NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions
Abstract
:1. Introduction
2. Structure, Generation, Comparison and Advantages
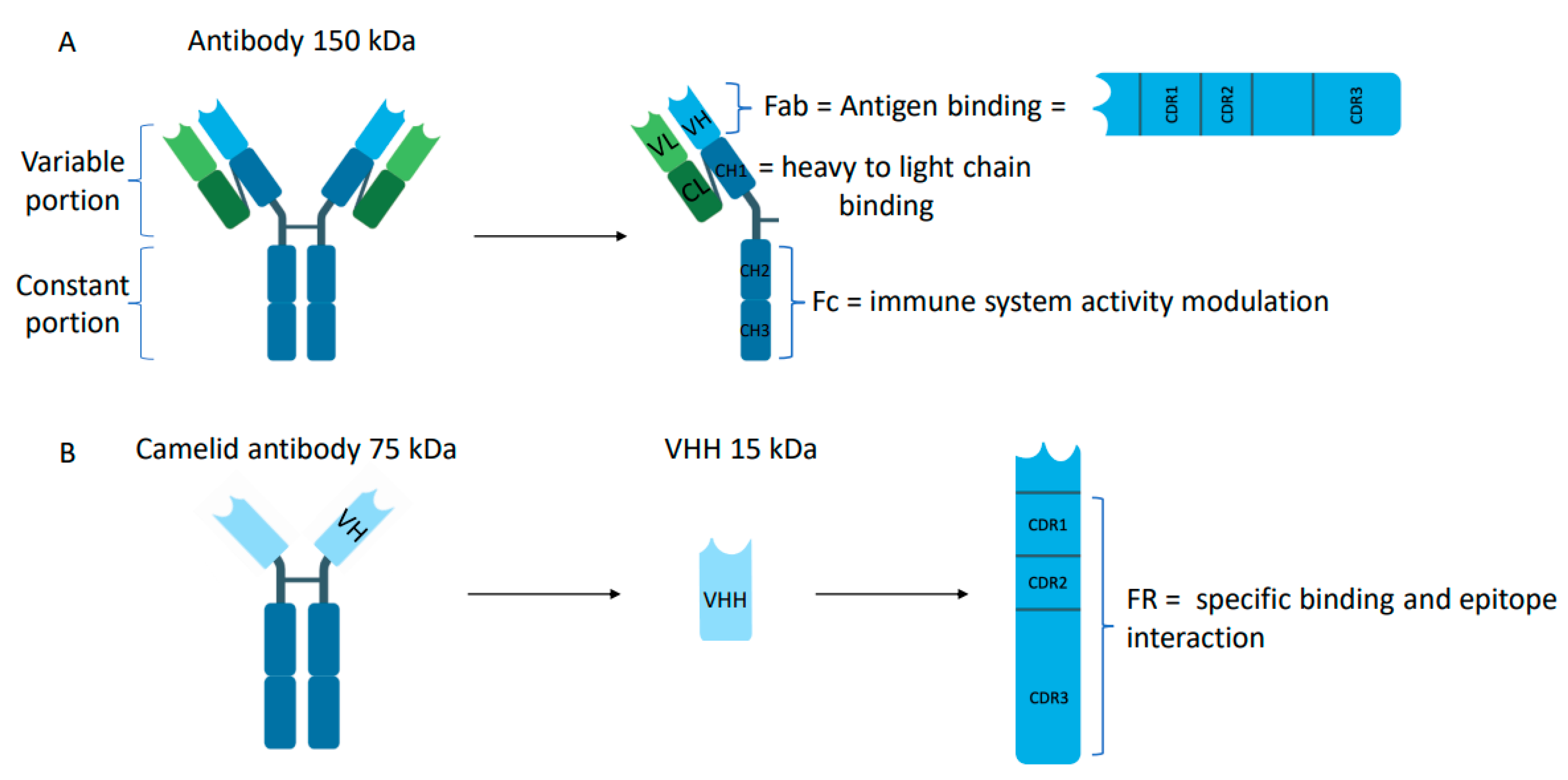
2.1. Structure of Nanobody® Molecules
2.2. Generation of Nanobody® Molecules
2.3. Comparison of Nanobody® Molecules with Other Types of Therapeutic Antibodies
2.4. Advantages of Nanobody® Molecules
3. Nanobody® Molecule, a Versatile Tool Emerging in Clinic
3.1. Nanobody® Molecules in Imaging
3.2. Nanobody® Molecules, from Bench to Bedside (or Path to the Way of Alternative Therapeutics)
3.2.1. Nanobody® Molecules in Central Nervous System Diseases
3.2.2. Nanobody® Molecules in Immune System Disorders
3.2.3. Nanobody® Molecules in Infectious and Viral Diseases
3.2.4. Nanobody® Molecules in Oncology
4. Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Räty, J.K.; Ylä-Herttuala, S. Challenges in Monoclonal Antibody-Based Therapies. Ann. Med. 2009, 41, 322–331. [Google Scholar] [CrossRef]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic Antibodies: Successes, Limitations and Hopes for the Future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, R.; de Geus, B.; Stok, W.; Bos, W.; van Wassenaar, D.; Verrips, T.; Frenken, L. Induction of Immune Responses and Molecular Cloning of the Heavy Chain Antibody Repertoire of Lama Glama. J. Immunol. Methods 2000, 240, 185–195. [Google Scholar] [CrossRef]
- Rothbauer, U.; Zolghadr, K.; Tillib, S.; Nowak, D.; Schermelleh, L.; Gahl, A.; Backmann, N.; Conrath, K.; Muyldermans, S.; Cardoso, M.C.; et al. Targeting and Tracing Antigens in Live Cells with Fluorescent Nanobodies. Nat. Methods 2006, 3, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Maass, D.R.; Sepulveda, J.; Pernthaner, A.; Shoemaker, C.B. Alpaca (Lama Pacos) as a Convenient Source of Recombinant Camelid Heavy Chain Antibodies (VHHs). J. Immunol. Methods 2007, 324, 13–25. [Google Scholar] [CrossRef] [PubMed]
- De Simone, E.A.; Saccodossi, N.; Ferrari, A.; Leoni, J. Development of ELISAs for the Measurement of IgM and IgG Subclasses in Sera from Llamas (Lama Glama) and Assessment of the Humoral Immune Response against Different Antigens. Vet. Immunol. Immunopathol. 2008, 126, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.R.; Anouassi, A.; Ahmed Abed, M.; Tsikis, G.; Canepa, S.; Labas, V.; Belghazi, M.; Bruneau, G. A One-Step Exclusion-Binding Procedure for the Purification of Functional Heavy-Chain and Mammalian-Type Gamma-Globulins from Camelid Sera. Biotechnol. Appl. Biochem. 2009, 54, 207–212. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Hamers, R.; Wyns, L.; Muyldermans, S. Camel Heavy-Chain Antibodies: Diverse Germline VHH and Specific Mechanisms Enlarge the Antigen-Binding Repertoire. EMBO J. 2000, 19, 921–930. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.R.G.; Hamers, R. Sequence and Structure of VH Domain from Naturally Occurring Camel Heavy Chain Immunoglobulins Lacking Light Chains. Protein Eng. Des. Sel. 1994, 7, 1129–1135. [Google Scholar] [CrossRef]
- Mitchell, L.S.; Colwell, L.J. Analysis of Nanobody Paratopes Reveals Greater Diversity than Classical Antibodies. Protein Eng. Des. Sel. 2018, 31, 267–275. [Google Scholar] [CrossRef]
- Mitchell, L.S.; Colwell, L.J. Comparative Analysis of Nanobody Sequence and Structure Data. Proteins Struct. Funct. Bioinform. 2018, 86, 697–706. [Google Scholar] [CrossRef]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of Llama VH Sequences from Conventional and Heavy Chain Antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Janssens, R.; Dekker, S.; Hendriks, R.W.; Panayotou, G.; Remoortere, A.V.; San, J.K.; Grosveld, F.; Drabek, D. Generation of Heavy-Chain-Only Antibodies in Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 15130–15135. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; De Vlaeminck, Y.; Hanssens, H.; D’Huyvetter, M.; Raes, G.; Goyvaerts, C.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. Theranostics in Immuno-Oncology Using Nanobody Derivatives. Theranostics 2019, 9, 7772–7791. [Google Scholar] [CrossRef]
- Yan, J.; Wang, P.; Zhu, M.; Li, G.; Romão, E.; Xiong, S.; Wan, Y. Characterization and Applications of Nanobodies against Human Procalcitonin Selected from a Novel Naïve Nanobody Phage Display Library. J. Nanobiotechnology 2015, 13, 1–11. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, Y.; Sivaccumar, J.P.; An, Z.; Xia, N.; Luo, W. Research Progress and Applications of Nanobody in Human Infectious Diseases. Front. Pharmacol. 2022, 13, 963978. [Google Scholar] [CrossRef]
- Fleetwood, F.; Devoogdt, N.; Pellis, M.; Wernery, U.; Muyldermans, S.; Ståhl, S.; Löfblom, J. Surface Display of a Single-Domain Antibody Library on Gram-Positive Bacteria. Cell. Mol. Life Sci. 2013, 70, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Koide, S. Affinity Maturation of Single-Domain Antibodies by Yeast Surface Display. Methods Mol. Biol. 2012, 911, 431–443. [Google Scholar] [CrossRef]
- Ryckaert, S.; Pardon, E.; Steyaert, J.; Callewaert, N. Isolation of Antigen-Binding Camelid Heavy Chain Antibody Fragments (Nanobodies) from an Immune Library Displayed on the Surface of Pichia Pastoris. J. Biotechnol. 2010, 145, 93–98. [Google Scholar] [CrossRef]
- Ferrari, D.; Garrapa, V.; Locatelli, M.; Bolchi, A. A Novel Nanobody Scaffold Optimized for Bacterial Expression and Suitable for the Construction of Ribosome Display Libraries. Mol. Biotechnol. 2020, 62, 43–55. [Google Scholar] [CrossRef]
- Rahbarnia, L.; Farajnia, S.; Babaei, H.; Majidi, J.; Veisi, K.; Ahmadzadeh, V.; Akbari, B. Evolution of Phage Display Technology: From Discovery to Application. J. Drug Target. 2017, 25, 216–224. [Google Scholar] [CrossRef]
- Wang, C.; Hong, J.; Yang, Z.; Zhou, X.; Yang, Y.; Kong, Y.; Chen, B.; Wu, H.; Qian, B.-Z.; Dimitrov, D.S.; et al. Design of a Novel Fab-Like Antibody Fragment with Enhanced Stability and Affinity for Clinical Use. Small Methods 2022, 6, e2100966. [Google Scholar] [CrossRef]
- Johnson, M. Antibody Structure and Antibody Fragments. Mater. Methods 2013, 3, 160. [Google Scholar] [CrossRef]
- Nelson, A.L. Antibody Fragments. mAbs 2010, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-Cell Engagers for Cancer Immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; Rahimi-Jamnani, F. Single-Chain Variable Fragment-Based Bispecific Antibodies: Hitting Two Targets with One Sophisticated Arrow. Mol. Ther. Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A Comprehensive Comparison between Camelid Nanobodies and Single Chain Variable Fragments. Biomark. Res. 2021, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Monnier, P.P.; Vigouroux, R.J.; Tassew, N.G. In Vivo Applications of Single Chain Fv (Variable Domain) (ScFv) Fragments. Antibodies 2013, 2, 193–208. [Google Scholar] [CrossRef]
- Ackaert, C.; Smiejkowska, N.; Xavier, C.; Sterckx, Y.G.J.; Denies, S.; Stijlemans, B.; Elkrim, Y.; Devoogdt, N.; Caveliers, V.; Lahoutte, T.; et al. Immunogenicity Risk Profile of Nanobodies. Front. Immunol. 2021, 12, 632687. [Google Scholar] [CrossRef]
- Van der Linden, R.H.; de Geus, B.; Frenken, L.G.; Peters, H.; Verrips, C.T. Improved production and function of llama heavy chain antibody fragments by molecular evolution. J. Biotechnol. 2000, 80, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Arbabi Ghahroudi, M.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and Identification of Single Domain Antibody Fragments from Camel Heavy-Chain Antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef]
- Kunz, P.; Zinner, K.; Mücke, N.; Bartoschik, T.; Muyldermans, S.; Hoheisel, J.D. The Structural Basis of Nanobody Unfolding Reversibility and Thermoresistance. Sci. Rep. 2018, 8, 7934. [Google Scholar] [CrossRef] [PubMed]
- Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K. A Comprehensive Screening Platform for Aerosolizable Protein Formulations for Intranasal and Pulmonary Drug Delivery. Int. J. Pharm. 2017, 532, 537–546. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An Ultrapotent Synthetic Nanobody Neutralizes SARS-CoV-2 by Stabilizing Inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef]
- Subrahmanyam, N.; Ghandehari, H. Harnessing Extracellular Matrix Biology for Tumor Drug Delivery. J. Pers. Med. 2021, 11, 88. [Google Scholar] [CrossRef]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and Affinity Kinetics of Nanobodies Influence Targeting and Penetration of Solid Tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pothin, E.; Lesuisse, D.; Lafaye, P. Brain Delivery of Single-Domain Antibodies: A Focus on VHH and VNAR. Pharmaceutics 2020, 12, 937. [Google Scholar] [CrossRef]
- Davies, J.; Riechmann, L. Single Antibody Domains as Small Recognition Units: Design and in Vitro Antigen Selection of Camelized, Human VH Domains with Improved Protein Stability. Protein Eng. Des. Sel. 1996, 9, 531–537. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.J.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-Domain Antibody Fragments with High Conformational Stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef]
- Harmsen, M.M.; De Haard, H.J. Properties, Production, and Applications of Camelid Single-Domain Antibody Fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies for Extended Serum Half-Life of Protein Therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef]
- Wunder, A.; Müller-Ladner, U.; Stelzer, E.H.K.; Funk, J.; Neumann, E.; Stehle, G.; Pap, T.; Sinn, H.; Gay, S.; Fiehn, C. Albumin-Based Drug Delivery as Novel Therapeutic Approach for Rheumatoid Arthritis. J. Immunol. 2003, 170, 4793–4801. [Google Scholar] [CrossRef]
- Fishburn, C.S. The Pharmacology of PEGylation: Balancing PD with PK to Generate Novel Therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Kesh, K.; Gupta, V.K.; Durden, B.; Garrido, V.; Mateo-Victoriano, B.; Lavania, S.P.; Banerjee, S. Therapy Resistance, Cancer Stem Cells and ECM in Cancer: The Matrix Reloaded. Cancers 2020, 12, 3067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting Focal Adhesion Kinase Renders Pancreatic Cancers Responsive to Checkpoint Immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen Promotes Anti-PD-1/PD-L1 Resistance in Cancer through LAIR1-Dependent CD8+ T Cell Exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, S.; Ben Abderrazek, R.; Loustau, T.; Abou-Faycal, C.; Ksouri, A.; Erne, W.; Murdamoothoo, D.; Mörgelin, M.; Kungl, A.; Jung, A.; et al. Novel Human Tenascin-C Function-Blocking Camel Single Domain Nanobodies. Front. Immunol. 2021, 12, 635166. [Google Scholar] [CrossRef] [PubMed]
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive Imaging of Tumor Progression, Metastasis, and Fibrosis Using a Nanobody Targeting the Extracellular Matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [Google Scholar] [CrossRef]
- Muruganandam, A.; Tanha, J.; Narang, S.; Stanimirovic, D. Selection of Phage-Displayed Llama Single-Domain Antibodies That Transmigrate across Human Blood-Brain Barrier Endothelium. FASEB J. 2002, 16, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bourgeois, J.-P.; Celli, S.; Glacial, F.; Le Sourd, A.-M.; Mecheri, S.; Weksler, B.; Romero, I.; Couraud, P.-O.; Rougeon, F.; et al. Cell-Penetrating Anti-GFAP VHH and Corresponding Fluorescent Fusion Protein VHH-GFP Spontaneously Cross the Blood-Brain Barrier and Specifically Recognize Astrocytes: Application to Brain Imaging. FASEB J. 2012, 26, 3969–3979. [Google Scholar] [CrossRef]
- Selection of Phage-Displayed Llama Single-Domain Antibodies that Transmigrate across Human blood—Brain Barrier Endothelium—Muruganandam—2002—The FASEB Journal—Wiley Online Library. Available online: https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj.01-0343fje (accessed on 5 August 2023).
- Kim, T.Y.; Park, J.H.; Shim, H.E.; Choi, D.S.; Lee, D.-E.; Song, J.-J.; Kim, H.-S. Prolonged Half-Life of Small-Sized Therapeutic Protein Using Serum Albumin-Specific Protein Binder. J. Control. Release 2019, 315, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hoefman, S.; Ottevaere, I.; Baumeister, J.; Sargentini-Maier, M.L. Pre-Clinical Intravenous Serum Pharmacokinetics of Albumin Binding and Non-Half-Life Extended Nanobodies®. Antibodies 2015, 4, 141–156. [Google Scholar] [CrossRef]
- Valenzuela Nieto, G.; Jara, R.; Watterson, D.; Modhiran, N.; Amarilla, A.A.; Himelreichs, J.; Khromykh, A.A.; Salinas-Rebolledo, C.; Pinto, T.; Cheuquemilla, Y.; et al. Potent Neutralization of Clinical Isolates of SARS-CoV-2 D614 and G614 Variants by a Monomeric, Sub-Nanomolar Affinity Nanobody. Sci. Rep. 2021, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Mikolajek, H.; Le Bas, A.; Clark, J.J.; Sharma, P.; Kipar, A.; Dormon, J.; Norman, C.; Weckener, M.; Clare, D.K.; et al. A Potent SARS-CoV-2 Neutralising Nanobody Shows Therapeutic Efficacy in the Syrian Golden Hamster Model of COVID-19. Nat. Commun. 2021, 12, 5469. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Liu, X.; Zhang, J.; Yin, Y.; Luan, L.; Jiang, D.; Yang, X.; Li, L.; Xiong, H.; et al. A Potent Synthetic Nanobody with Broad-Spectrum Activity Neutralizes SARS-CoV-2 Virus and the Omicron Variant BA.1 through a Unique Binding Mode. J. Nanobiotechnology 2022, 20, 31–39. [Google Scholar] [CrossRef]
- Aria, H.; Mahmoodi, F.; Ghaheh, H.S.; Zare, H.; Heiat, M.; Bakherad, H. Outlook of Therapeutic and Diagnostic Competency of Nanobodies against SARS-CoV-2: A Systematic Review. Anal. Biochem. 2022, 640, 114546. [Google Scholar] [CrossRef]
- Horne, C.; Klein, M.; Polidoulis, I.; Dorrington, K.J. Noncovalent Association of Heavy and Light Chains of Human Immunoglobulins. III. Specific Interactions between VH and VL. J. Immunol. 1982, 129, 660–664. [Google Scholar] [CrossRef]
- Berry, M.J.; Davies, J. Use of Antibody Fragments in Immunoaffinity Chromatography: Comparison of FV Fragments, VH Fragments and Paralog Peptides. J. Chromatogr. A 1992, 597, 239–245. [Google Scholar] [CrossRef]
- Borrebaeck, C.A.; Malmborg, A.C.; Furebring, C.; Michaelsson, A.; Ward, S.; Danielsson, L.; Ohlin, M. Kinetic Analysis of Recombinant Antibody-Antigen Interactions: Relation between Structural Domains and Antigen Binding. Biotechnology 1992, 10, 697–698. [Google Scholar] [CrossRef]
- Ward, E.S.; Güssow, D.; Griffiths, A.D.; Jones, P.T.; Winter, G. Binding Activities of a Repertoire of Single Immunoglobulin Variable Domains Secreted from Escherichia Coli. Nature 1989, 341, 544–546. [Google Scholar] [CrossRef]
- Pérez, J.M.; Renisio, J.G.; Prompers, J.J.; van Platerink, C.J.; Cambillau, C.; Darbon, H.; Frenken, L.G. Thermal Unfolding of a Llama Antibody Fragment: A Two-State Reversible Process. Biochemistry 2001, 40, 74–83. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.; Devoogdt, N.; Lahoutte, T.; Muyldermans, S. Camelid Single-Domain Antibody-Fragment Engineering for (Pre)Clinical in Vivo Molecular Imaging Applications: Adjusting the Bullet to Its Target. Expert Opin. Biol. Ther. 2013, 13, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Thrane, S.W.; Gram, L.; Muyldermans, S.; Laustsen, A.H. Orally Delivered Single-Domain Antibodies against Gastrointestinal Pathogens. Trends Biotechnol. 2023, 41, 875–886. [Google Scholar] [CrossRef]
- Hussack, G.; Hirama, T.; Ding, W.; MacKenzie, R.; Tanha, J. Engineered Single-Domain Antibodies with High Protease Resistance and Thermal Stability. PLoS ONE 2011, 6, e28218. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; Piedra, P.A.; Martinon-Torres, F.; Szymanski, H.; Brackeva, B.; Dombrecht, E.; Detalle, L.; Fleurinck, C.; Cunningham, S.; Piedra, P.A.; et al. Nebulised ALX-0171 for Respiratory Syncytial Virus Lower Respiratory Tract Infection in Hospitalised Children: A Double-Blind, Randomised, Placebo-Controlled, Phase 2b Trial. Lancet Respir. Med. 2021, 9, 21–32. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Backmann, N.; Senter, P.D.; Wernery, U.; De Baetselier, P.; Muyldermans, S.; Revets, H. Efficient Cancer Therapy with a Nanobody-Based Conjugate. Cancer Res. 2004, 64, 2853–2857. [Google Scholar] [CrossRef]
- Bartunek, J.; Barbato, E.; Heyndrickx, G.; Vanderheyden, M.; Wijns, W.; Holz, J.-B. Novel Antiplatelet Agents: ALX-0081, a Nanobody Directed towards von Willebrand Factor. J. Cardiovasc. Transl. Res. 2013, 6, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Holz, J.-B.; Sargentini-Maier, L.; Bruyn, S.D.; Gachályi, B.; Udvaros, I.; Rojkovich, B.; Bruk, S.; Sramek, P.; Korkosz, M.; Krause, K.; et al. OP0043 Twenty-Four Weeks of Treatment with a Novel Anti-IL-6 Receptor Nanobody® (ALX-0061) Resulted in 84% ACR20 Improvement and 58% DAS28 Remission in a Phase I/Ii Study in RA. Ann. Rheum. Dis. 2013, 72 (Suppl. 3), A64. [Google Scholar] [CrossRef]
- Holland, M.C.; Wurthner, J.U.; Morley, P.J.; Birchler, M.A.; Lambert, J.; Albayaty, M.; Serone, A.P.; Wilson, R.; Chen, Y.; Forrest, R.M.; et al. Autoantibodies to Variable Heavy (VH) Chain Ig Sequences in Humans Impact the Safety and Clinical Pharmacology of a VH Domain Antibody Antagonist of TNF-α Receptor 1. J. Clin. Immunol. 2013, 33, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Isaacs, R.; Bilic, S.; Kentsch, K.; Huet, H.A.; Hofmann, M.; Rasco, D.; Kundamal, N.; Tang, Z.; Cooksey, J.; et al. Unexpected Hepatotoxicity in a Phase I Study of TAS266, a Novel Tetravalent Agonistic Nanobody® Targeting the DR5 Receptor. Cancer Chemother. Pharmacol. 2015, 75, 887–895. [Google Scholar] [CrossRef]
- Rossotti, M.A.; Bélanger, K.; Henry, K.A.; Tanha, J. Immunogenicity and Humanization of Single-Domain Antibodies. FEBS J. 2022, 289, 4304–4327. [Google Scholar] [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Boulenouar, H.; .Amar, Y.; Bouchoutrouch, N.; Faouzi, M.E.A.; Cherrah, Y.; Sefrioui, H. Nanobodies and Their Medical Applications. Genet. Mol. Res. 2020, 19, gmr18452. [Google Scholar] [CrossRef]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Jin, B.; Odongo, S.; Radwanska, M.; Magez, S. Nanobodies: A Review of Generation, Diagnostics and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef]
- Berland, L.; Kim, L.; Abousaway, O.; Mines, A.; Mishra, S.; Clark, L.; Hofman, P.; Rashidian, M. Nanobodies for Medical Imaging: About Ready for Prime Time? Biomolecules 2021, 11, 637. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical Screening of Anti-HER2 Nanobodies for Molecular Imaging of Breast Cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; de Muynck, L.D.A.N.; Baart, V.M.; Bhairosingh, S.; Debie, P.; Vahrmeijer, A.L.; Hernot, S.; Mieog, J.S.D. Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using a Fluorescently Labelled Anti-CEA Nanobody Probe: A Preclinical Study. Biomolecules 2023, 13, 618. [Google Scholar] [CrossRef] [PubMed]
- de Geus, S.W.L.; Boogerd, L.S.F.; Swijnenburg, R.-J.; Mieog, J.S.D.; Tummers, W.S.F.J.; Prevoo, H.A.J.M.; Sier, C.F.M.; Morreau, H.; Bonsing, B.A.; van de Velde, C.J.H.; et al. Selecting Tumor-Specific Molecular Targets in Pancreatic Adenocarcinoma: Paving the Way for Image-Guided Pancreatic Surgery. Mol. Imaging Biol. 2016, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; Groen, J.V.; Putter, H.; Pichler, M.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.D. Stage-Specific Value of Carbohydrate Antigen 19-9 and Carcinoembryonic Antigen Serum Levels on Survival and Recurrence in Pancreatic Cancer: A Single Center Study and Meta-Analysis. Cancers 2020, 12, 2970. [Google Scholar] [CrossRef]
- van Manen, L.; Groen, J.V.; Putter, H.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; Bonsing, B.A.; Mieog, J.S.D. Elevated CEA and CA19-9 Serum Levels Independently Predict Advanced Pancreatic Cancer at Diagnosis. Biomarkers 2020, 25, 186–193. [Google Scholar] [CrossRef]
- Bannas, P.; Well, L.; Lenz, A.; Rissiek, B.; Haag, F.; Schmid, J.; Hochgräfe, K.; Trepel, M.; Adam, G.; Ittrich, H.; et al. In Vivo Near-Infrared Fluorescence Targeting of T Cells: Comparison of Nanobodies and Conventional Monoclonal Antibodies. Contrast Media Mol. Imaging 2014, 9, 135–142. [Google Scholar] [CrossRef]
- Lwin, T.M.; Turner, M.A.; Nishino, H.; Amirfakhri, S.; Hernot, S.; Hoffman, R.M.; Bouvet, M. Fluorescent Anti-CEA Nanobody for Rapid Tumor-Targeting and Imaging in Mouse Models of Pancreatic Cancer. Biomolecules 2022, 12, 711. [Google Scholar] [CrossRef]
- van Brussel, A.S.A.; Adams, A.; Oliveira, S.; Dorresteijn, B.; El Khattabi, M.; Vermeulen, J.F.; van der Wall, E.; Mali, W.P.T.M.; Derksen, P.W.B.; van Diest, P.J.; et al. Hypoxia-Targeting Fluorescent Nanobodies for Optical Molecular Imaging of Pre-Invasive Breast Cancer. Mol. Imaging Biol. 2016, 18, 535–544. [Google Scholar] [CrossRef]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the Response to CTLA-4 Blockade by Longitudinal Noninvasive Monitoring of CD8 T Cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef]
- Lecocq, Q.; Debie, P.; Puttemans, J.; Awad, R.M.; De Beck, L.; Ertveldt, T.; De Vlaeminck, Y.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; et al. Evaluation of Single Domain Antibodies as Nuclear Tracers for Imaging of the Immune Checkpoint Receptor Human Lymphocyte Activation Gene-3 in Cancer. EJNMMI Res. 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Zeven, K.; De Vlaeminck, Y.; Martens, S.; Massa, S.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; Breckpot, K.; Devoogdt, N. Noninvasive Imaging of the Immune Checkpoint LAG-3 Using Nanobodies, from Development to Pre-Clinical Use. Biomolecules 2019, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s Disease: Recent Treatment Strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, K.; Iqbal, U.; Tanha, J.; MacKenzie, R.; Moreno, M.; Stanimirovic, D. Single-Domain Antibodies as Therapeutic and Imaging Agents for the Treatment of CNS Diseases. Antibodies 2019, 8, 27. [Google Scholar] [CrossRef]
- Li, T.; Vandesquille, M.; Koukouli, F.; Dudeffant, C.; Youssef, I.; Lenormand, P.; Ganneau, C.; Maskos, U.; Czech, C.; Grueninger, F.; et al. Camelid Single-Domain Antibodies: A Versatile Tool for in Vivo Imaging of Extracellular and Intracellular Brain Targets. J. Control. Release 2016, 243, 1–10. [Google Scholar] [CrossRef]
- Danis, C.; Dupré, E.; Zejneli, O.; Caillierez, R.; Arrial, A.; Bégard, S.; Mortelecque, J.; Eddarkaoui, S.; Loyens, A.; Cantrelle, F.-X.; et al. Inhibition of Tau Seeding by Targeting Tau Nucleation Core within Neurons with a Single Domain Antibody Fragment. Mol. Ther. 2022, 30, 1484–1499. [Google Scholar] [CrossRef]
- Marino, M.; Holt, M.G. AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System. Front. Neurol. 2022, 13, 870799. [Google Scholar] [CrossRef]
- Marino, M.; Zhou, L.; Rincon, M.Y.; Callaerts-Vegh, Z.; Verhaert, J.; Wahis, J.; Creemers, E.; Yshii, L.; Wierda, K.; Saito, T.; et al. AAV-Mediated Delivery of an Anti-BACE1 VHH Alleviates Pathology in an Alzheimer’s Disease Model. EMBO Mol. Med. 2022, 14, e09824. [Google Scholar] [CrossRef] [PubMed]
- Butler, Y.R.; Liu, Y.; Kumbhar, R.; Zhao, P.; Gadhave, K.; Wang, N.; Li, Y.; Mao, X.; Wang, W. α-Synuclein Fibril-Specific Nanobody Reduces Prion-like α-Synuclein Spreading in Mice. Nat. Commun. 2022, 13, 4060. [Google Scholar] [CrossRef]
- Butler, D.C.; Joshi, S.N.; Genst, E.D.; Baghel, A.S.; Dobson, C.M.; Messer, A. Bifunctional Anti-Non-Amyloid Component α-Synuclein Nanobodies Are Protective In Situ. PLoS ONE 2016, 11, e0165964. [Google Scholar] [CrossRef]
- Leemans, M.; Galicia, C.; Deyaert, E.; Daems, E.; Krause, L.; Paesmans, J.; Pardon, E.; Steyaert, J.; Kortholt, A.; Sobott, F.; et al. Allosteric Modulation of the GTPase Activity of a Bacterial LRRK2 Homolog by Conformation-Specific Nanobodies. Biochem. J. 2020, 477, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Vultaggio, A.; Danesi, R. The Use of Intravenous versus Subcutaneous Monoclonal Antibodies in the Treatment of Severe Asthma: A Review. Respir. Res. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Steeland, S.; Puimège, L.; Vandenbroucke, R.E.; Van Hauwermeiren, F.; Haustraete, J.; Devoogdt, N.; Hulpiau, P.; Leroux-Roels, G.; Laukens, D.; Meuleman, P.; et al. Generation and Characterization of Small Single Domain Antibodies Inhibiting Human Tumor Necrosis Factor Receptor 1. J. Biol. Chem. 2015, 290, 4022–4037. [Google Scholar] [CrossRef] [PubMed]
- Formatted Anti–Tumor Necrosis Factor α VHH Proteins Derived From Camelids Show Superior Potency and Targeting to Inflamed Joints in a Murine Model of Collagen-Induced Arthritis—Coppieters—2006—Arthritis & Rheumatism—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/art.21827 (accessed on 5 August 2023).
- Nurbhai, S.; Roberts, K.J.; Carlton, T.M.; Maggiore, L.; Cubitt, M.F.; Ray, K.P.; Reckless, J.; Mohammed, H.; Irving, P.; MacDonald, T.T.; et al. Oral Anti-Tumour Necrosis Factor Domain Antibody V565 Provides High Intestinal Concentrations, and Reduces Markers of Inflammation in Ulcerative Colitis Patients. Sci. Rep. 2019, 9, 14042. [Google Scholar] [CrossRef] [PubMed]
- Baral, T.N.; Magez, S.; Stijlemans, B.; Conrath, K.; Vanhollebeke, B.; Pays, E.; Muyldermans, S.; De Baetselier, P. Experimental Therapy of African Trypanosomiasis with a Nanobody-Conjugated Human Trypanolytic Factor. Nat. Med. 2006, 12, 580–584. [Google Scholar] [CrossRef]
- Hempelmann, A.; Hartleb, L.; van Straaten, M.; Hashemi, H.; Zeelen, J.P.; Bongers, K.; Papavasiliou, F.N.; Engstler, M.; Stebbins, C.E.; Jones, N.G. Nanobody-Mediated Macromolecular Crowding Induces Membrane Fission and Remodeling in the African Trypanosome. Cell Rep. 2021, 37, 109923. [Google Scholar] [CrossRef]
- Scally, S.W.; Triglia, T.; Evelyn, C.; Seager, B.A.; Pasternak, M.; Lim, P.S.; Healer, J.; Geoghegan, N.D.; Adair, A.; Tham, W.-H.; et al. PCRCR Complex Is Essential for Invasion of Human Erythrocytes by Plasmodium Falciparum. Nat. Microbiol. 2022, 7, 2039–2053. [Google Scholar] [CrossRef]
- Dietrich, M.H.; Gabriela, M.; Reaksudsan, K.; Dixon, M.W.A.; Chan, L.-J.; Adair, A.; Trickey, S.; O’Neill, M.T.; Tan, L.L.; Lopaticki, S.; et al. Nanobodies against Pfs230 Block Plasmodium Falciparum Transmission. Biochem. J. 2022, 479, 2529–2546. [Google Scholar] [CrossRef]
- Ukegbu, C.V.; Giorgalli, M.; Tapanelli, S.; Rona, L.D.P.; Jaye, A.; Wyer, C.; Angrisano, F.; Blagborough, A.M.; Christophides, G.K.; Vlachou, D. PIMMS43 Is Required for Malaria Parasite Immune Evasion and Sporogonic Development in the Mosquito Vector. Proc. Natl. Acad. Sci. USA. 2020, 117, 7363–7373. [Google Scholar] [CrossRef]
- Valenzuela-Nieto, G.; Miranda-Chacon, Z.; Salinas-Rebolledo, C.; Jara, R.; Cuevas, A.; Berking, A.; Rojas-Fernandez, A. Nanobodies: COVID-19 and Future Perspectives. Front. Drug Discov. 2022, 2, 927164. [Google Scholar] [CrossRef]
- Xiang, Y.; Nambulli, S.; Xiao, Z.; Liu, H.; Sang, Z.; Duprex, W.P.; Schneidman-Duhovny, D.; Zhang, C.; Shi, Y. Versatile and Multivalent Nanobodies Efficiently Neutralize SARS-CoV-2. Science 2020, 370, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zeng, W.; Meng, X.; Huang, X.; Yang, Y.; Zhao, D.; Zhou, P.; Wang, X.; Zhao, C.; Sun, Y.; et al. Potent Neutralization of SARS-CoV-2 by Hetero-Bivalent Alpaca Nanobodies Targeting the Spike Receptor-Binding Domain. J. Virol. 2021, 95, e02438-20. [Google Scholar] [CrossRef]
- Dong, J.; Huang, B.; Wang, B.; Titong, A.; Gallolu Kankanamalage, S.; Jia, Z.; Wright, M.; Parthasarathy, P.; Liu, Y. Development of Humanized Tri-Specific Nanobodies with Potent Neutralization for SARS-CoV-2. Sci. Rep. 2020, 10, 17806. [Google Scholar] [CrossRef] [PubMed]
- Koenig, P.-A.; Das, H.; Liu, H.; Kümmerer, B.M.; Gohr, F.N.; Jenster, L.-M.; Schiffelers, L.D.J.; Tesfamariam, Y.M.; Uchima, M.; Wuerth, J.D.; et al. Structure-Guided Multivalent Nanobodies Block SARS-CoV-2 Infection and Suppress Mutational Escape. Science 2021, 371, eabe6230. [Google Scholar] [CrossRef] [PubMed]
- Pymm, P.; Redmond, S.J.; Dolezal, O.; Mordant, F.; Lopez, E.; Cooney, J.P.; Davidson, K.C.; Haycroft, E.R.; Tan, C.W.; Seneviratna, R.; et al. Biparatopic Nanobodies Targeting the Receptor Binding Domain Efficiently Neutralize SARS-CoV-2. iScience 2022, 25, 105259. [Google Scholar] [CrossRef] [PubMed]
- Hanke, L.; Vidakovics Perez, L.; Sheward, D.J.; Das, H.; Schulte, T.; Moliner-Morro, A.; Corcoran, M.; Achour, A.; Karlsson Hedestam, G.B.; Hällberg, B.M.; et al. An Alpaca Nanobody Neutralizes SARS-CoV-2 by Blocking Receptor Interaction. Nat. Commun. 2020, 11, 4420. [Google Scholar] [CrossRef]
- Moliner-Morro, A.; Sheward, D.J.; Karl, V.; Perez Vidakovics, L.; Murrell, B.; McInerney, G.M.; Hanke, L. Picomolar SARS-CoV-2 Neutralization Using Multi-Arm PEG Nanobody Constructs. Biomolecules 2020, 10, 1661. [Google Scholar] [CrossRef]
- Gai, J.; Ma, L.; Li, G.; Zhu, M.; Qiao, P.; Li, X.; Zhang, H.; Zhang, Y.; Chen, Y.; Ji, W.; et al. A Potent Neutralizing Nanobody against SARS-CoV-2 with Inhaled Delivery Potential. MedComm 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Tang, Z.; Chen, Q.; Liu, X. Development of a Biotin-Streptavidin-Amplified Nanobody-Based ELISA for Ochratoxin A in Cereal. Ecotoxicol. Environ. Saf. 2019, 171, 382–388. [Google Scholar] [CrossRef]
- Gu, K.; Song, Z.; Zhou, C.; Ma, P.; Li, C.; Lu, Q.; Liao, Z.; Huang, Z.; Tang, Y.; Li, H.; et al. Development of Nanobody-Horseradish Peroxidase-Based Sandwich ELISA to Detect Salmonella Enteritidis in Milk and in Vivo Colonization in Chicken. J. Nanobiotechnology 2022, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zakri, A.M.; Al-Doss, A.A.; Ali, A.A.; Samara, E.M.; Ahmed, B.S.; Al-Saleh, M.A.; Idris, A.M.; Abdalla, O.A.; Sack, M. Generation and Characterization of Nanobodies Against Tomato Leaf Curl Sudan Virus. Plant Dis. 2021, 105, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, C.; Djennane, S.; Ackerer, L.; Hleibieh, K.; Marmonier, A.; Gersch, S.; Garcia, S.; Vigne, E.; Komar, V.; Perrin, M.; et al. Nanobody-Mediated Resistance to Grapevine Fanleaf Virus in Plants. Plant Biotechnol. J. 2018, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ding, C.; Zheng, D.; Ma, X.; Yi, L.; Tong, X.; Wu, C.; Xue, C.; Yu, Y.; Zhou, Q. Nanobody Conjugates for Targeted Cancer Therapy and Imaging. Technol. Cancer Res. Treat. 2021, 20, 15330338211010117. [Google Scholar] [CrossRef] [PubMed]
- Roovers, R.C.; Laeremans, T.; Huang, L.; De Taeye, S.; Verkleij, A.J.; Revets, H.; de Haard, H.J.; van Bergen en Henegouwen, P.M.P. Efficient Inhibition of EGFR Signaling and of Tumour Growth by Antagonistic Anti-EFGR Nanobodies. Cancer Immunol. Immunother. 2007, 56, 303–317. [Google Scholar] [CrossRef]
- Roovers, R.C.; Vosjan, M.J.W.D.; Laeremans, T.; el Khoulati, R.; de Bruin, R.C.G.; Ferguson, K.M.; Verkleij, A.J.; van Dongen, G.A.M.S.; van Bergen en Henegouwen, P.M.P. A Biparatopic Anti-EGFR Nanobody Efficiently Inhibits Solid Tumour Growth. Int. J. Cancer 2011, 129, 2013–2024. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, X.; Yang, X.; Xu, Y.; Zhou, Z.; Pan, L.; Chen, S. A Multivalent Biparatopic EGFR-Targeting Nanobody Drug Conjugate Displays Potent Anticancer Activity in Solid Tumor Models. Signal Transduct. Target. Ther. 2021, 6, 320. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Ding, L.; Tian, C.; Feng, S.; Fida, G.; Zhang, C.; Ma, Y.; Ai, G.; Achilefu, S.; Gu, Y. Small Sized EGFR1 and HER2 Specific Bifunctional Antibody for Targeted Cancer Therapy. Theranostics 2015, 5, 378–398. [Google Scholar] [CrossRef]
- Hassanzadeh Eskafi, A.; Oghalaei, A.; Mahboudi, F.; Ghaderi, H.; Behdani, M.; Shoari, A.; Kazemi-Lomedasht, F. Investigation of the Therapeutic Potential of Recombinant Bispecific Bivalent Anti-PD-L1/VEGF Nanobody in Inhibition of Angiogenesis. Immunopharmacol. Immunotoxicol. 2023, 45, 197–202. [Google Scholar] [CrossRef]
- de Bruin, R.C.G.; Veluchamy, J.P.; Lougheed, S.M.; Schneiders, F.L.; Lopez-Lastra, S.; Lameris, R.; Stam, A.G.; Sebestyen, Z.; Kuball, J.; Molthoff, C.F.M.; et al. A Bispecific Nanobody Approach to Leverage the Potent and Widely Applicable Tumor Cytolytic Capacity of Vγ9Vδ2-T Cells. OncoImmunology 2018, 7, e1375641. [Google Scholar] [CrossRef] [PubMed]
- De Munter, S.; Ingels, J.; Goetgeluk, G.; Bonte, S.; Pille, M.; Weening, K.; Kerre, T.; Abken, H.; Vandekerckhove, B. Nanobody Based Dual Specific CARs. Int. J. Mol. Sci. 2018, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Brown, S. Antibody–Drug Conjugates as Novel Anti-Cancer Chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef]
- Gheibi hayat, S.M.; Sahebkar, A.H. Antibody Drug Conjugates for Cancer Therapy. J. Babol Univ. Med. Sci. 2017, 19, 20–27. [Google Scholar] [CrossRef]
- Matsuda, Y.; Mendelsohn, B.A. An Overview of Process Development for Antibody-Drug Conjugates Produced by Chemical Conjugation Technology. Expert Opin. Biol. Ther. 2021, 21, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, E.R.; Woodham, A.W.; Ploegh, H.L. Nanobodies in Cancer. Semin. Immunol. 2021, 52, 101425. [Google Scholar] [CrossRef]
- Panikar, S.S.; Banu, N.; Haramati, J.; del Toro-Arreola, S.; Riera Leal, A.; Salas, P. Nanobodies as Efficient Drug-Carriers: Progress and Trends in Chemotherapy. J. Control. Release 2021, 334, 389–412. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Zhou, P.; Qiao, Y.; Li, Y.; Xu, Y.; Shi, X. Generation of a High-Affinity Nanobody Against CD147 for Tumor Targeting and Therapeutic Efficacy Through Conjugating Doxorubicin. Front. Immunol. 2022, 13, 852700. [Google Scholar] [CrossRef]
- Stenton, B.J.; Oliveira, B.L.; Matos, M.J.; Sinatra, L.; Bernardes, G.J.L. A Thioether-Directed Palladium-Cleavable Linker for Targeted Bioorthogonal Drug Decaging. Chem. Sci. 2018, 9, 4185–4189. [Google Scholar] [CrossRef]
- Ma, J.; Xu, X.; Fu, C.; Xia, P.; Tian, M.; Zheng, L.; Chen, K.; Liu, X.; Li, Y.; Yu, L.; et al. CDH17 Nanobodies Facilitate Rapid Imaging of Gastric Cancer and Efficient Delivery of Immunotoxin. Biomater. Res. 2022, 26, 64. [Google Scholar] [CrossRef]
- Behdani, M.; Zeinali, S.; Karimipour, M.; Khanahmad, H.; Schoonooghe, S.; Aslemarz, A.; Seyed, N.; Moazami-Godarzi, R.; Baniahmad, F.; Habibi-Anbouhi, M.; et al. Development of VEGFR2-Specific Nanobody Pseudomonas Exotoxin A Conjugated to Provide Efficient Inhibition of Tumor Cell Growth. New Biotechnol. 2013, 30, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, L.; Grivel, J.-C.; Farag, E.A.B.A.; Pavlovski, I.; Maacha, S.; Sathappan, A.; Al-Romaihi, H.E.; Abuaqel, S.W.J.; Ata, M.M.A.; Chouchane, A.I.; et al. Dromedary Camels as a Natural Source of Neutralizing Nanobodies against SARS-CoV-2. JCI Insight 2021, 6, e145785. [Google Scholar] [CrossRef] [PubMed]
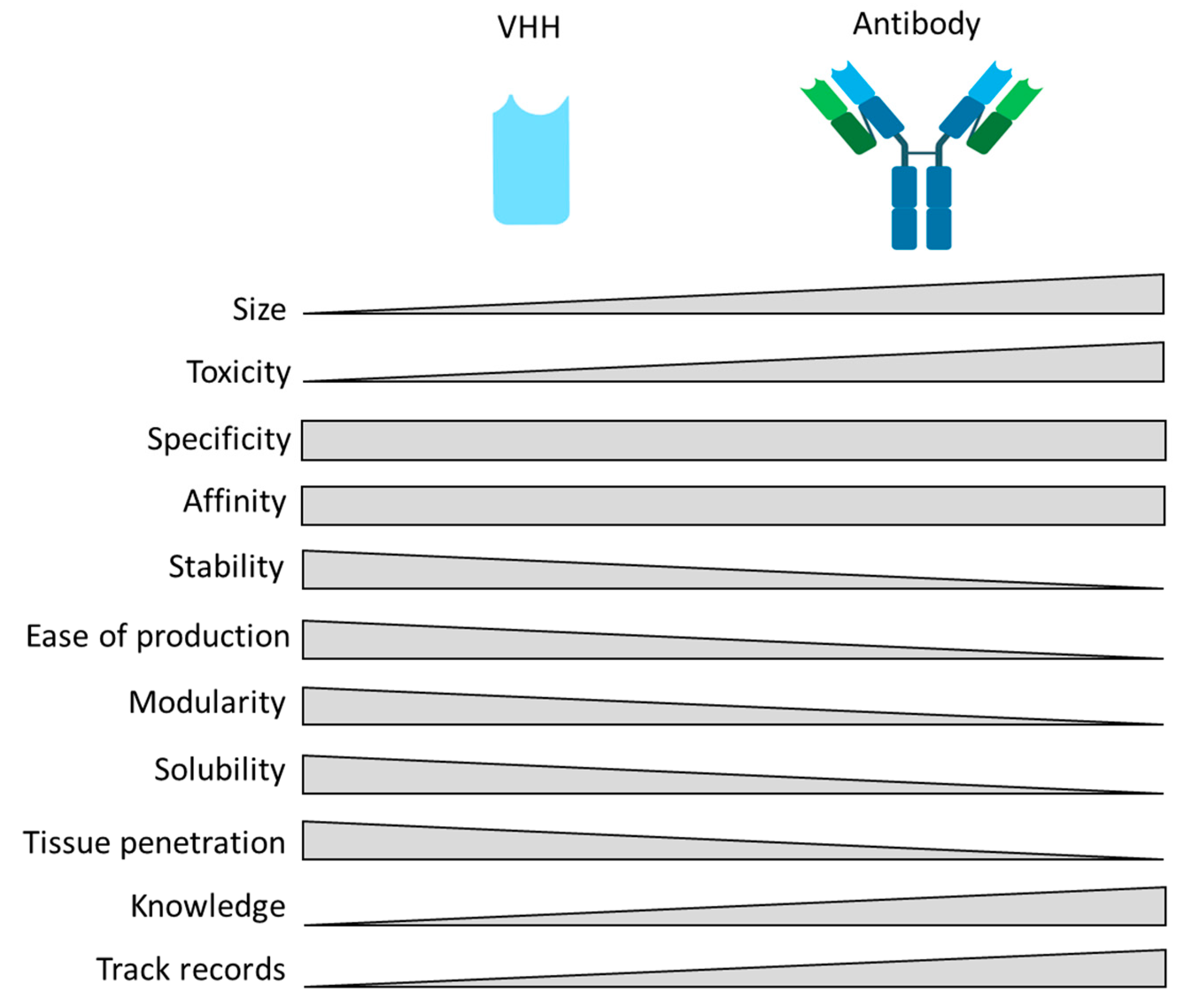


| Properties | VHH | Conventional Antibody | References |
|---|---|---|---|
| Immunogenicity | Not yet well documented, needs to be more investigated. | Often | Ackaert, C., et al., 2021 [31] |
| Thermostability | Up to 90 °C | <90 °C | Van der Linder, R., et al. 2000 [32]; Arbabi Ghahroudi, M., et al. 1997 [33]; Kunz, P., et al., 2018 [34] |
| Nasal administration | Aerosol | No | Rohm, M et al., 2017 [35]; Schoof, M., et al., 2020 [36] |
| Size | 15 kDa | 150 kDa | Hamers-Casterman, C., et al., 1993 [3] |
| Tissue penetration | BBB *, ECM **, grooves, clefts | No | Subrahmanyam, N., et al., 2021 [37]; Debie, P., et al. 2020 [38]; Pothin, E., et al., 2020 [39] |
| Stability/solubility | High stability and solubility | Soluble and stable with risk of aggregation | Davies, J., et al., 1996 [40]; Dumoulin, M., et al., 2009 [41]; Harmsen, M., et al., 2007 [42] |
| Half life | Fast clearance but could be modulated by e.g., adding an albumin-binding VHH, an Fc domain, or Polyethylene glycol ((PEG)-ylation) | Long (Fc-mediated) | Konterman, R.E., et al., 2011 [43]; Wunder, A., et al., 2003 [44]; Fishburn, C.S., et al., 2008 [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunz, S.; Durandy, M.; Seguin, L.; Feral, C.C. NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions. Int. J. Mol. Sci. 2023, 24, 13229. https://doi.org/10.3390/ijms241713229
Kunz S, Durandy M, Seguin L, Feral CC. NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions. International Journal of Molecular Sciences. 2023; 24(17):13229. https://doi.org/10.3390/ijms241713229
Chicago/Turabian StyleKunz, Sarah, Manon Durandy, Laetitia Seguin, and Chloe C. Feral. 2023. "NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions" International Journal of Molecular Sciences 24, no. 17: 13229. https://doi.org/10.3390/ijms241713229





