A Common Molecular Signature Indicates the Pre-Meristematic State of Plant Calli
Abstract
:1. Introduction
2. Callus Formation
2.1. Tissue Regeneration and Wound Healing
2.1.1. In the Root
2.1.2. In the Shoot
2.2. Exogenous Hormone-Induced Callus Formation
2.3. Hormone Gradients in Regeneration and Callus Initiation
3. Molecular Signature of Callus Tissues
3.1. The Induction Phase
3.2. Young Calli
3.3. Established Callus Cultures
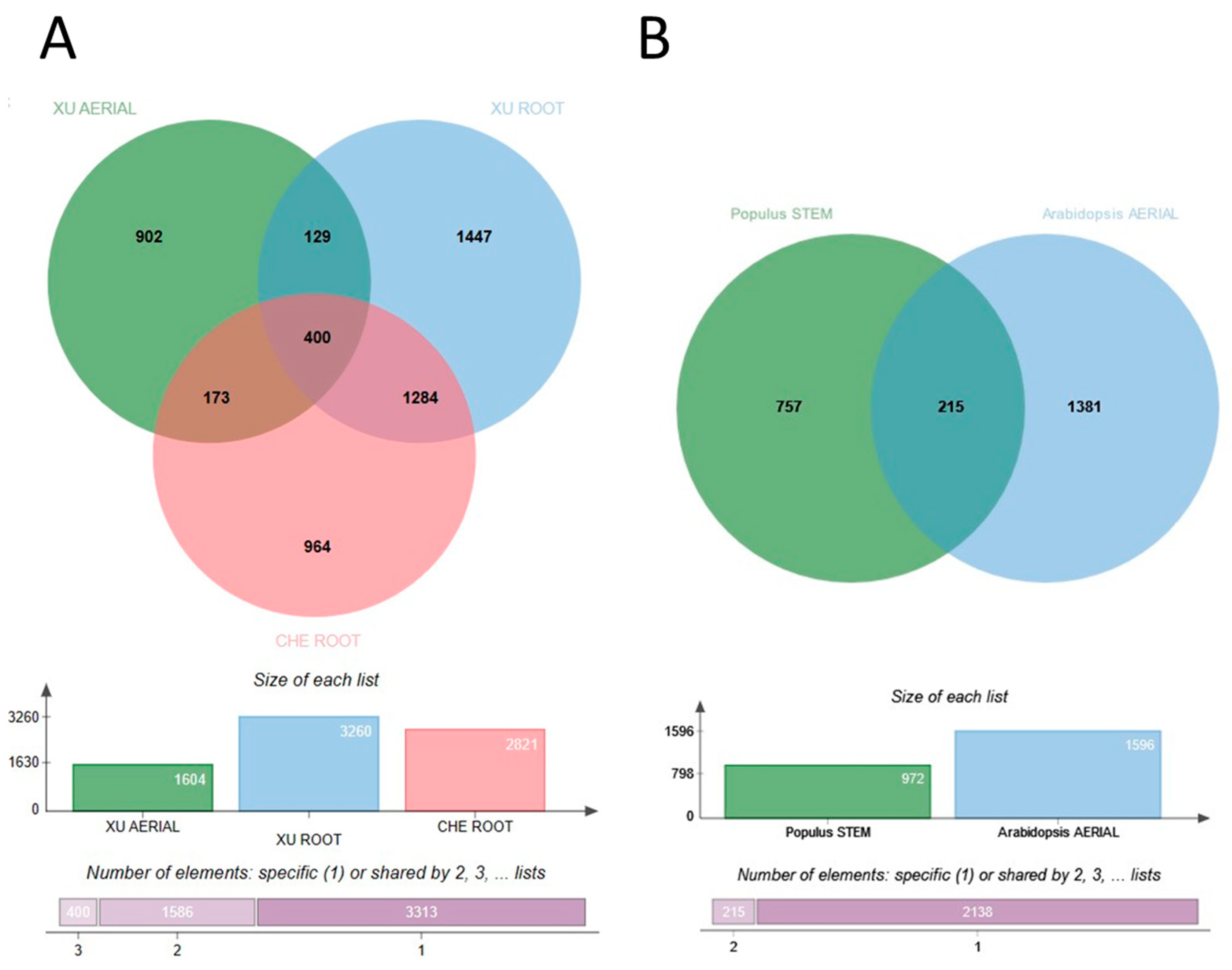
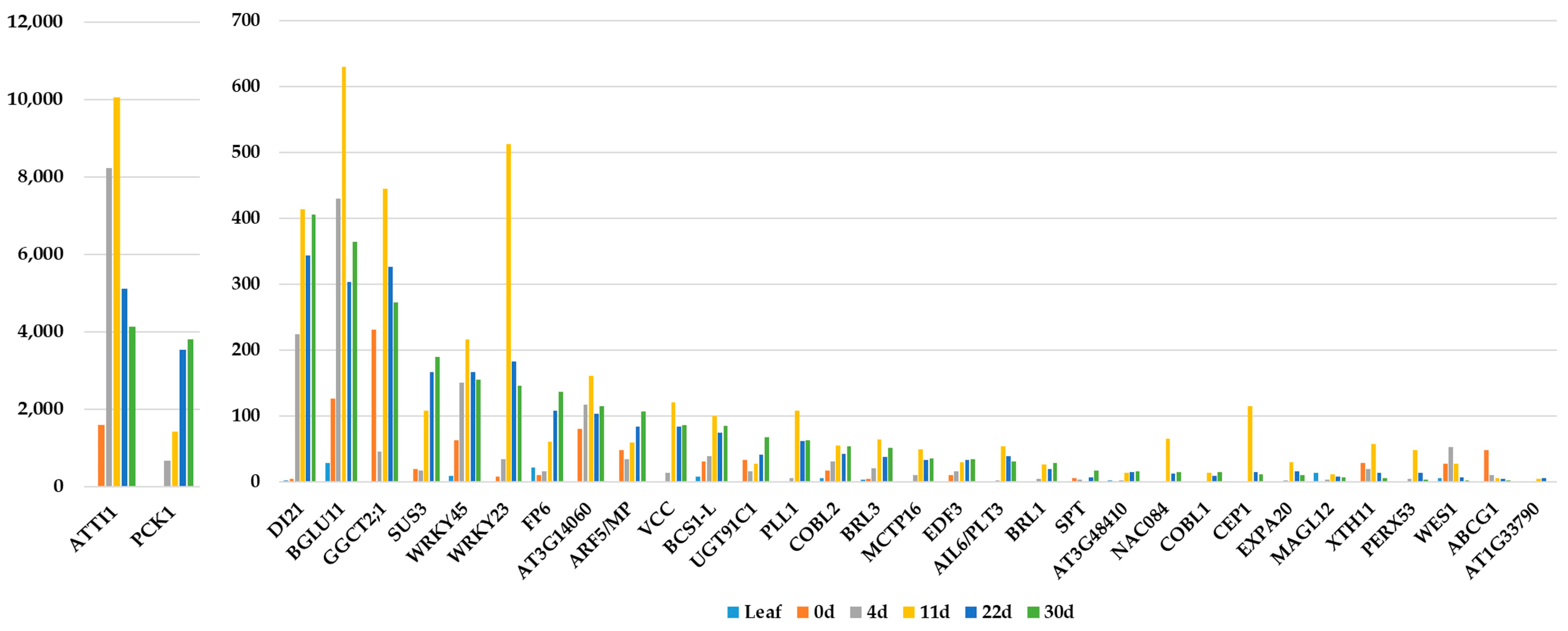
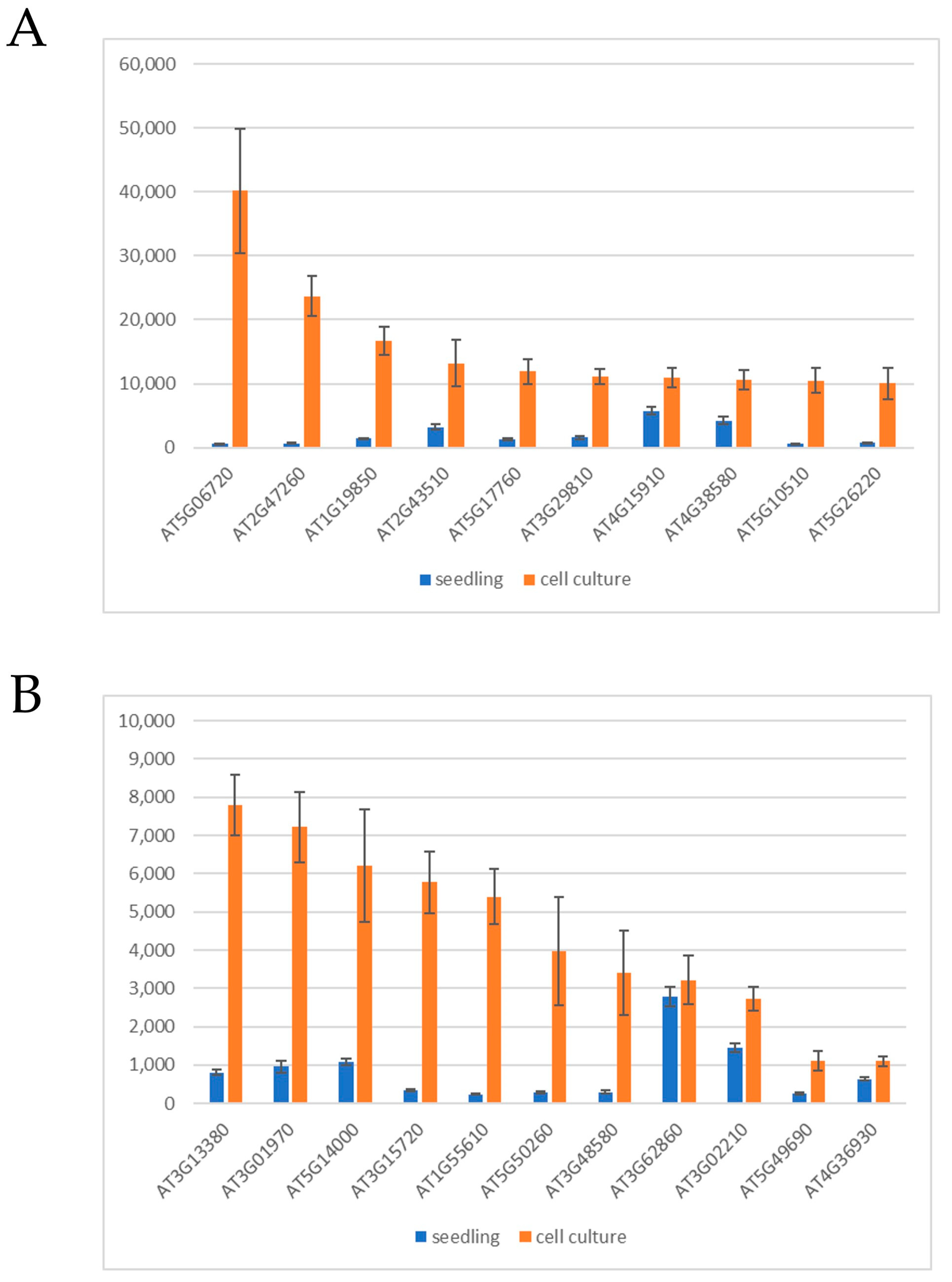
4. What Can Callus-Expressed Genes Teach Us?
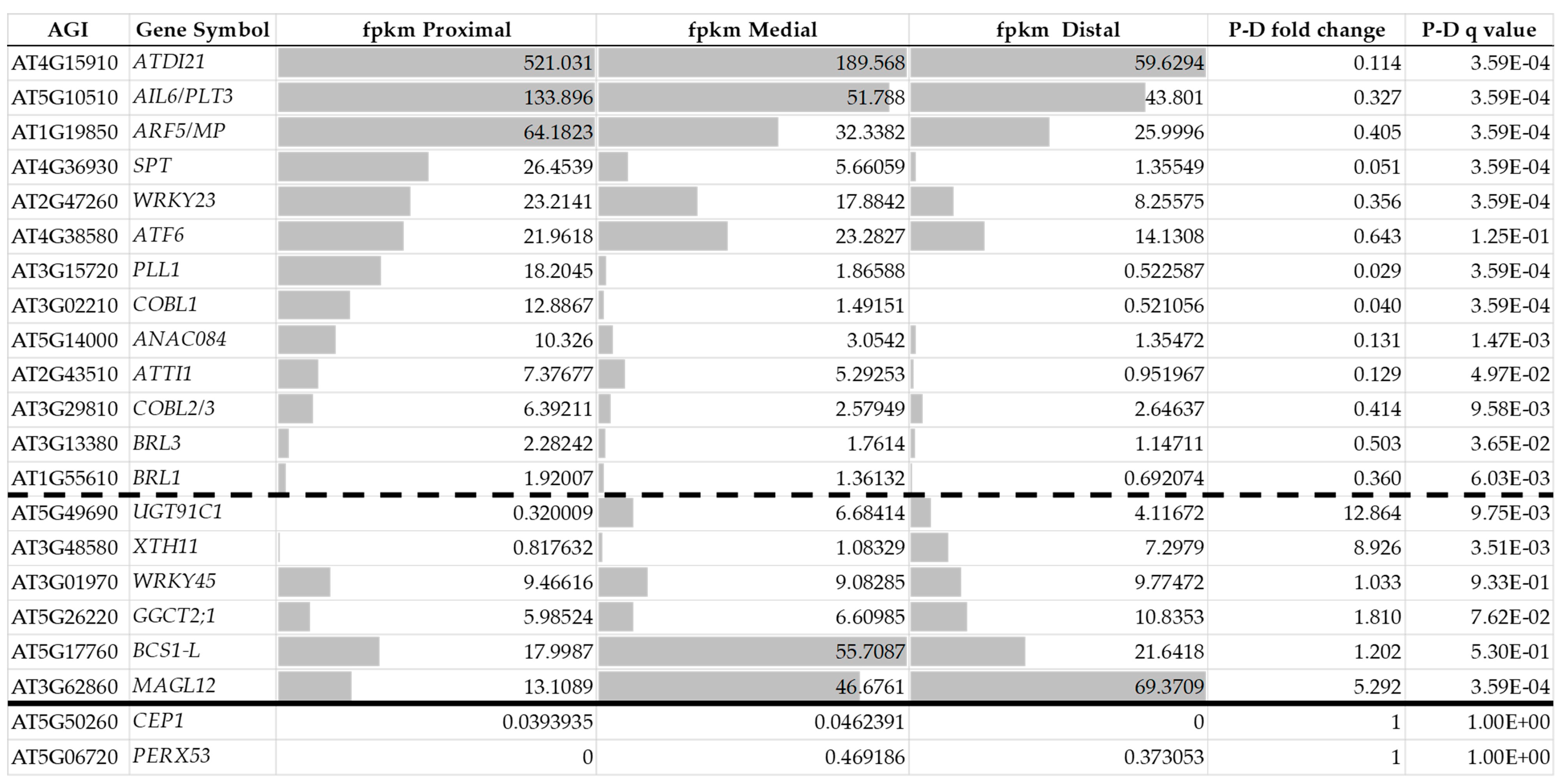


5. What Is a Plant Callus?
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikeuchi, M.; Rymen, B.; Sugimoto, K. How Do Plants Transduce Wound Signals to Induce Tissue Repair and Organ Regeneration? Curr. Opin. Plant Biol. 2020, 57, 72–77. [Google Scholar] [CrossRef]
- Christiaens, F.; Canher, B.; Lanssens, F.; Bisht, A.; Stael, S.; De Veylder, L.; Heyman, J. Pars Pro Toto: Every Single Cell Matters. Front. Plant Sci. 2021, 12, 656825. [Google Scholar] [CrossRef]
- Buck-Sorlin, G. Phytomer. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 1713–1714. ISBN 978-1-4419-9863-7. [Google Scholar]
- Bennett, T.; Leyser, O. Something on the Side: Axillary Meristems and Plant Development. Plant Mol. Biol. 2006, 60, 843–854. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching Out in Roots: Uncovering Form, Function, and Regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef]
- Feldman, L.J. The de Novo Origin of the Quiescent Center Regenerating Root Apices of Zea Mays. Planta 1976, 128, 207–212. [Google Scholar] [CrossRef]
- Reinhardt, D.; Frenz, M.; Mandel, T.; Kuhlemeier, C. Microsurgical and Laser Ablation Analysis of Interactions between the Zones and Layers of the Tomato Shoot Apical Meristem. Development 2003, 130, 4073–4083. [Google Scholar] [CrossRef]
- Sena, G.; Wang, X.; Liu, H.-Y.; Hofhuis, H.; Birnbaum, K.D. Organ Regeneration Does Not Require a Functional Stem Cell Niche in Plants. Nature 2009, 457, 1150–1153. [Google Scholar] [CrossRef]
- Marhava, P.; Hoermayer, L.; Yoshida, S.; Marhavý, P.; Benková, E.; Friml, J. Re-Activation of Stem Cell Pathways for Pattern Restoration in Plant Wound Healing. Cell 2019, 177, 957–969. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhang, J.; He, X.-Q. Plant in Situ Tissue Regeneration: Dynamics, Mechanisms and Implications for Forestry Research. For. Res. 2023, 3, 8. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. Adventitious Regeneration. In Plant Propagation by Tissue Culture: Volume 1. The Background; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 355–401. ISBN 978-1-4020-5005-3. [Google Scholar]
- Takebe, I.; Labib, G.; Melchers, G. Regeneration of Whole Plants from Isolated Mesophyll Protoplasts of Tobacco. Naturwissenschaften 1971, 58, 318–320. [Google Scholar] [CrossRef]
- Fehér, A.; Dudits, D. Plant Protoplasts for Cell Fusion and Direct DNA Uptake: Culture and Regeneration Systems. In Plant Cell and Tissue Culture; Springer Netherlands: Dordrecht, The Netherlands, 1994; pp. 71–118. ISBN 90-481-4327-6. [Google Scholar]
- Chupeau, M.-C.; Granier, F.; Pichon, O.; Renou, J.-P.; Gaudin, V.; Chupeau, Y. Characterization of the Early Events Leading to Totipotency in an Arabidopsis Protoplast Liquid Culture by Temporal Transcript Profiling. Plant Cell 2013, 25, 2444–2463. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, K. Chapter 14—Plant Tissue Culture-Based Industries. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia, S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 405–417. ISBN 978-0-12-802221-4. [Google Scholar]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. Methods Mol. Biol. 2018, 1815, 17–46. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E.; Jogam, P.; Babu, K.H.; Jain, S.M.; Suprasanna, P. Chapter 13—Improving Crops through Transgenic Breeding—Technological Advances and Prospects. In Advances in Plant Tissue Culture; Chandra Rai, A., Kumar, A., Modi, A., Singh, M., Eds.; Academic Press: Boston, MA, USA, 2022; pp. 295–324. ISBN 978-0-323-90795-8. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant Regeneration: Cellular Origins and Molecular Mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Mohan Jain, S. Cellular, Molecular, and Physiological Aspects of in Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, S.; Carneros, E.; Testillano, P.S.; Pérez-Pérez, J.M. Advances in Plant Regeneration: Shake, Rattle and Roll. Plants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Tavakoli Dinanai, E.; Abiri, R. Bioreactor-Based Advances in Plant Tissue and Cell Culture: Challenges and Prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Bloch, R. Wound Healing in Higher Plants. Bot. Rev. 1941, 7, 110–146. [Google Scholar] [CrossRef]
- Bloch, R. Wound Healing in Higher Plants: II. Bot. Rev. 1952, 18, 655–679. [Google Scholar] [CrossRef]
- Dodueva, I.E.; Lebedeva, M.A.; Kuznetsova, K.A.; Gancheva, M.S.; Paponova, S.S.; Lutova, L.L. Plant Tumors: A Hundred Years of Study. Planta 2020, 251, 82. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Skoog, F.; Miller, C.O. Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured in Vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar]
- Feldmann, K.A.; Marks, M.D. Rapid and Efficient Regeneration of Plants from Explants of Arabidopsis thaliana. Plant Sci. 1986, 47, 63–69. [Google Scholar] [CrossRef]
- Asahina, M.; Satoh, S. Molecular and Physiological Mechanisms Regulating Tissue Reunion in Incised Plant Tissues. J Plant Res 2015, 128, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lup, S.D.; Tian, X.; Xu, J.; Pérez-Pérez, J.M. Wound Signaling of Regenerative Cell Reprogramming. Plant Sci. 2016, 250, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Heyman, J.; Canher, B.; Bisht, A.; Christiaens, F.; De Veylder, L. Emerging Role of the Plant ERF Transcription Factors in Coordinating Wound Defense Responses and Repair. J. Cell Sci. 2018, 131, jcs208215. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Gu, Z.; Wu, S.; Yilan, E.; Zhou, W.; Lin, J.; Xu, L. Roles of the Wound Hormone Jasmonate in Plant Regeneration. J. Exp. Bot. 2021, 74, erab508. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.-X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-Mediated Wound Signalling Promotes Plant Regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956. [Google Scholar] [CrossRef]
- Heyman, J.; Cools, T.; Canher, B.; Shavialenka, S.; Traas, J.; Vercauteren, I.; Van den Daele, H.; Persiau, G.; De Jaeger, G.; Sugimoto, K.; et al. The Heterodimeric Transcription Factor Complex ERF115–PAT1 Grants Regeneration Competence. Nat. Plants 2016, 2, 16165. [Google Scholar] [CrossRef] [PubMed]
- Canher, B.; Heyman, J.; Savina, M.; Devendran, A.; Eekhout, T.; Vercauteren, I.; Prinsen, E.; Matosevich, R.; Xu, J.; Mironova, V.; et al. Rocks in the Auxin Stream: Wound-Induced Auxin Accumulation and ERF115 Expression Synergistically Drive Stem Cell Regeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 16667–16677. [Google Scholar] [CrossRef] [PubMed]
- Canher, B.; Lanssens, F.; Zhang, A.; Bisht, A.; Mazumdar, S.; Heyman, J.; Wolf, S.; Melnyk, C.W.; De Veylder, L. The Regeneration Factors ERF114 and ERF115 Regulate Auxin-Mediated Lateral Root Development in Response to Mechanical Cues. Mol. Plant 2022, 15, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Hoermayer, L.; Montesinos, J.C.; Marhava, P.; Benková, E.; Yoshida, S.; Friml, J. Wounding-Induced Changes in Cellular Pressure and Localized Auxin Signalling Spatially Coordinate Restorative Divisions in Roots. Proc. Natl. Acad. Sci. USA 2020, 117, 15322–15331. [Google Scholar] [CrossRef]
- Matosevich, R.; Cohen, I.; Gil-Yarom, N.; Modrego, A.; Friedlander-Shani, L.; Verna, C.; Scarpella, E.; Efroni, I. Local Auxin Biosynthesis Is Required for Root Regeneration after Wounding. Nat. Plants 2020, 6, 1020–1030. [Google Scholar] [CrossRef]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF Transcription Factor WIND1 Controls Cell Dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef]
- Iwase, A.; Mitsuda, N.; Ikeuchi, M.; Ohnuma, M.; Koizuka, C.; Kawamoto, K.; Imamura, J.; Ezura, H.; Sugimoto, K. Arabidopsis WIND1 Induces Callus Formation in Rapeseed, Tomato, and Tobacco. Plant Signal. Behav. 2013, 8, e27432. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Durgaprasad, K.; Roy, M.V.; Venugopal, M.A.; Kareem, A.; Raj, K.; Willemsen, V.; Mähönen, A.P.; Scheres, B.; Prasad, K. Gradient Expression of Transcription Factor Imposes a Boundary on Organ Regeneration Potential in Plants. Cell Rep. 2019, 29, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Mähönen, A.P.; Tusscher, K.T.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA Gradient Formation Mechanism Separates Auxin Responses. Nature 2014, 515, 125–129. [Google Scholar] [CrossRef]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Adibi, M.; Yoshida, S.; Weijers, D.; Fleck, C. Centering the Organizing Center in the Arabidopsis thaliana Shoot Apical Meristem by a Combination of Cytokinin Signaling and Self-Organization. PLoS ONE 2016, 11, e0147830. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Kondo, Y.; Laohavisit, A.; Takebayashi, A.; Ikeuchi, M.; Matsuoka, K.; Asahina, M.; Mitsuda, N.; Shirasu, K.; Fukuda, H.; et al. WIND Transcription Factors Orchestrate Wound-Induced Callus Formation, Vascular Reconnection and Defense Response in Arabidopsis. N. Phytol. 2021, 232, 734–752. [Google Scholar] [CrossRef]
- Iwase, A.; Harashima, H.; Ikeuchi, M.; Rymen, B.; Ohnuma, M.; Komaki, S.; Morohashi, K.; Kurata, T.; Nakata, M.; Ohme-Takagi, M.; et al. WIND1 Promotes Shoot Regeneration through Transcriptional Activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 2017, 29, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Asahina, M.; Azuma, K.; Pitaksaringkarn, W.; Yamazaki, T.; Mitsuda, N.; Ohme-Takagi, M.; Yamaguchi, S.; Kamiya, Y.; Okada, K.; Nishimura, T.; et al. Spatially Selective Hormonal Control of RAP2.6L and ANAC071 Transcription Factors Involved in Tissue Reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16128–16132. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome Dynamics at Arabidopsis Graft Junctions Reveal an Intertissue Recognition Mechanism That Activates Vascular Regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2447–E2456. [Google Scholar] [CrossRef]
- Omary, M.; Matosevich, R.; Efroni, I. Systemic Control of Plant Regeneration and Wound Repair. N. Phytol. 2023, 237, 408–413. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Ito, T.; Tanaka, H.; Favero, D.S.; Kawamura, A.; Sakamoto, S.; Wakazaki, M.; Tameshige, T.; Fujii, H.; et al. Wound-Inducible WUSCHEL-RELATED HOMEOBOX 13 Is Required for Callus Growth and Organ Reconnection. Plant Physiol. 2022, 188, 425–441. [Google Scholar] [CrossRef]
- Tanaka, H.; Hashimoto, N.; Kawai, S.; Yumoto, E.; Shibata, K.; Tameshige, T.; Yamamoto, Y.; Sugimoto, K.; Asahina, M.; Ikeuchi, M. Auxin-Induced WUSCHEL-RELATED HOMEOBOX13 Mediates Asymmetric Activity of Callus Formation upon Cutting. Plant Cell Physiol. 2022, 64, pcac146. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.-S.; Lee, H.G.; Seo, P.J. The ASHR3 SET-Domain Protein Is a Pivotal Upstream Coordinator for Wound-Induced Callus Formation in Arabidopsis. J. Plant Biol. 2020, 63, 361–368. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Y.; Sheng, L.; Liu, J.; Huang, H.; Xu, L. A Simple Method Suitable to Study de Novo Root Organogenesis. Front Plant Sci 2014, 5, 208. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-B.; Shang, G.-D.; Pan, Y.; Xu, Z.-G.; Zhou, C.-M.; Mao, Y.-B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.-W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration[OPEN]. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. De Novo Root Regeneration from Leaf Explants: Wounding, Auxin, and Cell Fate Transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, Y. The Molecular Regulation of Cell Pluripotency in Plants. aBIOTECH 2020, 1, 169–177. [Google Scholar] [CrossRef]
- Kamínek, M. Tracking the Story of Cytokinin Research. J. Plant Growth Regul. 2015, 34, 723–739. [Google Scholar] [CrossRef]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis Xylem Pericycle Underlies Shoot Regeneration from Root and Hypocotyl Explants Grown in Vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef]
- Del Pozo, J.C.; Lopez-Matas, M.A.; Ramirez-Parra, E.; Gutierrez, C. Hormonal Control of the Plant Cell Cycle. Physiol. Plant. 2005, 123, 173–183. [Google Scholar] [CrossRef]
- Perianez-Rodriguez, J.; Manzano, C.; Moreno-Risueno, M.A. Post-Embryonic Organogenesis and Plant Regeneration from Tissues: Two Sides of the Same Coin? Front. Plant Sci. 2014, 5, 219. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, F.; Li, J. Promotive Effect of Brassinosteroids on Cell Division Involves a Distinct CycD3-Induction Pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Goren, R.; Altman, A.; Giladi, I. Role of Ethylene in Abscisic Acid-Induced Callus Formation in Citrus Bud Cultures 1. Plant Physiol. 1979, 63, 280–282. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, H.; Liu, W.; Hu, X.; Han, N.; Qian, Q.; Xu, L.; Bian, H. Callus Initiation from Root Explants Employs Different Strategies in Rice and Arabidopsis. Plant Cell Physiol. 2018, 59, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral Root Formation Is Blocked by a Gain-of-Function Mutation in the SOLITARY-ROOT/IAA14 Gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Nakao, Y.; Okushima, Y.; Theologis, A.; Tasaka, M. Tissue-Specific Expression of Stabilized SOLITARY-ROOT/IAA14 Alters Lateral Root Development in Arabidopsis. Plant J. 2005, 44, 382–395. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Xu, C.; Xu, K.; Hu, Y. LATERAL ORGAN BOUNDARIES DOMAIN Transcription Factors Direct Callus Formation in Arabidopsis Regeneration. Cell Res. 2012, 22, 1169–1180. [Google Scholar] [CrossRef]
- Berckmans, B.; Vassileva, V.; Schmid, S.P.C.; Maes, S.; Parizot, B.; Naramoto, S.; Magyar, Z.; Kamei, C.L.A.; Koncz, C.; Bögre, L.; et al. Auxin-Dependent Cell Cycle Reactivation through Transcriptional Regulation of Arabidopsis E2Fa by Lateral Organ Boundary Proteins. Plant Cell 2011, 23, 3671–3683. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, M.-J.; Kim, N.Y.; Lee, S.H.; Kim, J. LBD18 Acts as a Transcriptional Activator That Directly Binds to the EXPANSIN14 Promoter in Promoting Lateral Root Emergence of Arabidopsis. Plant J. 2013, 73, 212–224. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Zhang, Q.; Wang, H.; Xin, W.; Xu, E.; Zhang, S.; Yu, R.; Yu, D.; Hu, Y. Control of Auxin-Induced Callus Formation by BZIP59–LBD Complex in Arabidopsis Regeneration. Nat. Plants 2018, 4, 108–115. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Xu, E.; Zhang, S.; Hu, Y. Genome-Wide Identification of Arabidopsis LBD29 Target Genes Reveals the Molecular Events behind Auxin-Induced Cell Reprogramming during Callus Formation. Plant Cell Physiol. 2018, 59, 749–760. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 Are Involved in the First-Step Cell Fate Transition during de Novo Root Organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Qin, P.; Prasad, K.; Hu, Y.; Xu, L. The WOX11–LBD16 Pathway Promotes Pluripotency Acquisition in Callus Cells During De Novo Shoot Regeneration in Tissue Culture. Plant Cell Physiol. 2018, 59, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, G.; Liu, W.; Shi, J.; Wang, H.; Qi, M.; Li, J.; Qin, P.; Ruan, Y.; Huang, H.; et al. Divergent Regeneration-Competent Cells Adopt a Common Mechanism for Callus Initiation in Angiosperms. Regeneration 2017, 4, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Shanmukhan, A.P.; Mathew, M.M.; Aiyaz, M.; Varaparambathu, V.; Kareem, A.; Radhakrishnan, D.; Prasad, K. Regulation of Touch-Stimulated de Novo Root Regeneration from Arabidopsis Leaves. Plant Physiol. 2021, 187, 52–58. [Google Scholar] [CrossRef]
- Shin, S.Y.; Choi, Y.; Kim, S.-G.; Park, S.-J.; Park, J.-S.; Moon, K.-B.; Kim, H.-S.; Jeon, J.H.; Cho, H.S.; Lee, H.-J. Submergence Promotes Auxin-Induced Callus Formation through Ethylene-Mediated Post-Transcriptional Control of Auxin Receptors. Mol. Plant 2022, 15, 1947–1961. [Google Scholar] [CrossRef]
- Alabadí, D.; Blázquez, M.A.; Carbonell, J.; Ferrándiz, C.; Pérez-Amador, M.A. Instructive Roles for Hormones in Plant Development. Int. J. Dev. Biol. 2009, 53, 1597–1608. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Chandler, J.W.; Werr, W. Cytokinin–Auxin Crosstalk in Cell Type Specification. Trends Plant Sci. 2015, 20, 291–300. [Google Scholar] [CrossRef]
- Scheres, B. Plant Cell Identity. The Role of Position and Lineage. Plant Physiol. 2001, 125, 112–114. [Google Scholar] [CrossRef]
- Hoermayer, L.; Friml, J. Targeted Cell Ablation-Based Insights into Wound Healing and Restorative Patterning. Curr. Opin. Plant Biol. 2019, 52, 124–130. [Google Scholar] [CrossRef]
- Lakehal, A.; Dob, A.; Rahneshan, Z.; Novák, O.; Escamez, S.; Alallaq, S.; Strnad, M.; Tuominen, H.; Bellini, C. ETHYLENE RESPONSE FACTOR 115 Integrates Jasmonate and Cytokinin Signaling Machineries to Repress Adventitious Rooting in Arabidopsis. N. Phytol. 2020, 228, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Che, P.; Lall, S.; Nettleton, D.; Howell, S.H. Gene Expression Programs during Shoot, Root, and Callus Development in Arabidopsis Tissue Culture. Plant Physiol. 2006, 141, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, J.; Fan, M.; Xin, W.; Hu, Y.; Xu, C. A Genome-Wide Transcriptome Profiling Reveals the Early Molecular Events during Callus Initiation in Arabidopsis Multiple Organs. Genomics 2012, 100, 116–124. [Google Scholar] [CrossRef]
- Bao, Y.; Dharmawardhana, P.; Mockler, T.C.; Strauss, S.H. Genome Scale Transcriptome Analysis of Shoot Organogenesis in Populus. BMC Plant Biol. 2009, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Pasha, A.; Subramaniam, S.; Cleary, A.; Chen, X.; Berardini, T.; Farmer, A.; Town, C.; Provart, N. Araport Lives: An Updated Framework for Arabidopsis Bioinformatics. Plant Cell 2020, 32, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Ishii, H.; Aoyagi, H.; Ohme-Takagi, M.; Tanaka, H. Comparative Analyses of the Gene Expression Profiles of Arabidopsis Intact Plant and Cultured Cells. Biotechnol. Lett. 2005, 27, 1097–1103. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.-S.; Seo, P.J. RNA-Seq Analysis of the Arabidopsis Transcriptome in Pluripotent Calli. Mol. Cells 2016, 39, 484–494. [Google Scholar] [CrossRef]
- Xu, M.; Du, Q.; Tian, C.; Wang, Y.; Jiao, Y. Stochastic Gene Expression Drives Mesophyll Protoplast Regeneration. Sci. Adv. 2021, 7, eabg8466. [Google Scholar] [CrossRef]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator v3: A Reference Expression Database for the Meta-Analysis of Transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Möller, B.; Lokerse, A.S.; Llavata-Peris, C.I.; van den Berg, W.; Weijers, D. A Cellular Expression Map of the Arabidopsis AUXIN RESPONSE FACTOR Gene Family. Plant J. 2011, 68, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.K.; ten Hove, C.A.; Xiang, D.; Williams, N.; López, L.G.; Yoshida, S.; Smit, M.; Datla, R.; Weijers, D. Auxin Response Cell-Autonomously Controls Ground Tissue Initiation in the Early Arabidopsis Embryo. Proc. Natl. Acad. Sci. USA 2017, 114, E2533–E2539. [Google Scholar] [CrossRef] [PubMed]
- Hardtke, C.S.; Berleth, T. The Arabidopsis Gene MONOPTEROS Encodes a Transcription Factor Mediating Embryo Axis Formation and Vascular Development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, A.; Möller, B.; Liu, W.; Kientz, M.; Flipse, J.; Rademacher, E.H.; Schmid, M.; Jürgens, G.; Weijers, D. MONOPTEROS Controls Embryonic Root Initiation by Regulating a Mobile Transcription Factor. Nature 2010, 464, 913–916. [Google Scholar] [CrossRef]
- Malamy, J.E.; Benfey, P.N. Organization and Cell Differentiation in Lateral Roots of Arabidopsis thaliana. Development 1997, 124, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, J.; Goh, T.; Guyomarc’h, S.; Hill, K.; Lucas, M.; Voß, U.; Kenobi, K.; Wilson, M.H.; Farcot, E.; Hagen, G.; et al. Inference of the Arabidopsis Lateral Root Gene Regulatory Network Suggests a Bifurcation Mechanism That Defines Primordia Flanking and Central Zones. Plant Cell 2015, 27, 1368–1388. [Google Scholar] [CrossRef]
- De Smet, I.; Lau, S.; Voß, U.; Vanneste, S.; Benjamins, R.; Rademacher, E.H.; Schlereth, A.; De Rybel, B.; Vassileva, V.; Grunewald, W.; et al. Bimodular Auxin Response Controls Organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 2705–2710. [Google Scholar] [CrossRef]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal Control of the Shoot Stem-Cell Niche. Nature 2010, 465, 1089–1092. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, J.; Wu, H.; Tian, Z.; Zhao, Z. A Molecular Framework for Auxin-Controlled Homeostasis of Shoot Stem Cells in Arabidopsis. Mol. Plant 2018, 11, 899–913. [Google Scholar] [CrossRef]
- Cole, M.; Chandler, J.; Weijers, D.; Jacobs, B.; Comelli, P.; Werr, W. DORNRÖSCHEN Is a Direct Target of the Auxin Response Factor MONOPTEROS in the Arabidopsis Embryo. Development 2009, 136, 1643–1651. [Google Scholar] [CrossRef]
- Bhatia, N.; Bozorg, B.; Larsson, A.; Ohno, C.; Jönsson, H.; Heisler, M.G. Auxin Acts through MONOPTEROS to Regulate Plant Cell Polarity and Pattern Phyllotaxis. Curr. Biol. 2016, 26, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Gaillochet, C.; Stiehl, T.; Wenzl, C.; Ripoll, J.-J.; Bailey-Steinitz, L.J.; Li, L.; Pfeiffer, A.; Miotk, A.; Hakenjos, J.P.; Forner, J.; et al. Control of Plant Cell Fate Transitions by Transcriptional and Hormonal Signals. eLife 2017, 6, e30135. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.T.; Berleth, T. A Dominant Mutation Reveals Asymmetry in MP/ARF5 Function along the Adaxial-Abaxial Axis of Shoot Lateral Organs. Plant Signal. Behav. 2012, 7, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Ckurshumova, W.; Smirnova, T.; Marcos, D.; Zayed, Y.; Berleth, T. Irrepressible MONOPTEROS/ARF5 Promotes de Novo Shoot Formation. N. Phytol. 2014, 204, 556–566. [Google Scholar] [CrossRef]
- Krogan, N.T.; Marcos, D.; Weiner, A.I.; Berleth, T. The Auxin Response Factor MONOPTEROS Controls Meristem Function and Organogenesis in Both the Shoot and Root through the Direct Regulation of PIN Genes. N. Phytol. 2016, 212, 42–50. [Google Scholar] [CrossRef]
- Horstman, A.; Willemsen, V.; Boutilier, K.; Heidstra, R. AINTEGUMENTA-LIKE Proteins: Hubs in a Plethora of Networks. Trends Plant Sci. 2014, 19, 146–157. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA Proteins as Dose-Dependent Master Regulators of Arabidopsis Root Development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. PLETHORA Transcription Factors Orchestrate de Novo Organ Patterning during Arabidopsis Lateral Root Outgrowth. Proc. Natl. Acad. Sci. USA 2017, 114, 11709–11714. [Google Scholar] [CrossRef]
- Hofhuis, H.; Laskowski, M.; Du, Y.; Prasad, K.; Grigg, S.; Pinon, V.; Scheres, B. Phyllotaxis and Rhizotaxis in Arabidopsis Are Modified by Three PLETHORA Transcription Factors. Curr. Biol. 2013, 23, 956–962. [Google Scholar] [CrossRef]
- Mudunkothge, J.S.; Krizek, B.A. Three Arabidopsis AIL/PLT Genes Act in Combination to Regulate Shoot Apical Meristem Function. Plant J. 2012, 71, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Grigg, S.P.; Barkoulas, M.; Yadav, R.K.; Sanchez-Perez, G.F.; Pinon, V.; Blilou, I.; Hofhuis, H.; Dhonukshe, P.; Galinha, C.; et al. Arabidopsis PLETHORA Transcription Factors Control Phyllotaxis. Curr. Biol. 2011, 21, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Pinon, V.; Prasad, K.; Grigg, S.P.; Sanchez-Perez, G.F.; Scheres, B. Local Auxin Biosynthesis Regulation by PLETHORA Transcription Factors Controls Phyllotaxis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Krizek, B. AINTEGUMENTA and AINTEGUMENTA-LIKE6 Act Redundantly to Regulate Arabidopsis Floral Growth and Patterning. Plant Physiol. 2009, 150, 1916–1929. [Google Scholar] [CrossRef]
- Krizek, B.A.; Eaddy, M. AINTEGUMENTA-LIKE6 Regulates Cellular Differentiation in Flowers. Plant Mol. Biol. 2012, 78, 199–209. [Google Scholar] [CrossRef]
- Krizek, B.A.; Bequette, C.J.; Xu, K.; Blakley, I.C.; Fu, Z.Q.; Stratmann, J.W.; Loraine, A.E. RNA-Seq Links the Transcription Factors AINTEGUMENTA and AINTEGUMENTA-LIKE6 to Cell Wall Remodeling and Plant Defense Pathways. Plant Physiol. 2016, 171, 2069–2084. [Google Scholar] [CrossRef]
- Scheres, B.; Krizek, B.A. Coordination of Growth in Root and Shoot Apices by AIL/PLT Transcription Factors. Curr. Opin. Plant Biol. 2018, 41, 95–101. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Wu, M.-F.; Winter, C.M.; Berns, M.C.; Nole-Wilson, S.; Yamaguchi, A.; Coupland, G.; Krizek, B.A.; Wagner, D. A Molecular Framework for Auxin-Mediated Initiation of Flower Primordia. Dev. Cell 2013, 24, 271–282. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Jeong, C.W.; Nole-Wilson, S.; Krizek, B.A.; Wagner, D. AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 Induce LEAFY Expression in Response to Auxin to Promote the Onset of Flower Formation in Arabidopsis. Plant Physiol. 2016, 170, 283–293. [Google Scholar] [CrossRef]
- Heisler, M.G.; Atkinson, A.; Bylstra, Y.H.; Walsh, R.; Smyth, D.R. SPATULA, a Gene That Controls Development of Carpel Margin Tissues in Arabidopsis, Encodes a BHLH Protein. Development 2001, 128, 1089–1098. [Google Scholar] [CrossRef]
- Penfield, S.; Josse, E.-M.; Kannangara, R.; Gilday, A.D.; Halliday, K.J.; Graham, I.A. Cold and Light Control Seed Germination through the BHLH Transcription Factor SPATULA. Curr. Biol. 2005, 15, 1998–2006. [Google Scholar] [CrossRef]
- Makkena, S.; Lamb, R.S. The BHLH Transcription Factor SPATULA Regulates Root Growth by Controlling the Size of the Root Meristem. BMC Plant Biol. 2013, 13, 1. [Google Scholar] [CrossRef]
- Wendrich, J.R.; Möller, B.K.; Uddin, B.; Radoeva, T.; Lokerse, A.S.; De Rybel, B.; Weijers, D. A Set of Domain-Specific Markers in the Arabidopsis Embryo. Plant Reprod 2015, 28, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Bylstra, Y.; Lampugnani, E.R.; Smyth, D.R. Regulation of Tissue-Specific Expression of SPATULA, a BHLH Gene Involved in Carpel Development, Seedling Germination, and Lateral Organ Growth in Arabidopsis. J. Exp. Bot 2010, 61, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Chávez Montes, R.A.; Coello, G.; González-Aguilera, K.L.; Marsch-Martínez, N.; de Folter, S.; Alvarez-Buylla, E.R. ARACNe-Based Inference, Using Curated Microarray Data, of Arabidopsis thaliana Root Transcriptional Regulatory Networks. BMC Plant Biol. 2014, 14, 97. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L.P.M.; et al. The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Horiguchi, G.; Gleissberg, S.; Tsukaya, H. The BHLH Transcription Factor SPATULA Controls Final Leaf Size in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 252–261. [Google Scholar] [CrossRef]
- Josse, E.-M.; Gan, Y.; Bou-Torrent, J.; Stewart, K.L.; Gilday, A.D.; Jeffree, C.E.; Vaistij, F.E.; Martínez-García, J.F.; Nagy, F.; Graham, I.A.; et al. A DELLA in Disguise: SPATULA Restrains the Growth of the Developing Arabidopsis Seedling. Plant Cell 2011, 23, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, Y.; Tsukaya, H. Behavior of Leaf Meristems and Their Modification. Front. Plant Sci. 2015, 6, 1060. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Oelmüller, R. WRKY Transcription Factors. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef]
- Grunewald, W.; Karimi, M.; Wieczorek, K.; Van de Cappelle, E.; Wischnitzki, E.; Grundler, F.; Inzé, D.; Beeckman, T.; Gheysen, G. A Role for AtWRKY23 in Feeding Site Establishment of Plant-Parasitic Nematodes. Plant Physiol. 2008, 148, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; De Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Vanden Bossche, R.; Karimi, M.; De Rybel, B.; Vanholme, B.; et al. Transcription Factor WRKY23 Assists Auxin Distribution Patterns during Arabidopsis Root Development through Local Control on Flavonol Biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; De Smet, I.; De Rybel, B.; Robert, H.S.; van de Cotte, B.; Willemsen, V.; Gheysen, G.; Weijers, D.; Friml, J.; Beeckman, T. Tightly Controlled WRKY23 Expression Mediates Arabidopsis Embryo Development. EMBO Rep. 2013, 14, 1136–1142. [Google Scholar] [CrossRef]
- Prát, T.; Hajný, J.; Grunewald, W.; Vasileva, M.; Molnár, G.; Tejos, R.; Schmid, M.; Sauer, M.; Friml, J. WRKY23 Is a Component of the Transcriptional Network Mediating Auxin Feedback on PIN Polarity. PLoS Genet. 2018, 14, e1007177. [Google Scholar] [CrossRef]
- Hajný, J.; Prát, T.; Rydza, N.; Rodriguez, L.; Tan, S.; Verstraeten, I.; Domjan, D.; Mazur, E.; Smakowska-Luzan, E.; Smet, W.; et al. Receptor Kinase Module Targets PIN-Dependent Auxin Transport during Canalization. Science 2020, 370, 550–557. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression Profiles of the Arabidopsis WRKY Gene Superfamily during Plant Defense Response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Li, B.; Gaudinier, A.; Tang, M.; Taylor-Teeples, M.; Nham, N.T.; Ghaffari, C.; Benson, D.S.; Steinmann, M.; Gray, J.A.; Brady, S.M.; et al. Promoter-Based Integration in Plant Defense Regulation. Plant Physiol. 2014, 166, 1803–1820. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, S.; Chen, Y.; Li, D.; Yu, D. Arabidopsis WRKY45 Interacts with the DELLA Protein RGL1 to Positively Regulate Age-Triggered Leaf Senescence. Mol. Plant 2017, 10, 1174–1189. [Google Scholar] [CrossRef]
- Barros, J.A.S.; Cavalcanti, J.H.F.; Pimentel, K.G.; Medeiros, D.B.; Silva, J.C.F.; Condori-Apfata, J.A.; Lapidot-Cohen, T.; Brotman, Y.; Nunes-Nesi, A.; Fernie, A.R.; et al. The Significance of WRKY45 Transcription Factor in Metabolic Adjustments during Dark-Induced Leaf Senescence. Plant Cell Environ. 2022, 45, 2682–2695. [Google Scholar] [CrossRef]
- Smit, M.E.; McGregor, S.R.; Sun, H.; Gough, C.; Bågman, A.-M.; Soyars, C.L.; Kroon, J.T.; Gaudinier, A.; Williams, C.J.; Yang, X.; et al. A PXY-Mediated Transcriptional Network Integrates Signaling Mechanisms to Control Vascular Development in Arabidopsis[OPEN]. Plant Cell 2020, 32, 319–335. [Google Scholar] [CrossRef]
- Müller, C.J.; Valdés, A.E.; Wang, G.; Ramachandran, P.; Beste, L.; Uddenberg, D.; Carlsbecker, A. PHABULOSA Mediates an Auxin Signaling Loop to Regulate Vascular Patterning in Arabidopsis. Plant Physiol. 2016, 170, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Carlsbecker, A.; Etchells, J.P. Class III HD-ZIPs Govern Vascular Cell Fate: An HD View on Patterning and Differentiation. J. Exp. Bot. 2017, 68, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC Transcription Factors: Structurally Distinct, Functionally Diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.-L.; Cao, Y.-R.; Liu, Y.-F.; Lei, G.; Zou, H.-F.; Liao, Y.; Wang, H.-W.; Zhang, W.-K.; Ma, B.; Du, J.-Z.; et al. An R2R3-Type Transcription Factor Gene AtMYB59 Regulates Root Growth and Cell Cycle Progression in Arabidopsis. Cell Res. 2009, 19, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Fleury, D.; Himanen, K.; Cnops, G.; Nelissen, H.; Boccardi, T.M.; Maere, S.; Beemster, G.T.S.; Neyt, P.; Anami, S.; Robles, P.; et al. The Arabidopsis thaliana Homolog of Yeast BRE1 Has a Function in Cell Cycle Regulation during Early Leaf and Root Growth. Plant Cell 2007, 19, 417–432. [Google Scholar] [CrossRef]
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-García, S.; Cheng, J.-C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 Are Novel Brassinosteroid Receptors That Function in Vascular Differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, N.; Li, N.; Boeren, S.; Nash, T.E.; Goshe, M.B.; Clouse, S.D.; de Vries, S.; Caño-Delgado, A.I. The Brassinosteroid Insensitive1-Like3 Signalosome Complex Regulates Arabidopsis Root Development. Plant Cell 2013, 25, 3377–3388. [Google Scholar] [CrossRef]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid Perception in the Epidermis Controls Root Meristem Size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) Proteins and Their Encoding Genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Cao, S.; He, C.; Zhao, X.; Yu, R.; Li, Y.; Fang, W.; Zhang, C.-Y.; Yan, W.; Chen, D. Comprehensive Integration of Single-Cell Transcriptomic Data Illuminates the Regulatory Network Architecture of Plant Cell Fate Specification. bioRxiv 2022. [Google Scholar] [CrossRef]
- Suzuki, M.; Kato, A.; Komeda, Y. An RNA-Binding Protein, AtRBP1, Is Expressed in Actively Proliferative Regions in Arabidopsis thaliana. Plant Cell Physiol. 2000, 41, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Fukuda, A.; Saito, T.; Ito, H.; Kato, A. AtRBP1, Which Encodes an RNA-Binding Protein Containing RNA-Recognition Motifs, Regulates Root Growth in Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 92, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gandotra, N.; Coughlan, S.J.; Nelson, T. The Arabidopsis Leaf Provascular Cell Transcriptome Is Enriched in Genes with Roles in Vein Patterning. Plant J. 2013, 74, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; van Nocker, S. Analysis of Promoter Activity of Members of the PECTATE LYASE-LIKE (PLL) Gene Family in Cell Separation in Arabidopsis. BMC Plant Biol. 2010, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.; Schon, M.A.; Mosiolek, M.; Enugutti, B.; Nodine, M.D. Gene Expression Variation in Arabidopsis Embryos at Single-Nucleus Resolution. Development 2021, 148, dev199589. [Google Scholar] [CrossRef]
- Nawy, T.; Lee, J.-Y.; Colinas, J.; Wang, J.Y.; Thongrod, S.C.; Malamy, J.E.; Birnbaum, K.; Benfey, P.N. Transcriptional Profile of the Arabidopsis Root Quiescent Center. Plant Cell 2005, 17, 1908–1925. [Google Scholar] [CrossRef]
- Scofield, S.; Murison, A.; Jones, A.; Fozard, J.; Aida, M.; Band, L.R.; Bennett, M.; Murray, J.A.H. Coordination of Meristem and Boundary Functions by Transcription Factors in the SHOOT MERISTEMLESS Regulatory Network. Development 2018, 145, dev157081. [Google Scholar] [CrossRef]
- Stratilová, B.; Kozmon, S.; Stratilová, E.; Hrmova, M. Plant Xyloglucan Xyloglucosyl Transferases and the Cell Wall Structure: Subtle but Significant. Molecules 2020, 25, 5619. [Google Scholar] [CrossRef]
- Jansweijer, V.M.A. Role of SCHIZORIZA in Asymmetric Cell Division, Cell Fate Segregation and Specification in Arabidopsis Root Development. Ph.D. Thesis, Utrecht University: Utrecht, The Netherlands, 2013. [Google Scholar]
- Pernas, M.; Ryan, E.; Dolan, L. SCHIZORIZA Controls Tissue System Complexity in Plants. Curr. Biol. 2010, 20, 818–823. [Google Scholar] [CrossRef]
- Roudier, F.; Schindelman, G.; DeSalle, R.; Benfey, P.N. The COBRA Family of Putative GPI-Anchored Proteins in Arabidopsis. A New Fellowship in Expansion. Plant Physiol. 2002, 130, 538–548. [Google Scholar] [CrossRef]
- Ben-Tov, D.; Idan-Molakandov, A.; Hugger, A.; Ben-Shlush, I.; Günl, M.; Yang, B.; Usadel, B.; Harpaz-Saad, S. The Role of COBRA-LIKE 2 Function, as Part of the Complex Network of Interacting Pathways Regulating Arabidopsis Seed Mucilage Polysaccharide Matrix Organization. Plant J. 2018, 94, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.M.; Song, S.; Dhugga, K.S.; Rafalski, J.A.; Benfey, P.N. Combining Expression and Comparative Evolutionary Analysis. The COBRA Gene Family. Plant Physiol. 2007, 143, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Teilum, K.; Mirza, O.; Mattsson, O.; Petersen, M.; Welinder, K.G.; Mundy, J.; Gajhede, M.; Henriksen, A. Arabidopsis ATP A2 Peroxidase. Expression and High-Resolution Structure of a Plant Peroxidase with Implications for Lignification. Plant Mol. Biol. 2000, 44, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hewezi, T.; Baum, T.J. Arabidopsis Peroxidase AtPRX53 Influences Cell Elongation and Susceptibility to Heterodera Schachtii. Plant Signal. Behav. 2011, 6, 1778–1786. [Google Scholar] [CrossRef]
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive Comparison of Auxin-Regulated and Brassinosteroid-Regulated Genes in Arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef]
- Ehlting, J.; Mattheus, N.; Aeschliman, D.S.; Li, E.; Hamberger, B.; Cullis, I.F.; Zhuang, J.; Kaneda, M.; Mansfield, S.D.; Samuels, L.; et al. Global Transcript Profiling of Primary Stems from Arabidopsis thaliana Identifies Candidate Genes for Missing Links in Lignin Biosynthesis and Transcriptional Regulators of Fiber Differentiation. Plant J. 2005, 42, 618–640. [Google Scholar] [CrossRef]
- Dang, T.V.T.; Lee, S.; Cho, H.; Choi, K.; Hwang, I. The LBD11-ROS Feedback Regulatory Loop Modulates Vascular Cambium Proliferation and Secondary Growth in Arabidopsis. Mol. Plant 2023, 16, 1131–1145. [Google Scholar] [CrossRef]
- Ramachandran, V.; Tobimatsu, Y.; Masaomi, Y.; Sano, R.; Umezawa, T.; Demura, T.; Ohtani, M. Plant-Specific Dof Transcription Factors VASCULAR-RELATED DOF1 and VASCULAR-RELATED DOF2 Regulate Vascular Cell Differentiation and Lignin Biosynthesis in Arabidopsis. Plant Mol. Biol. 2020, 104, 263–281. [Google Scholar] [CrossRef]
- Joshi, N.C.; Meyer, A.J.; Bangash, S.A.K.; Zheng, Z.-L.; Leustek, T. Arabidopsis γ-Glutamylcyclotransferase Affects Glutathione Content and Root System Architecture during Sulfur Starvation. N. Phytol. 2019, 221, 1387–1397. [Google Scholar] [CrossRef]
- Gadjev, I.; Vanderauwera, S.; Gechev, T.S.; Laloi, C.; Minkov, I.N.; Shulaev, V.; Apel, K.; Inzé, D.; Mittler, R.; Van Breusegem, F. Transcriptomic Footprints Disclose Specificity of Reactive Oxygen Species Signaling in Arabidopsis. Plant Physiol. 2006, 141, 436–445. [Google Scholar] [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen Remobilization during Leaf Senescence: Lessons from Arabidopsis to Crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, J.M.; Salinas-Mondragon, R.; Boss, W.F.; Brown, C.S.; Sederoff, H.W. The Fast and Transient Transcriptional Network of Gravity and Mechanical Stimulation in the Arabidopsis Root Apex. Plant Physiol. 2004, 136, 2790–2805. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A.H. Cell Cycle-Regulated Gene Expression InArabidopsis *. J. Biol. Chem. 2002, 277, 41987–42002. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress Induced and Nuclear Localized HIPP26 from Arabidopsis thaliana Interacts via Its Heavy Metal Associated Domain with the Drought Stress Related Zinc Finger Transcription Factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Kim, R.J.; Kim, H.J.; Shim, D.; Suh, M.C. Molecular and Biochemical Characterizations of the Monoacylglycerol Lipase Gene Family of Arabidopsis thaliana. Plant J. 2016, 85, 758–771. [Google Scholar] [CrossRef]
- Huang, X.-X.; Zhao, S.-M.; Zhang, Y.-Y.; Li, Y.-J.; Shen, H.-N.; Li, X.; Hou, B.-K. A Novel UDP-Glycosyltransferase 91C1 Confers Specific Herbicide Resistance through Detoxification Reaction in Arabidopsis. Plant Physiol. Biochem. 2021, 159, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Takahashi, H.; Kojima, S.; Sato, N.; Ohga, K.; Cha, B.Y.; Woo, J.-T.; Nagai, K.; Horiguchi, G.; Tsukaya, H.; et al. Berberine Enhances Defects in the Establishment of Leaf Polarity in Asymmetric Leaves1 and Asymmetric Leaves2 of Arabidopsis thaliana. Plant Mol. Biol. 2012, 79, 569–581. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Mitsuda, N.; Ohtani, M.; Ohme-Takagi, M.; Kato, K.; Demura, T. VASCULAR-RELATED NAC-DOMAIN 7 Directly Regulates the Expression of a Broad Range of Genes for Xylem Vessel Formation. Plant J. 2011, 66, 579–590. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Lv, X.; Wang, Y.; Xun, Z.; Liu, Z.; Li, F.; Lu, H. The Cysteine Protease CEP1, a Key Executor Involved in Tapetal Programmed Cell Death, Regulates Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2939–2961. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Yin, B.; Zhang, Y.; Liu, Y.; Cheng, Z.; Liu, D.; Lu, H. The Papain-like Cysteine Protease CEP1 Is Involved in Programmed Cell Death and Secondary Wall Thickening during Xylem Development in Arabidopsis. J. Exp. Bot. 2019, 70, 205–215. [Google Scholar] [CrossRef]
- Höwing, T.; Dann, M.; Müller, B.; Helm, M.; Scholz, S.; Schneitz, K.; Hammes, U.Z.; Gietl, C. The Role of KDEL-Tailed Cysteine Endopeptidases of Arabidopsis (AtCEP2 and AtCEP1) in Root Development. PLoS ONE 2018, 13, e0209407. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, D.R.; Marzol, E.; del Carmen Rondón Guerrero, Y.; Ferrero, L.; Rossi, A.H.; Miglietta, E.A.; Aptekmann, A.A.; Pacheco, J.M.; Carignani, M.; Gabarain, V.B.; et al. NAC1 Directs CEP1-CEP3 Peptidase Expression and Decreases Cell Wall Extensins Linked to Root Hair Growth in Arabidopsis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ogura, T.; Wilkinson, A.J. AAA+ Superfamily ATPases: Common Structure–Diverse Function. Genes Cells 2001, 6, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Viñegra de la Torre, N.; Vayssières, A.; Obeng-Hinneh, E.; Neumann, U.; Zhou, Y.; Lázaro, A.; Roggen, A.; Sun, H.; Stolze, S.C.; Nakagami, H.; et al. FLOWERING REPRESSOR AAA+ ATPase 1 Is a Novel Regulator of Perennial Flowering in Arabis Alpina. N. Phytol. 2022, 236, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, J.R.; Möller, B.K.; Li, S.; Saiga, S.; Sozzani, R.; Benfey, P.N.; De Rybel, B.; Weijers, D. Framework for Gradual Progression of Cell Ontogeny in the Arabidopsis Root Meristem. Proc. Natl. Acad. Sci. USA 2017, 114, E8922–E8929. [Google Scholar] [CrossRef]
- Yadav, R.K.; Tavakkoli, M.; Xie, M.; Girke, T.; Reddy, G.V. A High-Resolution Gene Expression Map of the Arabidopsis Shoot Meristem Stem Cell Niche. Development 2014, 141, 2735–2744. [Google Scholar] [CrossRef]
- Zhang, T.-Q.; Chen, Y.; Wang, J.-W. A Single-Cell Analysis of the Arabidopsis Vegetative Shoot Apex. Dev. Cell 2021, 56, 1056–1074.e8. [Google Scholar] [CrossRef]
- Wendrich, J.R.; Yang, B.; Vandamme, N.; Verstaen, K.; Smet, W.; Van de Velde, C.; Minne, M.; Wybouw, B.; Mor, E.; Arents, H.E.; et al. Vascular Transcription Factors Guide Plant Epidermal Responses to Limiting Phosphate Conditions. Science 2020, 370, eaay4970. [Google Scholar] [CrossRef]
- Falasca, G.; Altamura, M.M. Histological Analysis of Adventitious Rooting in Arabidopsis thaliana (L.) Heynh Seedlings. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2003, 137, 265–273. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and Cytokinin Control Formation of the Quiescent Centre in the Adventitious Root Apex of Arabidopsis. Ann. Bot 2013, 112, 1395–1407. [Google Scholar] [CrossRef]
- Cao, X.; Jiao, Y. Control of Cell Fate during Axillary Meristem Initiation. Cell Mol. Life Sci. 2020, 77, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Rosspopoff, O.; Chelysheva, L.; Saffar, J.; Lecorgne, L.; Gey, D.; Caillieux, E.; Colot, V.; Roudier, F.; Hilson, P.; Berthomé, R.; et al. Direct Conversion of Root Primordium into Shoot Meristem Relies on Timing of Stem Cell Niche Development. Development 2017, 144, 1187–1200. [Google Scholar] [CrossRef] [PubMed]

| GO Term | p-Value | Matches |
|---|---|---|
| mitotic cell cycle phase transition [GO:0044772] | 1.113797 × 10−4 | 12 |
| hormone-mediated signaling pathway [GO:0009755] | 1.531419 × 10−4 | 43 |
| cell cycle phase transition [GO:0044770] | 1.660908 × 10−4 | 13 |
| auxin-activated signaling pathway [GO:0009734] | 2.028745 × 10−4 | 20 |
| cellular response to auxin stimulus [GO:0071365] | 2.297677 × 10−4 | 20 |
| response to auxin [GO:0009733] | 3.177196 × 10−4 | 26 |
| cellular response to hormone stimulus [GO:0032870] | 3.547532 × 10−4 | 41 |
| cellular response to endogenous stimulus [GO:0071495] | 8.612505 × 10−4 | 41 |
| response to hormone [GO:0009725] | 0.001567 | 54 |
| response to endogenous stimulus [GO:0009719] | 0.002949 | 54 |
| pattern specification process [GO:0007389] | 0.014219 | 15 |
| root system development [GO:0022622] | 0.014762 | 26 |
| root development [GO:0048364] | 0.016967 | 27 |
| cellular response to organic substance [GO:0071310] | 0.022434 | 42 |
| rRNA processing [GO:0006364] | 0.022969 | 17 |
| ribonucleoprotein complex biogenesis [GO:0022613] | 0.029372 | 23 |
| regionalization [GO:0003002] | 0.033701 | 13 |
| rRNA metabolic process [GO:0016072] | 0.037409 | 17 |
| ribosome biogenesis [GO:0042254] | 0.048866 | 20 |
| GO Term | p-Value | Matches |
|---|---|---|
| response to stimulus [GO:0050896] | 4.533972 × 10−11 | 110 |
| response to chemical [GO:0042221] | 1.392826 × 10−9 | 68 |
| response to hypoxia [GO:0001666] | 4.483907 × 10−9 | 19 |
| cellular response to hypoxia [GO:0071456] | 5.461530 × 10−9 | 18 |
| response to stress [GO:0006950] | 5.658327 × 10−9 | 79 |
| response to decreased oxygen levels [GO:0036293] | 6.189549 × 10−9 | 19 |
| cellular response to decreased oxygen levels [GO:0036294] | 6.509485 × 10−9 | 18 |
| cellular response to oxygen levels [GO:0071453] | 6.509485 × 10−9 | 18 |
| response to oxygen levels [GO:0070482] | 6.699425 × 10−9 | 19 |
| response to abiotic stimulus [GO:0009628] | 5.633967 × 10−5 | 48 |
| response to salt [GO:1902074] | 6.862141 × 10−5 | 22 |
| response to organic substance [GO:0010033] | 8.356267 × 10−5 | 50 |
| cellular response to chemical stimulus [GO:0070887] | 1.689590 × 10−4 | 39 |
| response to acid chemical [GO:0001101] | 6.834915 × 10−4 | 19 |
| response to water [GO:0009415] | 0.001198 | 18 |
| response to endogenous stimulus [GO:0009719] | 0.001254 | 38 |
| cellular response to stress [GO:0033554] | 0.001323 | 32 |
| response to hormone [GO:0009725] | 0.002230 | 37 |
| response to water deprivation [GO:0009414] | 0.003922 | 17 |
| the biological process involved in interspecies interaction between organisms [GO:0044419] | 0.004448 | 35 |
| response to external biotic stimulus [GO:0043207] | 0.010234 | 34 |
| response to other organisms [GO:0051707] | 0.010234 | 34 |
| cellular response to stimulus [GO:0051716] | 0.014815 | 54 |
| response to biotic stimulus [GO:0009607] | 0.015728 | 34 |
| response to oxygen-containing compound [GO:1901700] | 0.032401 | 36 |
| GO Term | p-Value | Matches |
|---|---|---|
| response to stimulus [GO:0050896] | 9.093059 × 10−5 | 66 |
| response to stress [GO:0006950] | 0.001507 | 47 |
| response to chemical [GO:0042221] | 0.005759 | 38 |
| response to hypoxia [GO:0001666] | 0.006900 | 10 |
| response to decreased oxygen levels [GO:0036293] | 0.008117 | 10 |
| response to oxygen levels [GO:0070482] | 0.008448 | 10 |
| cellular response to hypoxia [GO:0071456] | 0.020639 | 9 |
| cellular response to decreased oxygen levels [GO:0036294] | 0.022433 | 9 |
| cellular response to oxygen levels [GO:0071453] | 0.022433 | 9 |
| ID | Name | Abbreviation |
|---|---|---|
| AT1G19850 | AUXIN RESPONSE FACTOR 5/MONOPTEROS | ARF5/MP |
| AT1G55610 | BRI1-LIKE 1 | BRL1 |
| AT2G43510 | TRYPSIN INHIBITOR PROTEIN 1 | ATTI1 |
| AT2G47260 | ATWRKY23 | WRKY23 |
| AT3G01970 | ATWRKY45 | WRKY45 |
| AT3G02210 | COBRA-LIKE PROTEIN 1 PRECURSOR | COBL1 |
| AT3G13380 | BRI1-LIKE 3 | BRL3 |
| AT3G15720 | PECTIN LYASE-LIKE SUPERFAMILY PROTEIN | PLL1 |
| AT3G29810 | COBRA-LIKE PROTEIN 2/3 PRECURSOR | COBL2/3 |
| AT3G48580 | XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 11 | XTH11 |
| AT3G62860 | MONOACYLGLYCEROL LIPASE 12 | MAGL12 |
| AT4G15910 | DROUGHT-INDUCED 21 | ATDI21 |
| AT4G36930 | SPATULA | SPT |
| AT4G38580 | FARNESYLATED PROTEIN 6 | ATF6 |
| AT5G06720 | PEROXIDASE 2/PEROXIDASE 53 | PERX53 |
| AT5G10510 | AINTEGUMENTA-LIKE 6/PLETHORA 3 | AIL6/PLT3 |
| AT5G14000 | ANAC084 | ANAC084 |
| AT5G17760 | BCS1-LIKE PROTEIN | BCS1-L |
| AT5G26220 | GAMMA-GLUTAMYL CYCLOTRANSFERASE 2;1 | GGCT2;1 |
| AT5G49690 | UDP-GLYCOSYLTRANSFERASE SUPERFAMILY PROTEIN | UGT91C1 |
| AT5G50260 | CYSTEINE PROTEINASES SUPERFAMILY PROTEIN | CEP1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehér, A. A Common Molecular Signature Indicates the Pre-Meristematic State of Plant Calli. Int. J. Mol. Sci. 2023, 24, 13122. https://doi.org/10.3390/ijms241713122
Fehér A. A Common Molecular Signature Indicates the Pre-Meristematic State of Plant Calli. International Journal of Molecular Sciences. 2023; 24(17):13122. https://doi.org/10.3390/ijms241713122
Chicago/Turabian StyleFehér, Attila. 2023. "A Common Molecular Signature Indicates the Pre-Meristematic State of Plant Calli" International Journal of Molecular Sciences 24, no. 17: 13122. https://doi.org/10.3390/ijms241713122






