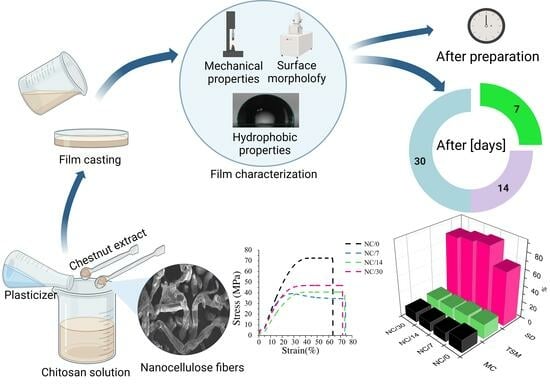
Effect of Time on the Properties of Bio-Nanocomposite Films Based on Chitosan with Bio-Based Plasticizer Reinforced with Nanofiber Cellulose
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mechanical Properties
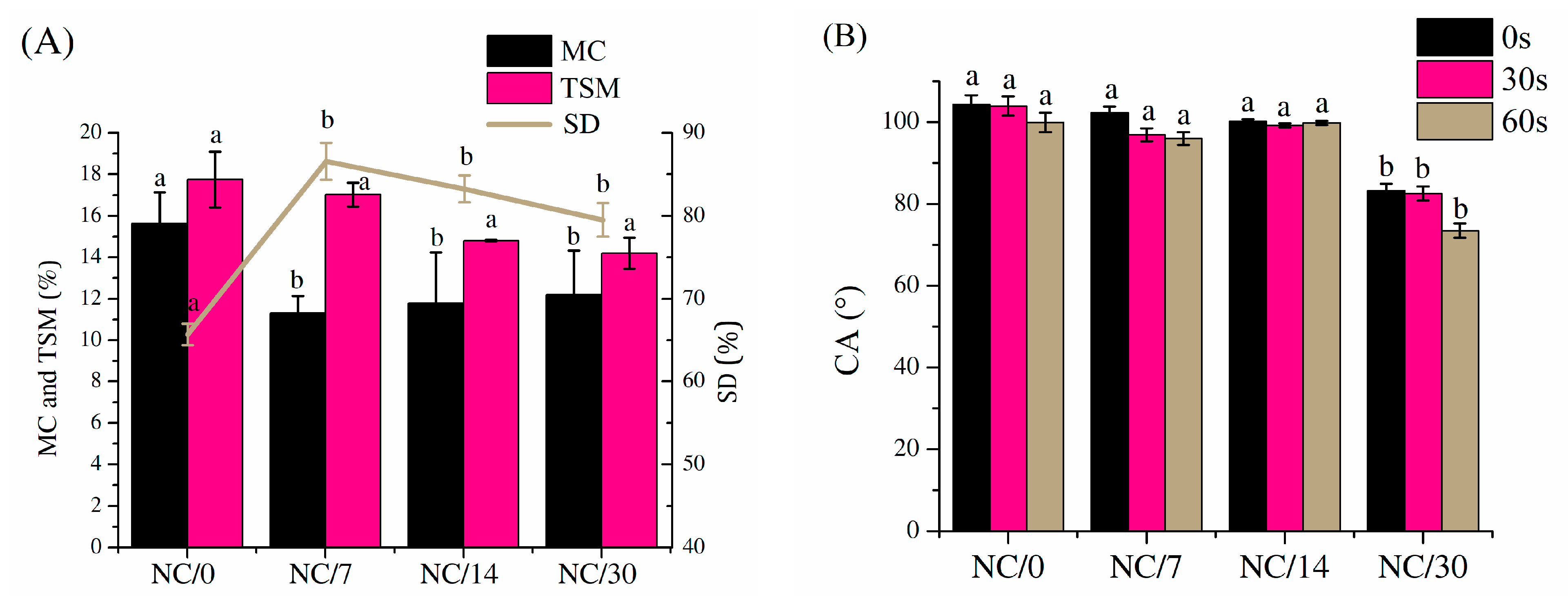
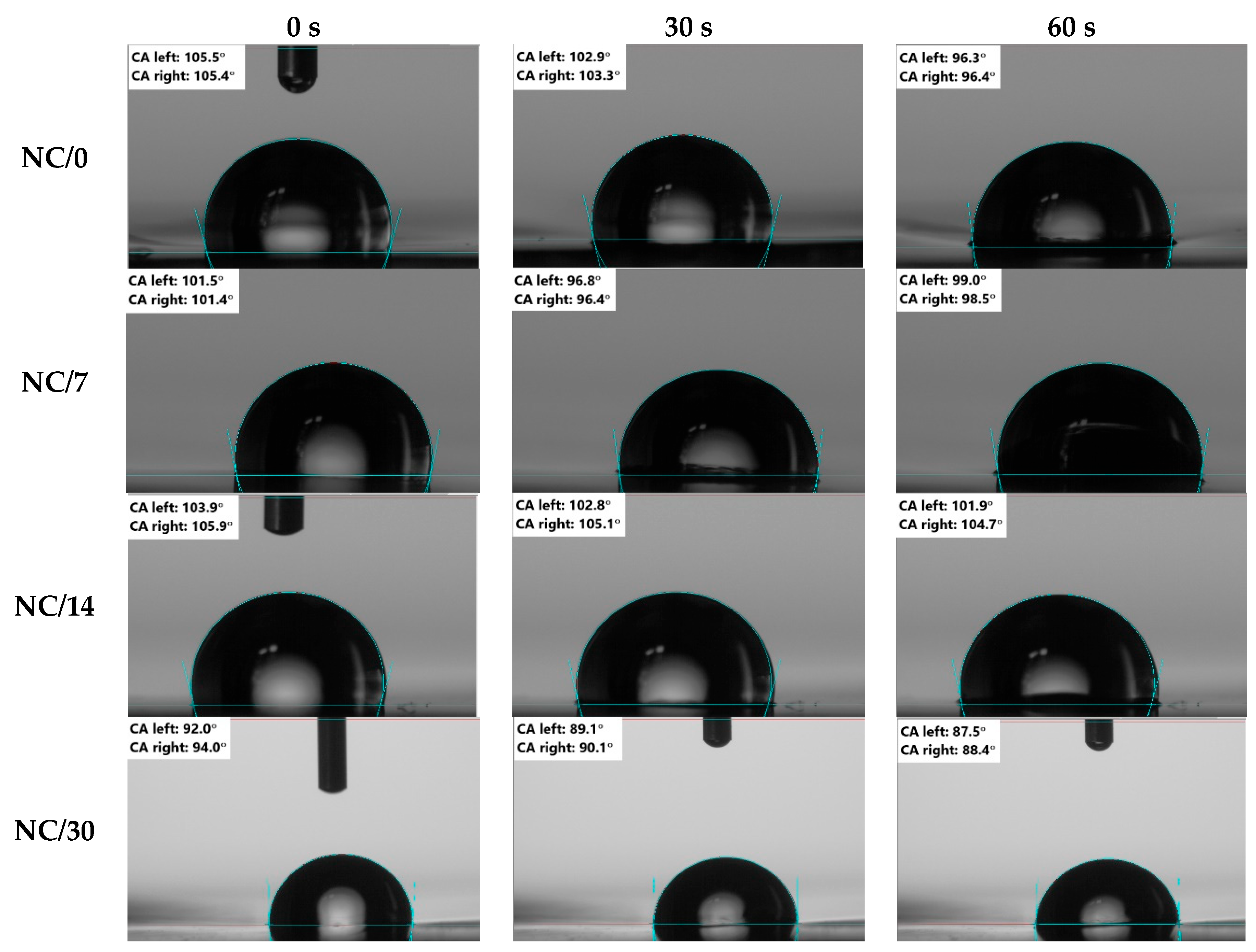
2.2. Hydrophobic Properties
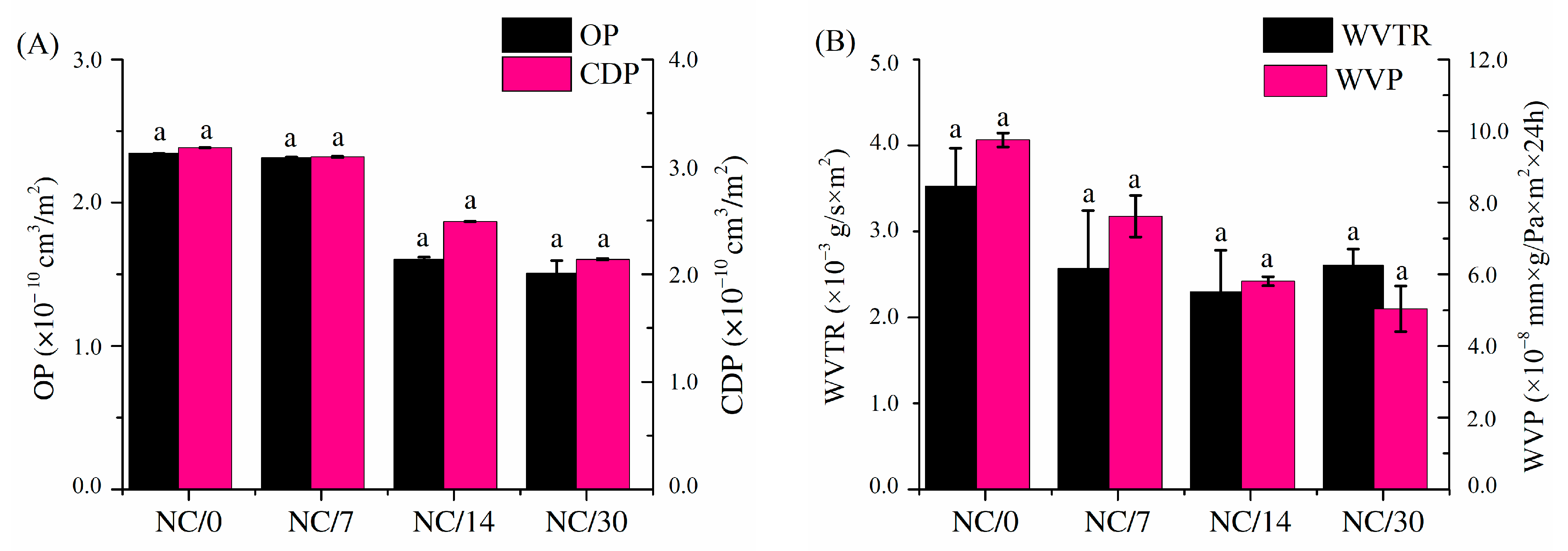
2.3. Gas Permeability
2.4. Morphology
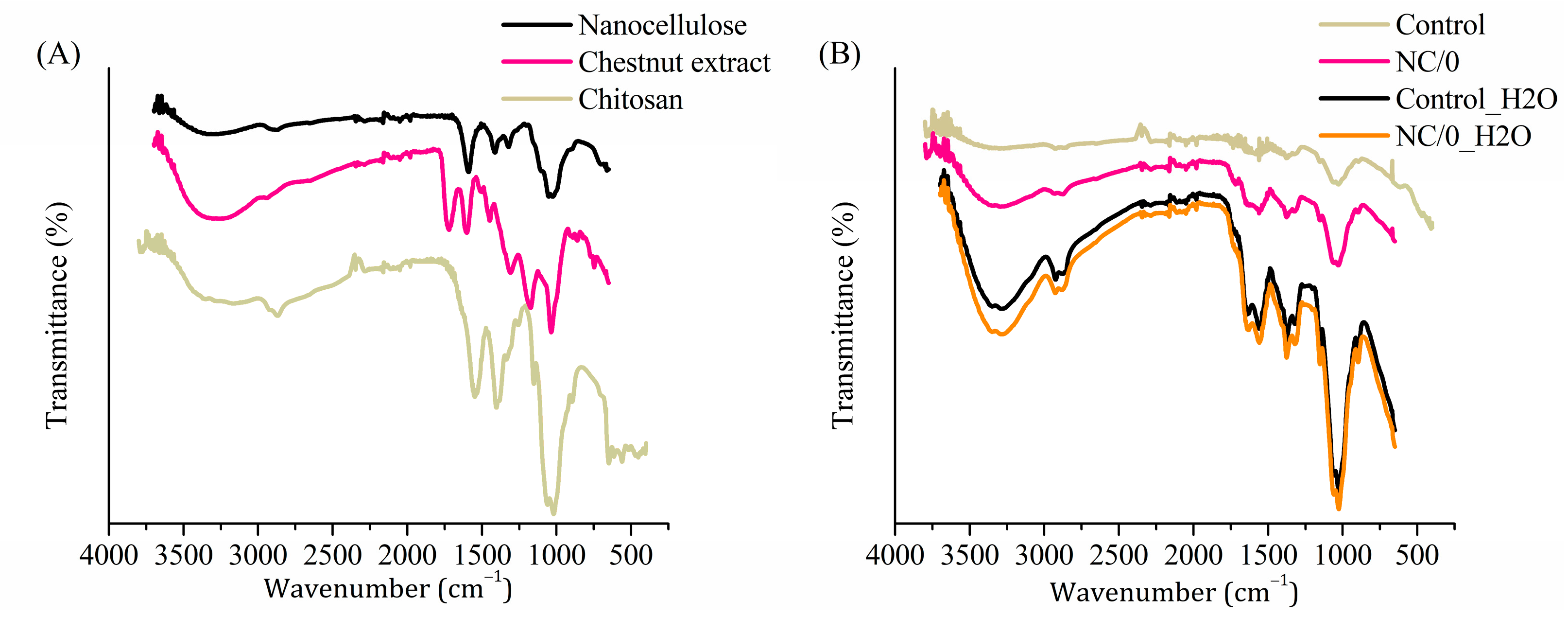
2.5. Fourier Transform Infrared Spectroscopy
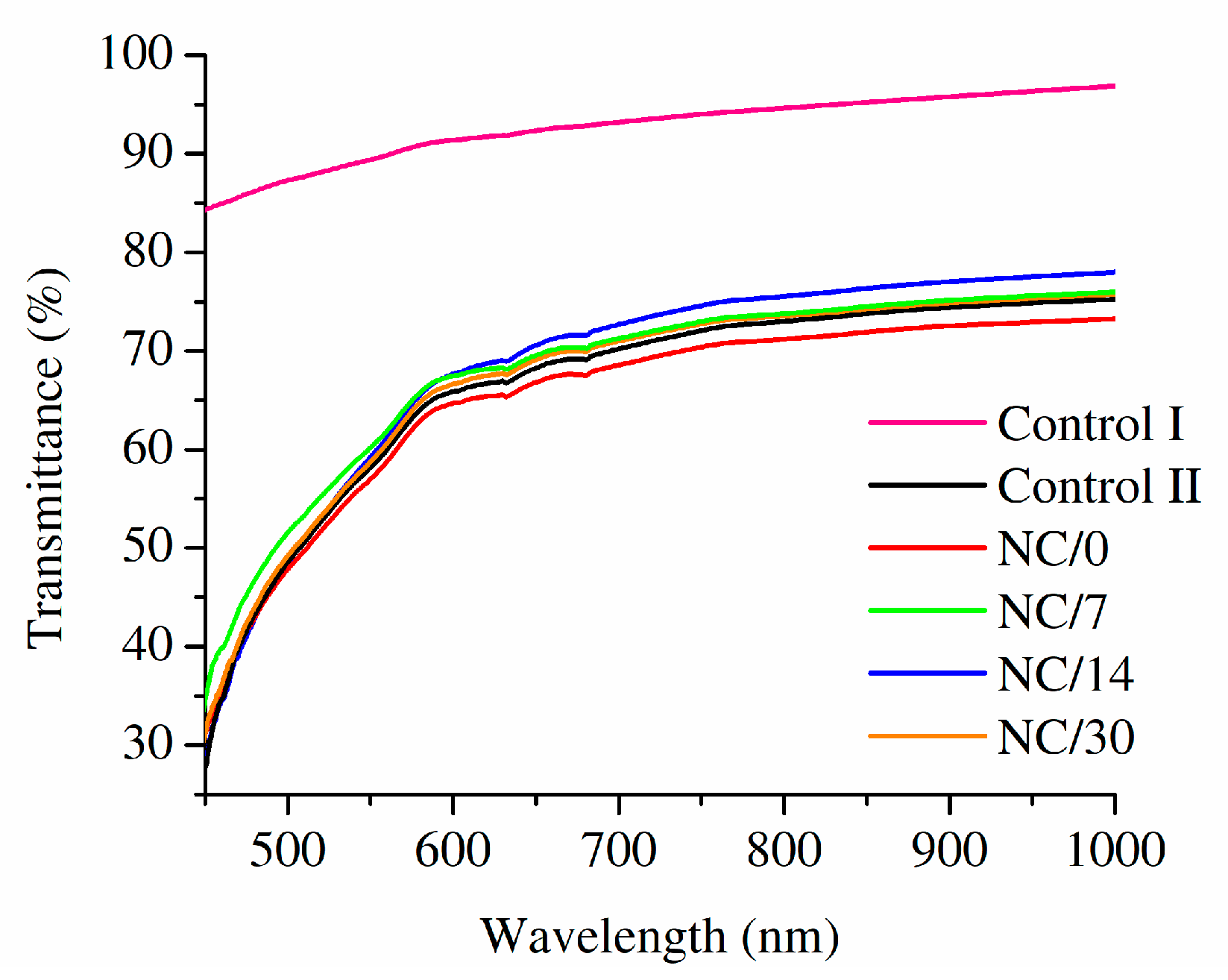
2.6. Optical Properties
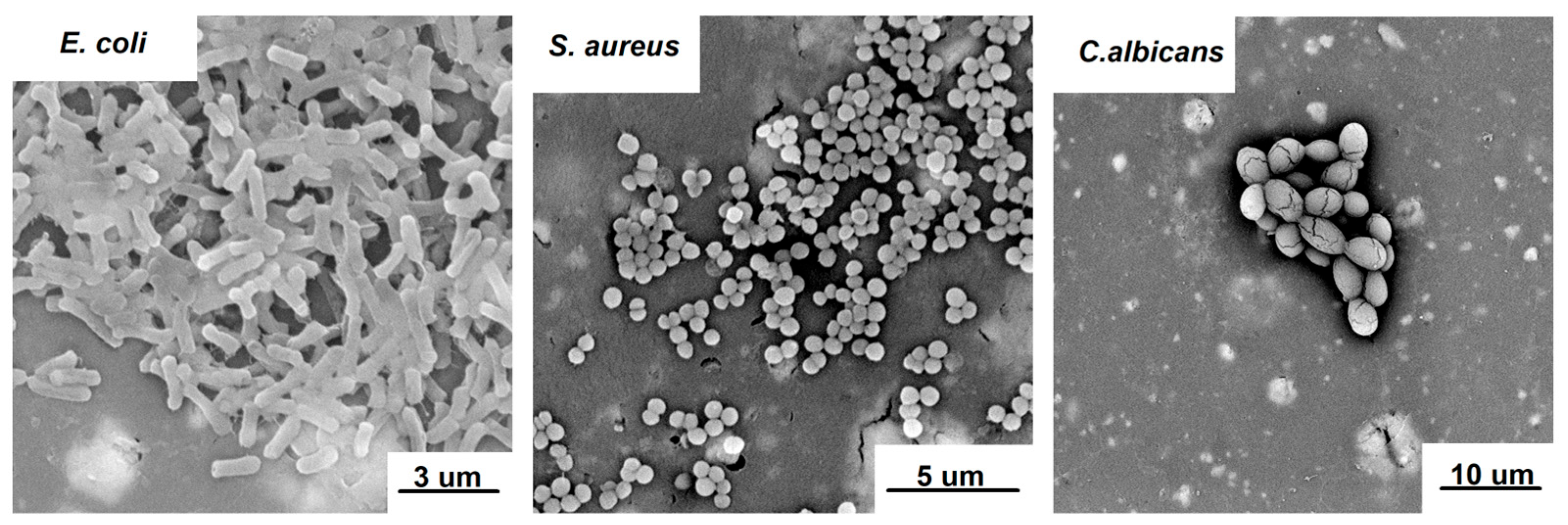
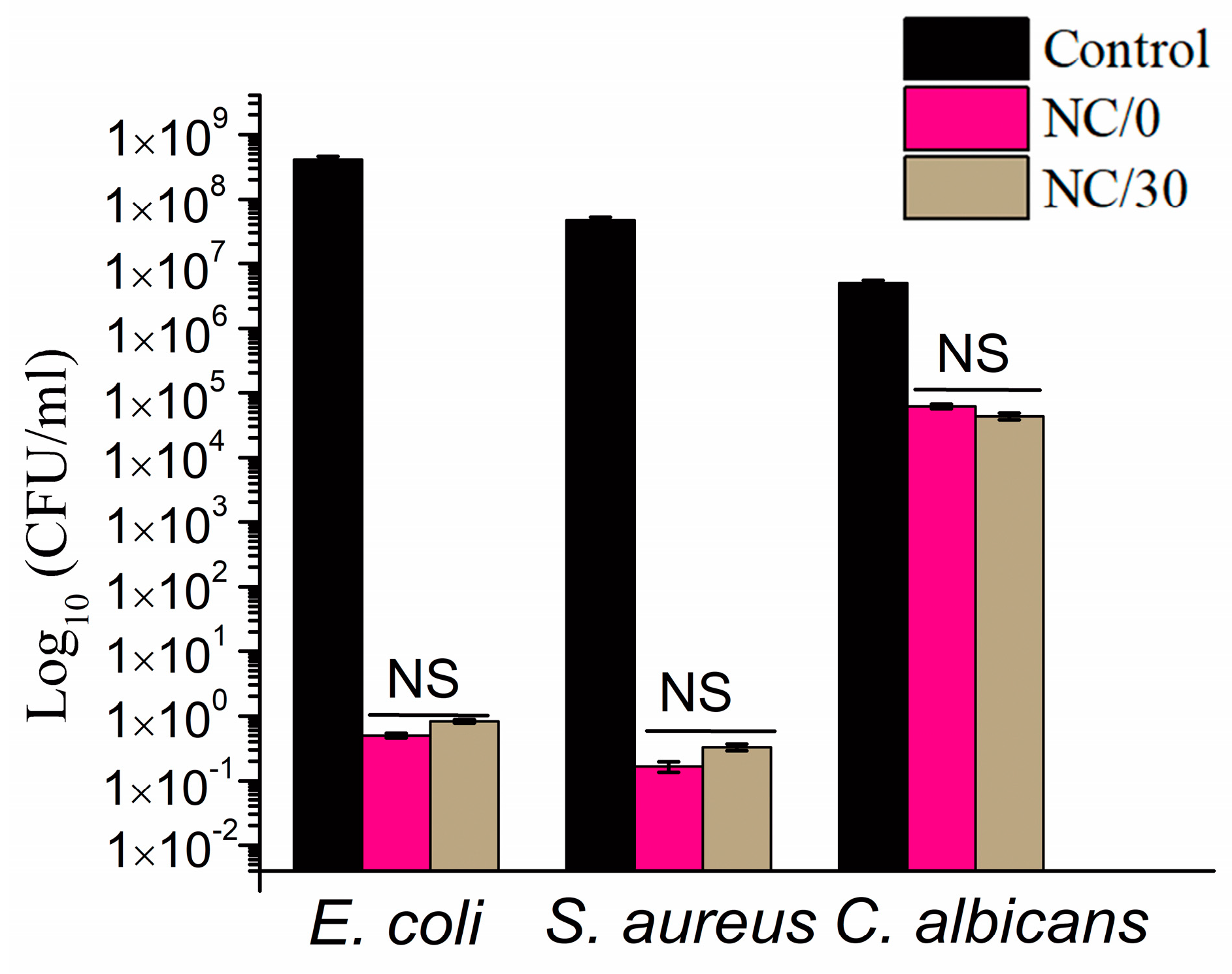
2.7. Antimicrobial Activity
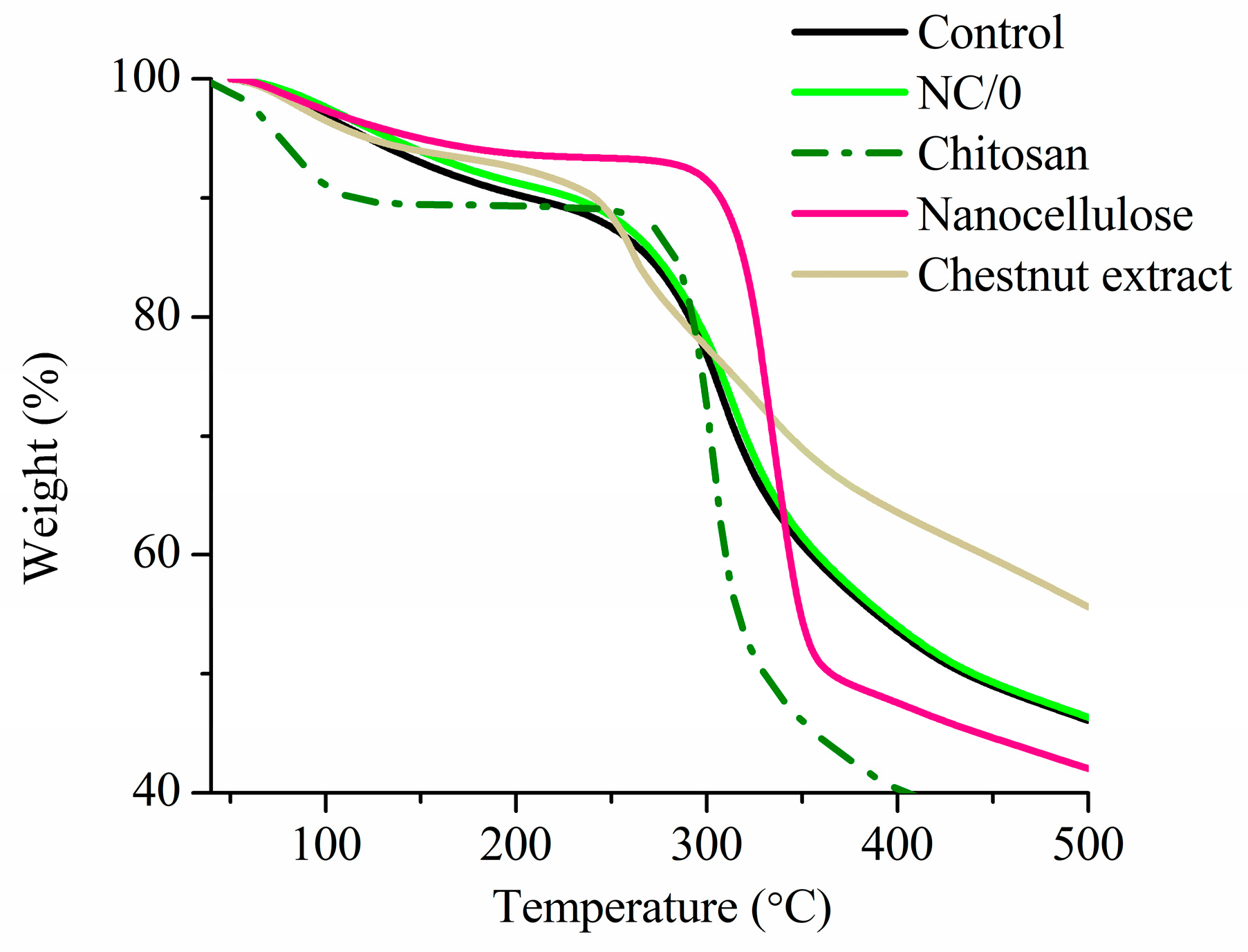
2.8. Thermal Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Bio-Based Plasticizer
3.3. Preparation of the Bio-Nanocomposite Films
3.4. Mechanical Properties
3.5. Hydrophilic Properties
3.6. Gas Permeability
3.7. Morphology
3.8. Fourier Transform Infrared Spectroscopy
3.9. Transparency
3.10. Antimicrobial Activity
3.11. Thermal Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Lavrič, G.; Oberlintner, A.; Filipova, I.; Novak, U.; Likozar, B.; Vrabič-Brodnjak, U. Functional Nanocellulose, Alginate and Chitosan Nanocomposites Designed as Active Film Packaging Materials. Polymers 2021, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Willis, S.; Jordan, K.; Sismour, E. Chitosan Nanocomposite Films Incorporating Cellulose Nanocrystals and Grape Pomace Extracts. Packag. Technol. Sci. 2018, 31, 631–638. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Membrane Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose Reinforced Chitosan Composite Films as Affected by Nanofiller Loading and Plasticizer Content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.-W. Antimicrobial Nanofillers Reinforced Biopolymer Composite Films for Active Food Packaging Applications—A Review. Sustain. Mater. Technol. 2022, 32, e00353. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-Aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing Mechanical, Barrier and Antimicrobial Properties of Nanocellulose/CMC and Nanochitosan/CMC Composite Films. Int. J. Biol. Macromol. 2020, 164, 126187. [Google Scholar] [CrossRef]
- Isogai, A. Cellulose Nanofibers: Recent Progress and Future Prospects. J. Fiber Sci. Technol. 2020, 76, 310–326. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in Food Packaging: A Review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of Flax (Linum Usitatissimum) Cellulose Nanocrystals as Reinforcing Material for Chitosan Films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef]
- Fahma, F.; Febiyanti, I.; Lisdayana, N.; Arnata, I.; Sartika, D. Nanocellulose as a New Sustainable Material for Various Applications: A Review. Arch. Mater. Sci. Eng. 2021, 2, 49–64. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and Antimicrobial Properties of Chitosan–Nanocellulose Films for Extending Shelf Life of Ground Meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Janik, W.; Ledniowska, K.; Nowotarski, M.; Kudła, S.; Knapczyk-Korczak, J.; Stachewicz, U.; Nowakowska-Bogdan, E.; Sabura, E.; Nosal-Kovalenko, H.; Turczyn, R.; et al. Chitosan-Based Films with Alternative Eco-Friendly Plasticizers: Preparation, Physicochemical Properties and Stability. Carbohydr. Polym. 2022, 301, 120277. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Ledniowska, K.; Shyntum, D.Y.; Krukiewicz, K.; Turczyn, R.; Sabura, E.; Furgoł, S.; Kudła, S.; Dudek, G. Modulation of Physicochemical Properties and Antimicrobial Activity of Sodium Alginate Films through the Use of Chestnut Extract and Plasticizers. Sci. Rep. 2023, 13, 11530. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging Chitosan-Essential Oil Films and Coatings for Food Preservation—A Review of Advances and Applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 Nanoparticle/Cymbopogon Citratus Essential Oil Film as Food Packaging Material: Physico-Mechanical Properties and Its Effects on Microbial, Chemical, and Organoleptic Quality of Minced Meat during Refrigeration. J. Food Process. Preserv. 2020, 44, e14536. [Google Scholar] [CrossRef]
- Šupová, M.; Simha Martynková, G.; Cech Barabaszova, K. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2010, 3, 1–25. [Google Scholar] [CrossRef]
- Bajić, M.; Oberlintner, A.; Kõrge, K.; Likozar, B.; Novak, U. Formulation of Active Food Packaging by Design: Linking Composition of the Film-Forming Solution to Properties of the Chitosan-Based Film by Response Surface Methodology (RSM) Modelling. Int. J. Biol. Macromol. 2020, 160, 971–978. [Google Scholar] [CrossRef]
- Kõrge, K.; Šeme, H.; Bajić, M.; Likozar, B.; Novak, U. Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods 2020, 9, 1645. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and Properties of Rice Starch–Chitosan Blend Biodegradable Film. LWT—Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Water Vapor Absorption and Permeability of Films Based on Chitosan and Sodium Caseinate. J. Appl. Polym. Sci. 2009, 111, 2777–2784. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Mechanical and Barrier Properties of Chitosan Combined with Other Components as Food Packaging Film. Environ. Chem. Lett. 2020, 18, 257–267. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Shyntum, D.Y.; Banaś, A.; Krukiewicz, K.; Kudła, S.; Dudek, G. Antibacterial and Biodegradable Polysaccharide-Based Films for Food Packaging Applications: Comparative Study. Materials 2022, 15, 3236. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional Properties of Chitosan-Based Films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and Barrier Properties of Edible Chitosan Films as Affected by Composition and Storage. J. Food Sci. 1996, 61, 953–956. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Galić, K.; Debeaufort, F. Effect of Relative Humidity on Carvacrol Release and Permeation Properties of Chitosan Based Films and Coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef]
- Masclaux, C.; Gouanvé, F.; Espuche, E. Experimental and Modelling Studies of Transport in Starch Nanocomposite Films as Affected by Relative Humidity. J. Membr. Sci. 2010, 363, 221–231. [Google Scholar] [CrossRef]
- Stading, M.; Rindlav-Westling, Å.; Gatenholm, P. Humidity-Induced Structural Transitions in Amylose and Amylopectin Films. Carbohydr. Polym. 2001, 45, 209–217. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of Plasticizers and Fatty Acids on Mechanical and Permeability Characteristics of Chitosan Films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of Equilibrium Moisture Content on Barrier, Mechanical and Thermal Properties of Chitosan Films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Borys, P.; Pawelek, K.; Grzywna, Z.J. On the Magnetic Channels in Polymer Membranes. Phys. Chem. Chem. Phys. 2011, 13, 17122–17129. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G.; Korkhov, V. Effect of Storage Time and Temperature on Structure, Mechanical and Barrier Properties of Chitosan-Based Films. Eur. Food Res. Technol. 2011, 232, 17–22. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and Barrier Properties of Nanocrystalline Cellulose Reinforced Chitosan Based Nanocomposite Films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Helmiyati, H.; Budiman, Y.; Abbas, G.H.; Dini, F.; Khalil, M. Highly Efficient Synthesis of Biodiesel Catalyzed by a Cellulose@hematite-Zirconia Nanocomposite. Heliyon 2021, 7, e06622. [Google Scholar] [CrossRef]
- Li, M.; He, B.; Chen, Y.; Zhao, L. Physicochemical Properties of Nanocellulose Isolated from Cotton Stalk Waste. ACS Omega 2021, 6, 25162–25169. [Google Scholar] [CrossRef]
- Wulandari, W.; Rochliadi, A.; Arcana, I.M. Nanocellulose Prepared by Acid Hydrolysis of Isolated Cellulose from Sugarcane Bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Development of Methodology for Identification the Nature of the Polyphenolic Extracts by FTIR Associated with Multivariate Analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Fernández, K.; Agosin, E. Quantitative Analysis of Red Wine Tannins Using Fourier-Transform Mid-Infrared Spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef]
- Indrani, D.J.; Lukitowati, F.; Yulizar, Y. Preparation of Chitosan/Collagen Blend Membranes for Wound Dressing: A Study on FTIR Spectroscopy and Mechanical Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012020. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of Polymeric Food Packaging Materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Assessment of Stability of a Spray Dried Extract from the Medicinal Plant Bidens pilosa L. J. King Saud Univ. Eng. Sci. 2016, 28, 141–146. [Google Scholar] [CrossRef]
- Fetsch, A.; Johler, S. Staphylococcus Aureus as a Foodborne Pathogen. Curr. Clin. Microbiol. Rep. 2018, 5, 88–96. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia Coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Gassem, M.A.; Athinarayanan, J.; Periyasamy, V.S.; Prasad, S.; Alshatwi, A.A. Antifungal Activity of Nanoemulsion from Cleome Viscosa Essential Oil against Food-Borne Pathogenic Candida Albicans. Saudi J. Biol. Sci. 2021, 28, 286–293. [Google Scholar] [CrossRef]
- Czerwińska-Główka, D.; Przystaś, W.; Zabłocka-Godlewska, E.; Student, S.; Cwalina, B.; Łapkowski, M.; Krukiewicz, K. Bacterial Surface Colonization of Sputter-Coated Platinum Films. Materials 2020, 13, 2674. [Google Scholar] [CrossRef]
- Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Appl. Sci. 2023, 13, 2494. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef]
- Kõrge, K.; Bajić, M.; Likozar, B.; Novak, U. Active Chitosan–Chestnut Extract Films Used for Packaging and Storage of Fresh Pasta. Int. J. Food Sci. Technol. 2020, 55, 3043–3052. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced Physico-Chemical Characterization of Chitosan by Means of TGA Coupled on-Line with FTIR and GCMS: Thermal Degradation and Water Adsorption Capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Abdullah, M.F.b.; Omar, M.F. Thermal Properties of Nanocellulose-Reinforced Composites: A Review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Çiçek Özkan, B.; Güner, M. Isolation, Characterization, and Comparison of Nanocrystalline Cellulose from Solid Wastes of Horse Chestnut and Chestnut Seed Shell. Cellulose 2022, 29, 6629–6644. [Google Scholar] [CrossRef]
- Ledniowska, K.; Nosal-Kovalenko, H.; Janik, W.; Krasuska, A.; Stańczyk, D.; Sabura, E.; Bartoszewicz, M.; Rybak, A. Effective, Environmentally Friendly PVC Plasticizers Based on Succinic Acid. Polymers 2022, 14, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zulfa, Z.; Chia, C.T.; Rukayadi, Y. In Vitro Antimicrobial Activity of Cymbopogon Citratus (Lemongrass) Extracts against Selected Foodborne Pathogens. Int. Food Res. J. 2016, 23, 1262–1267. [Google Scholar]
- Becerril, R.; Gómez-Lus, R.; Goñi, P.; López, P.; Nerín, C. Combination of Analytical and Microbiological Techniques to Study the Antimicrobial Activity of a New Active Food Packaging Containing Cinnamon or Oregano against E. Coli and S. Aureus. Anal. Bioanal. Chem. 2007, 388, 1003–1011. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, W.; Nowotarski, M.; Ledniowska, K.; Biernat, N.; Abdullah; Shyntum, D.Y.; Krukiewicz, K.; Turczyn, R.; Gołombek, K.; Dudek, G. Effect of Time on the Properties of Bio-Nanocomposite Films Based on Chitosan with Bio-Based Plasticizer Reinforced with Nanofiber Cellulose. Int. J. Mol. Sci. 2023, 24, 13205. https://doi.org/10.3390/ijms241713205
Janik W, Nowotarski M, Ledniowska K, Biernat N, Abdullah, Shyntum DY, Krukiewicz K, Turczyn R, Gołombek K, Dudek G. Effect of Time on the Properties of Bio-Nanocomposite Films Based on Chitosan with Bio-Based Plasticizer Reinforced with Nanofiber Cellulose. International Journal of Molecular Sciences. 2023; 24(17):13205. https://doi.org/10.3390/ijms241713205
Chicago/Turabian StyleJanik, Weronika, Michał Nowotarski, Kerstin Ledniowska, Natalia Biernat, Abdullah, Divine Yufetar Shyntum, Katarzyna Krukiewicz, Roman Turczyn, Klaudiusz Gołombek, and Gabriela Dudek. 2023. "Effect of Time on the Properties of Bio-Nanocomposite Films Based on Chitosan with Bio-Based Plasticizer Reinforced with Nanofiber Cellulose" International Journal of Molecular Sciences 24, no. 17: 13205. https://doi.org/10.3390/ijms241713205