Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila
Abstract
:1. Introduction
2. Results
2.1. Droj2 Is Required for Neurite Remodeling of C4da Sensory Neuron
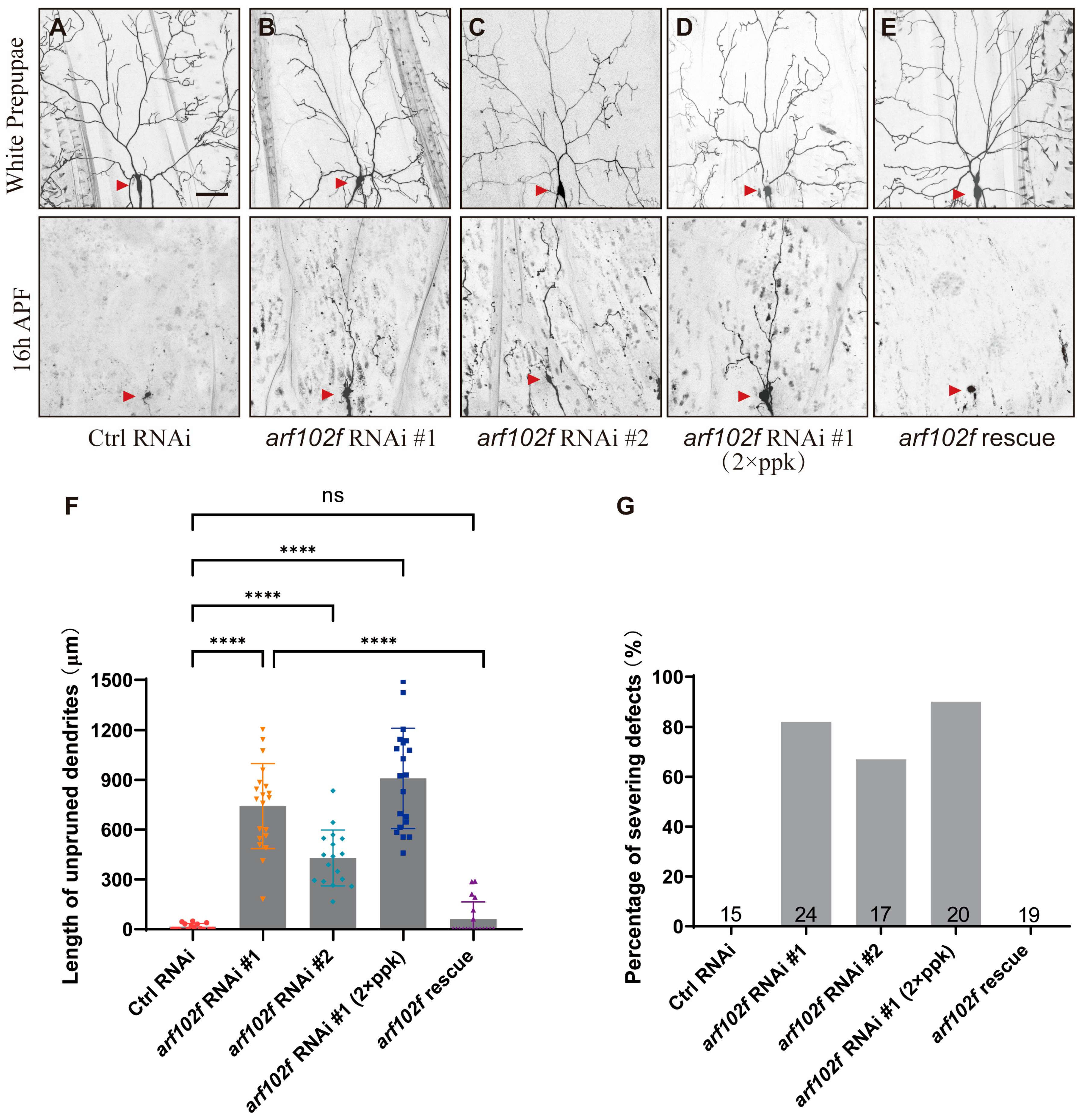
2.2. Loss of Arf102F Function Leads to Dendrite Remodeling Defects in C4da Neuron
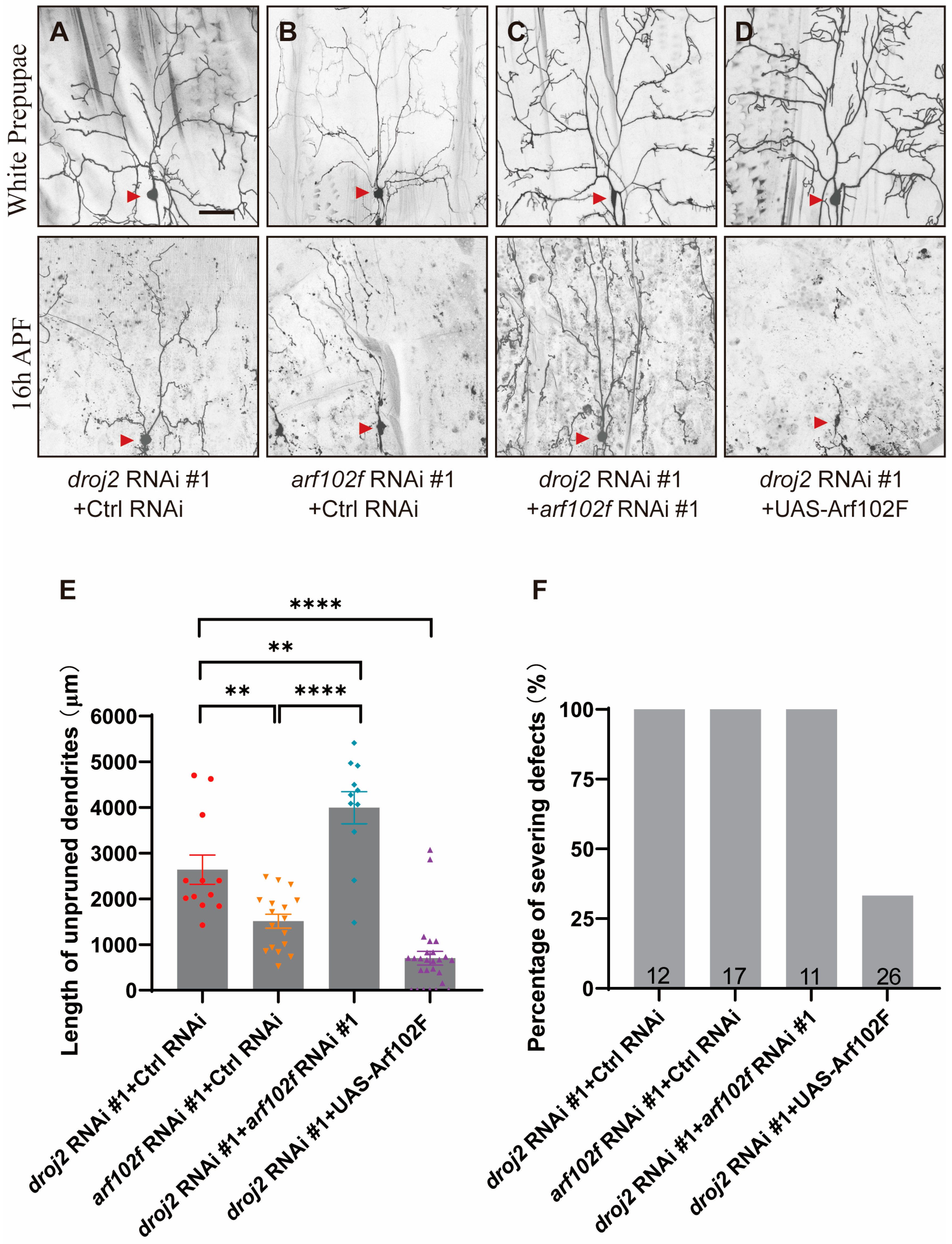
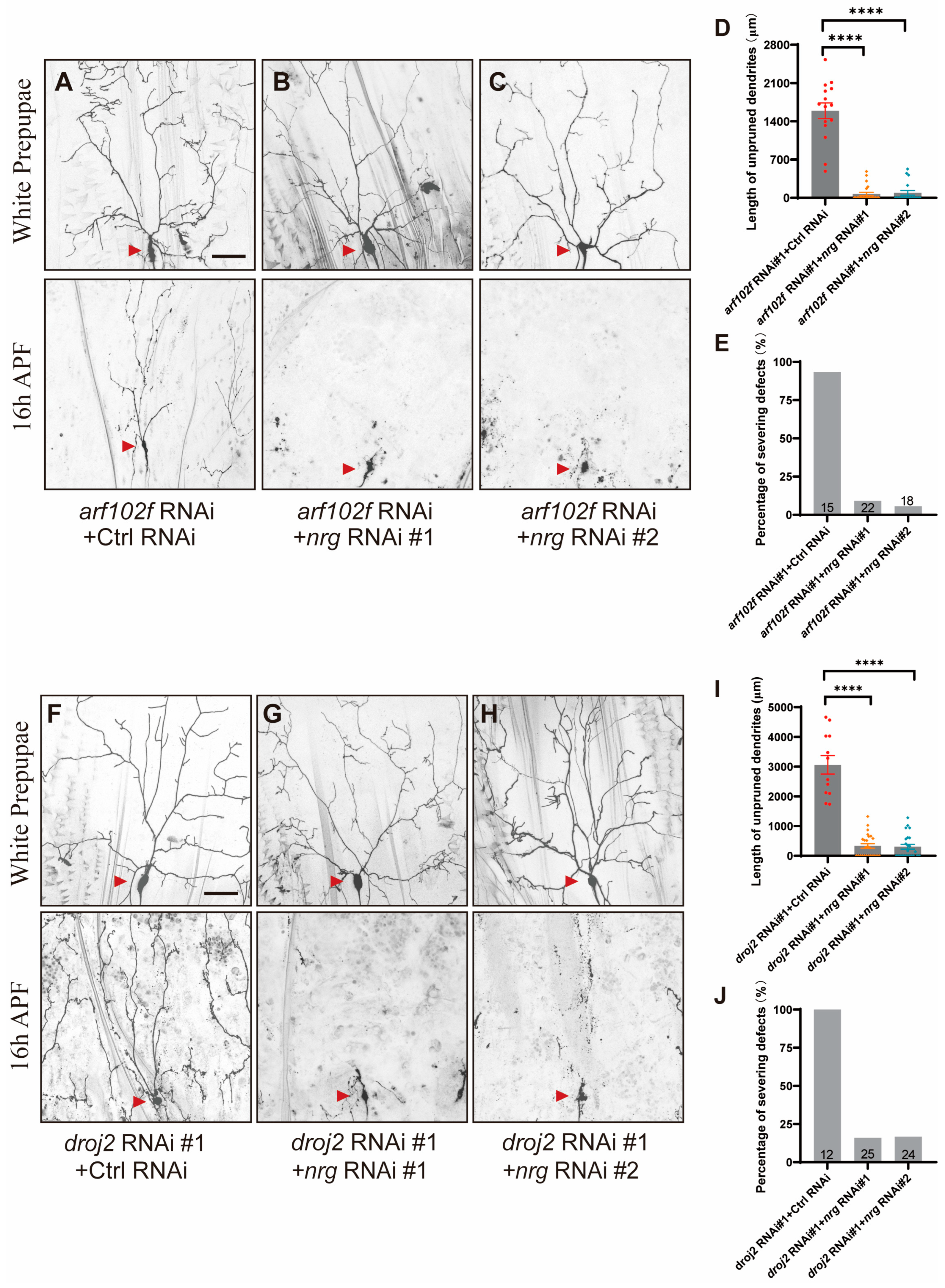
2.3. Droj2 Genetically Interacts with Arf102F to Promote Neurite Elimination
2.4. Arf102F Localizes on Golgi Compartments and Regulates the Integrity of Golgi Apparatus in C4da Neuron
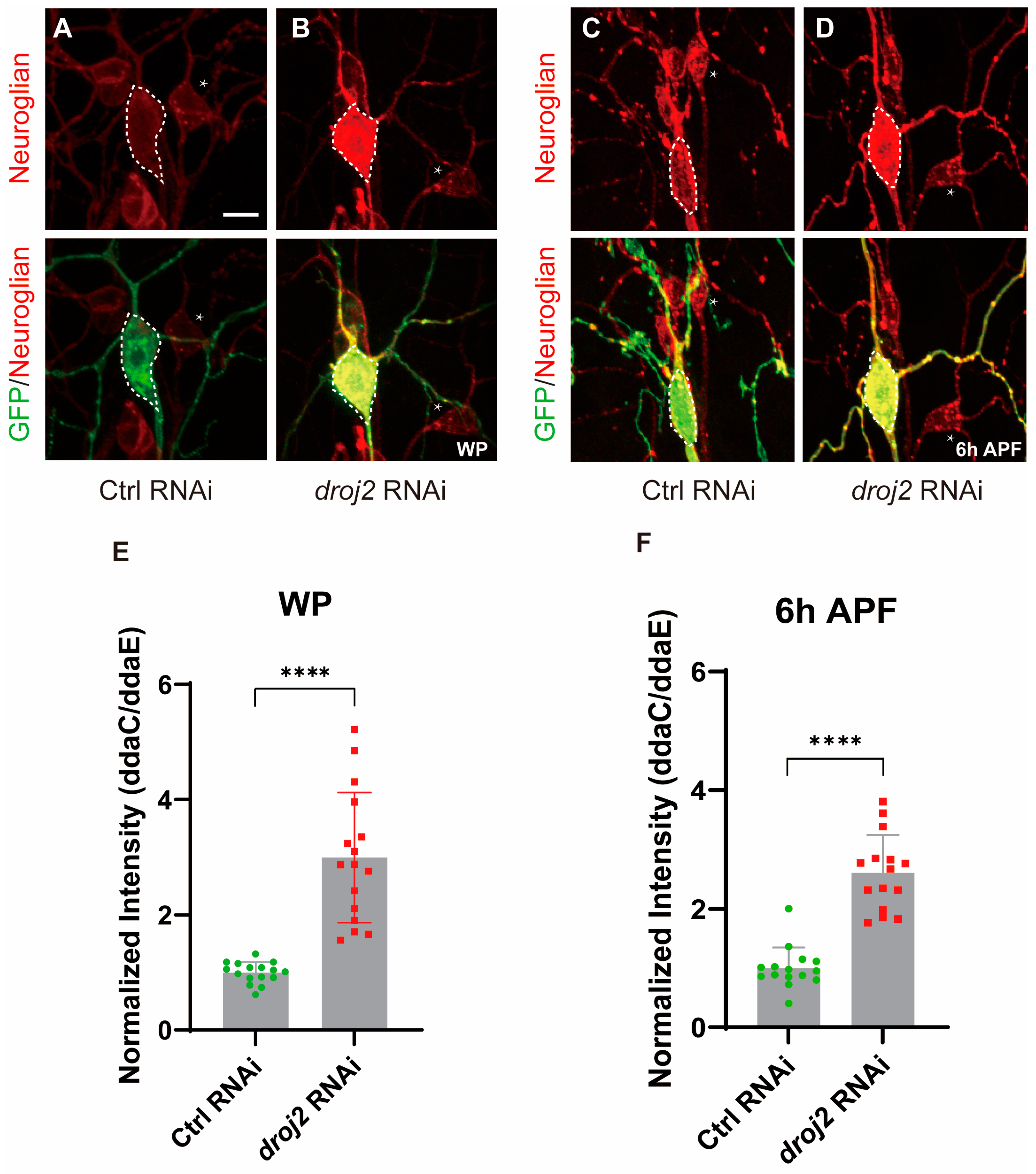
2.5. Droj2 and Arf102F Are Indispensable for the Distribution of Neuroglian
2.6. Droj2 and Arf102F Regulate Neurite Pruning by Downregulating Neuroglian
3. Discussion
4. Materials and Methods
4.1. Fly Strains
4.2. Generation of Droj2 Transgene
4.3. Live Imaging Analysis
4.4. Immunohistochemistry
4.5. Quantification of Dendrites
4.6. RNAi Screen
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, L.; O’Leary, D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005, 28, 127–156. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Kolodkin, A.L. Sculpting Neural Circuits by Axon and Dendrite Pruning. Annu. Rev. Cell Dev. Biol. 2015, 31, 779–805. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, O.; Yaron, A. Mechanisms of developmental neurite pruning. Cell. Mol. Life Sci. 2015, 72, 101–119. [Google Scholar] [CrossRef]
- Truman, J.W. Metamorphosis of the central nervous system of Drosophila. J. Neurobiol. 1990, 21, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.D.; Koester, S.E. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron 1993, 10, 991–1006. [Google Scholar] [CrossRef]
- Furusawa, K.; Emoto, K. Spatiotemporal regulation of developmental neurite pruning: Molecular and cellular insights from Drosophila models. Neurosci. Res. 2020, 167, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Schuldiner, O. Axon and dendrite pruning in Drosophila. Curr. Opin. Neurobiol. 2014, 27, 192–198. [Google Scholar] [CrossRef]
- Lee, T.; Marticke, S.; Sung, C.; Robinow, S.; Luo, L. Cell-Autonomous Requirement of the USP_EcR-B Ecdysone Receptor for Mushroom Body Neuronal Remodeling in Drosophila. Neuron 2000, 28, 807–818. [Google Scholar] [CrossRef]
- Rabinovich, D.; Yaniv, S.P.; Alyagor, I.; Schuldiner, O. Nitric Oxide as a Switching Mechanism between Axon Degeneration and Regrowth during Developmental Remodeling. Cell 2016, 164, 170–182. [Google Scholar] [CrossRef]
- Watts, R.J.; Hoopfer, E.D.; Luo, L. Axon Pruning during Drosophila Metamorphosis: Evidence for Local Degeneration and Requirement of the Ubiquitin-Proteasome System. Neuron 2003, 38, 871–885. [Google Scholar] [CrossRef]
- Kuo, C.T.; Jan, L.Y.; Jan, Y.N. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 15230–15235. [Google Scholar] [CrossRef]
- Germann, M.; Brederoo, S.G.; Sommer, I.E. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr. Opin. Psychiatry 2021, 34, 222–227. [Google Scholar] [CrossRef]
- Issman-Zecharya, N.; Schuldiner, O. The PI3K Class III Complex Promotes Axon Pruning by Downregulating a Ptc-Derived Signal via Endosome-Lysosomal Degradation. Dev. Cell 2014, 31, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rui, M.; Tang, Q.; Bu, S.; Yu, F. Patronin governs minus-end-out orientation of dendritic microtubules to promote dendrite pruning in Drosophila. Elife 2019, 8, e39964. [Google Scholar] [CrossRef] [PubMed]
- Glantz, L.A.; Lewis, D.A. Decreased Dendritic Spine Density on Prefrontal Cortical Pyramidal Neurons in Schizophrenia. Arch. Gen. Psychiatry 2000, 57, 65–73. [Google Scholar] [CrossRef]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.-E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, A.; Dura, J.-M. Nuclear receptors and Drosophila neuronal remodeling. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2015, 1849, 187–195. [Google Scholar] [CrossRef]
- Kirilly, D.; Wong, J.J.L.; Lim, E.K.H.; Wang, Y.; Zhang, H.; Wang, C.; Liao, Q.; Wang, H.; Liou, Y.-C.; Wang, H.; et al. Intrinsic Epigenetic Factors Cooperate with the Steroid Hormone Ecdysone to Govern Dendrite Pruning in Drosophila. Neuron 2011, 72, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Kirilly, D.; Gu, Y.; Huang, Y.; Wu, Z.; Bashirullah, A.; Low, B.C.; Kolodkin, A.L.; Wang, H.; Yu, F. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat. Neurosci. 2009, 12, 1497–1505. [Google Scholar] [CrossRef]
- Wong, J.J.L.; Li, S.; Lim, E.K.H.; Wang, Y.; Wang, C.; Zhang, H.; Kirilly, D.; Wu, C.; Liou, Y.-C.; Wang, H.; et al. A Cullin1-Based SCF E3 Ubiquitin Ligase Targets the InR/PI3K/TOR Pathway to Regulate Neuronal Pruning. PLoS Biol. 2013, 11, e1001657. [Google Scholar] [CrossRef]
- Kuo, C.T.; Zhu, S.; Younger, S.; Jan, L.Y.; Jan, Y.N. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron 2006, 51, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Kondo, S.; Krzyzanowska, A.; Hiromi, Y.; Truman, J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 2006, 9, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, T.; Kanai, M.I.; Dairyo, Y.; Yasunaga, K.-I.; Morikawa, R.K.; Emoto, K. Compartmentalized Calcium Transients Trigger Dendrite Pruning in Drosophila Sensory Neurons. Science 2013, 340, 1475–1478. [Google Scholar] [CrossRef]
- Kanamori, T.; Yoshino, J.; Yasunaga, K.-I.; Dairyo, Y.; Emoto, K. Local endocytosis triggers dendritic thinning and pruning in Drosophila sensory neurons. Nat. Commun. 2015, 6, 6515. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Yong, W.L.; Lim, B.J.W.; Kondo, S.; Yu, F. A systematic analysis of microtubule-destabilizing factors during dendrite pruning in Drosophila. EMBO Rep. 2021, 22, e52679. [Google Scholar] [CrossRef]
- Herzmann, S.; Götzelmann, I.; Reekers, L.-F.; Rumpf, S. Spatial regulation of microtubule disruption during dendrite pruning in Drosophila. Development 2018, 145, dev156950. [Google Scholar] [CrossRef]
- Herzmann, S.; Krumkamp, R.; Rode, S.; Kintrup, C.; Rumpf, S. PAR-1 promotes microtubule breakdown during dendrite pruning in Drosophila. EMBO J. 2017, 36, 1981–1991. [Google Scholar] [CrossRef]
- Lee, H.-H.; Jan, L.Y.; Jan, Y.-N. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc. Natl. Acad. Sci. USA 2009, 106, 6363–6368. [Google Scholar] [CrossRef]
- Rui, M.; Ng, K.S.; Tang, Q.; Bu, S.; Yu, F. Protein phosphatase PP2A regulates microtubule orientation and dendrite pruning in Drosophila. EMBO Rep. 2020, 21, e48843. [Google Scholar] [CrossRef]
- Tang, Q.; Rui, M.; Bu, S.; Wang, Y.; Chew, L.Y.; Yu, F. A microtubule polymerase is required for microtubule orientation and dendrite pruning in Drosophila. EMBO J. 2020, 39, e103549. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, R.; Soba, P.; Jan, Y.-N. JNK signaling coordinates with ecdysone signaling to promote pruning of Drosophila sensory neuron dendrites. Development 2019, 146, dev163592. [Google Scholar] [CrossRef]
- Marzano, M.; Herzmann, S.; Elsbroek, L.; Sanal, N.; Tarbashevich, K.; Raz, E.; Krahn, M.P.; Rumpf, S. AMPK adapts metabolism to developmental energy requirement during dendrite pruning in Drosophila. Cell Rep. 2021, 37, 110024. [Google Scholar] [CrossRef] [PubMed]
- Krämer, R.; Wolterhoff, N.; Galic, M.; Rumpf, S. Developmental pruning of sensory neurites by mechanical tearing in Drosophila. J. Cell Biol. 2023, 222, e202205004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wong, J.J.L.; Lim, K.-L.; Liou, Y.-C.; Wang, H.; Yu, F. Endocytic Pathways Downregulate the L1-type Cell Adhesion Molecule Neuroglian to Promote Dendrite Pruning in Drosophila. Dev. Cell 2014, 30, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Shi, M.; Liou, Y.-C.; Lu, L.; Yu, F. Sec71 functions as a GEF for the small GTPase Arf1 to govern dendrite pruning of Drosophila sensory neurons. Development 2017, 144, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Yu, F. Yif1 associates with Yip1 on Golgi and regulates dendrite pruning in sensory neurons during Drosophila metamorphosis. Development 2018, 145, dev164475. [Google Scholar] [CrossRef]
- Rui, M.; Bu, S.; Chew, L.Y.; Wang, Q.; Yu, F. The membrane protein Raw regulates dendrite pruning via the secretory pathway. Development 2020, 147, dev191155. [Google Scholar] [CrossRef]
- Han, C.; Song, Y.; Xiao, H.; Wang, D.; Franc, N.C.; Jan, L.Y.; Jan, Y.-N. Epidermal Cells Are the Primary Phagocytes in the Fragmentation and Clearance of Degenerating Dendrites in Drosophila. Neuron 2014, 81, 544–560. [Google Scholar] [CrossRef]
- Momiuchi, Y.; Kumada, K.; Kuraishi, T.; Takagaki, T.; Aigaki, T.; Oshima, Y.; Kurata, S. The Role of the Phylogenetically Conserved Cochaperone Protein Droj2/DNAJA3 in NF-kappa B Signaling. J. Biol. Chem. 2015, 290, 23816–23825. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Kametaka, S.; Kametaka, A.; Yonekura, S.; Haruta, M.; Takenoshita, S.; Goto, S.; Waguri, S. AP-1 clathrin adaptor and CG8538/Aftiphilin are involved in Notch signaling during eye development in Drosophila melanogaster. J. Cell Sci. 2012, 125 Pt 3, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Zeledon, C.; Sun, X.; Plutoni, C.; Emery, G. The ArfGAP Drongo Promotes Actomyosin Contractility during Collective Cell Migration by Releasing Myosin Phosphatase from the Trailing Edge. Cell Rep. 2019, 28, 3238–3248.e3. [Google Scholar] [CrossRef] [PubMed]
- Baylies, M.; Dw, W.; Jw, T. Faculty Opinions recommendation of Cellular mechanisms of dendrite pruning in Drosophila: Insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development 2005, 132, 3631–3642. [Google Scholar] [CrossRef]
- Guruharsha, K.; Rual, J.-F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A Protein Complex Network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Krämer, R.; Rode, S.; Rumpf, S. Rab11 is required for neurite pruning and developmental membrane protein degradation in Drosophila sensory neurons. Dev. Biol. 2019, 451, 68–78. [Google Scholar] [CrossRef]
- Cardozo, P.L.; de Lima, I.B.Q.; Maciel, E.M.A.; Silva, N.C.; Dobransky, T.; Ribeiro, F.M. Synaptic Elimination in Neurological Disorders. Curr. Neuropharmacol. 2019, 17, 1071–1095. [Google Scholar] [CrossRef]
- Hutsler, J.J.; Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef]
- Lloyd, T.E.; Verstreken, P.; Ostrin, E.J.; Phillippi, A.; Lichtarge, O.; Bellen, H.J. A Genome-Wide Search for Synaptic Vesicle Cycle Proteins in Drosophila. Neuron 2000, 26, 45–50. [Google Scholar] [CrossRef]
- Ring, J.; Tadic, J.; Ristic, S.; Poglitsch, M.; Bergmann, M.; Radic, N.; Mossmann, D.; Liang, Y.; Maglione, M.; Jerkovic, A.; et al. The HSP40 chaperone Ydj1 drives amyloid beta 42 toxicity. EMBO Mol. Med. 2022, 14, e13952. [Google Scholar] [CrossRef]
- Tadic, J.; Ring, J.; Jerkovic, A.; Ristic, S.; Maglione, M.; Dengjel, J.; Sigrist, S.J.; Eisenberg, T. A pathological role of the Hsp40 protein Ydj1/DnaJA1 in models of Alzheimer’s disease. Cell Stress 2022, 6, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Yoon, S.Y.; Zhu, L.; Brodbeck, J.; Dai, J.; Walker, D.; Huang, Y. Arf4 Determines Dentate Gyrus-Mediated Pattern Separation by Regulating Dendritic Spine Development. PLoS ONE 2012, 7, e46340. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rui, M.; Kong, W.; Wang, W.; Zheng, T.; Wang, S.; Xie, W. Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila. Int. J. Mol. Sci. 2023, 24, 13213. https://doi.org/10.3390/ijms241713213
Rui M, Kong W, Wang W, Zheng T, Wang S, Xie W. Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila. International Journal of Molecular Sciences. 2023; 24(17):13213. https://doi.org/10.3390/ijms241713213
Chicago/Turabian StyleRui, Menglong, Weiyu Kong, Wanting Wang, Ting Zheng, Su Wang, and Wei Xie. 2023. "Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila" International Journal of Molecular Sciences 24, no. 17: 13213. https://doi.org/10.3390/ijms241713213
APA StyleRui, M., Kong, W., Wang, W., Zheng, T., Wang, S., & Xie, W. (2023). Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila. International Journal of Molecular Sciences, 24(17), 13213. https://doi.org/10.3390/ijms241713213





