Picrasidine J, a Dimeric β-Carboline-Type Alkaloid from Picrasma quassioides, Inhibits Metastasis of Head and Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
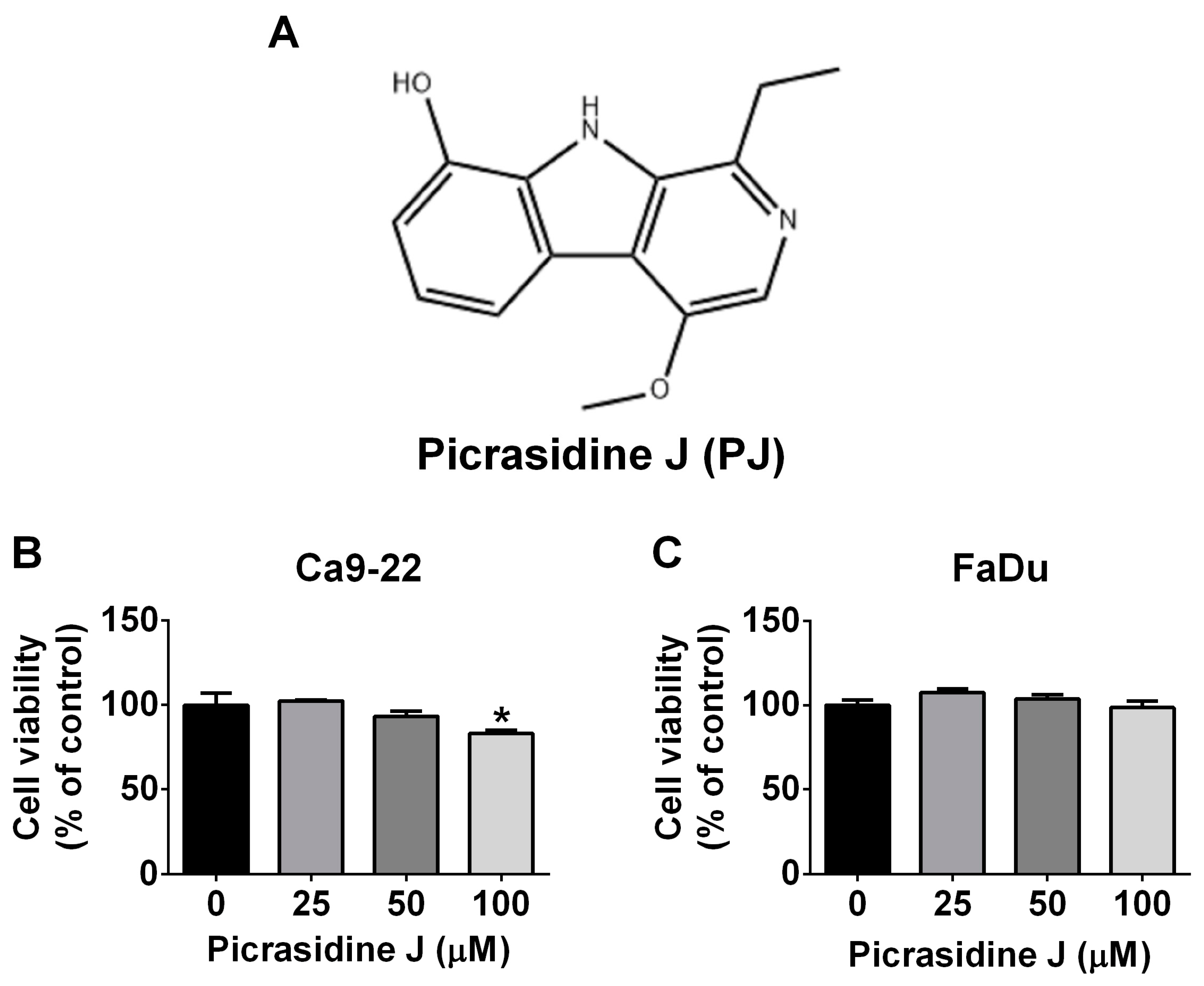
2.1. Cytotoxic Effect of Picrasidine J in HNSCC Cells
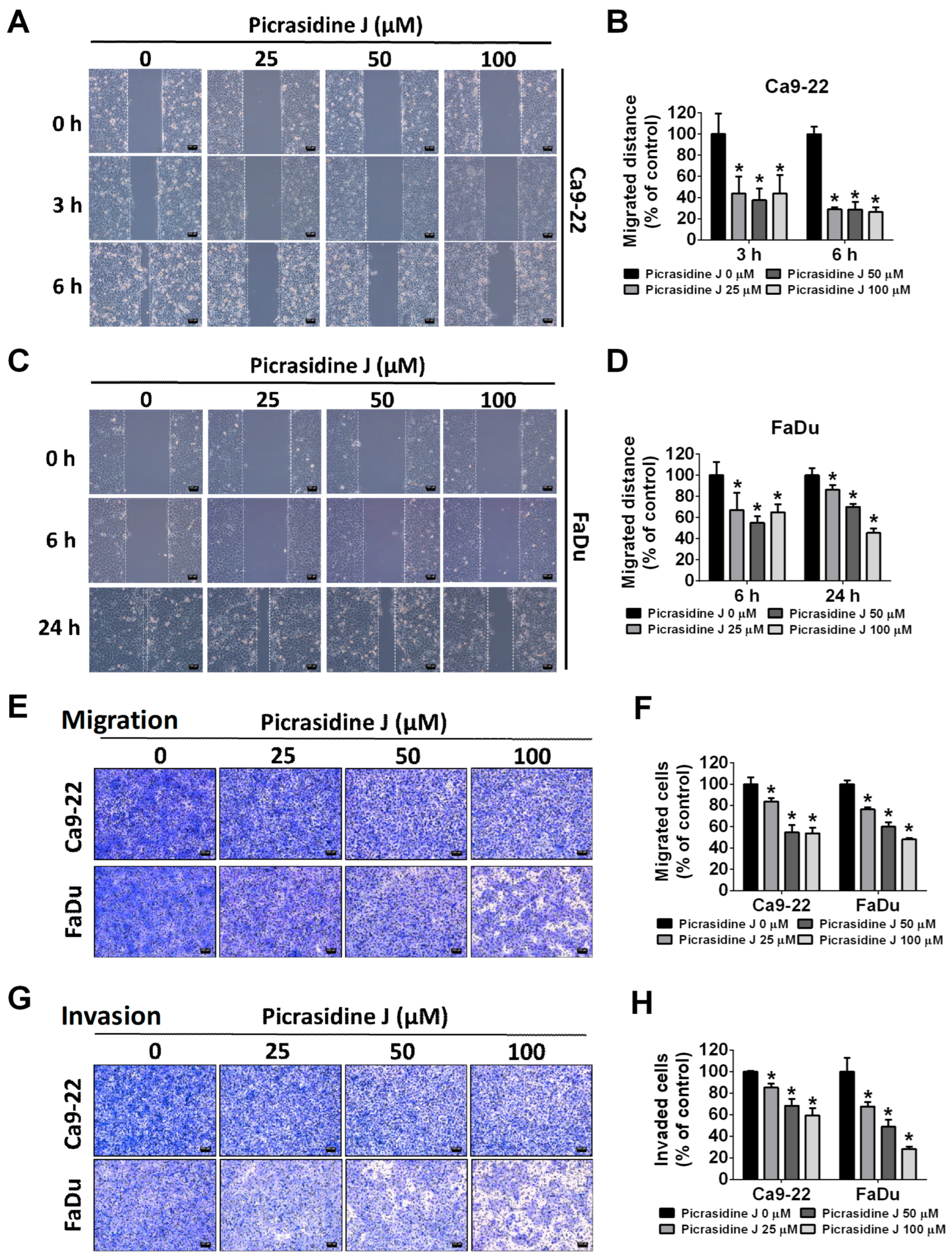
2.2. Effect of Picrasidine J on Migration and Invasion of HNSCC Cells
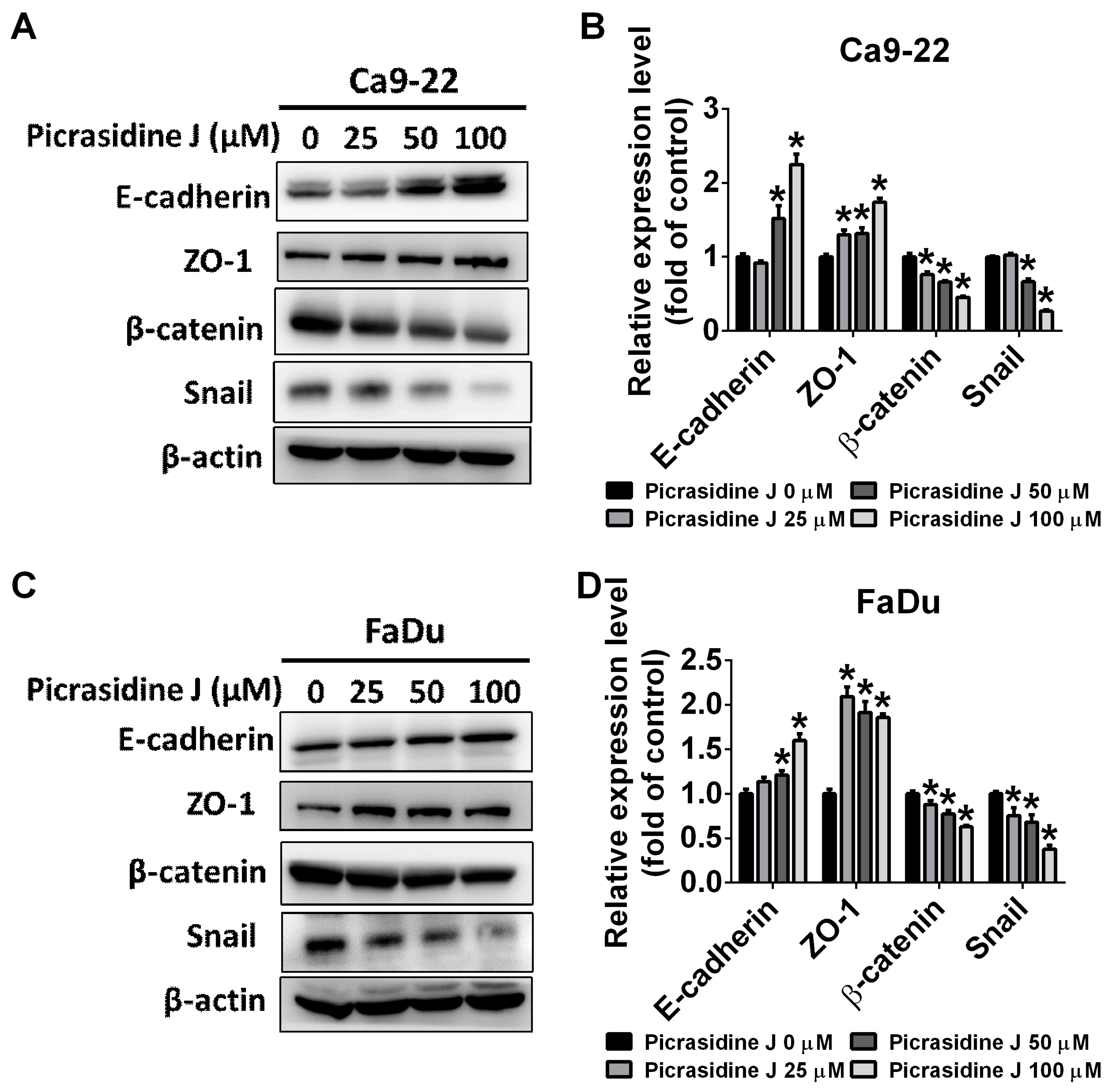
2.3. Effect of Picrasidine J on Epithelial–Mesenchymal Transition of HNSCC Cells
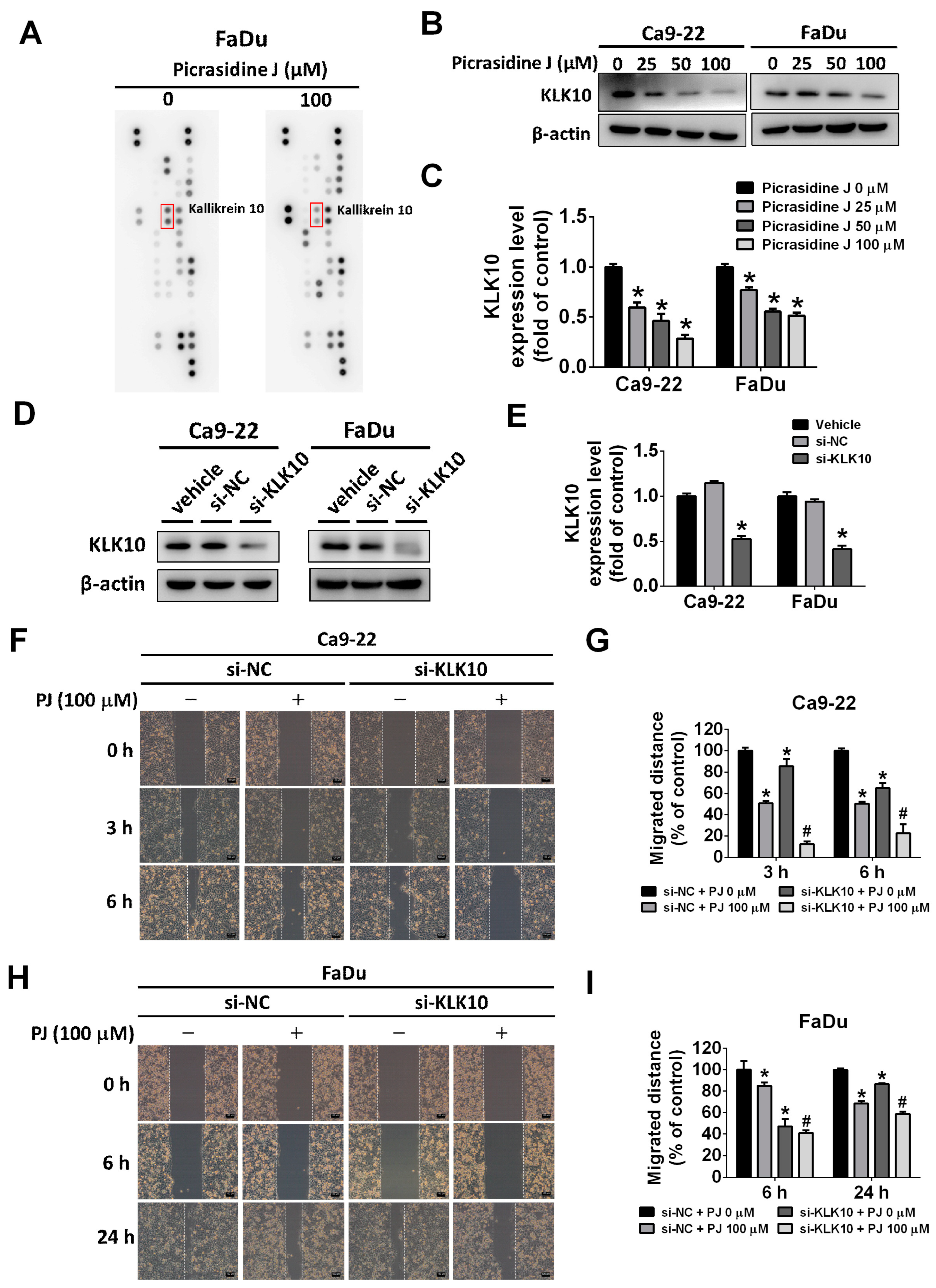
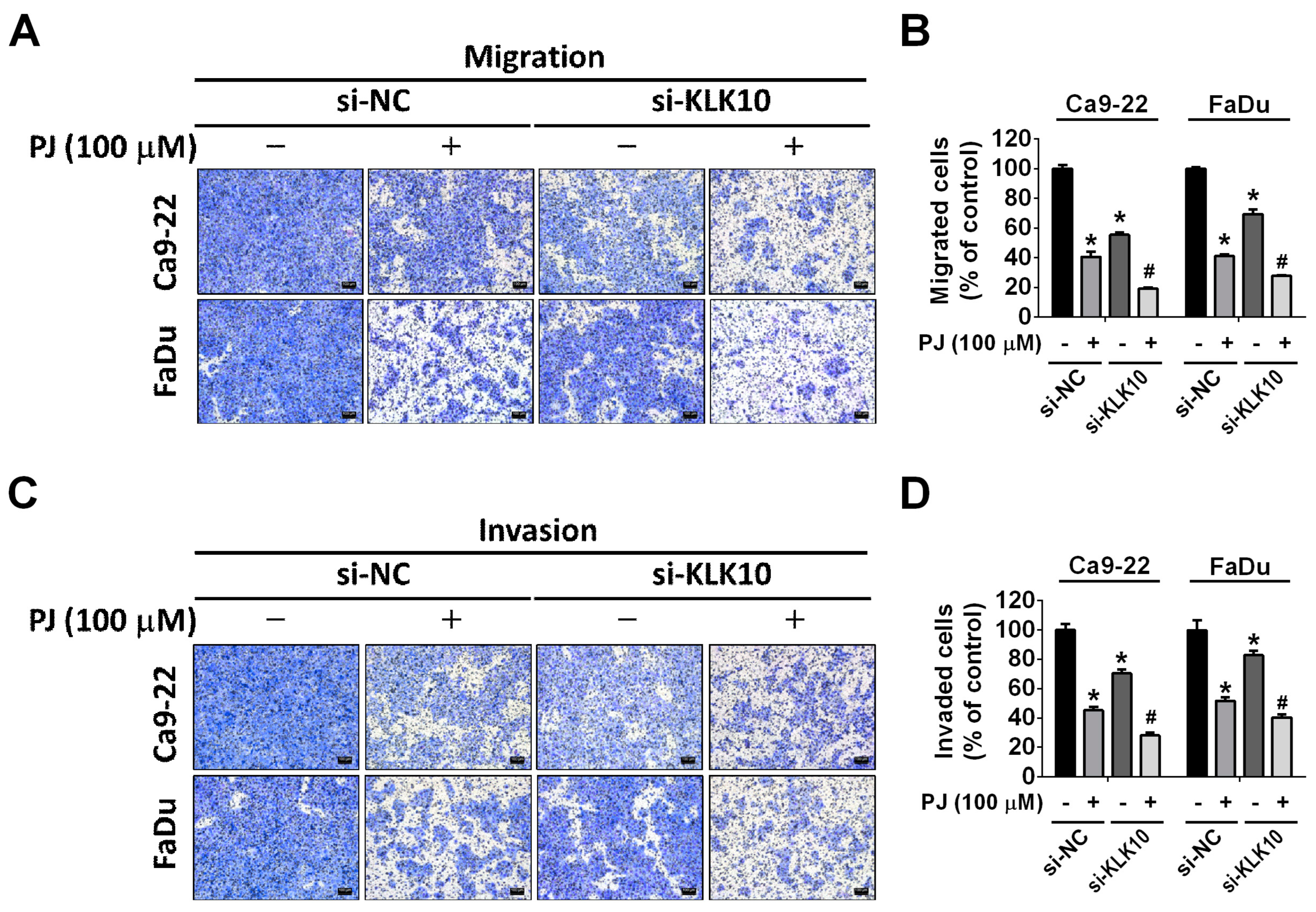
2.4. Effect of Picrasidine J on Kallikrein-10 Expression in HNSCC Cells
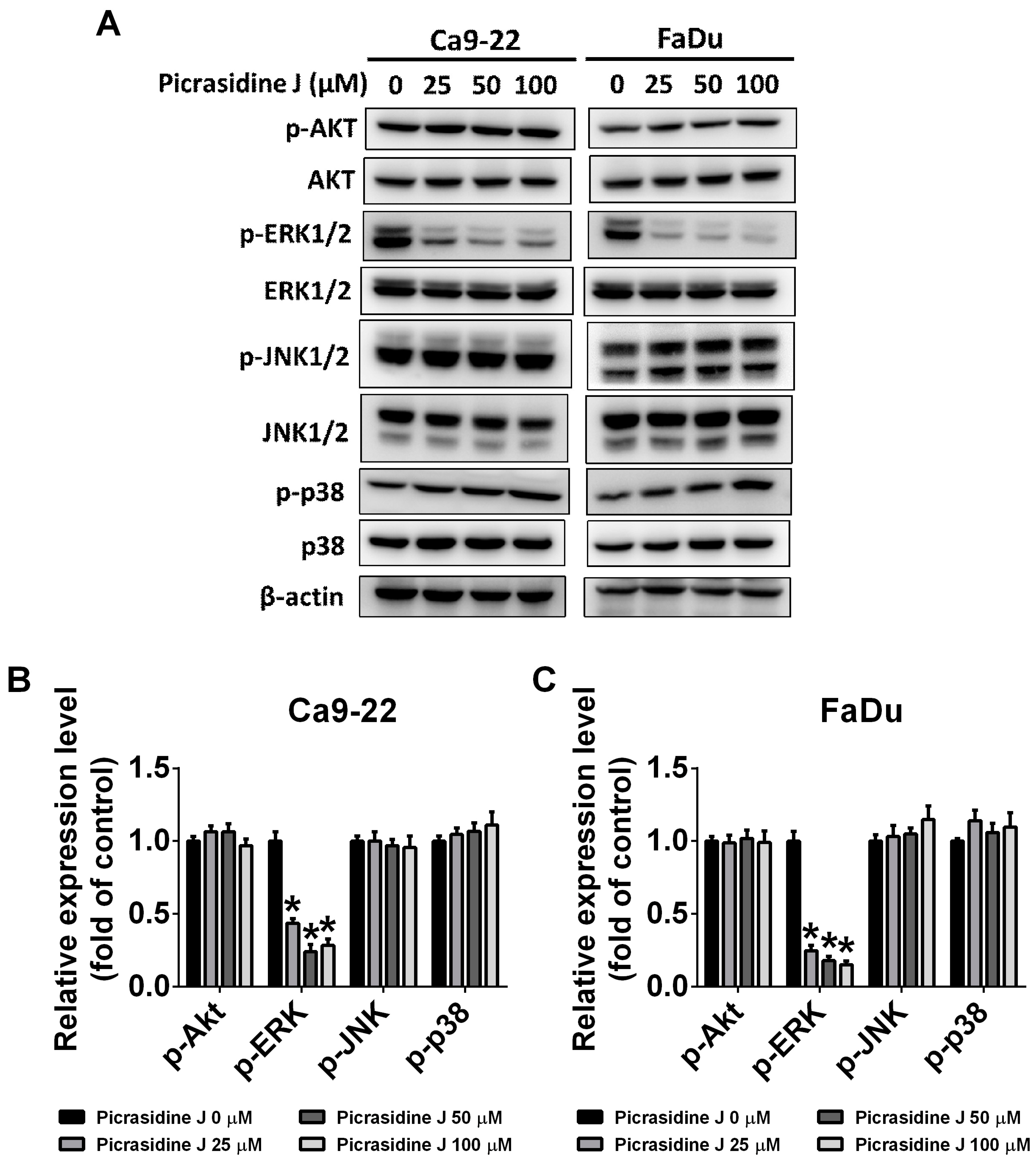
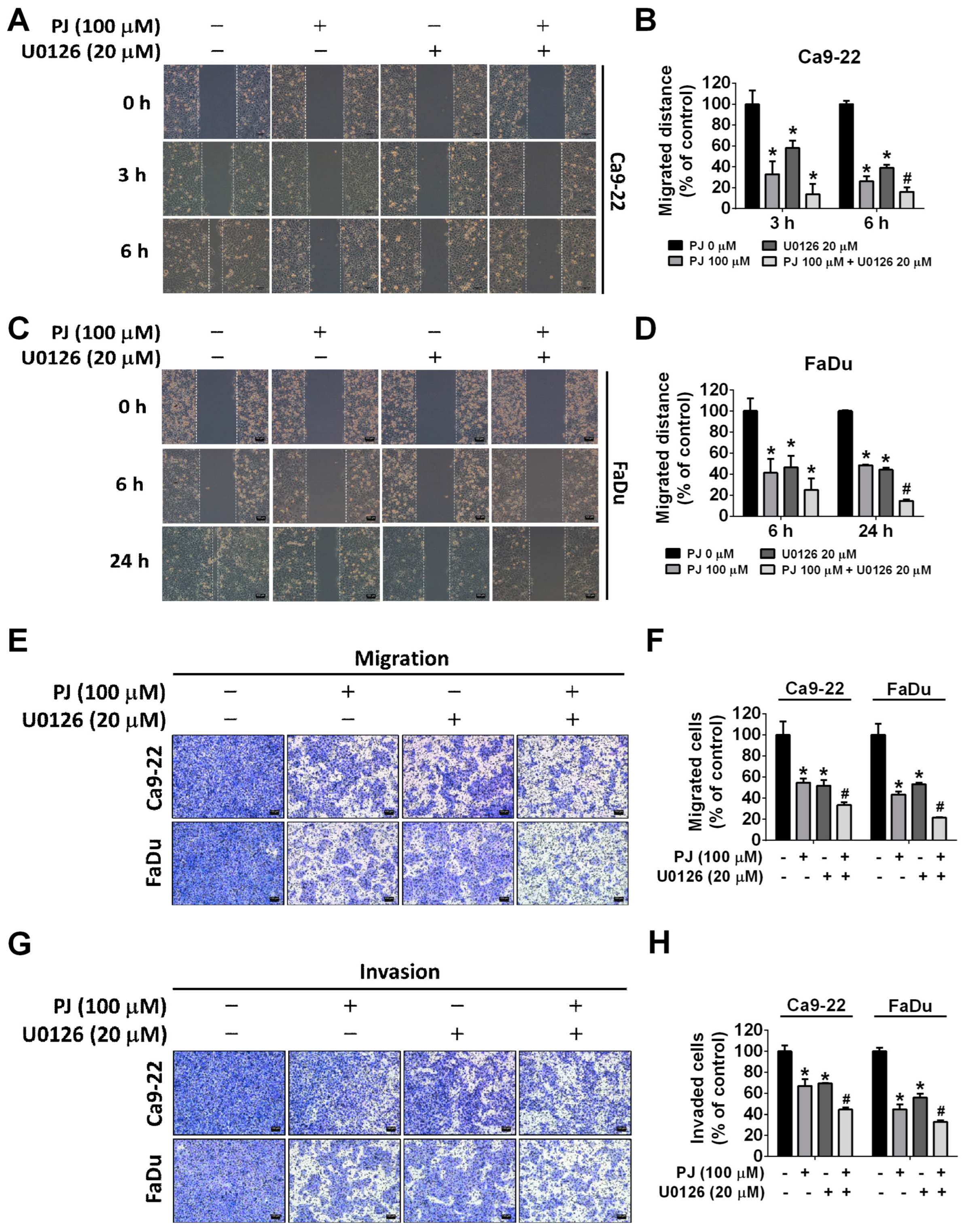
2.5. Picrasidine J Inhibits Cell Metastasis via ERK Signaling Pathway in HNSCC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Picrasidine J Treatments
4.3. MTT Assay
4.4. Wound Healing Assay
4.5. Cell Migration and Invasion Assay
4.6. Western Blot Assay
4.7. Gene Silencing and Transfection
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Diana, G.; Corica, C. Human Papilloma Virus vaccine and prevention of head and neck cancer, what is the current evidence? Oral. Oncol. 2021, 115, 105168. [Google Scholar] [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Tolstonog, G.; Simon, C. Trends in Surgical Research in Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 38. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Trotti, A.; Pajak, T.F.; Gwede, C.K.; Paulus, R.; Cooper, J.; Forastiere, A.; Ridge, J.A.; Watkins-Bruner, D.; Garden, A.S.; Ang, K.K.; et al. TAME: Development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007, 8, 613–624. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Saada-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Hsieh, M.J.; Chuang, Y.C.; Lin, C.C.; Lo, Y.S.; Ho, H.Y.; Kumar, V.B.; Ko, J.L. Anticancer effects of picrasidine I on oral squamous cell carcinoma. Environ. Toxicol. 2022, 37, 627–636. [Google Scholar] [CrossRef]
- Zhao, S.; Kanno, Y.; Li, W.; Sasaki, T.; Zhang, X.; Wang, J.; Cheng, M.; Koike, K.; Nemoto, K.; Li, H. Identification of Picrasidine C as a Subtype-Selective PPARalpha Agonist. J. Nat. Prod. 2016, 79, 3127–3133. [Google Scholar] [CrossRef]
- Zhao, S.; Kanno, Y.; Li, W.; Wakatabi, H.; Sasaki, T.; Koike, K.; Nemoto, K.; Li, H. Picrasidine N Is a Subtype-Selective PPARbeta/delta Agonist. J. Nat. Prod. 2016, 79, 879–885. [Google Scholar] [CrossRef]
- Yamashita, N.; Kondo, M.; Zhao, S.; Li, W.; Koike, K.; Nemoto, K.; Kanno, Y. Picrasidine G decreases viability of MDA-MB 468 EGFR-overexpressing triple-negative breast cancer cells through inhibition of EGFR/STAT3 signaling pathway. Bioorg Med. Chem. Lett. 2017, 27, 2608–2612. [Google Scholar] [CrossRef]
- Ohmoto, T.; Koike, K.; Higuchi, T.; Ikeda, K. Studies on the Alkaloids from Picrasma quassioides BENNET. IV. Structures of Picrasidines I, J, and K. Chem. Pharm. Bull. 1985, 33, 3356–3360. [Google Scholar] [CrossRef]
- Ho, H.Y.; Chen, P.J.; Chuang, Y.C.; Lo, Y.S.; Lin, C.C.; Hsieh, M.J.; Chen, M.K. Picrasidine I Triggers Heme Oxygenase-1-Induced Apoptosis in Nasopharyngeal Carcinoma Cells via ERK and Akt Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 6103. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Worsham, M.J.; Chen, K.M.; Meduri, V.; Nygren, A.O.; Errami, A.; Schouten, J.P.; Benninger, M.S. Epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2006, 132, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Sharma, V.P.; Condeelis, J.; Harris, T.; Ow, T.J.; Prystowsky, M.B.; Childs, G.; Segall, J.E. MicroRNA-375 Suppresses Extracellular Matrix Degradation and Invadopodial Activity in Head and Neck Squamous Cell Carcinoma. Arch. Pathol. Lab. Med. 2015, 139, 1349–1361. [Google Scholar] [CrossRef]
- Vachani, A.; Nebozhyn, M.; Singhal, S.; Alila, L.; Wakeam, E.; Muschel, R.; Powell, C.A.; Gaffney, P.; Singh, B.; Brose, M.S.; et al. A 10-gene classifier for distinguishing head and neck squamous cell carcinoma and lung squamous cell carcinoma. Clin. Cancer Res. 2007, 13, 2905–2915. [Google Scholar] [CrossRef]
- Dasgupta, S.; Tripathi, P.K.; Qin, H.; Bhattacharya-Chatterjee, M.; Valentino, J.; Chatterjee, S.K. Identification of molecular targets for immunotherapy of patients with head and neck squamous cell carcinoma. Oral. Oncol. 2006, 42, 306–316. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecinska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Fredimoses, M.; Song, M.; Chen, H.; Liu, K.; Lee, M.H.; Dong, Z. FGFR2 regulation by picrasidine Q inhibits the cell growth and induces apoptosis in esophageal squamous cell carcinoma. J. Cell Biochem. 2018, 119, 2231–2239. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Lin, J.T.; Mahalakshmi, B.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Ho, H.Y.; Hsieh, M.J.; Chen, M.K. Dehydrocrenatidine inhibits head and neck cancer cells invasion and migration by modulating JNK1/2 and ERK1/2 pathway and decreases MMP-2 expression. Environ. Toxicol. 2021, 36, 1848–1856. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, F.; Huang, J.; Xiong, C. Mechanical transmission enables EMT cancer cells to drive epithelial cancer cell migration to guide tumor spheroid disaggregation. Sci. China Life Sci. 2022, 65, 2031–2049. [Google Scholar] [CrossRef]
- Dave, B.; Mittal, V.; Tan, N.M.; Chang, J.C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Borgono, C.A.; Diamandis, E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 2004, 4, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.C.; Grass, L.; Soosaipillai, A.; Sotiropoulou, G.; Diamandis, E.P. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 2004, 25, 193–199. [Google Scholar] [CrossRef]
- Michael, I.P.; Sotiropoulou, G.; Pampalakis, G.; Magklara, A.; Ghosh, M.; Wasney, G.; Diamandis, E.P. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 2005, 280, 14628–14635. [Google Scholar] [CrossRef]
- Cao, X.Y.; Zhang, X.X.; Yang, M.W.; Hu, L.P.; Jiang, S.H.; Tian, G.A.; Zhu, L.L.; Li, Q.; Sun, Y.W.; Zhang, Z.G. Aberrant upregulation of KLK10 promotes metastasis via enhancement of EMT and FAK/SRC/ERK axis in PDAC. Biochem. Biophys. Res. Commun. 2018, 499, 584–593. [Google Scholar] [CrossRef]
- Ho, H.Y.; Ho, Y.C.; Hsieh, M.J.; Yang, S.F.; Chuang, C.Y.; Lin, C.W.; Hsin, C.H. Hispolon suppresses migration and invasion of human nasopharyngeal carcinoma cells by inhibiting the urokinase-plasminogen activator through modulation of the Akt signaling pathway. Environ. Toxicol. 2017, 32, 645–655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-Y.; Lin, C.-C.; Lo, Y.-S.; Chuang, Y.-C.; Abomughaid, M.M.; Hsieh, M.-J. Picrasidine J, a Dimeric β-Carboline-Type Alkaloid from Picrasma quassioides, Inhibits Metastasis of Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 13230. https://doi.org/10.3390/ijms241713230
Ho H-Y, Lin C-C, Lo Y-S, Chuang Y-C, Abomughaid MM, Hsieh M-J. Picrasidine J, a Dimeric β-Carboline-Type Alkaloid from Picrasma quassioides, Inhibits Metastasis of Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(17):13230. https://doi.org/10.3390/ijms241713230
Chicago/Turabian StyleHo, Hsin-Yu, Chia-Chieh Lin, Yu-Sheng Lo, Yi-Ching Chuang, Mosleh Mohammad Abomughaid, and Ming-Ju Hsieh. 2023. "Picrasidine J, a Dimeric β-Carboline-Type Alkaloid from Picrasma quassioides, Inhibits Metastasis of Head and Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 24, no. 17: 13230. https://doi.org/10.3390/ijms241713230




