Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering
Abstract
1. Introduction
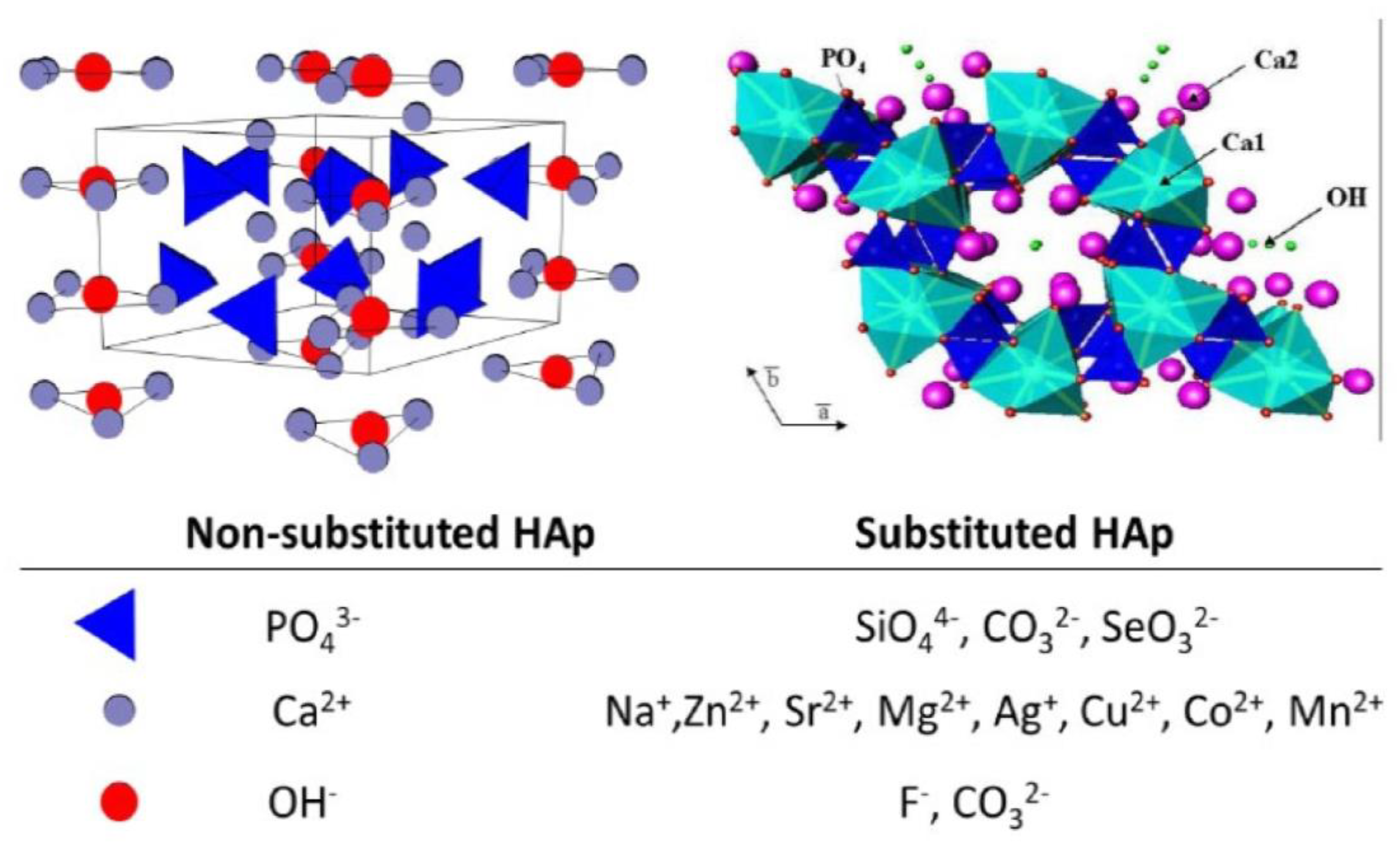
- replacing the Ca2+ sites with Zn2+, Sr2+, Mg2+, and Na+ ions;
- replacing the PO43− sites with CO32−, or SiO32− ions;
- replacing the −OH group with Cl−, F−, and CO32− ions.
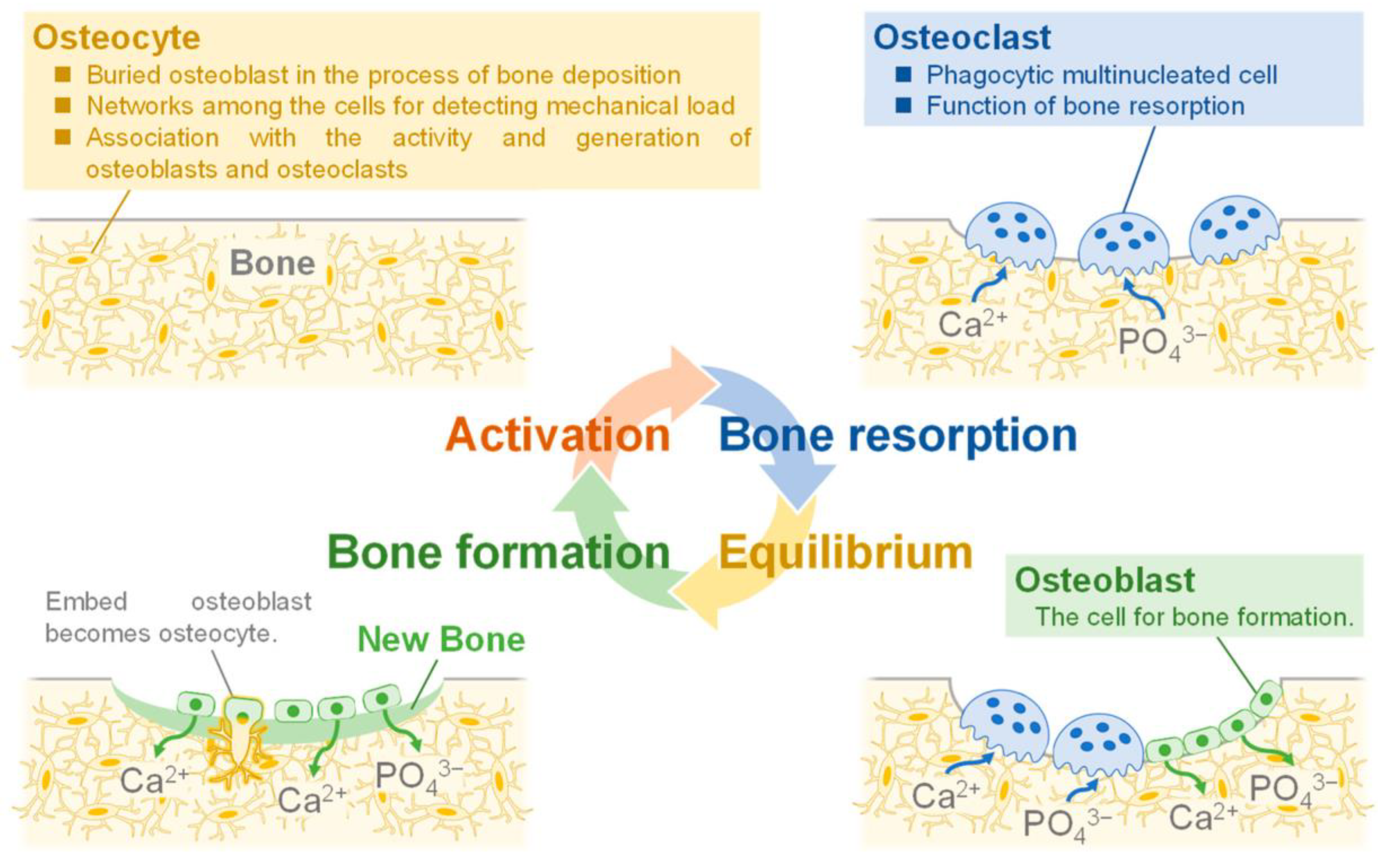
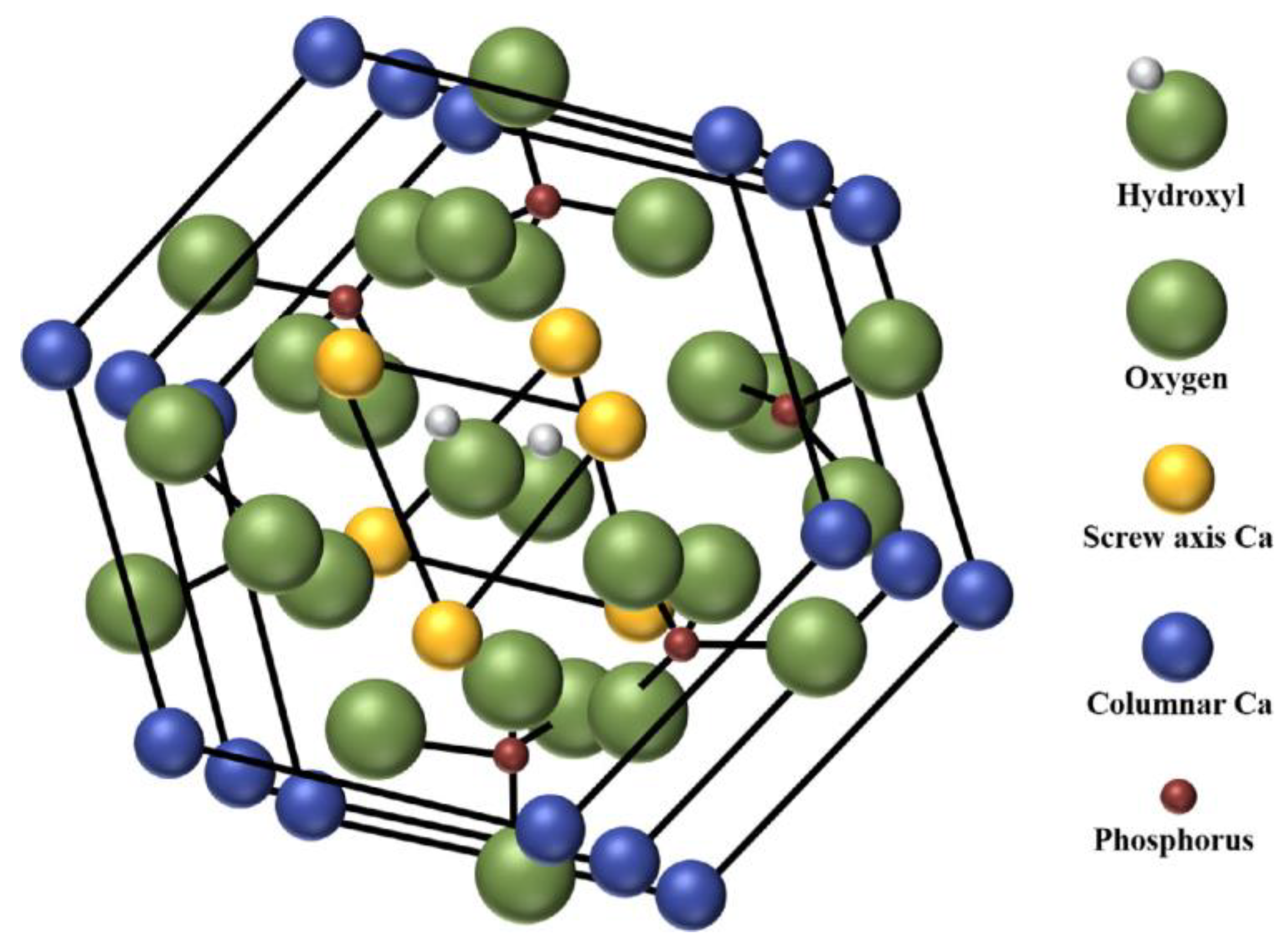
2. General Aspects of Hydroxyapatite Applied in Bone Tissue Engineering
2.1. Physical and Mechanical Properties
2.2. Synthesis Methods
2.3. Hydroxyapatite Applications
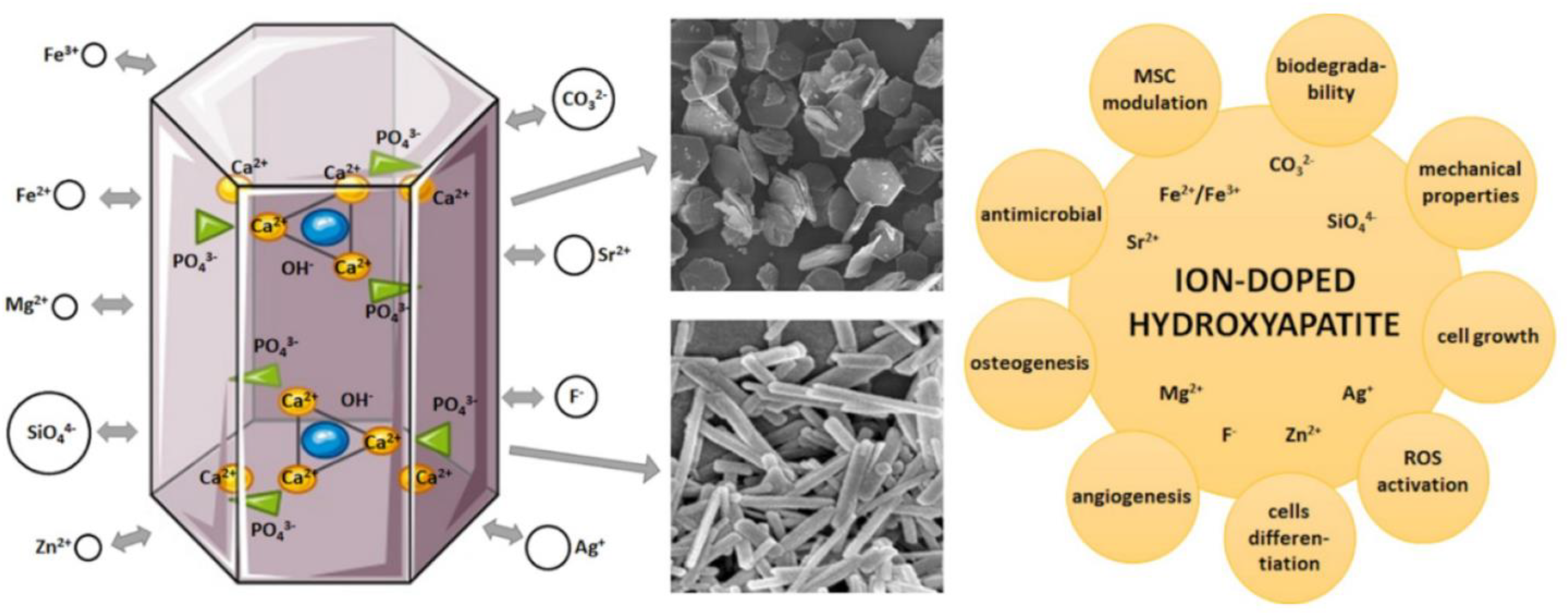
3. Comparison of Non-Doped and Doped Hydroxyapatite: Properties, Structure, and Applicability in Bone Tissue Engineering
4. Doped Hydroxyapatite: Doping Material and Methods, Synthesis Method, and Properties
4.1. Zn2+-Doped Hydroxyapatite: Doping Method, Properties, and Biologic Activity
4.2. Mg2+-Doped Hydroxyapatite: Doping Method, Properties, and Biologic Activity
4.3. Cu2+-Doped Hydroxyapatite: Doping Method, Properties, and Biologic Activity
4.4. Sr2+-Doped Hydroxyapatite: Doping Method, Properties, and Biologic Activity
4.5. Ag+-Doped Hydroxyapatite: Doping Method, Properties, and Biologic Activity
4.6. Hydroxyapatite Doped with Other Metals: Doping Method, Properties, and Biologic Activity
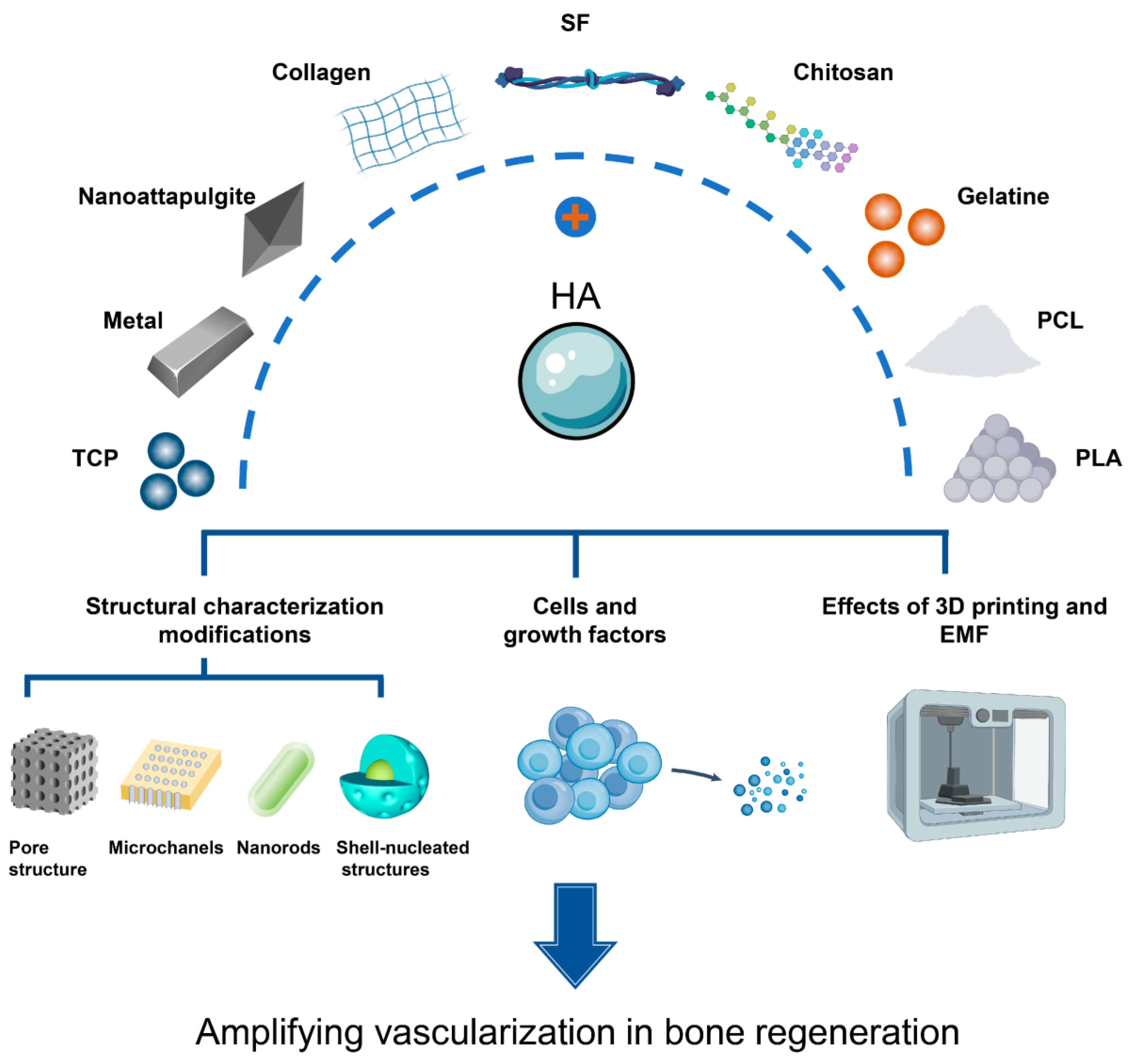
5. Latest Applications of Doped Hydroxyapatite in Bone Tissue Engineering
5.1. Applicability of Doped Hydroxyapatite in Dental Applications
5.2. Applicability of Doped Hydroxyapatite in Drug Delivery Systems
5.3. Applicability of Doped Hydroxyapatite in Bone Tissue Engineering
6. Future Challenges, Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdem, U.; Dogan, D.; Bozer, B.M.; Turkoz, M.B.; Yıldırım, G.; Metin, A.U. Fabrication of mechanically advanced polydopamine decorated hydroxyapatite/polyvinyl alcohol bio-composite for biomedical applications: In-vitro physicochemical and biological evaluation. J. Mech. Behav. Biomed. Mater. 2022, 136, 105517. [Google Scholar] [CrossRef]
- Sagadevan, S.; Schirhagl, R.; Rahman, M.Z.; Bin Ismail, M.F.; Lett, J.A.; Fatimah, I.; Mohd Kaus, N.H.; Oh, W.-C. Recent advancements in polymer matrix nanocomposites for bone tissue engineering applications. J. Drug Deliv. Sci. Technol. 2023, 82, 104313. [Google Scholar] [CrossRef]
- Sugimoto, K.; Zhou, Y.; Galindo, T.G.P.; Kimura, R.; Tagaya, M. Investigation of Surface Layers on Biological and Synthetic Hydroxyapatites Based on Bone Mineralization Process. Biomimetics 2023, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Uddin, M.N.; Sarkar, S.; Ahmed, S. Crystallographic dependency of waste cow bone, hydroxyapatite, and β-tricalcium phosphate for biomedical application. J. Saudi Chem. Soc. 2022, 26, 101559. [Google Scholar] [CrossRef]
- Cao, Z.; Bian, Y.; Hu, T.; Yang, Y.; Cui, Z.; Wang, T.; Yang, S.; Weng, X.; Liang, R.; Tan, C. Recent advances in two-dimensional nanomaterials for bone tissue engineering. J. Mater. 2023, 9, 930–958. [Google Scholar] [CrossRef]
- Nisar, A.; Iqbal, S.; Atiq Ur Rehman, M.; Mahmood, A.; Younas, M.; Hussain, S.Z.; Tayyaba, Q.; Shah, A. Study of physico-mechanical and electrical properties of cerium doped hydroxyapatite for biomedical applications. Mater. Chem. Phys. 2023, 299, 127511. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Govindaraj, S.K.; Mariyappan, K.; Boobalan, S. CTAB enabled microwave-hydrothermal assisted mesoporous Zn-doped hydroxyapatite nanorods synthesis using bio-waste Nodipecten nodosus scallop for biomedical implant applications. Environ. Res. 2023, 216, 114683. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Li, W.; Jiang, L.; Li, X. Recent progress of rare earth doped hydroxyapatite nanoparticles: Luminescence properties, synthesis and biomedical applications. Acta Biomater. 2022, 148, 22–43. [Google Scholar] [CrossRef]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- de Lima, C.O.; de Oliveira, A.L.M.; Chantelle, L.; Silva Filho, E.C.; Jaber, M.; Fonseca, M.G. Zn-doped mesoporous hydroxyapatites and their antimicrobial properties. Colloids Surf. B Biointerfaces 2021, 198, 111471. [Google Scholar] [CrossRef]
- Murugesan, V.; Vaiyapuri, M.; Murugeasan, A. Fabrication and characterization of strontium substituted chitosan modify hydroxyapatite for biomedical applications. Inorg. Chem. Commun. 2022, 142, 109653. [Google Scholar] [CrossRef]
- Ruffini, A.; Sandri, M.; Dapporto, M.; Campodoni, E.; Tampieri, A.; Sprio, S. Nature-Inspired Unconventional Approaches to Develop 3D Bioceramic Scaffolds with Enhanced Regenerative Ability. Biomedicines 2021, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- El-Bassyouni, G.T.; Kenawy, S.H.; El-Aty, A.A.A.; Hamzawy, E.M.A.; Turky, G.M. Influence of ZnO doped into hydroxyapatite: Structural, electrical, biocompatibility, and antimicrobial assessment. J. Mol. Struct. 2022, 1268, 133700. [Google Scholar] [CrossRef]
- Babaei, P.; Safai-Ghomi, J.; Rashki, S. Engineered dual-purpose Ta-doped ZnO/Hydroxyapatite nanocomposites: Antibacterial activity and robust catalyst in MW-Induced synthesis of chromopyrimidines. Ceram. Int. 2022, 48, 8359–8373. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng. C 2021, 128, 112298. [Google Scholar] [CrossRef]
- Zhang, A.-M.; Lenin, P.; Zeng, R.-C.; Kannan, M.B. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J. Magnes. Alloys 2022, 10, 1154–1170. [Google Scholar] [CrossRef]
- Ahmed, L.O.; Bulut, N.; Osmanlıoğlu, F.; Tatar, B.; Kebiroglu, H.; Ates, T.; Koytepe, S.; Ates, B.; Keser, S.; Kaygili, O. Theoretical and experimental investigation of the effects of Pr dopant on the electronic band structure, thermal, structural, in vitro biocompatibility of Er-based hydroxyapatites. J. Mol. Struct. 2023, 1280, 135095. [Google Scholar] [CrossRef]
- Jose, S.; Senthilkumar, M.; Elayaraja, K.; Haris, M.; George, A.; Raj, A.D.; Sundaram, S.J.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Preparation and characterization of Fe doped n-hydroxyapatite for biomedical application. Surf. Interfaces 2021, 25, 101185. [Google Scholar] [CrossRef]
- Beh, C.Y.; Cheng, E.M.; Mohd Nasir, N.F.; Khor, S.F.; Eng, S.K.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Lee, K.Y. Low Frequency Dielectric and Optical Behavior on Physicochemical Properties of Hydroxyapatite/Cornstarch Composite. J. Colloid Interface Sci. 2021, 600, 187–198. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Yeong, C.H.; Thein, W.M. Development of the third generation of bioceramics: Doping hydroxyapatite with s-, p-, d-, and f-blocks cations and their potential applications in bone regeneration and void filling. Ceram. Int. 2023, 49, 7142–7179. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wang, S.-F.; Chien, R.R.; Tu, C.-S.; Feng, K.-C.; Chen, C.-S.; Hung, K.-Y.; Schmidt, V.H. Evolution of the microstructural and mechanical properties of hydroxyapatite bioceramics with varying sintering temperature. Ceram. Int. 2019, 45, 16226–16233. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the Biological and Mechanical Performances of Sintered Hydroxyapatite by Multiple Ions Doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Obada, D.O.; Abolade, S.A.; Akande, A.; Csaki, S.; Dodoo-Arhin, D. Datasets on the elastic and mechanical properties of hydroxyapatite: A first principle investigation, experiments, and pedagogical perspective. Data Brief 2023, 48, 109075. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. [Google Scholar] [CrossRef] [PubMed]
- Abere, D.V.; Ojo, S.A.; Oyatogun, G.M.; Paredes-Epinosa, M.B.; Niluxsshun, M.C.D.; Hakami, A. Mechanical and morphological characterization of nano-hydroxyapatite (nHA) for bone regeneration: A mini review. Biomed. Eng. Adv. 2022, 4, 100056. [Google Scholar] [CrossRef]
- Benaqqa, C.; Chevalier, J.; Saâdaoui, M.; Fantozzi, G. Investigation of Crack Growth Process in Dense Hydroxyapatite Using the Double Torsion Method. In Fracture Mechanics of Ceramics; Springer: Boston, MA, USA, 2005; pp. 387–397. [Google Scholar]
- Ingole, V.H.; Ghule, S.S.; Vuherer, T.; Kokol, V.; Ghule, A.V. Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration. Minerals 2021, 11, 1390. [Google Scholar] [CrossRef]
- Ferreira, C.R.D.; Santiago, A.A.G.; Vasconcelos, R.C.; Paiva, D.F.F.; Pirih, F.Q.; Araújo, A.A.; Motta, F.V.; Bomio, M.R.D. Study of microstructural, mechanical, and biomedical properties of zirconia/hydroxyapatite ceramic composites. Ceram. Int. 2022, 48, 12376–12386. [Google Scholar] [CrossRef]
- Albulescu, R.; Popa, A.-C.; Enciu, A.-M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.-R.; et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials 2019, 12, 3704. [Google Scholar] [CrossRef]
- Zamiri, A.; De, S. Mechanical properties of hydroxyapatite single crystals from nanoindentation data. J. Mech. Behav. Biomed. Mater. 2011, 4, 146–152. [Google Scholar] [CrossRef]
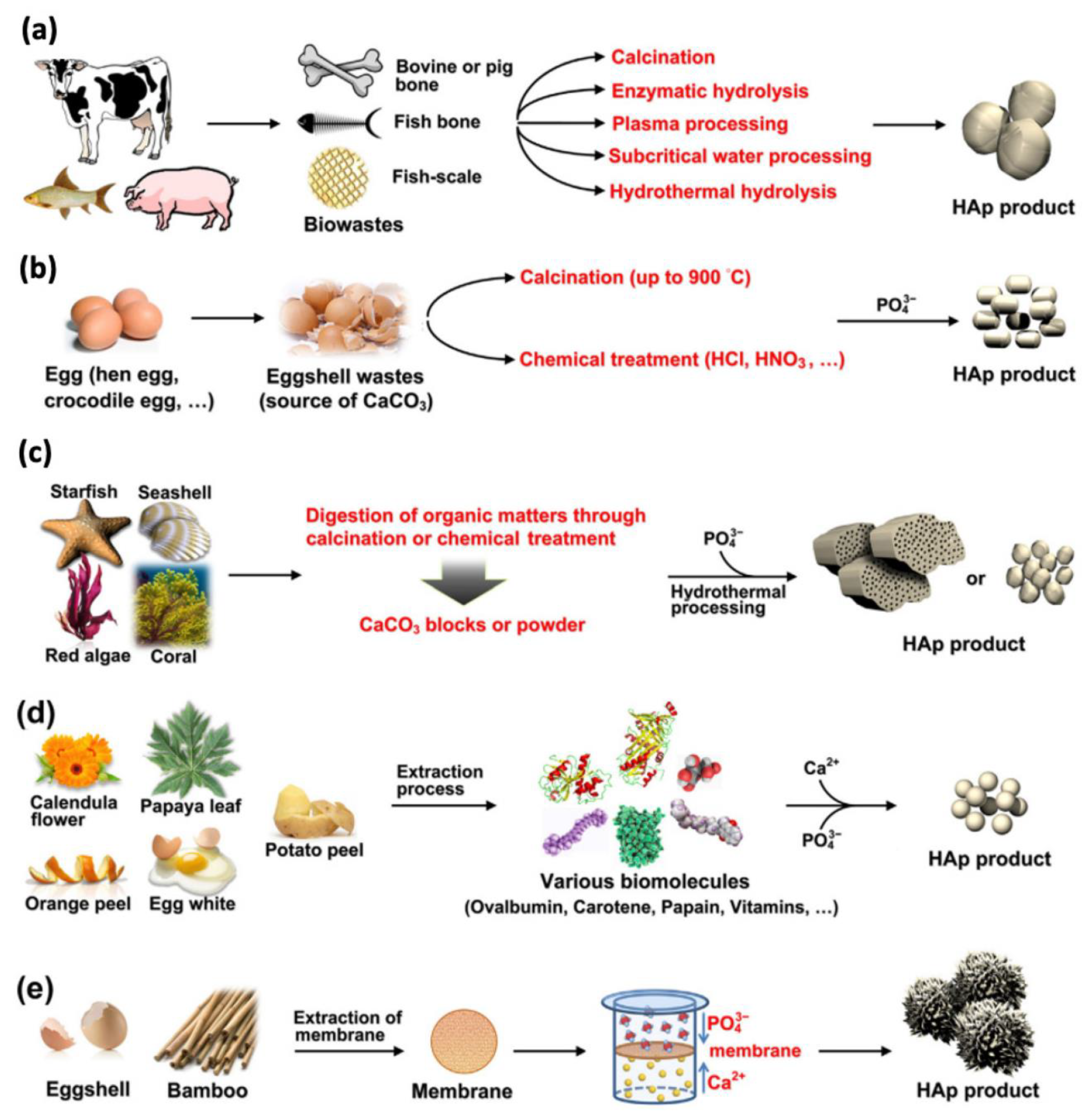
- Arokiasamy, P.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Mehrvarz, A.; Khalil-Allafi, J.; Motallebzadeh, A.; Khalili, V. The effect of ZnO nanoparticles on nanomechanical behavior of Hydroxyapatite electrodeposited on NiTi biomedical alloy. Ceram. Int. 2022, 48, 35039–35049. [Google Scholar] [CrossRef]
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef] [PubMed]
- Hincapie-Bedoya, J.; Poblano-Salas, C.A.; Moreno-Murguia, B.; Gutierrez-Perez, A.I.; Henao, J.; Espinosa-Arbelaez, D.G.; Giraldo-Betancur, A.L. Effect of the modification of spray drying parameters on the fabrication of bovine-derived hydroxyapatite microspheres for biomedical applications. Mater. Today Commun. 2022, 31, 103838. [Google Scholar] [CrossRef]
- Zastulka, A.; Clichici, S.; Tomoaia-Cotisel, M.; Mocanu, A.; Roman, C.; Olteanu, C.-D.; Culic, B.; Mocan, T. Recent Trends in Hydroxyapatite Supplementation for Osteoregenerative Purposes. Materials 2023, 16, 1303. [Google Scholar] [CrossRef]
- Firdaus Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Abdul Wahap, M.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef]
- Egan, E.P., Jr.; Wakefield, Z.T.; Elmore, K.L. High-Temperature Heat Content of Hydroxyapatite. J. Am. Chem. Soc. 1950, 72, 2418–2421. [Google Scholar] [CrossRef]
- Blakeslee, K.C.; Condrate, R.A., Sr. Vibrational Spectra of Hydrothermally Prepared Hydroxyapatites. J. Am. Ceram. Soc. 1971, 54, 559–563. [Google Scholar] [CrossRef]
- Joris, S.J.; Amberg, C.H. Nature of deficiency in nonstoichiometric hydroxyapatites. II. Spectroscopic studies of calcium and strontium hydroxyapatites. J. Phys. Chem. 1971, 75, 3172–3178. [Google Scholar] [CrossRef]
- Pereira, M.M.; Clark, A.E.; Hench, L.L. Effect of Texture on the Rate of Hydroxyapatite Formation on Gel-Silica Surface. J. Am. Ceram. Soc. 1995, 78, 2463–2468. [Google Scholar] [CrossRef]
- Hench, L.L. Sol-gel materials for bioceramic applications. Curr. Opin. Solid State Mater. Sci. 1997, 2, 604–610. [Google Scholar] [CrossRef]
- Mann, S.; Calvert, P.D. Biotechnological horizons in biomineralization. Trends Biotechnol. 1987, 5, 309–314. [Google Scholar] [CrossRef]
- Calvert, P. Biomimetic Ceramics. MRS Online Proc. Libr. 1990, 180, 619. [Google Scholar] [CrossRef]
- Collin, R.L. Strontium-Calcium Hydroxyapatite Solid Solutions: Preparation and Lattice Constant Measurements1. J. Am. Chem. Soc. 1959, 81, 5275–5278. [Google Scholar] [CrossRef]
- Eanes, E.D.; Posner, A.S. A note on the crystal growth of hydroxyapatite precipitated from aqueous solutions. Mater. Res. Bull. 1970, 5, 377–383. [Google Scholar] [CrossRef]
- Aizawa, M.; Hanazawa, T.; Itatani, K.; Howell, F.S.; Kishioka, A. Characterization of hydroxyapatite powders prepared by ultrasonic spray-pyrolysis technique. J. Mater. Sci. 1999, 34, 2865–2873. [Google Scholar] [CrossRef]
- Aizawa, M.; Itatani, K.; Howell, F.S.; Kishioka, A. Some Properties of Carbonate-Containing Hydroxyapatite Powder Prepared by Spray-Pyrolysis Technique Using Urea as a Foaming Agent. J. Ceram. Soc. Jpn. 1995, 103, 1214–1219. [Google Scholar] [CrossRef][Green Version]
- Kalpana, M.; Nagalakshmi, R. Effect of reaction temperature and pH on structural and morphological properties of hydroxyapatite from precipitation method. J. Indian Chem. Soc. 2023, 100, 100947. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Ahmad, R.N.; Mohd Rosli, N.L.; Abdul Manan, M.S.; Marzuki, M.; Wahi, A. Sol gel deposited hydroxyapatite-based coating technique on porous titanium niobium for biomedical applications: A mini review. Mater. Today Proc. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Choi, G.; Choi, A.H.; Evans, L.A.; Akyol, S.; Ben-Nissan, B. A review: Recent advances in sol-gel-derived hydroxyapatite nanocoatings for clinical applications. J. Am. Ceram. Soc. 2020, 103, 5442–5453. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Kamonwannasit, S.; Khemthong, P. Structural characterization and antibacterial activity of hydroxyapatite synthesized via sol-gel method using glutinous rice as a template. J. Sol-Gel Sci. Technol. 2019, 89, 764–775. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Mendoza-Anaya, D.; Sánchez-Campos, D.; Fernandez-García, M.E.; Salinas-Rodríguez, E.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. The pH Effect on the Growth of Hexagonal and Monoclinic Hydroxyapatite Synthesized by the Hydrothermal Method. J. Nanomater. 2020, 2020, 5912592. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Deng, H.; Yao, S.; Wang, Y. Regulatory synthesis and characterization of hydroxyapatite nanocrystals by a microwave-assisted hydrothermal method. Ceram. Int. 2020, 46, 2185–2193. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, E.; Jiang, F.; Hu, Z.; Fu, H.; Li, Y.; Meng, H.; Wu, Q.; Liu, J.; Yang, Q.; et al. Preparation of novel porous hydroxyapatite sheets with high Pb2+ adsorption properties by self-assembly non-aqueous precipitation method. Ceram. Int. 2023, 49, 30603–30612. [Google Scholar] [CrossRef]
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Adediran, A.A.; Olasukanmi, O.O.; Tanimola, M.O. Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Moradi, M.; Pakseresht, A.; Asl, M.S.; Karimi-Maleh, H.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Biocompatibility and mechanical properties of pigeon bone waste extracted natural nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. B 2021, 264, 114950. [Google Scholar] [CrossRef]
- Huang, A.; Dai, H.; Wu, X.; Zhao, Z.; Wu, Y. Synthesis and characterization of mesoporous hydroxyapatite powder by microemulsion technique. J. Mater. Res. Technol. 2019, 8, 3158–3166. [Google Scholar] [CrossRef]
- Collins Arun Prakash, V.; Venda, I.; Thamizharasi, V. Synthesis and characterization of surfactant assisted hydroxyapatite powder using microemulsion method. Mater. Today Proc. 2022, 51, 1788–1792. [Google Scholar] [CrossRef]
- Arun Prakash, V.C.; Venda, I.; Thamizharasi, V.; Sathya, E. A new attempt on synthesis of spherical nano hydroxyapatite powders prepared by dimethyl sulfoxide—Poly vinyl alcohol assisted microemulsion method. Mater. Chem. Phys. 2021, 259, 124097. [Google Scholar] [CrossRef]
- Batista, H.A.; Silva, F.N.; Lisboa, H.M.; Costa, A.C.F.M. Modeling and optimization of combustion synthesis for hydroxyapatite production. Ceram. Int. 2020, 46, 11638–11646. [Google Scholar] [CrossRef]
- Angioni, D.; Orrù, R.; Cao, G.; Garroni, S.; Ricci, P.C.; Manukyan, K.V. Combustion synthesis and spark plasma sintering of apatite-tricalcium phosphate nanocomposites. Ceram. Int. 2023, 49, 26825–26833. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Prog. Org. Coat. 2020, 147, 105858. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Indira, J.; Malathi, K.S. Comparison of template mediated ultrasonic and microwave irradiation method on the synthesis of hydroxyapatite nanoparticles for biomedical applications. Mater. Today Proc. 2022, 51, 1765–1769. [Google Scholar] [CrossRef]
- Herradi, S.; Adouar, I.; Bouhazma, S.; Chajri, S.; Khaldi, M.; Bali, B.E.; Lachkar, M. A physicochemical study of a modified sol–gel derived neodymium-hydroxyapatite. Mater. Today Proc. 2022, 53, 386–391. [Google Scholar] [CrossRef]
- Nellis, B.A.; Satcher, J.H.; Risbud, S.H. Phospholipid bilayer formation on hydroxyapatite sol–gel synthesized films. Colloids Surf. B Biointerfaces 2011, 82, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Afshar, A. In vitro study: Bond strength, electrochemical and biocompatibility evaluations of TiO2/Al2O3 reinforced hydroxyapatite sol–gel coatings on 316L SS. Surf. Coat. Technol. 2021, 405, 126594. [Google Scholar] [CrossRef]
- Poovendran, K.; Josephwilson, K.S.; Sakthipandi, K.; Ramanujam, N.R. Assimilation of manganese metal ion doped hydroxyapatite by Co-Precipitation technique. J. Indian Chem. Soc. 2022, 99, 100779. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Apátiga-Castro, M.; Soto, K.M.; Manzano-Ramírez, A.; Zamora-Antuñano, M.; Gonzalez-Gutierrez, C. Effect of temperature on crystallite size of hydroxyapatite powders obtained by wet precipitation process. J. Saudi Chem. Soc. 2022, 26, 101513. [Google Scholar] [CrossRef]
- Rajhi, F.Y.; Yahia, I.S.; Zahran, H.Y.; Kilany, M. Synthesis, structural, optical, dielectric properties, gamma radiation attenuation, and antimicrobial activity of V-doped hydroxyapatite nanorods. Mater. Today Commun. 2021, 26, 101981. [Google Scholar] [CrossRef]
- Balu, S.K.; Sampath, V.; Andra, S.; Alagar, S.; Manisha Vidyavathy, S. Fabrication of carbon and silver nanomaterials incorporated hydroxyapatite nanocomposites: Enhanced biological and mechanical performances for biomedical applications. Mater. Sci. Eng. C 2021, 128, 112296. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Rathi, M.A.; Almaary, K.S.; Alkhattaf, F.S.; Elbadawi, Y.B.; Chang, S.W.; Ravindran, B. Preparation and antibacterial application of hydroxyapatite doped Silver nanoparticles derived from chicken bone. J. King Saud Univ. Sci. 2022, 34, 101749. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Martínez-Macias, M.d.R.; Correa-Murrieta, M.A.; Sanches-Silva, A. Hydroxyapatite recovery from fish byproducts for biomedical applications. Sustain. Chem. Pharm. 2022, 28, 100726. [Google Scholar] [CrossRef]
- Kumar Yadav, M.; Hiren Shukla, R.; Prashanth, K.G. A comprehensive review on development of waste derived hydroxyapatite (HAp) for tissue engineering application. Mater. Today Proc. 2023, in press. [CrossRef]
- Ramachandran, R.; Shinyjoy, E.; Ramya, S.; Kavitha, L.; Gopi, D. Leucas aspera assisted green synthesis of mineralized hydroxyapatite/polycaprolactone: A potential composite for biomedical applications. Mater. Lett. 2022, 326, 132972. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, K.; Balázsi, C. Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends. Coatings 2023, 13, 360. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Etinosa, P.O.; Obada, D.O. A comparative study of the mechanical properties of sol-gel derived hydroxyapatite produced from a novel mixture of two natural biowastes for biomedical applications. Mater. Chem. Phys. 2023, 297, 127434. [Google Scholar] [CrossRef]
- Mathina, M.; Shinyjoy, E.; Ramya, S.; Kavitha, L.; Gopi, D. Multifunctional crab shell derived hydroxyapatite/metal oxide/polyhydroxybutyrate composite coating on 316L SS for biomedical applications. Mater. Lett. 2022, 313, 131701. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Obada, D.O. Synthesis and characterization of sol–gel derived hydroxyapatite from a novel mix of two natural biowastes and their potentials for biomedical applications. Mater. Today Proc. 2022, 62, 4182–4187. [Google Scholar] [CrossRef]
- Ojo, S.A.; Abere, D.V.; Adejo, H.O.; Robert, R.A.; Oluwasegun, K.M. Additive manufacturing of hydroxyapatite-based composites for bioengineering applications. Bioprinting 2023, 32, e00278. [Google Scholar] [CrossRef]
- Elyaderani, A.K.; De Lama-Odría, M.d.C.; Valle, L.J.d.; Puiggalí, J. Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 15016. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.-E.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers 2022, 14, 899. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, L.; Wu, T. Recent Advances in Bioengineering Bone Revascularization Based on Composite Materials Comprising Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 12492. [Google Scholar] [CrossRef]
- Almulhim, K.S.; Syed, M.R.; Alqahtani, N.; Alamoudi, M.; Khan, M.; Ahmed, S.Z.; Khan, A.S. Bioactive Inorganic Materials for Dental Applications: A Narrative Review. Materials 2022, 15, 6864. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; Algahtani, F.D.; Alshammari, H.F.; Kolsi, L.; Dkhil, M.A.; Abd El-Rahman, G.I.; El-Morsy, M.A.; Menazea, A.A. Improvement of mechanical and antibacterial features of hydroxyapatite/chromium oxide/graphene oxide nanocomposite for biomedical utilizations. Surf. Coat. Technol. 2022, 440, 128476. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, S.; Yao, K.; Sun, Y.; Liu, Y.; Wang, X.; Huang, W. A novel La3+ doped MIL spherical analogue used as antibacterial and anticorrosive additives for hydroxyapatite coating on titanium dioxide nanotube array. Appl. Surf. Sci. 2021, 551, 149425. [Google Scholar] [CrossRef]
- Dragomir, L.; Antoniac, A.; Manescu, V.; Robu, A.; Dinu, M.; Pana, I.; Cotrut, C.M.; Kamel, E.; Antoniac, I.; Rau, J.V.; et al. Preparation and characterization of hydroxyapatite coating by magnetron sputtering on Mg–Zn–Ag alloys for orthopaedic trauma implants. Ceram. Int. 2023, 49, 26274–26288. [Google Scholar] [CrossRef]
- Zawisza, K.; Sobierajska, P.; Nowak, N.; Kedziora, A.; Korzekwa, K.; Pozniak, B.; Tikhomirov, M.; Miller, J.; Mrowczynska, L.; Wiglusz, R.J. Preparation and preliminary evaluation of bio-nanocomposites based on hydroxyapatites with antibacterial properties against anaerobic bacteria. Mater. Sci. Eng. C 2020, 106, 110295. [Google Scholar] [CrossRef]
- Izzetti, R.; Gennai, S.; Nisi, M.; Gulia, F.; Miceli, M.; Giuca, M.R. Clinical Applications of Nano-Hydroxyapatite in Dentistry. Appl. Sci. 2022, 12, 10762. [Google Scholar] [CrossRef]
- Fadli, A.; Prabowo, A.; Reni Yenti, S.; Huda, F.; Annisa Liswani, A.; Lamsinar Br Hutauruk, D. High performance of coating hydroxyapatite layer on 316L stainless steel using ultrasonically and alkaline pretreatment. J. King Saud Univ.Sci. 2023, 35, 102681. [Google Scholar] [CrossRef]
- Sheykholeslami, S.O.R.; Khalil-Allafi, J.; Etminanfar, M.; Khalili, V.; Parsa, A.B. Synthesis and development of novel spherical mesoporous SiO2/HA particles and incorporating them in electrodeposited hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2023, 459, 129410. [Google Scholar] [CrossRef]
- González-Estrada, O.A.; Pertuz Comas, A.D.; Ospina, R. Characterization of hydroxyapatite coatings produced by pulsed-laser deposition on additive manufacturing Ti6Al4V ELI. Thin Solid Film. 2022, 763, 139592. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Croes, M.; Akhavan, B.; Jahanmard, F.; Eigenhuis, C.C.; Dadbakhsh, S.; Vogely, H.C.; Bilek, M.M.; Fluit, A.C.; Boel, C.H.E.; et al. Layer by layer coating for bio-functionalization of additively manufactured meta-biomaterials. Addit. Manuf. 2020, 32, 100991. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Deev, R.V.; Bozo, I.I.; Fedotov, A.Y.; Gurin, A.N.; Mamonov, V.E.; Kravchuk, A.D.; Popov, V.K.; Egorov, A.A.; Komlev, V.S. Octacalcium phosphate coating for 3D printed cranioplastic porous titanium implants. Surf. Coat. Technol. 2020, 383, 125192. [Google Scholar] [CrossRef]
- Ambrogi, V.; Quaglia, G.; Pietrella, D.; Nocchetti, M.; Di Michele, A.; Bolli, E.; Kaciulis, S.; Mezzi, A.; Padeletti, G.; Latterini, L. Silver@Hydroxyapatite functionalized calcium carbonate composites: Characterization, antibacterial and antibiofilm activities and cytotoxicity. Appl. Surf. Sci. 2022, 586, 152760. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Ghazanfar, U.; Idrees, M.; Irshad, M.S.; Haq, Z.; Javed, M.Q.; Hassan, S.Z.; Rizwan, M. In vitro controlled drug delivery of cationic substituted hydroxyapatite nanoparticles; enhanced anti-chelating and antibacterial response. Kuwait J. Sci. 2023, 50, 97–104. [Google Scholar] [CrossRef]
- Ghosh, R.; Das, S.; Mallick, S.P.; Beyene, Z. A review on the antimicrobial and antibiofilm activity of doped hydroxyapatite and its composites for biomedical applications. Mater. Today Commun. 2022, 31, 103311. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent advances in hydroxyapatite-based electrochemical biosensors: Applications and future perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542. [Google Scholar] [CrossRef]
- Anushika; Sharma, P.; Trivedi, A.; Begam, H. Synthesis and characterization of pure and titania doped hydroxyapatite. Mater. Today Proc. 2019, 16, 302–307. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Hussain, R.; Dalgic, A.D.; Evis, Z. Bactericidal and in vitro osteogenic activity of nano sized cobalt-doped silicate hydroxyapatite. Ceram. Int. 2022, 48, 28231–28239. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Weiserová, M.; Jurek, K.; Mikšovský, J.; Strnad, J.; Galandáková, A.; Ulrichová, J. Antibacterial, cytotoxicity and physical properties of laser—Silver doped hydroxyapatite layers. Mater. Sci. Eng. C 2013, 33, 1242–1246. [Google Scholar] [CrossRef]
- Shokri, M.; Kharaziha, M.; Tafti, H.A.; Eslaminejad, M.B.; Aghdam, R.M. Synergic role of zinc and gallium doping in hydroxyapatite nanoparticles to improve osteogenesis and antibacterial activity. Biomater. Adv. 2022, 134, 112684. [Google Scholar] [CrossRef]
- Yahia, I.S.; Shkir, M.; AlFaify, S.; Ganesh, V.; Zahran, H.Y.; Kilany, M. Facile microwave-assisted synthesis of Te-doped hydroxyapatite nanorods and nanosheets and their characterizations for bone cement applications. Mater. Sci. Eng. C 2017, 72, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mater. Sci. Eng. C 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Aksakal, B.; Say, Y.; Sinirlioglu, Z.A. Effects of silver/selenium/chitosan doped hydroxyapatite coatings on REX-734 alloy: Morphology, antibacterial activity, and cell viability. Mater. Today Commun. 2022, 33, 104246. [Google Scholar] [CrossRef]
- De Lama-Odría, M.D.; Valle, L.J.d.; Puiggalí, J. Lanthanides-Substituted Hydroxyapatite for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 3446. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of physico-chemical properties of ions-doped hydroxyapatite on adsorption and release performance of doxorubicin as a model anticancer drug. Mater. Chem. Phys. 2022, 276, 125440. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Padmanabhan, V.P.; Kulandaivelu, R.; Sankara Narayanan Nellaiappan, T.S.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Obulapuram, P.K.; Oh, W.C. Influence of iron doping towards the physicochemical and biological characteristics of hydroxyapatite. Ceram. Int. 2021, 47, 5061–5070. [Google Scholar] [CrossRef]
- Manzoor, F.; Golbang, A.; Dixon, D.; Mancuso, E.; Azhar, U.; Manolakis, I.; Crawford, D.; McIlhagger, A.; Harkin-Jones, E. 3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants. Polymers 2022, 14, 1376. [Google Scholar] [CrossRef]
- Aryal, S.; Matsunaga, K.; Ching, W.-Y. Ab initio simulation of elastic and mechanical properties of Zn- and Mg-doped hydroxyapatite (HAP). J. Mech. Behav. Biomed. Mater. 2015, 47, 135–146. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.-Y.; Xiang, Y.-Y.; Qian, Y.-P.; Ren, J.-N.; Jia, R. How does fluoride enhance hydroxyapatite? A theoretical understanding. Appl. Surf. Sci. 2022, 586, 152753. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, characterization and in vitro evaluation of zinc and strontium binary doped hydroxyapatite for biomedical application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Matić, T.; Zebić, M.L.; Miletić, V.; Cvijović-Alagić, I.; Petrović, R.; Janaćković, D.; Veljović, D. Sr,Mg co-doping of calcium hydroxyapatite: Hydrothermal synthesis, processing, characterization and possible application as dentin substitutes. Ceram. Int. 2022, 48, 11155–11165. [Google Scholar] [CrossRef]
- Cao, J.; Lian, R.; Jiang, X. Magnesium and fluoride doped hydroxyapatite coatings grown by pulsed laser deposition for promoting titanium implant cytocompatibility. Appl. Surf. Sci. 2020, 515, 146069. [Google Scholar] [CrossRef]
- Sridevi, S.; Sutha, S.; Kavitha, L.; Gopi, D. Valorization of biowaste derived nanophase yttrium substituted hydroxyapatite/citrate cellulose/ opuntia mucilage biocomposite: A template assisted synthesis for potential biomedical applications. Mater. Chem. Phys. 2021, 273, 125144. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-Rad, H.R.; Alsakkaf, A.; Kamil, A.; Abdul Kadir, M.R.; Idris, M.H.; Raghav, H.B. Zinc-doped hydroxyapatite—Zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferrous Met. Soc. China 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Saranya, S.; Prema Rani, M. Sol gel synthesis of Niobium influence on Hydroxyapatite: A view of invitro, structural, morphological and studies for Biomedical Applications. Mater. Today Proc. 2021, 46, 1441–1450. [Google Scholar] [CrossRef]
- Sukhodub, L.; Kumeda, M.; Sukhodub, L.; Bielai, V.; Lyndin, M. Metal ions doping effect on the physicochemical, antimicrobial, and wound healing profiles of alginate-based composite. Carbohydr. Polym. 2023, 304, 120486. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Alturki, A.M. Physicochemical studies of iron/vanadate doped hydroxyapatite/polycaprolactone nanofibers scaffolds. J. Mol. Struct. 2022, 1260, 132835. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Prosanov, I.Y.; Vorobyev, A.M.; Gerasimov, K.B.; Borodulina, I.A.; Pryadko, A.; Botvin, V.V.; Surmeneva, M.A.; Surmenev, R.A. Mechanochemical synthesis of strontium- and magnesium-substituted and cosubstituted hydroxyapatite powders for a variety of biomedical applications. Ceram. Int. 2022, 48, 35217–35226. [Google Scholar] [CrossRef]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstić, A.; Jovanović, D.; Raičević, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowders for bone tissue engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Hssain, A.H.; Bulut, N.; Ates, T.; Koytepe, S.; Kuruçay, A.; Kebiroglu, H.; Kaygili, O. Experimental characterization and theoretical investigation of Zn/Sm co-doped hydroxyapatites. Mater. Today Commun. 2022, 31, 103850. [Google Scholar] [CrossRef]
- Kapoor, S.; Batra, U.; Kohli, S.; Kumar, R. Structural, thermal and in-vitro analysis of sol-gel derived zinc and fluorine co-substituted nanodimensional hydroxyapatite for biomedical applications. Mater. Today Proc. 2022, 57, 761–767. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Huri, P.Y.; Schmidt, J.; Pana, I.; Dinu, M.; Vitelaru, C.; Kiss, A.E.; Tamay, D.G.; Hasirci, V.; Vladescu, A.; et al. In vitro cytotoxicity, corrosion and antibacterial efficiencies of Zn doped hydroxyapatite coated Ti based implant materials. Ceram. Int. 2023, 49, 12570–12584. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Candidato, R.T.; Sergi, R.; Jouin, J.; Noguera, O.; Pawłowski, L. Advanced microstructural study of solution precursor plasma sprayed Zn doped hydroxyapatite coatings. J. Eur. Ceram. Soc. 2018, 38, 2134–2144. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wang, T.; Yang, G.; Xi, W.; Gan, Y.; Lu, W.; Hu, J. Enhanced corrosion resistance and bioactivity of Mg alloy modified by Zn-doped nanowhisker hydroxyapatite coatings. Colloids Surf. B Biointerfaces 2020, 186, 110710. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Kim, H.; Mondal, S.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Oh, J. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram. Int. 2018, 44, 6062–6071. [Google Scholar] [CrossRef]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu co-substituted hydroxyapatite—Biocompatibility, mechanical properties and antimicrobial activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Pana, I.; Parau, A.C.; Cotrut, C.M.; Dinu, M.; Vranceanu, D.M.; Kiss, A.E.; Serratore, G.; Böhner, D.A.; Vitelaru, C.; Ambrogio, G.; et al. Influence of deposition temperature on the structure and functional properties of Mg doped hydroxyapatite coatings deposited on manufactured AZ31B alloy substrates by RF magnetron sputtering. Ceram. Int. 2023, 49, 22340–22354. [Google Scholar] [CrossRef]
- Monte, J.P.; Fontes, A.; Pereira, G.A.L.; Pereira, G.; Santos, B.S. Preparation and characterization of Mg(II) doped hydroxyapatite biocomposites. Results Chem. 2022, 4, 100625. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Y.; Zheng, Y.; Li, Q.; Lei, T.; Yin, P. Evaluation of the antibacterial properties and in-vitro cell compatibilities of doped copper oxide/hydroxyapatite composites. Colloids Surf. B Biointerfaces 2022, 209, 112194. [Google Scholar] [CrossRef]
- Daood, U.; Fawzy, A. Development of a bioactive dentin adhesive resin modified with magnesium-doped synthetic hydroxyapatite crystals. J. Mech. Behav. Biomed. Mater. 2023, 140, 105737. [Google Scholar] [CrossRef]
- Jenifer, A.; Senthilarasan, K.; Arumugam, S.; Sivaprakash, P.; Sagadevan, S.; Sakthivel, P. Investigation on antibacterial and hemolytic properties of magnesium-doped hydroxyapatite nanocomposite. Chem. Phys. Lett. 2021, 771, 138539. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, S.; Yang, Z.; Yang, P.; Liu, A. High-strength and tough bioactive Mg-doped hydroxyapatite bioceramics with oriented microchannels. Ceram. Int. 2022, 48, 13494–13507. [Google Scholar] [CrossRef]
- Ling, L.; Cai, S.; Zuo, Y.; Tian, M.; Meng, T.; Tian, H.; Bao, X.; Xu, G. Copper-doped zeolitic imidazolate frameworks-8/hydroxyapatite composite coating endows magnesium alloy with excellent corrosion resistance, antibacterial ability and biocompatibility. Colloids Surf. B Biointerfaces 2022, 219, 112810. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Ercan, I.; Kaygili, O.; Kayed, T.; Bulut, N.; Tombuloğlu, H.; İnce, T.; Al Ahmari, F.; Kebiroglu, H.; Ates, T.; Almofleh, A.; et al. Structural, spectroscopic, dielectric, and magnetic properties of Fe/Cu co-doped hydroxyapatites prepared by a wet-chemical method. Phys. B Condens. Matter 2022, 625, 413486. [Google Scholar] [CrossRef]
- Unabia, R.B.; Bonebeau, S.; Candidato, R.T.; Jouin, J.; Noguera, O.; Pawłowski, L. Investigation on the structural and microstructural properties of copper-doped hydroxyapatite coatings deposited using solution precursor plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 4255–4263. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Bulina, N.V.; Eremina, N.V.; Vinokurova, O.B.; Ishchenko, A.V.; Chaikina, M.V. Diffusion of Copper Ions in the Lattice of Substituted Hydroxyapatite during Heat Treatment. Materials 2022, 15, 5759. [Google Scholar] [CrossRef]
- Alasvand, N.; Simorgh, S.; Malekzadeh Kebria, M.; Bozorgi, A.; Moradi, S.; Hosseinpour Sarmadi, V.; Ebrahimzadeh, K.; Amini, N.; Kermani, F.; Kargozar, S.; et al. Copper / cobalt doped strontium-bioactive glasses for bone tissue engineering applications. Open Ceram. 2023, 14, 100358. [Google Scholar] [CrossRef]
- Park, S.; Choi, J.; Mondal, S.; Vo, T.M.T.; Pham, V.H.; Lee, H.; Nam, S.Y.; Kim, C.-S.; Oh, J. The impact of Cu(II) ions doping in nanostructured hydroxyapatite powder: A finite element modelling study for physico-mechanical and biological property evaluation. Adv. Powder Technol. 2022, 33, 103405. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Bazin, T.; Cardinal, T.; Chung, U.C.; Catros, S.; Bertrand, C.; Gaudon, M.; Vignoles, G. Powder bed laser sintering of copper-doped hydroxyapatite: Numerical and experimental parametric analysis. Addit. Manuf. 2021, 46, 102044. [Google Scholar] [CrossRef]
- Sasireka, A.; Renji, R.; Mohan Raj, R.; Vignesh, S.; Raj, V.; Ashraf, I.M.; Shkir, M. Exploration on in vitro bioactivity, antibacterial activity and corrosion behavior of Strontium doped Hydroxyapatite reinforced chitosan-polypyrrole/TNT for bone regeneration. Inorg. Chem. Commun. 2022, 142, 109621. [Google Scholar] [CrossRef]
- Szyszka, K.; Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Ledwa, K.A.; Piecuch, A.; Giersig, M.; Drulis-Kawa, Z.; Wiglusz, R.J. Structural modification of nanohydroxyapatite Ca10(PO4)6(OH)2 related to Eu3+ and Sr2+ ions doping and its spectroscopic and antimicrobial properties. J. Inorg. Biochem. 2020, 203, 110884. [Google Scholar] [CrossRef]
- Baldassarre, F.; Altomare, A.; Mesto, E.; Lacalamita, M.; Dida, B.; Mele, A.; Bauer, E.M.; Puzone, M.; Tempesta, E.; Capelli, D.; et al. Structural Characterization of Low-Sr-Doped Hydroxyapatite Obtained by Solid-State Synthesis. Crystals 2023, 13, 117. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, P.; Wang, Y. Construction and properties of an osteogenic-antibacterial functionalised drug delivery system based on hydroxyapatite microspheres. Inorg. Chem. Commun. 2022, 140, 109419. [Google Scholar] [CrossRef]
- Wang, T.; Yang, G.; Zhou, W.; Hu, J.; Jia, W.; Lu, W. One-pot hydrothermal synthesis, in vitro biodegradation and biocompatibility of Sr-doped nanorod/nanowire hydroxyapatite coatings on ZK60 magnesium alloy. J. Alloys Compd. 2019, 799, 71–82. [Google Scholar] [CrossRef]
- Xu, W.-L.; Ci, L.-J.; Qi, M.-L.; Xiao, G.-Y.; Chen, X.; Xu, W.-H.; Lu, Y.-P. Sr2+-dependent microstructure regulation of biodegradable Sr-doped hydroxyapatite microspheres with interconnected porosity for sustained drug delivery. Ceram. Int. 2023, 49, 17148–17157. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Krokhicheva, P.A.; Goldberg, M.A.; Fomin, A.S.; Khayrutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Konovalov, A.A.; Leonov, A.V.; Mikheev, I.V.; Merzlyak, E.M.; et al. Enhanced bone repair by silver-doped magnesium calcium phosphate bone cements. Ceram. Int. 2023, 49, 19249–19264. [Google Scholar] [CrossRef]
- Phatai, P.; Prachumrak, N.; Kamonwannasit, S.; Kamcharoen, A.; Roschat, W.; Phewphong, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Youngjan, S.; et al. Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity. Sustainability 2022, 14, 11756. [Google Scholar] [CrossRef]
- Ressler, A.; Ivanković, T.; Polak, B.; Ivanišević, I.; Kovačić, M.; Urlić, I.; Hussainova, I.; Ivanković, H. A multifunctional strontium/silver-co-substituted hydroxyapatite derived from biogenic source as antibacterial biomaterial. Ceram. Int. 2022, 48, 18361–18373. [Google Scholar] [CrossRef]
- Nenen, A.; Maureira, M.; Neira, M.; Orellana, S.L.; Covarrubias, C.; Moreno-Villoslada, I. Synthesis of antibacterial silver and zinc doped nano-hydroxyapatite with potential in bone tissue engineering applications. Ceram. Int. 2022, 48, 34750–34759. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Chand Reger, N.; Das, H.; Balla, V.K.; Devi, K.B.; Roy, M.; Nandi, S.K.; Kundu, B. In vivo osteogenesis of plasma sprayed ternary-ion doped hydroxyapatite coatings on Ti6Al4V for orthopaedic applications. Ceram. Int. 2022, 48, 11475–11488. [Google Scholar] [CrossRef]
- Monte, J.P.; Fontes, A.; Santos, B.S.; Pereira, G.A.L.; Pereira, G. Recent advances in hydroxyapatite/polymer/silver nanoparticles scaffolds with antimicrobial activity for bone regeneration. Mater. Lett. 2023, 338, 134027. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ghosh, M.; Maji, M.; Ghosh, M.; Pradhan, S.K.; Meikap, A.K. Study of microstructural and electrical properties of silver substituted hydroxyapatite for drug delivery applications. Mater. Today Commun. 2022, 31, 103360. [Google Scholar] [CrossRef]
- Citradewi, P.W.; Hidayat, H.; Purwiandono, G.; Fatimah, I.; Sagadevan, S. Clitorea ternatea-mediated silver nanoparticle-doped hydroxyapatite derived from cockle shell as antibacterial material. Chem. Phys. Lett. 2021, 769, 138412. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124081. [Google Scholar] [CrossRef]
- Bhatt, A.; Sakai, K.; Madhyastha, R.; Murayama, M.; Madhyastha, H.; Rath, S.N. Biosynthesis and characterization of nano magnetic hydroxyapatite (nMHAp): An accelerated approach using simulated body fluid for biomedical applications. Ceram. Int. 2020, 46, 27866–27876. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K.J.; Yadav, A.K.; Kaur, S.; Kaur, R.; Kaur, S. Growth of bone like hydroxyapatite and cell viability studies on CeO2 doped CaO–P2O5–MgO–SiO2 bioceramics. Mater. Chem. Phys. 2020, 243, 122352. [Google Scholar] [CrossRef]
- Sousa, R.B.; Dametto, A.C.; Sábio, R.M.; de Carvalho, R.A.; Vieira, E.G.; Oliveira, A.F.d.A.; Ribeiro, L.K.; Barud, H.S.; Silva-Filho, E.C. Cerium-doped calcium phosphates precipitated on bacterial cellulose platform by mineralization. Ceram. Int. 2020, 46, 26985–26990. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Nellaiappan, S.N.T.S.; Lakshmipathy, M.; Sagadevan, S.; Johan, M.R. Facile fabrication of phase transformed cerium (IV) doped hydroxyapatite for biomedical applications—A health care approach. Ceram. Int. 2020, 46, 2510–2522. [Google Scholar] [CrossRef]
- Panda, S.; Behera, B.P.; Bhutia, S.K.; Biswas, C.K.; Paul, S. Rare transition metal doped hydroxyapatite coating prepared via microwave irradiation improved corrosion resistance, biocompatibility and anti-biofilm property of titanium alloy. J. Alloys Compd. 2022, 918, 165662. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Lima, T.A.R.M.; Brito, N.S.; Peixoto, J.A.; Valerio, M.E.G. The incorporation of chromium (III) into hydroxyapatite crystals. Mater. Lett. 2015, 140, 187–191. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, M.; ul Hassan, M.; Ryu, H.J.; Anjum, M.A.R.; Farhan, M.A.; Nadeem, M.; Yun, J.-I. Electronic, electrical and dielectric analysis of Cr-doped hydroxyapatite. Chem. Phys. Lett. 2021, 771, 138507. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Roy, B.; Agarwal, T.; Giri, S.; Pramanik, K.; Pal, K.; Ray, S.S.; Maiti, T.K.; Banerjee, I. Cobalt doped proangiogenic hydroxyapatite for bone tissue engineering application. Mater. Sci. Eng. C 2016, 58, 648–658. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Yahia, I.S. Novel and facile microwave-assisted synthesis of Mo-doped hydroxyapatite nanorods: Characterization, gamma absorption coefficient, and bioactivity. Mater. Sci. Eng. C 2017, 78, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of solid-phase mechanochemical synthesis of hydroxyapatite doped by copper and zinc ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Tautkus, S.; Ishikawa, K.; Ramanauskas, R.; Kareiva, A. Zinc and chromium co-doped calcium hydroxyapatite: Sol-gel synthesis, characterization, behaviour in simulated body fluid and phase transformations. J. Solid State Chem. 2020, 284, 121202. [Google Scholar] [CrossRef]
- Mathew, R.; Hegde, S.; Mathew, S.; Shruthi, N.; Geevarghese, S. Antimicrobial activity of a remineralizing paste containing Strontium doped Nano hydroxyapatite (Sr-nHAp) with Non Collagenous Protein (NCP) analogue Chitosan—An in vitro study. Mater. Today Proc. 2021, 46, 5975–5979. [Google Scholar] [CrossRef]
- Sauro, S.; Spagnuolo, G.; Del Giudice, C.; Neto, D.M.A.; Fechine, P.B.A.; Chen, X.; Rengo, S.; Chen, X.; Feitosa, V.P. Chemical, structural and cytotoxicity characterisation of experimental fluoride-doped calcium phosphates as promising remineralising materials for dental applications. Dent. Mater. 2023, 39, 391–401. [Google Scholar] [CrossRef]
- Teerakanok, S.; Zhao, M.; Giordano, R.; Fan, Y. Interaction of doped magnesium, zinc and fluoride ions on hydroxyapatite crystals grown on etched human enamel. J. Cryst. Growth 2021, 571, 126262. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Kadhim, M.M.; AlMashhadani, H.A.; Hashim, R.D.; Khadom, A.A.; Salih, K.A.; Salman, A.W. Effect of Sr/Mg co-substitution on corrosion resistance properties of hydroxyapatite coated on Ti–6Al–4V dental alloys. J. Phys. Chem. Solids 2022, 161, 110450. [Google Scholar] [CrossRef]
- Farkas, N.-I.; Turdean, G.L.; Bizo, L.; Marincaș, L.; Cadar, O.; Barbu-Tudoran, L.; Réka, B. The effect of chemical composition and morphology on the drug delivery properties of hydroxyapatite-based biomaterials. Ceram. Int. 2023, 49, 25156–25169. [Google Scholar] [CrossRef]
- Singh, G.; Jolly, S.S.; Singh, R.P. Cerium substituted hydroxyapatite mesoporous nanorods: Synthesis and characterization for drug delivery applications. Mater. Today Proc. 2020, 28, 1460–1466. [Google Scholar] [CrossRef]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Domingos, M.; Zhang, X. Synthesis and Characterization of Sintered Sr/Fe-Modified Hydroxyapatite Bioceramics for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 375–388. [Google Scholar] [CrossRef] [PubMed]
- De Lama-Odría, M.D.; del Valle, L.J.; Puiggalí, J. Hydroxyapatite Biobased Materials for Treatment and Diagnosis of Cancer. Int. J. Mol. Sci. 2022, 23, 1352. [Google Scholar] [CrossRef]
- Febrian, M.B.; Mahendra, I.; Kurniawan, A.; Setiadi, Y.; Ambar Wibawa, T.H.; Lesmana, R.; Syarif, D.G. Zirconium doped hydroxyapatite nanoparticle as a potential design for lung cancer therapy. Ceram. Int. 2021, 47, 27890–27897. [Google Scholar] [CrossRef]
- Jose, S.; Joy, A.; Devi, P.; Unnikrishnan, G.; Megha, M.; Haris, M.; Elayaraja, K.; Senthilkumar, M. Synthesis of luminescent Mg-incorporated hydroxyapatite by reflux condensation method: Photoluminescence, in-vitro drug release and kinetic studies. Mater. Today Proc. 2022, 58, 836–845. [Google Scholar] [CrossRef]
- Zheng, D.; Pu, Z.; Fan, H.; Zhu, P.; Gao, C.; Lu, Q. Facile synthesis of mesoporous strontium doped hydroxyapatite microspheres for drug-delivery applications. Mater. Lett. 2022, 311, 131553. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Y.; Fang, Z.; Pu, F.; Sun, R.; Chen, K.; Tang, Y. Preparation and characterization of Sr-substituted hydroxyapatite/reduced graphene oxide 3D scaffold as drug carrier for alendronate sodium delivery. Ceram. Int. 2022, 48, 36601–36608. [Google Scholar] [CrossRef]
- Ai, F.; Chen, L.; Yan, J.; Yang, K.; Li, S.; Duan, H.; Cao, C.; Li, W.; Zhou, K. Hydroxyapatite scaffolds containing copper for bone tissue engineering. J. Sol-Gel Sci. Technol. 2020, 95, 168–179. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Biswas, P.; Pal, K.; Thakur, P.; Das, K.; Karmakar, P.; et al. Antimicrobial and biocompatible fluorescent hydroxyapatite-chitosan nanocomposite films for biomedical applications. Colloids Surf. B Biointerfaces 2018, 171, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, Z.; Yang, Y.; Jian, Y.; Li, R.; Dai, X.; Wu, W.; Zhong, J.; Chen, C. Synthesis, characterization and biological performance study of Sr-doped hydroxyapatite/chitosan composite coatings. Mater. Chem. Phys. 2021, 270, 124752. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Al-Wafi, R.; Mansour, S.F.; El-dek, S.I.; Uskoković, V. Physical and biological changes associated with the doping of carbonated hydroxyapatite/polycaprolactone core-shell nanofibers dually, with rubidium and selenite. J. Mater. Res. Technol. 2020, 9, 3710–3723. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]

| Synthesis Method | Morphology | Size | Characteristics | References |
|---|---|---|---|---|
| Sol–gel Method | Spherical, rods, needle, tube | 5–1000 nm | Capacity to synthesize homogeneous materials, mechanical properties enhanced due to nanocrystalline grain structures and high purity. | [52,53,54,55] |
| Hydrothermal | Rod, needle, spherical, rollers, wire, spherulites, leaves, belts | 4–140 nm | Low costs, good homogenicity and solubility of the precursors, tailorable nucleation, and growth of the crystal. | [56,57,58] |
| Chemical Precipitation | Needle, whisker, rods, spherical, platelets | 5–130 nm | Low costs, controlled environment, and enhanced porosity. | [39,59,60] |
| Biosources | Irregular, sphere, flakes, needles, rods | 6–180 nm | Capacity to retain trace element, increase bioactivity, and be eco-friendly. | [61,62] |
| Microemulsion | Spherical, rods, irregular, needle, flakes | 5–100 nm | Good anti-aggregation property, outstanding stability, and self-controlled development of apertures. | [63,64,65] |
| Combustion | Needle, rods, irregular, spherical | 40–200 nm | Simplicity, high purity of the obtained products, low energy consumption, and high purity of synthesized material. | [56,66,67] |
| Doping Ion | Biologic Activity | |
|---|---|---|
| Cation | Na+ | Enhanced osteoconductivity and improved cell proliferation |
| K+ | Enhanced thermal stability | |
| Mg2+ | Improved crystal growth, crystallization, thermal stability, and dissolution rate | |
| Sr2+ | Enhanced bone regeneration and formation, and inhibited bone resorption | |
| Mn2+ | Enhanced cell adhesion | |
| Zn2+ | Enhanced bone formation and regeneration, increased antimicrobial activity | |
| Anion | ||
| CO32− | High specific surface area, reduced crystallite size, enhanced osteoconductive properties, increased solubility | |
| SiO44− | Enhanced bioactivity, improved dissolution rate | |
| F− | Improved stability, reduced solubility, and increased remineralization | |
| Cl− | Enhanced osteoconductive properties, and increased solubility | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, D.-E.; Vasile, O.R.; Andronescu, E.; Ficai, A. Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 13157. https://doi.org/10.3390/ijms241713157
Radulescu D-E, Vasile OR, Andronescu E, Ficai A. Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(17):13157. https://doi.org/10.3390/ijms241713157
Chicago/Turabian StyleRadulescu, Diana-Elena, Otilia Ruxandra Vasile, Ecaterina Andronescu, and Anton Ficai. 2023. "Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 17: 13157. https://doi.org/10.3390/ijms241713157
APA StyleRadulescu, D.-E., Vasile, O. R., Andronescu, E., & Ficai, A. (2023). Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering. International Journal of Molecular Sciences, 24(17), 13157. https://doi.org/10.3390/ijms241713157









