect. 2015, 21, 266.e5–266.e7. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jung, B.G.; Chen, J.; Samten, B.; Forrest, J.C.; Post, S.R.; Qin, Z. The potential impacts of early secreted antigenic target of 6 kDa of Mycobacterium tuberculosis on KSHV-infected cells. J. Med. Virol. 2021, 93, 4028–4032. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, K.; Webb, E.L.; Sebina, I.; Muhangi, L.; Miley, W.; Johnson, W.T.; Ndibazza, J.; Elliott, A.M.; Whitby, D.; Newton, R. Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect. Agents Cancer 2011, 6, 1–7. [Google Scholar] [CrossRef]
- Crispo, A.; Tamburini, M.; De Marco, M.; Ascierto, P.; Silvestro, P.; Ronga, D.; Tridente, V.; Desicato, S.; Carbone, S.; Fabbrocini, G. HHV-8 prevalence, immunosuppression and Kaposi’s sarcoma in South Italy. Int. J. Mol. Med. 2001, 7, 535–538. [Google Scholar] [CrossRef]
- Coluzzi, M.; Calabro, M.; Manno, D.; Chieco-Bianchi, L.; Schulz, T.; Ascoli, V. Reduced seroprevalence of Kaposi’s sarcoma-associated herpesvirus (KSHV), human herpesvirus 8 (HHV8), related to suppression of Anopheles density in Italy. Med. Vet. Entomol. 2003, 17, 461–464. [Google Scholar] [CrossRef]
- Sabourin, K.R.; Daud, I.; Ogolla, S.; Labo, N.; Miley, W.; Lamb, M.; Newton, R.; Whitby, D.; Rochford, R. Malaria Is Associated With Kaposi Sarcoma-Associated Herpesvirus Seroconversion in a Cohort of Western Kenyan Children. J. Infect. Dis. 2021, 224, 303–311. [Google Scholar] [CrossRef]
- Reese, T.; Wakeman, B.; Choi, H.; Hufford, M.; Huang, S.; Zhang, X.; Buck, M.; Jezewski, A.; Kambal, A.; Liu, C. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 2014, 345, 573–577. [Google Scholar] [CrossRef]
- Ruocco, V.; Ruocco, E.; Schwartz, R.A.; Janniger, C.K. Kaposi sarcoma and quinine: A potentially overlooked triggering factor in millions of Africans. J. Am. Acad. Dermatol. 2011, 64, 434–436. [Google Scholar] [CrossRef]
- Haldar, K.; Mohandas, N. Malaria, erythrocytic infection, and anemia. Hematology. ASH Educ. 2009, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jyotsana, N.; King, M.R. The impact of COVID-19 on cancer risk and treatment. Cell. Mol. Bioeng. 2020, 13, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.J.; Lambarey, H.; Chetram, A.; Riou, C.; Wilkinson, R.J.; Schäfer, G. Kaposi’s Sarcoma-Associated Herpesvirus, but Not Epstein-Barr Virus, Co-infection Associates With Coronavirus Disease 2019 Severity and Outcome in South African Patients. Front. Microbiol. 2022, 12, 795555. [Google Scholar] [CrossRef]
- Leoni, E.; Cerati, M.; Finzi, G.; Lombardo, M.; Sessa, F. COVID-19 and HHV8 first spotted together: An affair under electron microscopy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e311. [Google Scholar] [CrossRef] [PubMed]
- Saade, A.; Moratelli, G.; Azoulay, E.; Darmon, M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect. Dis. Now 2021, 51, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Adland, E.; Klenerman, P.; Goulder, P.; Matthews, P.C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front. Microbiol. 2015, 6, 1016. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Brantsaeter, A.B. Human cytomegalovirus (CMV) in Africa: A neglected but important pathogen. J. Virus Erad. 2016, 2, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Kim, Y.-E.; Seo, M.-R.; Lee, J.-R.; Lee, C.H.; Ahn, J.-H. Interactions among four proteins encoded by the human cytomegalovirus UL112-113 region regulate their intranuclear targeting and the recruitment of UL44 to prereplication foci. J. Virol. 2006, 80, 2718–2727. [Google Scholar] [CrossRef]
- Cheng, B.; Martínez, F.P.; Katano, H.; Tang, Q. Evidence of inability of human cytomegalovirus to reactivate Kaposi’s sarcoma-associated herpesvirus from latency in body cavity-based lymphocytes. J. Clin. Virol. 2009, 46, 244–248. [Google Scholar] [CrossRef]
- Qin, D.; Feng, N.; Fan, W.; Ma, X.; Yan, Q.; Lv, Z.; Zeng, Y.; Zhu, J.; Lu, C. Activation of PI3K/AKT and ERK MAPK signal pathways is required for the induction of lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus by herpes simplex virus type 1. BMC Microbiol. 2011, 11, 240. [Google Scholar] [CrossRef]
- Meeuwsen, S.; Persoon-Deen, C.; Bsibsi, M.; Bajramovic, J.J.; Ravid, R.; De Bolle, L.; van Noort, J.M. Modulation of the cytokine network in human adult astrocytes by human herpesvirus-6A. J. Neuroimmunol. 2005, 164, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, D.; Zhao, Y.; Zhang, L. Mutual inhibition between Kaposi’s sarcoma-associated herpesvirus and Epstein-Barr virus lytic replication initiators in dually-infected primary effusion lymphoma. PLoS ONE 2008, 3, e1569. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Heston, L.; Grogan, E.; Gradoville, L.; Rigsby, M.; Sun, R.; Shedd, D.; Kushnaryov, V.M.; Grossberg, S.; Chang, Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 1997, 71, 314–324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinna, P.; Bratl, K.; Lambarey, H.; Blumenthal, M.J.; Schäfer, G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. Int. J. Mol. Sci. 2023, 24, 13066. https://doi.org/10.3390/ijms241713066
Chinna P, Bratl K, Lambarey H, Blumenthal MJ, Schäfer G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. International Journal of Molecular Sciences. 2023; 24(17):13066. https://doi.org/10.3390/ijms241713066
Chicago/Turabian StyleChinna, Prishanta, Katrin Bratl, Humaira Lambarey, Melissa J. Blumenthal, and Georgia Schäfer. 2023. "The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies" International Journal of Molecular Sciences 24, no. 17: 13066. https://doi.org/10.3390/ijms241713066
APA StyleChinna, P., Bratl, K., Lambarey, H., Blumenthal, M. J., & Schäfer, G. (2023). The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. International Journal of Molecular Sciences, 24(17), 13066. https://doi.org/10.3390/ijms241713066






