Sympathetic Nervous System and Atherosclerosis
Abstract
:1. Introduction
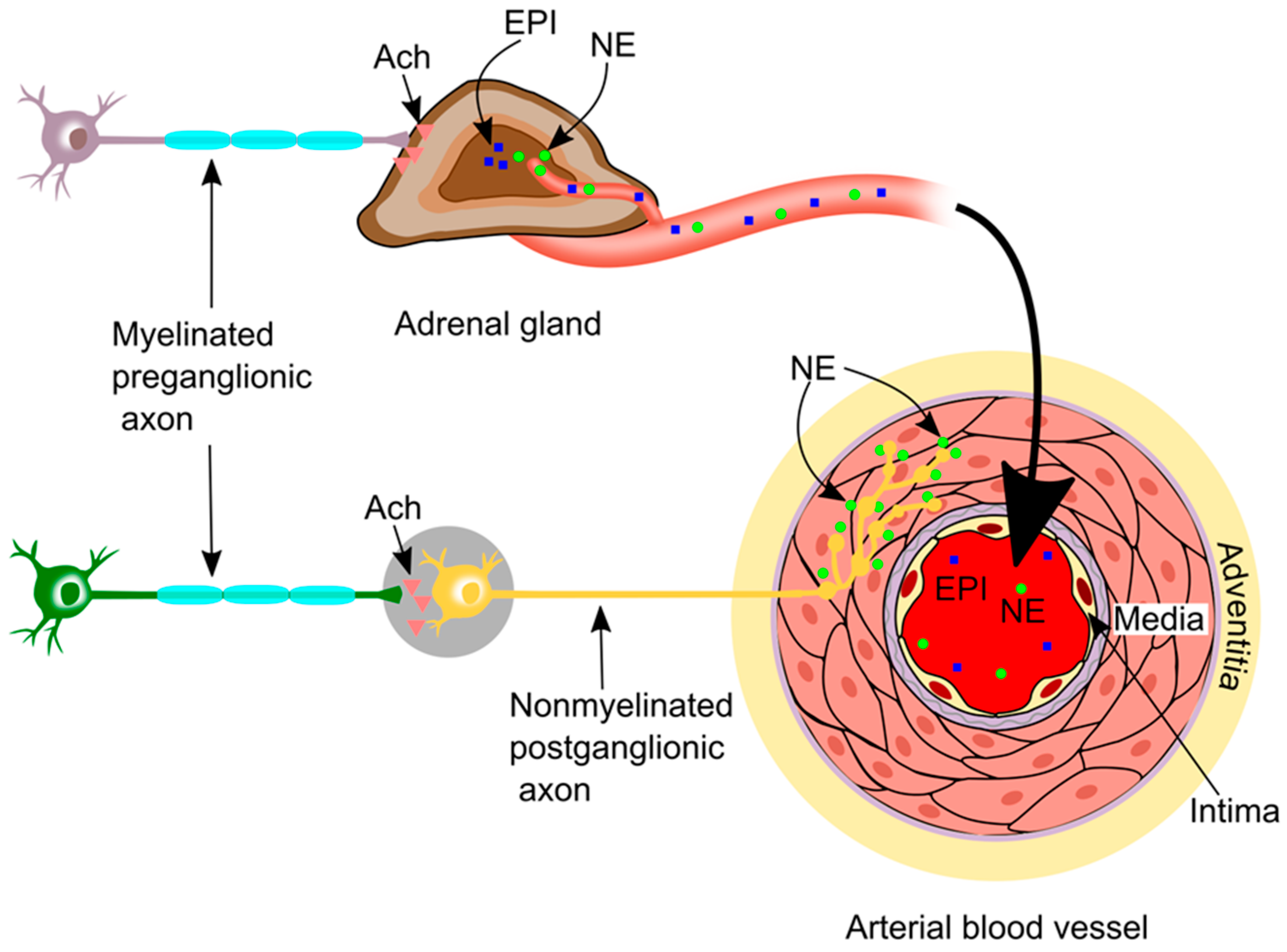
2. Sympathetic Innervation in the Vasculature
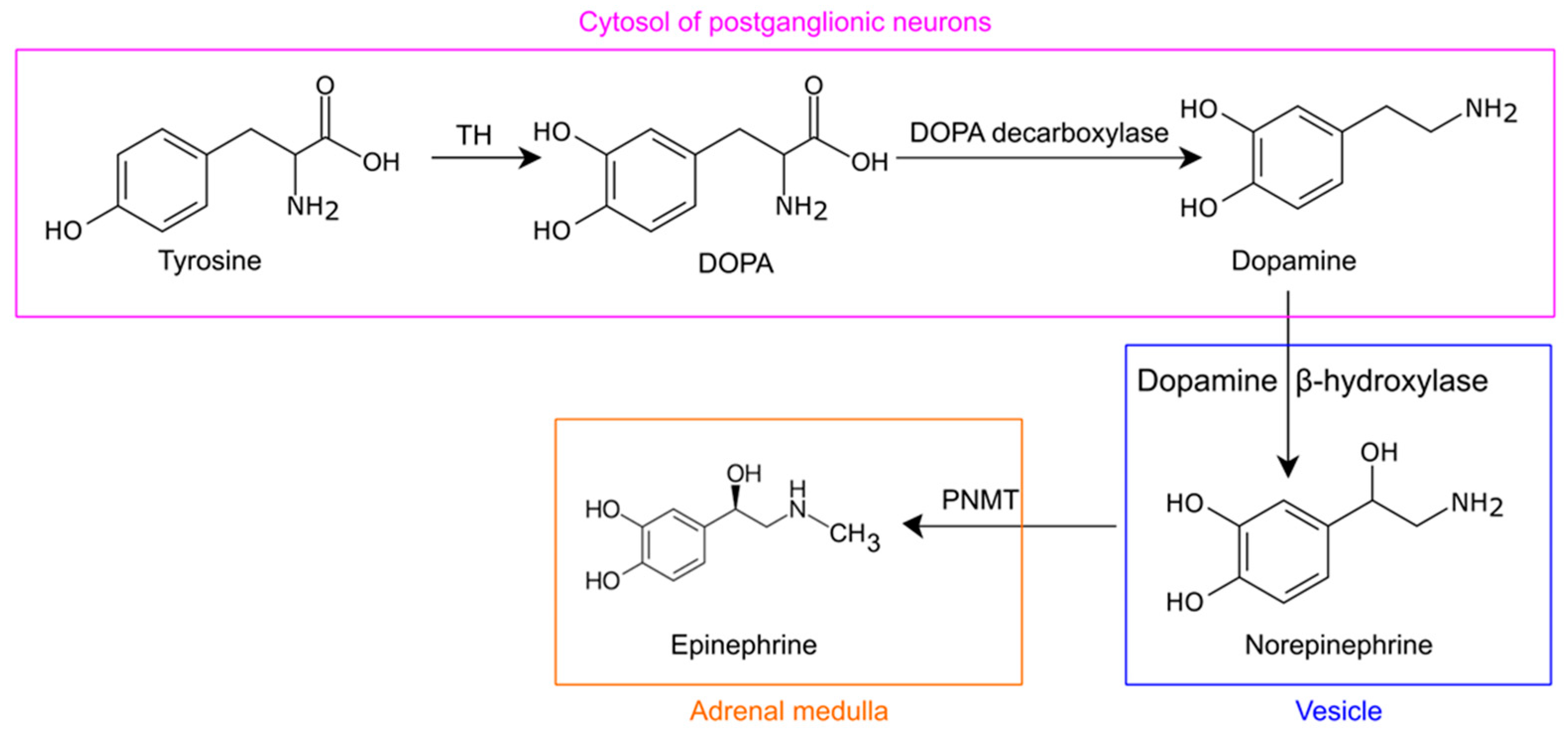
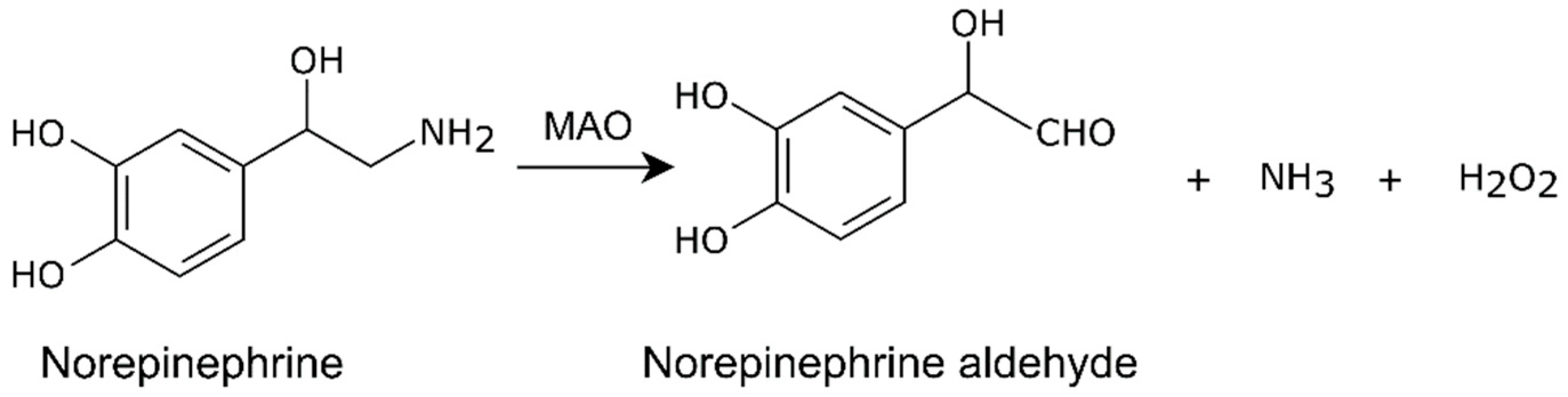
3. Norepinephrine Synthesis and Metabolism
4. Measurements of Sympathetic Nerve Activity
5. Adrenergic Receptors (Adrenoceptors) in the Vasculature
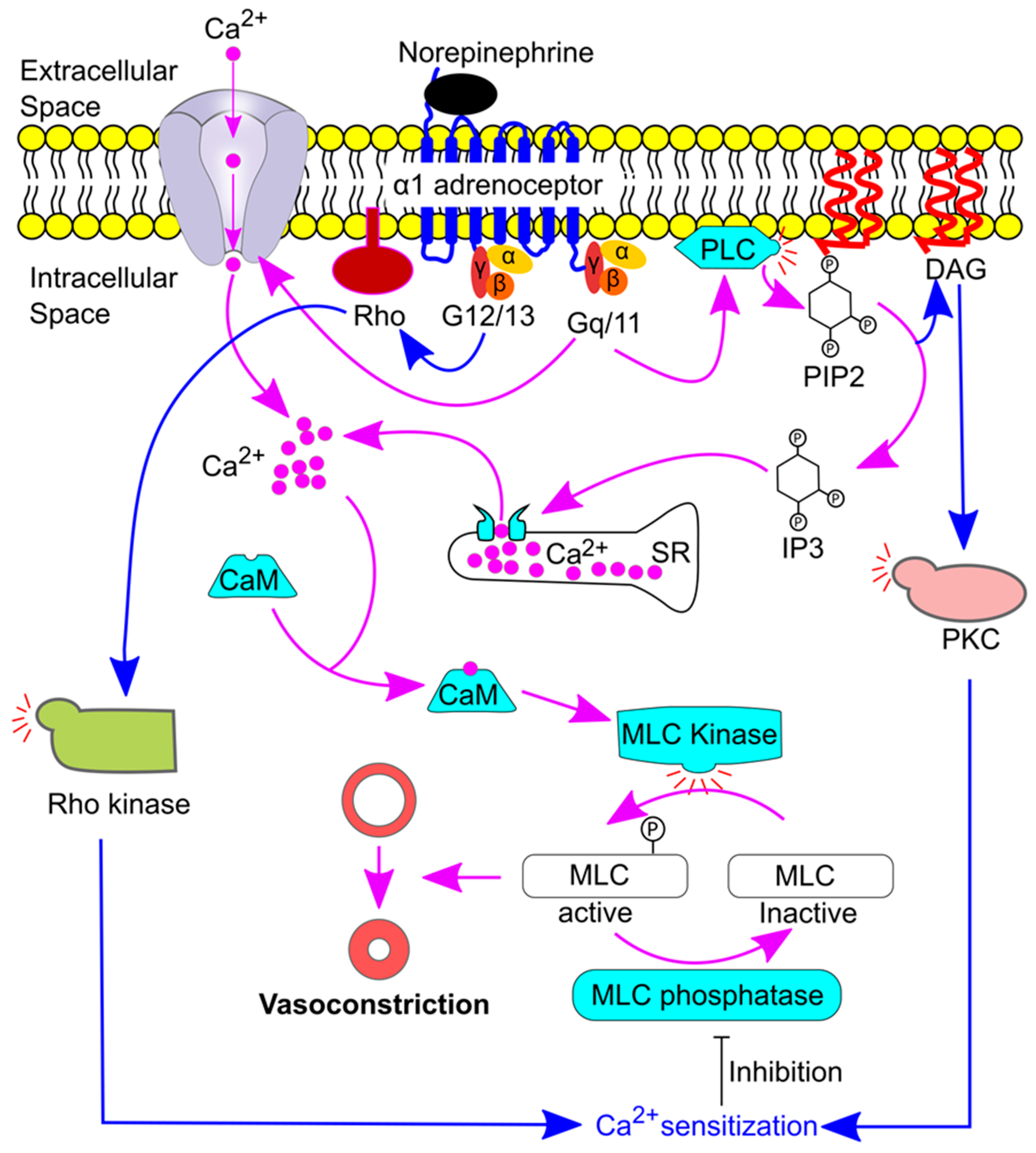
6. Molecular Pathways Underlying Sympathetic Activation-Induced Vasoconstriction and Relaxation
6.1. Sympathetic Activation-Induced Vasoconstriction under Physiological Conditions
6.2. Activation of β Adrenoceptors Induces Vasorelaxation
7. Roles of Adrenoceptors in Atherosclerosis
7.1. Role of α1 Adrenoceptors in Atherosclerosis
7.2. Role of α2 Adrenoceptors in Atherosclerosis
7.3. Role of β1 Adrenoceptors in Atherosclerosis
7.4. Role of β2 Adrenoceptors in Atherosclerosis
7.5. Role of β3 Adrenoceptors in Atherosclerosis
7.5.1. Preclinical Studies
7.5.2. Clinical Studies
8. Renal Denervation and Atherosclerosis
8.1. Preclinical Studies
8.2. Clinical Studies
| Patients/Animals | Effect on Atherosclerosis | Mechanisms | Reference |
|---|---|---|---|
| Preclinical Studies | |||
| ApoE−/−mice HFD for 20 weeks | ↓ | ↓ MAO-A ↓ CCL2, ICAM-1 ↓ Macrophage ↓ ROS ↓ NF-κB | [105] |
| ApoE−/−mice HFD for 6–12 weeks | ↓ | ↓ TNFα, IL-Iβ, etc ↓ Circulating neutrophils ↓ Circulating monocytes | [106] |
| ApoE−/−mice HFD for 10 weeks | ↓ | ↑ VSMC ↓ CCL2 and 8-isoprostane | [107] |
| ApoE−/−mice Angiotensin II fusion | ↑ | ↑ MMP-2 | [109] |
| Minipigs HFD for 6 months | ↑ | ↑ ET-1 ↑ ET-1 A and Breceptors ↑ NOX2 ↑ NF-κB ↑ 4-hydroxynonenal ↓ eNOS phosphorylation ↓ NO | [110] |
| Clinical studies | |||
| 39 patients with rHTN | ↔ | NR | [131] |
9. Artery–Brain Circuit and Atherosclerosis
9.1. Establishment of the Artery–Brain Circuit in Mice
9.2. Ganglionectomy and Atherosclerosis
10. Sympathetic Nervous System and Peripheral Artery Disease (PAD)
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Zhong, J.; Shen, J.; Zeng, Y. The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis. Front. Physiol. 2023, 14, 1179828. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ward, N.C.; Hodgson, J.M.; Puddey, I.B.; Wang, Y.; Zhang, D.; Maghzal, G.J.; Stocker, R.; Croft, K.D. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: A critical role for heme oxygenase-1. Free Radic. Biol. Med. 2013, 65, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Rached, F.H.; Miname, M.H. Insights into the Role of Inflammation in the Management of Atherosclerosis. J. Inflamm. Res. 2023, 16, 2223–2239. [Google Scholar] [CrossRef]
- Watts, G.F.; Gidding, S.S.; Hegele, R.A.; Raal, F.J.; Sturm, A.C.; Jones, L.K.; Sarkies, M.N.; Al-Rasadi, K.; Blom, D.J.; Daccord, M.; et al. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat. Rev. Cardiol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Eremin, I.I.; Nadelyaeva, I.I.; Gutyrchik, N.A.; Orekhov, A.N. Proprotein Convertase Subtilisin/Kexin 9 as a Modifier of Lipid Metabolism in Atherosclerosis. Biomedicines 2023, 11, 503. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Zou, Y.L.; Guo, S.D. Low-density lipoprotein particles in atherosclerosis. Front. Physiol. 2022, 13, 931931. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef]
- World Health Organisation. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27 June 2023).
- Santosa, S.M.; Guo, K.; Yamakawa, M.; Ivakhnitskaia, E.; Chawla, N.; Nguyen, T.; Han, K.Y.; Ema, M.; Rosenblatt, M.I.; Chang, J.H.; et al. Simultaneous fluorescence imaging of distinct nerve and blood vessel patterns in dual Thy1-YFP and Flt1-DsRed transgenic mice. Angiogenesis 2020, 23, 459–477. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Peng, L.; Li, Y.; Lu, S.; Sun, T.; Carnevale, L.; Perrotta, M.; Ma, Z.; Förstera, B.; Stanic, K.; et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 2022, 605, 152–159. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Weber, C.; Yin, C.; Habenicht, A.J.R. The dawn has come for new therapeutics to treat atherosclerosis: Targeting neuroimmune cardiovascular interfaces in artery brain circuits. Clin. Transl. Med. 2022, 12, e1040. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.E.; Phillips, J.K.; Sandow, S.L. Heterogeneous control of blood flow amongst different vascular beds. Med. Res. Rev. 2001, 21, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Shier, D.; Butler, J.; Lewis, R. Nervous System II: Divisions of the Nervous System. Holes Hum. Anat. Physiol. 2018, 389–443. [Google Scholar]
- Baizer, J.S.; Webster, C.J.; Witelson, S.F. Individual variability in the size and organization of the human arcuate nucleus of the medulla. Brain Struct. Funct. 2022, 227, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K. The Peripheral Nervous System and Reflex Activity. Hum. Anat. Physiol. 2018, 13, 521–562. [Google Scholar]
- Marieb, E.N.; Hoehn, K. The autonomic nervous system. Hum. Anat. Physiol. 2018, 14, 563–584. [Google Scholar]
- Bennett, M.R. Transmission at Sympathetic Varicosities. Physiology 1998, 13, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jimsheleishvili, S.; Marwaha, K.; Sherman, A.L. Physiology, Neuromuscular Transmission. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541133/ (accessed on 6 June 2023).
- Shier, D.; Butler, J.; Lewis, R. Endocrine System. Hole’s Hum. Anat. Physiol. 2018, 13, 489–528. [Google Scholar]
- Dey, S.K.; Saini, M.; Prabhakar, P.; Kundu, S. Dopamine β hydroxylase as a potential drug target to combat hypertension. Expert. Opin. Investig. Drugs 2020, 29, 1043–1057. [Google Scholar] [CrossRef]
- Shier, D.; Butler, J.; Lewis, R. Nervous System I: Basic Structure and Function. Hole’s Hum. Anat. Physiol. 2018, 10, 359–388. [Google Scholar]
- Chaudhary, A.; Kumar, P.; Rai, V. Catechol-O-methyltransferase (COMT) Val158Met Polymorphism and Susceptibility to Alcohol Dependence. Indian. J. Clin. Biochem. 2021, 36, 257–265. [Google Scholar] [CrossRef]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, M. Medical gallery of mikael haggstrom 2014. WikiJournal Med. 2014, 1, 1–53. [Google Scholar] [CrossRef]
- Padala, N.S.P.; Ajjala, D.R.; Boggavarapu, R.K.; Pantangi, H.R.; Thentu, J.B.; Mohammed, A.R.; Nirogi, R. LC-MS/MS method for quantification of 3,4-dihydroxyphenylglycol, a norepinephrine metabolite in plasma and brain regions. Bioanalysis 2019, 11, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Raffel, D.M.; Crawford, T.C.; Jung, Y.W.; Koeppe, R.A.; Gu, G.; Rothley, J.; Frey, K.A. Quantifying cardiac sympathetic denervation: First studies of (18)F-fluorohydroxyphenethylguanidines in cardiomyopathy patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 619–631. [Google Scholar] [CrossRef]
- Bylund, D.B.; Bylund, K.C. Norepinephrine. In Encyclopedia of the Neurological Sciences; Daroff, R.B., Aminoff, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 3, pp. 614–616. [Google Scholar]
- Grassi, G.; Quarti-Trevano, F.; Esler, M.D. Sympathetic activation in congestive heart failure: An updated overview. Heart Fail. Rev. 2021, 26, 173–182. [Google Scholar] [CrossRef]
- Dobrek, L. An Outline of Renal Artery Stenosis Pathophysiology-A Narrative Review. Life 2021, 11, 208. [Google Scholar] [CrossRef]
- Esler, M.; Lambert, G.; Brunner-La Rocca, H.P.; Vaddadi, G.; Kaye, D. Sympathetic nerve activity and neurotransmitter release in humans: Translation from pathophysiology into clinical practice. Acta Physiol. Scand. 2003, 177, 275–284. [Google Scholar] [CrossRef]
- Esler, M.; Jennings, G.; Lambert, G.; Meredith, I.; Horne, M.; Eisenhofer, G. Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol. Rev. 1990, 70, 963–985. [Google Scholar] [CrossRef]
- Esler, M. The sympathetic nervous system through the ages: From Thomas Willis to resistant hypertension. Exp. Physiol. 2011, 96, 611–622. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Arenare, F.; Buccianti, G.; Furiani, S.; Ilardo, V.; Bolla, G.; Mancia, G. Behaviour of regional adrenergic outflow in mild-to-moderate renal failure. J. Hypertens. 2009, 27, 562–566. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Arenare, F.; Facchetti, R.; Mancia, G. Reproducibility patterns of plasma norepinephrine and muscle sympathetic nerve traffic in human obesity. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 469–475. [Google Scholar] [CrossRef]
- Esler, M. Pivotal role of the sympathetic nerves of the human heart in mental stress responses and triggered cardiovascular catastrophes. Auton. Neurosci. 2022, 237, 102925. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Dawood, T.; Schlaich, M.; Straznicky, N.; Esler, M.; Lambert, G. Single-unit sympathetic discharge pattern in pathological conditions associated with elevated cardiovascular risk. Clin. Exp. Pharmacol. Physiol. 2008, 35, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Dimitriadis, K.; Dell’Oro, R.; Grassi, G. How to assess sympathetic nervous system activity in clinical practice. Curr. Clin. Pharmacol. 2013, 8, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, A.B.; Hagbarth, K.E.; Wallin, B.G. Microneurography: How the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 2004, 96, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Zelt, J.G.E.; deKemp, R.A.; Rotstein, B.H.; Nair, G.M.; Narula, J.; Ahmadi, A.; Beanlands, R.S.; Mielniczuk, L.M. Nuclear Imaging of the Cardiac Sympathetic Nervous System: A Disease-Specific Interpretation in Heart Failure. JACC Cardiovasc. Imaging 2020, 13, 1036–1054. [Google Scholar] [CrossRef]
- Grkovski, M.; Zanzonico, P.B.; Modak, S.; Humm, J.L.; Narula, J.; Pandit-Taskar, N. F-18 meta-fluorobenzylguanidine PET imaging of myocardial sympathetic innervation. J. Nucl. Cardiol. 2022, 29, 3179–3188. [Google Scholar] [CrossRef]
- Turnock, S.; Turton, D.R.; Martins, C.D.; Chesler, L.; Wilson, T.C.; Gouverneur, V.; Smith, G.; Kramer-Marek, G. (18)F-meta-fluorobenzylguanidine ((18)F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models. Sci. Rep. 2020, 10, 20918. [Google Scholar] [CrossRef]
- Docherty, J.R. Subtypes of functional alpha1-adrenoceptor. Cell Mol. Life Sci. 2010, 67, 405–417. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 17–28. [Google Scholar] [PubMed]
- Manzini, S.; Pinna, C.; Busnelli, M.; Cinquanta, P.; Rigamonti, E.; Ganzetti, G.S.; Dellera, F.; Sala, A.; Calabresi, L.; Franceschini, G.; et al. Beta2-adrenergic activity modulates vascular tone regulation in lecithin:cholesterol acyltransferase knockout mice. Vascul Pharmacol. 2015, 74, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Minneman, K.P. Recent progress in alpha1-adrenergic receptor research. Acta Pharmacol. Sin. 2005, 26, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Kaykı-Mutlu, G.; Papazisi, O.; Palmen, M.; Danser, A.H.J.; Michel, M.C.; Arioglu-Inan, E. Cardiac and Vascular α1-Adrenoceptors in Congestive Heart Failure: A Systematic Review. Cells 2020, 9, 2412. [Google Scholar] [CrossRef]
- Zalewska, E.; Kmieć, P.; Sworczak, K. Role of Catestatin in the Cardiovascular System and Metabolic Disorders. Front. Cardiovasc. Med. 2022, 9, 909480. [Google Scholar] [CrossRef]
- Chruscinski, A.; Brede, M.E.; Meinel, L.; Lohse, M.J.; Kobilka, B.K.; Hein, L. Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2)-adrenergic receptors. Mol. Pharmacol. 2001, 60, 955–962. [Google Scholar] [CrossRef]
- Berlan, M.; Galitzky, J.; Bousquet-Melou, A.; Lafontan, M.; Montastruc, J.L. Beta-3 adrenoceptor-mediated increase in cutaneous blood flow in the dog. J. Pharmacol. Exp. Ther. 1994, 268, 1444–1451. [Google Scholar]
- Taylor, B.N.; Cassagno, L.M. Alpha-Adrenergic Receptors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539830/ (accessed on 14 June 2023).
- Alhayek, S.P.C. Beta 1 Receptors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532904/ (accessed on 14 June 2023).
- Vrablik, M.; Corsini, A.; Tůmová, E. Beta-blockers for Atherosclerosis Prevention: A Missed Opportunity? Curr. Atheroscler. Rep. 2022, 24, 161–169. [Google Scholar] [CrossRef]
- Baskin, A.S.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Anflick-Chames, E.; Cero, C.; Johnson, J.W.; O’Mara, A.E.; Fletcher, L.A.; Leitner, B.P.; et al. Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a β3-Adrenergic Receptor Agonist. Diabetes 2018, 67, 2113–2125. [Google Scholar] [CrossRef]
- Larson, C.J. Translational pharmacology and physiology of brown adipose tissue in human disease and treatment. Handb. Exp. Pharmacol. 2019, 251, 381–424. [Google Scholar] [CrossRef]
- King, A.J.; Osborn, J.W.; Fink, G.D. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 2007, 50, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Navar, L.G. Physiology: Hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J. Am. Soc. Hypertens. 2014, 8, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hafen, B.B.; Shook, M.; Burns, B. Anatomy, Smooth Muscle. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532857/ (accessed on 15 June 2023).
- Kauffenstein, G.; Laher, I.; Matrougui, K.; Guérineau, N.C.; Henrion, D. Emerging role of G protein-coupled receptors in microvascular myogenic tone. Cardiovasc. Res. 2012, 95, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef]
- Zicha, J.; Behuliak, M.; Vavřínová, A.; Dobešová, Z.; Kuneš, J.; Rauchová, H.; Vaněčková, I. Cooperation of augmented calcium sensitization and increased calcium entry contributes to high blood pressure in salt-sensitive Dahl rats. Hypertens. Res. 2021, 44, 1067–1078. [Google Scholar] [CrossRef]
- Mueed, I.; Bains, P.; Zhang, L.; Macleod, K.M. Differential participation of protein kinase C and Rho kinase in alpha 1-adrenoceptor mediated contraction in rat arteries. Can. J. Physiol. Pharmacol. 2004, 82, 895–902. [Google Scholar] [CrossRef]
- Takashima, S.-I.; Sugimoto, N.; Takuwa, N.; Okamoto, Y.; Yoshioka, K.; Takamura, M.; Takata, S.; Kaneko, S.; Takuwa, Y. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc. Res. 2008, 79, 689–697. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Shao, X.M.; Lao, C.J.; Hasan, K.M.; Rivera, J.C.; Jordan, M.C.; Echeverria, V.; Roos, K.P.; Sinha-Hikim, A.P.; Friedman, T.C. Electronic Cigarette Use and the Risk of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 879726. [Google Scholar] [CrossRef]
- Shiina, S.; Kanemura, A.; Suzuki, C.; Yamaki, F.; Obara, K.; Chino, D.; Tanaka, Y. β-Adrenoceptor subtypes and cAMP role in adrenaline- and noradrenaline-induced relaxation in the rat thoracic aorta. J. Smooth Muscle Res. 2018, 54, 1–12. [Google Scholar] [CrossRef]
- White, R.E.; Kryman, J.P.; El-Mowafy, A.M.; Han, G.; Carrier, G.O. cAMP-Dependent Vasodilators Cross-Activate the cGMP-Dependent Protein Kinase to Stimulate BKCa Channel Activity in Coronary Artery Smooth Muscle Cells. Circ. Res. 2000, 86, 897–905. [Google Scholar] [CrossRef]
- Priest, R.M.; Hucks, D.; Ward, J.P. Noradrenaline, beta-adrenoceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997, 122, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Kowala, M.C.; Nunnari, J.J.; Durham, S.K.; Nicolosi, R.J. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis 1991, 91, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Swindell, A.C.; Krupp, M.N.; Twomey, T.M.; Reynolds, J.A.; Chichester, C.O. Effects of doxazosin on atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 1993, 99, 195–206. [Google Scholar] [CrossRef]
- Vashisht, R.; Sian, M.; Franks, P.J.; O’Malley, M.K. Long-term reduction of intimal hyperplasia by the selective alpha-1 adrenergic antagonist doxazosin. Br. J. Surg. 1992, 79, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Hoogerbrugge, N.; de Groot, E.; de Heide, L.H.; de Ridder, M.A.; Birkenhägeri, J.C.; Stijnen, T.; Jansen, H. Doxazosin and hydrochlorothiazide equally affect arterial wall thickness in hypertensive males with hypercholesterolaemia (the DAPHNE study). Doxazosin Atherosclerosis Progression Study in Hypertensives in the Netherlands. Neth. J. Med. 2002, 60, 354–361. [Google Scholar]
- Nafstad, I.; Tollersrud, S.; Eriksen, K.; Helgeland, A.; Solberg, L.A.; Bredesen, J.; Dale, O. The influence of atenolol and prazosin on serum lipids and atherosclerosis in minipigs fed a hyperlipidemic diet. Gen. Pharmacol. 1988, 19, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Shimazu, N.; Fujita, M.; Fujimaki, Y.; Kojima, K.; Mikuni, Y.; Horie, E.; Teramoto, T. Doxazosin, an alpha1-adrenergic antihypertensive agent, decreases serum oxidized LDL. Am. J. Hypertens. 2001, 14, 267–270. [Google Scholar] [CrossRef]
- Piller, L.B.; Davis, B.R.; Cutler, J.A.; Cushman, W.C.; Wright, J.T.; Williamson, J.D.; Leenen, F.H.H.; Einhorn, P.T.; Randall, O.S.; Golden, J.S.; et al. Validation of Heart Failure Events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants Assigned to Doxazosin and Chlorthalidone. Curr. Control. Trials Cardiovasc. Med. 2002, 3, 10. [Google Scholar] [CrossRef]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. G(i/o) protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, D.T.; Anesi, J.; Alramahi, A.; Witting, P.K.; Chai, Z.; Khan, A.W.; Kelly, J.; Denton, K.M.; Golledge, J. Moxonidine Increases Uptake of Oxidised Low-Density Lipoprotein in Cultured Vascular Smooth Muscle Cells and Inhibits Atherosclerosis in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2023, 24, 3857. [Google Scholar] [CrossRef]
- Al-Sharea, A.; Lee, M.K.S.; Whillas, A.; Michell, D.L.; Shihata, W.A.; Nicholls, A.J.; Cooney, O.D.; Kraakman, M.J.; Veiga, C.B.; Jefferis, A.M.; et al. Chronic sympathetic driven hypertension promotes atherosclerosis by enhancing hematopoiesis. Haematologica 2019, 104, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Venugopal, J.; Silaghi, P.; Su, E.J.; Guo, C.; Lawrence, D.A.; Eitzman, D.T. Beta1-receptor blockade attenuates atherosclerosis progression following traumatic brain injury in apolipoprotein E deficient mice. PLoS ONE 2023, 18, e0285499. [Google Scholar] [CrossRef] [PubMed]
- Ulleryd, M.A.; Bernberg, E.; Yang, L.J.; Bergström, G.M.; Johansson, M.E. Metoprolol reduces proinflammatory cytokines and atherosclerosis in ApoE−/−mice. Biomed. Res. Int. 2014, 2014, 548783. [Google Scholar] [CrossRef]
- Chen, S.J.; Tsui, P.F.; Chuang, Y.P.; Chiang, D.M.; Chen, L.W.; Liu, S.T.; Lin, F.Y.; Huang, S.M.; Lin, S.H.; Wu, W.L.; et al. Carvedilol Ameliorates Experimental Atherosclerosis by Regulating Cholesterol Efflux and Exosome Functions. Int. J. Mol. Sci. 2019, 20, 5202. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Hirano, E.; Kimura, T.; Fujita, M.; Kishimoto, C. Carvedilol reduces the severity of atherosclerosis in apolipoprotein E-deficient mice via reducing superoxide production. Exp. Biol. Med. 2012, 237, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- de Nigris, F.; Mancini, F.P.; Balestrieri, M.L.; Byrns, R.; Fiorito, C.; Williams-Ignarro, S.; Palagiano, A.; Crimi, E.; Ignarro, L.J.; Napoli, C. Therapeutic dose of nebivolol, a nitric oxide-releasing beta-blocker, reduces atherosclerosis in cholesterol-fed rabbits. Nitric Oxide 2008, 19, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.K.; Hayashi, T.; Sumi, D.; Kano, H.; Matsui-Hirai, H.; Tsunekawa, T.; Iguchi, A. Anti-atherosclerotic effect of beta-blocker with nitric oxide-releasing action on the severe atherosclerosis. J. Cardiovasc. Pharmacol. 2002, 39, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hedblad, B.; Wikstrand, J.; Janzon, L.; Wedel, H.; Berglund, G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the β-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS). Circulation 2001, 103, 1721–1726. [Google Scholar] [CrossRef]
- Wiklund, O.; Hulthe, J.; Wikstrand, J.; Schmidt, C.; Olofsson, S.O.; Bondjers, G. Effect of controlled release/extended release metoprolol on carotid intima-media thickness in patients with hypercholesterolemia: A 3-year randomized study. Stroke 2002, 33, 572–577. [Google Scholar] [CrossRef]
- Helgeland, A.; Eriksen, K.; Foss, P.O.; Nafstad, I.; Solberg, L.A.; Tollersrud, S. The influence of prazosin and propranolol on serum lipids and atherosclerosis in standard fed pigs. Acta Pharmacol. Toxicol. 1984, 54, 270–272. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Hoeks, S.E.; Sin, D.D.; Welten, G.M.; Schouten, O.; Witteveen, H.J.; Simsek, C.; Stam, H.; Mertens, F.W.; Bax, J.J.; et al. Impact of cardioselective beta-blockers on mortality in patients with chronic obstructive pulmonary disease and atherosclerosis. Am. J. Respir. Crit. Care Med. 2008, 178, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.H.; Mehaffey, J.H.; Cullen, J.M.; Hawkins, R.B.; Roy, R.; Upchurch, G.R., Jr.; Robinson, W.P. Preoperative beta blockade is associated with increased rates of 30-day major adverse cardiac events in critical limb ischemia patients undergoing infrainguinal revascularization. J. Vasc. Surg. 2019, 69, 1167–1172.e1. [Google Scholar] [CrossRef] [PubMed]
- Shavadia, J.S.; Zheng, Y.; Green, J.B.; Armstrong, P.W.; Westerhout, C.M.; McGuire, D.K.; Cornel, J.H.; Holman, R.R.; Peterson, E.D. Associations between β-blocker therapy and cardiovascular outcomes in patients with diabetes and established cardiovascular disease. Am. Heart J. 2019, 218, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, P.; Bernucci, D.; Cardelli, L.S.; Carone, A.; Scavone, G.; Manfrini, M.; Censi, S.; Calvi, S.; Ferrari, R.; Campo, G.; et al. Renin-Angiotensin-Aldosterone System Inhibitors, Statins, and Beta-Blockers in Diabetic Patients With Critical Limb Ischemia and Foot Lesions. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221101980. [Google Scholar] [CrossRef]
- Albiñana, V.; Recio-Poveda, L.; González-Peramato, P.; Martinez-Piñeiro, L.; Botella, L.M.; Cuesta, A.M. Blockade of β2-Adrenergic Receptor Reduces Inflammation and Oxidative Stress in Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 1325. [Google Scholar] [CrossRef]
- Hoeke, G.; Wang, Y.; van Dam, A.D.; Mol, I.M.; Gart, E.; Klop, H.G.; van den Berg, S.M.; Pieterman, E.H.; Princen, H.M.G.; Groen, A.K.; et al. Atorvastatin accelerates clearance of lipoprotein remnants generated by activated brown fat to further reduce hypercholesterolemia and atherosclerosis. Atherosclerosis 2017, 267, 116–126. [Google Scholar] [CrossRef]
- Zhou, E.; Li, Z.; Nakashima, H.; Liu, C.; Ying, Z.; Foks, A.C.; Berbée, J.F.P.; van Dijk, K.W.; Rensen, P.C.N.; Wang, Y. Hepatic Scavenger Receptor Class B Type 1 Knockdown Reduces Atherosclerosis and Enhances the Antiatherosclerotic Effect of Brown Fat Activation in APOE*3-Leiden.CETP Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1474–1486. [Google Scholar] [CrossRef]
- Zhou, E.; Hoeke, G.; Li, Z.; Eibergen, A.C.; Schonk, A.W.; Koehorst, M.; Boverhof, R.; Havinga, R.; Kuipers, F.; Coskun, T.; et al. Colesevelam enhances the beneficial effects of brown fat activation on hyperlipidaemia and atherosclerosis development. Cardiovasc. Res. 2020, 116, 1710–1720. [Google Scholar] [CrossRef]
- Zhou, E.; Li, Z.; Nakashima, H.; Choukoud, A.; Kooijman, S.; Berbée, J.F.P.; Rensen, P.C.N.; Wang, Y. Beneficial effects of brown fat activation on top of PCSK9 inhibition with alirocumab on dyslipidemia and atherosclerosis development in APOE*3-Leiden.CETP mice. Pharmacol. Res. 2021, 167, 105524. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elía, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; van Gelderen, E.M.; Lee, J.H.; Kowalski, D.L.; Yen, M.; Goldwater, R.; Mujais, S.K.; Schaddelee, M.P.; de Koning, P.; Kaibara, A.; et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: A randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin. Pharmacol. Ther. 2012, 92, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Y.L.; Tu, B.; Liu, S.Y.; Zhang, Z.H.; Hu, Z.; Yao, Y. Effect of renal denervation for patients with isolated systolic hypertension: A systematic review and meta-analysis. J. Geriatr. Cardiol. 2023, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Cheng, H.M.; Chia, Y.C.; Li, Y.; Van Minh, H.; Siddique, S.; Sukonthasarn, A.; Tay, J.C.; Turana, Y.; Verma, N.; et al. The role of renal nerve stimulation in percutaneous renal denervation for hypertension: A mini-review. J. Clin. Hypertens. 2022, 24, 1187–1193. [Google Scholar] [CrossRef]
- Rey-García, J.; Townsend, R.R. Renal Denervation: A Review. Am. J. Kidney Dis. 2022, 80, 527–535. [Google Scholar] [CrossRef]
- Wang, Y.; Seto, S.W.; Golledge, J. Therapeutic effects of renal denervation on renal failure. Curr. Neurovasc. Res. 2013, 10, 172–184. [Google Scholar] [CrossRef]
- Schmieder, R.E. Renal denervation in patients with chronic kidney disease: Current evidence and future perspectives. Nephrol. Dial. Transplant. 2023, 38, 1089–1096. [Google Scholar] [CrossRef]
- Nawar, K.; Mohammad, A.; Johns, E.J.; Abdulla, M.H. Renal denervation for atrial fibrillation: A comprehensive updated systematic review and meta-analysis. J. Hum. Hypertens. 2023, 37, 89–90. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Wang, L.; Li, C.; Xu, M.; Duan, Y.; Ma, L.; Li, T.; Chen, Q.; Wang, Y.; et al. Targeting mitochondria-inflammation circle by renal denervation reduces atheroprone endothelial phenotypes and atherosclerosis. Redox Biol. 2021, 47, 102156. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Xu, F.; Zang, T.; Ji, M.; Yin, J.; Chen, J.; Shen, L.; Ge, J. Renal denervation mitigates atherosclerosis in ApoE−/−mice via the suppression of inflammation. Am. J. Transl. Res. 2020, 12, 5362–5380. [Google Scholar]
- Wang, H.; Wang, J.; Guo, C.; Luo, W.; Kleiman, K.; Eitzman, D.T. Renal denervation attenuates progression of atherosclerosis in apolipoprotein E-deficient mice independent of blood pressure lowering. Hypertension 2015, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Visentin, V.; Boucher, J.; Bour, S.; Prévot, D.; Castan, I.; Carpéné, C.; Valet, P. Influence of high-fat diet on amine oxidase activity in white adipose tissue of mice prone or resistant to diet-induced obesity. J. Physiol. Biochem. 2005, 61, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dinh, T.N.; Nield, A.; Krishna, S.M.; Denton, K.; Golledge, J. Renal Denervation Promotes Atherosclerosis in Hypertensive Apolipoprotein E-Deficient Mice Infused with Angiotensin II. Front. Physiol. 2017, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; Zhao, L.; Yang, X.; Zhu, B.; Liu, Y.; Zhao, W.; Wang, X.; Qi, D.; Zhu, L.; Gao, C. Aggravated endothelial endocrine dysfunction and intimal thickening of renal artery in high-fat diet-induced obese pigs following renal denervation. BMC Cardiovasc. Disord. 2020, 20, 176. [Google Scholar] [CrossRef]
- Kuzuya, M.; Nakamura, K.; Sasaki, T.; Cheng, X.W.; Itohara, S.; Iguchi, A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1120–1125. [Google Scholar] [CrossRef]
- Kosowski, M.; Basiak, M.; Hachuła, M.; Okopień, B. Plasma Concentrations of New Biochemical Markers of Atherosclerosis in Patients with Dyslipidemia-A Pilot Study. Medicina 2022, 58, 717. [Google Scholar] [CrossRef]
- Tu, Y.; Ma, X.; Chen, H.; Fan, Y.; Jiang, L.; Zhang, R.; Cheng, Z. Molecular Imaging of Matrix Metalloproteinase-2 in Atherosclerosis Using a Smart Multifunctional PET/MRI Nanoparticle. Int. J. Nanomed. 2022, 17, 6773–6789. [Google Scholar] [CrossRef]
- D’Orléans-Juste, P.; Plante, M.; Honoré, J.C.; Carrier, E.; Labonté, J. Synthesis and degradation of endothelin-1. Can. J. Physiol. Pharmacol. 2003, 81, 503–510. [Google Scholar] [CrossRef]
- Abdalvand, A.; Morton, J.S.; Bourque, S.L.; Quon, A.L.; Davidge, S.T. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 2013, 61, 488–493. [Google Scholar] [CrossRef]
- Chen, S.; Mukherjee, S.; Chakraborty, C.; Chakrabarti, S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am. J. Physiol. Cell Physiol. 2003, 284, C263–C272. [Google Scholar] [CrossRef]
- Yu, H.; Alruwaili, N.; Kelly, M.R.; Zhang, B.; Liu, A.; Wang, Y.; Sun, D.; Wolin, M.S. Endothelin-1 depletion of cartilage oligomeric matrix protein modulates pulmonary artery superoxide and iron metabolism-associated mitochondrial heme biosynthesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L400–L409. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Mancia, G.; Schmieder, R.E.; Ruilope, L.; Narkiewicz, K.; Schlaich, M.; Williams, B.; Ribichini, F.; Weil, J.; Almerri, K.; et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension 2023, 80, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Barbato, E.; Azizi, M.; Schmieder, R.E.; Lauder, L.; Böhm, M.; Brouwers, S.; Bruno, R.M.; Dudek, D.; Kahan, T.; Kandzari, D.E.; et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2023, 44, 1313–1330. [Google Scholar] [CrossRef]
- Wang, Y. Renal denervation for resistant hypertension—The Symplicity HTN-1 study. Lancet 2014, 383, 1885. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, K.; Denton, K.M. Editorial: Function of Renal Sympathetic Nerves. Front. Physiol. 2017, 8, 642. [Google Scholar] [CrossRef]
- Wang, Y. What is the true incidence of renal artery stenosis after sympathetic denervation? Front. Physiol. 2014, 5, 311. [Google Scholar] [CrossRef]
- Wang, Y. Single-sided renal denervation may be not suitable for patients with significant renal artery stenosis. Clin. Res. Cardiol. 2014, 103, 950–951. [Google Scholar] [CrossRef]
- Wang, Y. It may be not suitable to perform renal denervation in renal arteries with significant stenosis. Int. J. Cardiol. 2014, 174, 750. [Google Scholar] [CrossRef]
- Wang, Y. Does Renal Denervation Inhibit Atherosclerosis in Humans? Austin J. Cardiovasc. Dis. Atheroscler. 2015, 2, 1013. [Google Scholar]
- Wang, Y. Patients with renal artery stenosis may not be suitable for renal denervation. Clin. Res. Cardiol. 2014, 103, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Renal artery stenosis may be responsible for the gradual return of high blood pressure after renal denervation. J. Clin. Hypertens 2014, 16, 313. [Google Scholar] [CrossRef] [PubMed]
- Dorr, O.; Liebetrau, C.; Mollmann, H.; Mahfoud, F.; Ewen, S.; Gaede, L.; Troidl, C.; Hoffmann, J.; Busch, N.; Laux, G.; et al. Beneficial effects of renal sympathetic denervation on cardiovascular inflammation and remodeling in essential hypertension. Clin. Res. Cardiol. 2015, 104, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, O.S.; Khan, S.Q.; Narayan, H.K.; Ng, K.H.; Mohammed, N.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Ng, L.L. Matrix metalloproteinase-2 predicts mortality in patients with acute coronary syndrome. Clin. Sci. 2009, 118, 249–257. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, K.; Jiang, F.L.; Zeng, L.X.; Jiang, W.H.; Wang, X.Y. The effects of catheter-based radiofrequency renal denervation on renal function and renal artery structure in patients with resistant hypertension. J. Clin. Hypertens. 2014, 16, 599–605. [Google Scholar] [CrossRef]
- Murphy, T.O.; Haglin, J.J.; Felder, D.A. The progression of experimental atherosclerosis after lumbar sympathectomy. Surg. Forum 1957, 7, 332–336. [Google Scholar]
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Canani, L.H.; Copstein, E.; Pecis, M.; Friedman, R.; Leitão, C.B.; Azevedo, M.J.; Triches, C.; Rados, D.R.; Moreas, R.S.; Gross, J.L. Cardiovascular autonomic neuropathy in type 2 diabetes mellitus patients with peripheral artery disease. Diabetol. Metab. Syndr. 2013, 5, 54. [Google Scholar] [CrossRef]
- Qin, L.; Cui, J.; Li, J. Sympathetic Nerve Activity and Blood Pressure Response to Exercise in Peripheral Artery Disease: From Molecular Mechanisms, Human Studies, to Intervention Strategy Development. Int. J. Mol. Sci. 2022, 23, 10622. [Google Scholar] [CrossRef]
- Li, J.; Xing, J. Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front. Physiol. 2012, 3, 247. [Google Scholar] [CrossRef]
- Qin, L.; Li, J. Nerve growth factor in muscle afferent neurons of peripheral artery disease and autonomic function. Neural Regen. Res. 2021, 16, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Nevitt, S.; Eleftheriadou, A.; Kanagala, P.; Esa, H.; Cuthbertson, D.J.; Tahrani, A.; Alam, U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: A meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, e002480. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.S.; Tarnow, L.; Rossing, P.; Hansen, B.V.; Hilsted, J.; Parving, H.H. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006, 29, 334–339. [Google Scholar] [CrossRef] [PubMed]


| NE in Blood/Urine | NE Spillover | Clinical Microneurography | Sympathetic Imaging | |
|---|---|---|---|---|
| Global/regional measurement | Global | Regional | Regional | Regional |
| Advantages | Convenient Little or no invasiveness | Measures the regional rate of NE spillover from the heart or kidneys | Is the only method for the direct measurement of adrenergic activity in humans Precisely assesses resting sympathetic activity and tracks the changes in cardiovascular regulation in response to stimuli | Demonstrates the anatomy of sympathetic innervation of an organ |
| Disadvantages | Lacks information on regional sympathetic responses | Is highly invasive Requires catheterization of veins draining internal organs | Requires a high degree of skill Requires several months of training | Is unable to differentiate the relative contribution of denervation and dysinnervation |
| References | [29,30,31] | [32,33] | [34,35,36,37,38,39] | [40,41,42] |
| β Blockers | Patients/Animals | Effect on Atherosclerosis | Mechanism | Reference |
|---|---|---|---|---|
| First Generation: Non-Selective β1 and β2 Blockers | ||||
| Propranolol | BPH/ApoE−/−mice | ↓ | ↓ HSPC proliferation in the BM ↓ GMPs in the BM ↓ Blood monocytes and neutrophils ↓ Macrophages in the lesion | [77] |
| Second Generation: β1-Selective Blockers | ||||
| Metoprolol | ApoE−/−mice | ↓ | N/R | [78] |
| ApoE−/−mice | ↓ | ↓ Circulating TNFα, CXCL1 ↓ Macrophages in the lesion | [79] | |
| Subjects without symptoms | ↓ | N/R | [84] | |
| Patients with hypercholesterolemia | ↓ | N/R | [85] | |
| Third Generation: Non-Selective β Blockers with Additional Properties | ||||
| Carvedilol With α1- blocking and antioxidant properties | Ldlr−/−mice | ↓ | ↑ ABCA1 in exosomes ↑ Cholesterol efflux ↓ Macrophages in the lesion | [80] |
| ApoE−/−mice | ↓ | ↓ Superoxide production ↓ Macrophage and T cell infiltration | [81] | |
| Rabbits | ↔ | ↓ LDL oxidation ↑ eNOS expression ↑ Endothelium-dependent relaxation | [82] | |
| Nipradilol With NO-releasing properties | Rabbits | ↓ | ↑ eNOS ↑ Endothelium-dependent relaxation ↓ Monocyte adhesion to EC ↓ Monocyte/macrophage infiltration | [83] |
| Third Generation: β1-Selective Blockers with Additional Properties | ||||
| Nebivolol With NO-releasing property | Rabbits | ↓ | ↓ LDL oxidation ↓ Inflammatory markers ↑ eNOS expression ↑ Endothelium-dependent relaxation | [82] |
| β3 Agonist | Animals | Effect on Atherosclerosis | Mechanisms | Reference |
|---|---|---|---|---|
| CL316,243 | E3L.CETP mice | ↓ | ↑ Energy expenditure ↑ Fat oxidation by activated BAT ↓ Total body fat mass ↓ Lipid droplet content in BAT ↓ Plasma TG, TC, and non-HDL cholesterol ↑ Plasma TRL clearance ↑ Hepatic cholesterol content ↑ HDL cholesterol | [92] |
| CL316,243 | E3L.CETP mice | ↓ | ↓ TC and TG ↑ VLDL clearance ↑ Liver uptake of VLDL core remnants ↑ Lipoprotein lipase lipolysis activity ↑ Transfer of VLDL to HDL cholesterol ↑ Plasma HDL cholesterol | [93] |
| CL316,243 | E3L.CETP mice | ↓ | ↓ Total fat mass ↓ Plasma TG and non-HDL cholesterol ↑ Plasma clearance and hepatic uptake of cholesterol-enriched TRL remnants. ↑ HDL cholesterol | [94] |
| CL316,243 | E3L.CETP mice | NR | ↓ Body fat masss and gonadal WAT ↓ Plasma TG, TC, and non-HDL cholesterol ↑ Clearance of TRL-like particles ↑ Hepatic uptake of TRL-like remnants ↑ Tranfer of TRL particles to HDL particles ↑ Plasma HDL cholesterol | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Anesi, J.; Maier, M.C.; Myers, M.A.; Oqueli, E.; Sobey, C.G.; Drummond, G.R.; Denton, K.M. Sympathetic Nervous System and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13132. https://doi.org/10.3390/ijms241713132
Wang Y, Anesi J, Maier MC, Myers MA, Oqueli E, Sobey CG, Drummond GR, Denton KM. Sympathetic Nervous System and Atherosclerosis. International Journal of Molecular Sciences. 2023; 24(17):13132. https://doi.org/10.3390/ijms241713132
Chicago/Turabian StyleWang, Yutang, Jack Anesi, Michelle C. Maier, Mark A. Myers, Ernesto Oqueli, Christopher G. Sobey, Grant R. Drummond, and Kate M. Denton. 2023. "Sympathetic Nervous System and Atherosclerosis" International Journal of Molecular Sciences 24, no. 17: 13132. https://doi.org/10.3390/ijms241713132
APA StyleWang, Y., Anesi, J., Maier, M. C., Myers, M. A., Oqueli, E., Sobey, C. G., Drummond, G. R., & Denton, K. M. (2023). Sympathetic Nervous System and Atherosclerosis. International Journal of Molecular Sciences, 24(17), 13132. https://doi.org/10.3390/ijms241713132







