Ganglionated Plexus Ablation Procedures to Treat Vasovagal Syncope
Abstract
:1. Introduction
2. Cardiac Innervation
3. GP Anatomy and Physiology
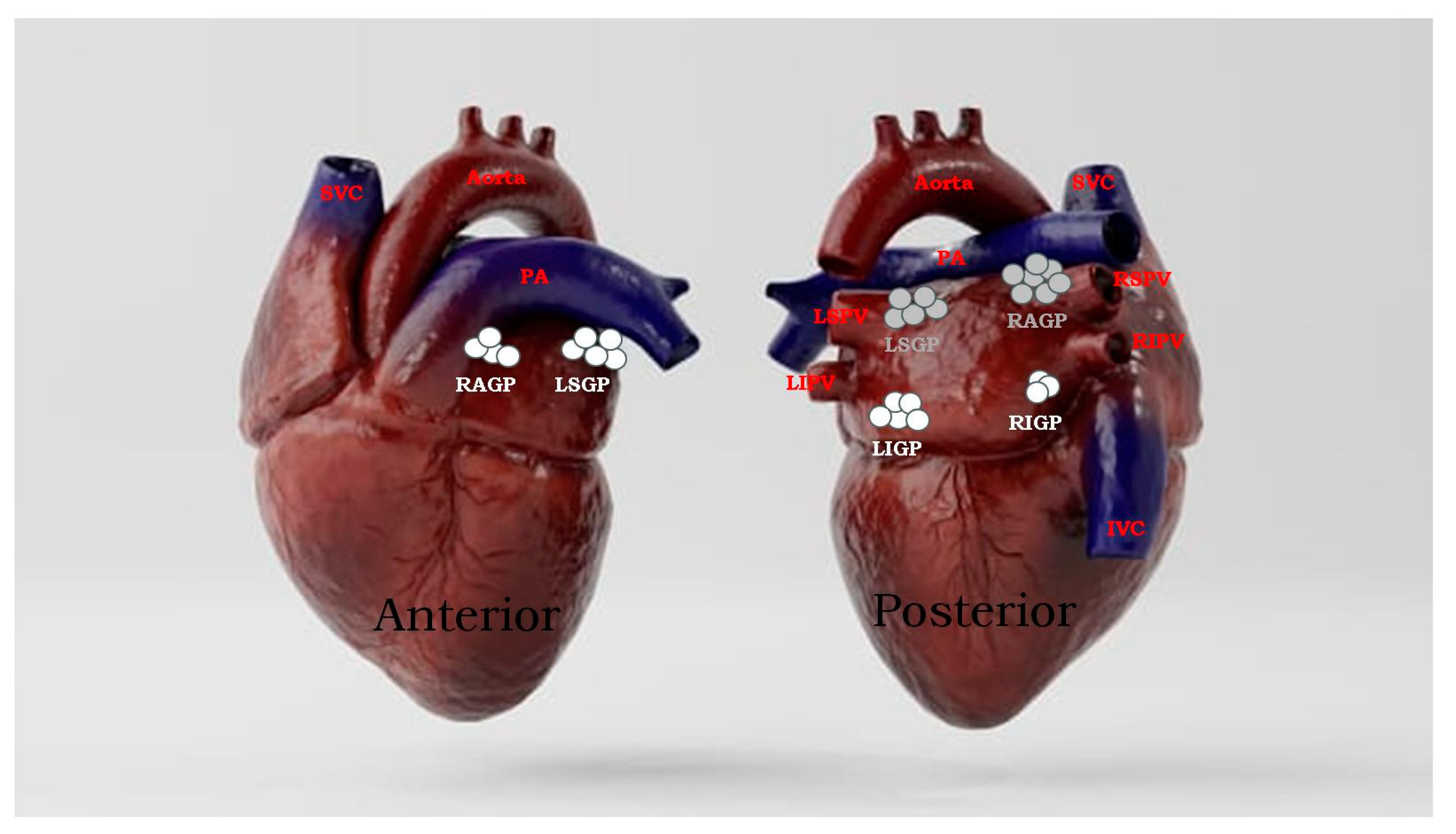
3.1. Anatomic Location of GPs
3.2. Physiology of GPs
3.3. The Vagal Nerve
4. Neurophysiology of Ablation Targets
5. GP Ablation as the Fundamental Treatment for VVS
6. Ganglia Detection Methods
6.1. Spectral Mapping Analysis (SA)
6.2. Anatomically-Guided Approach (AA) [21]
6.3. High Frequency Stimulation (HFS) Approach
6.4. Cardio-Neuromodulation (CardNM) Approach
6.5. Fractionation High-Density Mapping
7. Routes to Access Ablation
8. Post-Ablation Validations
9. Discussion
10. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Anatomical approach |
| ARGP | Anterior right ganglionated plexus |
| Ao-SVC | Aorta-superior vena cava |
| AF | Atrial fibrillation |
| AVB | Atrioventricular block |
| CANS | Cardiac autonomic nervous system |
| CNA | Cardio-neuroablation |
| CardNM | Cardio-neuromodulation |
| CNS | Central nervous system |
| FFT | Fourier transform |
| GP | Ganglionated plexus |
| HRV | Heart rate variability |
| HFS | High-frequency stimulation |
| ILGP | Inferior left ganglionated plexus |
| IRGP | Inferior right ganglionated plexus |
| ICANS | Intrinsic cardiac autonomic nervous system |
| LA | Left atrium |
| NMS | Neurally mediated syncope |
| PAG | Parasympathetic autonomic ganglia |
| PNS | Peripheral nervous system |
| PV | Pulmonary vein |
| PVI | pulmonary vein isolation |
| RA | Right atrium |
| SB | Sinus bradycardia |
| SR | Sinus rate |
| SA | Spectral mapping analysis |
| SDNN | Standard deviation of all normal R-R intervals |
| SLPG | Superior left ganglionated plexus |
| SVC | Superior vena cava |
| VR | Vagal response |
| VVS | Vasovagal syncope |
References
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya, A.; Sutton, R.; Ammirati, F.; Blanc, J.-J.; Brignole, M.; et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur. Heart J. 2009, 30, 2631–2671. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.-K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Ganzeboom, K.S.; Mairuhu, G.; Reitsma, J.B.; Linzer, M.; Wieling, W.; VAN Dijk, N. Lifetime Cumulative Incidence of Syncope in the General Population: A Study of 549 Dutch Subjects Aged 35–60 Years. J. Cardiovasc. Electrophysiol. 2006, 17, 1172–1176. [Google Scholar] [CrossRef]
- Serletis, A.; Rose, S.; Sheldon, A.G.; Sheldon, R.S. Vasovagal syncope in medical students and their first-degree relatives. Eur. Heart J. 2006, 27, 1965–1970. [Google Scholar] [CrossRef]
- Sutton, R.; Benditt, D.G. Epidemiology and economic impact of cardiac syncope in western countries. Future Cardiol. 2012, 8, 467–472. [Google Scholar] [CrossRef]
- Romme, J.J.; Reitsma, J.B.; Black, C.N.; Colman, N.; Scholten, R.J.; Wieling, W.; Van Dijk, N. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst. Rev. 2011, 5, CD004194. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Menozzi, C.; Moya, A.; Andresen, D.; Blanc, J.J.; Krahn, A.D.; Wieling, W.; Beiras, X.; Deharo, J.C.; Russo, V.; et al. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): A randomized trial. Circulation 2012, 125, 2566–2571. [Google Scholar] [CrossRef]
- Kimura, K.; Ieda, M.; Kanazawa, H.; Yagi, T.; Tsunoda, M.; Ninomiya, S.-I.; Kurosawa, H.; Yoshimi, K.; Mochizuki, H.; Yamazaki, K.; et al. Cardiac sympathetic rejuvenation: A link between nerve function and cardiac hypertrophy. Circ. Res. 2007, 100, 1755–1764. [Google Scholar] [CrossRef]
- Mj, S.; Dp, Z. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar]
- Mancia, G.; Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The Autonomic Nervous System and Heart Failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.-P.; Dimopoulos, K.; Okonko, D.; Uebing, A.; Broberg, C.S.; Babu-Narayan, S.; Bayne, S.; Poole-Wilson, P.A.; Sutton, R.; Francis, D.P.; et al. Heart Rate Response during Exercise Predicts Survival in Adults with Congenital Heart Disease. J. Am. Coll. Cardiol. 2006, 48, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W. Autonomic cardiac innervation: Development and adult plasticity. Organogenesis 2013, 9, 176–193. [Google Scholar] [CrossRef]
- Armour, J.A.; Murphy, D.A.; Yuan, B.-X.; MacDonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Pauza, D.H.; Skripka, V.; Pauziene, N.; Stropus, R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000, 259, 353–382. [Google Scholar] [CrossRef]
- Shivkumar, K.; Ajijola, O.A.; Anand, I.; Armour, J.A.; Chen, P.-S.; Esler, M.; De Ferrari, G.M.; Fishbein, M.C.; Goldberger, J.J.; Harper, R.M.; et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J. Physiol. 2016, 594, 3911–3954. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Hardwick, J.C.; Ryan, S.E.; Powers, E.N.; Southerland, E.M.; Gourine, A.; et al. Cardiac neuronal hierarchy in health and disease. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R262–R271. [Google Scholar] [CrossRef] [PubMed]
- Po, S.S.; Nakagawa, H.; Jackman, W.M. Localization of Left Atrial Ganglionated Plexi in Patients with Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 1186–1189. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, W.; Upadhyay, G.A.; Tung, R. Catheter-Based Cardio-Neural Ablation for Refractory Vasovagal Syncope: First U.S. Report. JACC Case Rep. 2020, 2, 1161–1165. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, L.; Qiao, Y.; Shi, R.; Hou, B.; Wu, L.; Guo, J.; Zhang, S.; Yao, Y. Catheter Ablation as a Treatment for Vasovagal Syncope: Long-Term Outcome of Endocardial Autonomic Modification of the Left Atrium. J. Am. Heart Assoc. 2016, 5, e003471. [Google Scholar] [CrossRef] [PubMed]
- Malcolme-Lawes, L.C.; Lim, P.B.; Wright, I.; Kojodjojo, P.; Koa-Wing, M.; Jamil-Copley, S.; Dehbi, H.-M.; Francis, D.P.; Davies, D.W.; Peters, N.S.; et al. Characterization of the Left Atrial Neural Network and its Impact on Autonomic Modification Procedures. Circ. Arrhythmia Electrophysiol. 2013, 6, 632–640. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, R.; Wong, T.; Zheng, L.; Chen, W.; Yang, L.; Huang, W.; Bao, J.; Zhang, S. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: An early experience in humans. Circ. Arrhythm Electrophysiol. 2012, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, W.; Zhou, L.; Wang, Y.; Zhang, X.; Wu, S.; Xu, K.; Liu, X. Atrial autonomic denervation for the treatment of long-standing symptomatic sinus bradycardia in non-elderly patients. J. Interv. Card. Electrophysiol. 2015, 43, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Eckberg, D.L.; Ellenbogen, K.A.; Beightol, L.A.; Hoag, J.B.; Tahvanainen, K.U.; Kuusela, T.A.; Diedrich, A.M. Vagal and Sympathetic Mechanisms in Patients with Orthostatic Vasovagal Syncope. Circulation 1997, 96, 2509–2513. [Google Scholar] [CrossRef]
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Wilson, R.F.; Laxson, D.D.; Christensen, B.V.; McGinn, A.L.; Kubo, S.H. Regional differences in sympathetic reinnervation after human orthotopic cardiac transplantation. Circulation 1993, 88, 165–171. [Google Scholar] [CrossRef]
- Bernardi, L.; Valenti, C.; Wdowczyck-Szulc, J.; Frey, A.W.; Rinaldi, M.; Spadacini, G.; Passino, C.; Martinelli, L.; Viganò, M.; Finardi, G. Influence of Type of Surgery on the Occurrence of Parasympathetic Reinnervation after Cardiac Transplantation. Circulation 1998, 97, 1368–1374. [Google Scholar] [CrossRef]
- Lemola, K.; Chartier, D.; Yeh, Y.-H.; Dubuc, M.; Cartier, R.; Armour, A.; Ting, M.; Sakabe, M.; Shiroshita-Takeshita, A.; Comtois, P.; et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: Role of pulmonary veins versus autonomic ganglia. Circulation 2008, 117, 470–477. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Sandhu, R.K. The Search for the Genes of Vasovagal Syncope. Front. Cardiovasc. Med. 2019, 6, 175. [Google Scholar] [CrossRef]
- Randall, W.C.; Milosavljevic, M.; Wurster, R.D.; Geis, G.S.; Ardell, J.L. Selective vagal innervation of the heart. Ann. Clin. Lab. Sci. 1986, 16, 198–208. [Google Scholar] [PubMed]
- Randall, W.C.; Ardell, J.L.; O’Toole, M.F.; Wurster, R.D. Differential autonomic control of SAN and AVN regions of the canine heart: Structure and function. Prog. Clin. Biol. Res. 1988, 275, 15–31. [Google Scholar] [PubMed]
- Chiou, C.-W.; Eble, J.N.; Zipes, D.P. Efferent Vagal Innervation of the Canine Atria and Sinus and Atrioventricular Nodes. The third fat pad. Circulation 1997, 95, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Scanavacca, M.I.; Pisani, C.F.; Hachul, D.; Trombetta, I.C.; Lara, S.; Hardy, C.; Grupi, C.; Darrieux, F.; Negrão, C.E.; Sosa, E.A. P5-70: Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Heart Rhythm 2006, 3, S283. [Google Scholar] [CrossRef]
- Jose, C.P.M.; Enrique, I.P.M.; Juan, C.P.M.; Lobo, T.J.; Pachon, M.Z.; Vargas, R.N.A.; Pachon, D.Q.V.; Lopez M, F.J.; Jatene, A.D. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace 2004, 6, 590–601. [Google Scholar]
- Lu, Z.; Scherlag, B.J.; Lin, J.; Niu, G.; Fung, K.-M.; Zhao, L.; Ghias, M.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Atrial fibrillation begets atrial fibrillation: Autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ. Arrhythm Electrophysiol. 2008, 1, 184–192. [Google Scholar] [CrossRef]
- Pachon, J.C.; Pachon, E.I.; Pachon, J.C.; Lobo, T.J.; Pachon, M.Z.; Vargas, R.N.A.; Jatene, A.D. ‘Cardioneuroablation’—New treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 2005, 7, 1–13. [Google Scholar] [CrossRef]
- Aksu, T.; Golcuk, E.; Yalin, K.; Guler, T.E.; Erden, I. Simplified Cardioneuroablation in the Treatment of Reflex Syncope, Functional AV Block, and Sinus Node Dysfunction. Pacing Clin. Electrophysiol. 2015, 39, 42–53. [Google Scholar] [CrossRef]
- Rivarola, E.; Hardy, C.; Sosa, E.; Hachul, D.; Furlan, V.; Raimundi, F.; Scanavacca, M. Selective atrial vagal denervation guided by spectral mapping to treat advanced atrioventricular block. Europace 2015, 18, 445–449. [Google Scholar] [CrossRef]
- Liang, Z.; Jiayou, Z.; Zonggui, W.; Dening, L. Selective Atrial Vagal Denervation Guided by Evoked Vagal Reflex to Treat Refractory Vasovagal Syncope. Pacing Clin. Electrophysiol. 2012, 35, e214–e218. [Google Scholar] [CrossRef]
- Scanavacca, M.; Hachul, D.; Pisani, C.; Sosa, E. Selective Vagal Denervation of the Sinus and Atrioventricular Nodes, Guided by Vagal Reflexes Induced by High Frequency Stimulation, to Treat Refractory Neurally Mediated Syncope. J. Cardiovasc. Electrophysiol. 2009, 20, 558–563. [Google Scholar] [CrossRef]
- Fukunaga, M.; Wichterle, D.; Peichl, P.; Aldhoon, B.; Čihák, R.; Kautzner, J. Differential effect of ganglionic plexi ablation in a patient with neurally mediated syncope and intermittent atrioventricular block. Europace 2016, 19, 119–126. [Google Scholar] [CrossRef]
- Stavrakis, S.; Scherlag, B.J.; Po, S.S. Autonomic modulation: An emerging paradigm for the treatment of cardiovascular diseases. Circ. Arrhythm Electrophysiol. 2012, 5, 247–248. [Google Scholar] [CrossRef]
- Pachon M, J.C.; Pachon M, E.I.; Santillana P, T.G.; Lobo, T.J.; Pachon, C.T.C.; Pachon M, J.C.; Albornoz V, R.N.; Zerpa A, J.C. Simplified Method for Vagal Effect Evaluation in Cardiac Ablation and Electrophysiological Procedures. JACC Clin. Electrophysiol. 2015, 1, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Sheldon, R.; Thorpe, K.E.; Roberts, R.S.; Ellenbogen, K.A.; Wilkoff, B.L.; Morillo, C.; Gent, M.; VPS II Investigators. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): A randomized trial. JAMA 2003, 289, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Santinelli, V.; Manguso, F.; Vicedomini, G.; Gugliotta, F.; Augello, G.; Mazzone, P.; Tortoriello, V.; Landoni, G.; Zangrillo, A.; et al. Pulmonary Vein Denervation Enhances Long-Term Benefit after Circumferential Ablation for Paroxysmal Atrial Fibrillation. Circulation 2004, 109, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Zdarska, J.; Osmancik, P.; Budera, P.; Herman, D.; Prochazkova, R.; Talavera, D.; Straka, Z. The absence of effect of ganglionated plexi ablation on heart rate variability parameters in patients after thoracoscopic ablation for atrial fibrillation. J. Thorac. Dis. 2017, 9, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.G.; Giazitzoglou, E.; Zografos, T.; Pokushalov, E.; Po, S.S.; Camm, A.J. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm 2011, 8, 672–678. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.C.M.; Po, S.S.; Camm, A.J.; Ioannidis, J.P.A. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef]
- Rivarola, E.W.; Hachul, D.; Wu, T.; Pisani, C.; Hardy, C.; Raimundi, F.; Melo, S.; Darrieux, F.; Scanavacca, M. Targets and End Points in Cardiac Autonomic Denervation Procedures. Circ. Arrhythm Electrophysiol. 2017, 10, e004638. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, W.; Qiao, Y.; Hou, B.; Guo, J.; Killu, A.; Yao, Y. Symptomatic Premature Ventricular Contractions in Vasovagal Syncope Patients: Autonomic Modulation and Catheter Ablation. Front. Physiol. 2021, 12, 653225. [Google Scholar] [CrossRef]
- Pachon, J.C.M.; Pachon, E.I.M.; Pachon, M.Z.C.; Lobo, T.J.; Pachon, J.C.M.; Santillana, T.G.P. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: Cardioneuroablation long-term results. Europace 2011, 13, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Ozcan, K.S.; Yalin, K.; Mutluer, F.O. Cardioneuroablation in the treatment of neurally mediated reflex syncope: A review of the current literature. Turk Kardiyol. Dern. Ars. 2016, 45, 4–41. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Mutluer, F.O.; Bozyel, S.; Golcuk, S.E.; Yalin, K. Electroanatomic-mapping-guided cardioneuroablation versus combined approach for vasovagal syncope: A cross-sectional observational study. J. Interv. Card. Electrophysiol. 2019, 54, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Pachon-M, J.C.; Pachon-M, E.I.; Pachon, C.T.C.; Santillana-P, T.G.; Lobo, T.J.; Pachon-M, J.C.; Zerpa-A, J.C.; Cunha-P, M.Z.; Higuti, C.; Ortencio, F.A.; et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008703. [Google Scholar] [CrossRef]
- Aksu, T.; Yalin, K.; Mutluer, F.O.; Farhat, K.; Tanboga, H.I.; Po, S.S.; Stavrakis, S. The impact of the clinical diagnosis on the vagal response and heart rate after ganglionated plexus ablation. J. Interv. Card. Electrophysiol. 2022, 1–8. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Duan, Y.; Wang, R.; Hou, J.; Wang, J.; Chen, B.; Yang, Y.; Xue, X.; Zhao, Y.; et al. Clinical Efficacy of Catheter Ablation in the Treatment of Vasovagal Syncope. J. Clin. Med. 2022, 11, 5371. [Google Scholar] [CrossRef]
- Hu, F.; Zheng, L.; Liu, S.; Shen, L.; Liang, E.; Liu, L.; Wu, L.; Ding, L.; Yao, Y. The impacts of the ganglionated plexus ablation sequence on the vagal response, heart rate, and blood pressure during cardioneuroablation. Auton. Neurosci. 2021, 233, 102812. [Google Scholar] [CrossRef]
- Hu, F.; Zheng, L.; Liang, E.; Ding, L.; Wu, L.; Chen, G.; Fan, X.; Yao, Y. Right anterior ganglionated plexus: The primary target of cardioneuroablation? Heart Rhythm 2019, 16, 1545–1551. [Google Scholar] [CrossRef]
- Calo, L.; Rebecchi, M.; Sette, A.; Sciarra, L.; Borrelli, A.; Scara, A.; Grieco, D.; Politano, A.; Sgueglia, M.; De Luca, L.; et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: A long-term follow-up prospective study. J. Interv. Card. Electrophysiol. 2021, 61, 499–510. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Y.; Liu, X.; Jiang, W.-F.; Wu, S.-H.; Po, S. Atrial Ganglionated Plexus Modification: A Novel Approach to Treat Symptomatic Sinus Bradycardia. JACC Clin. Electrophysiol. 2017, 3, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Padmanabhan, D.; Shenthar, J.; Yalin, K.; Gautam, S.; Valappil, S.P.; Banavalikar, B.; Guler, T.E.; Bozyel, S.; Tanboga, I.H.; et al. The benefit of cardioneuroablation to reduce syncope recurrence in vasovagal syncope patients: A case-control study. J. Interv. Card. Electrophysiol. 2021, 63, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Wu, L.; Hu, F.; Fan, S.; Liu, S.; Liu, L.; Ding, L.; Zheng, L.; Yao, Y. Cardiac deceleration capacity as an indicator for cardioneuroablation in patients with refractory vasovagal syncope. Heart Rhythm 2022, 19, 562–569. [Google Scholar] [CrossRef]
- Debruyne, P.; Rossenbacker, T.; Collienne, C.; Roosen, J.; Ector, B.; Janssens, L.; Charlier, F.; Vankelecom, B.; Dewilde, W.; Wijns, W. Unifocal Right-Sided Ablation Treatment for Neurally Mediated Syncope and Functional Sinus Node Dysfunction under Computed Tomographic Guidance. Circ. Arrhythmia Electrophysiol. 2018, 11, e006604. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, P.; Rossenbacker, T.; Janssens, L.; Collienne, C.; Ector, J.; Haemers, P.; Waroux, J.-B.l.P.d.; Bazelmans, C.; Boussy, T.; Wijns, W. Durable Physiological Changes and Decreased Syncope Burden 12 Months after Unifocal Right-Sided Ablation Under Computed Tomographic Guidance in Patients with Neurally Mediated Syncope or Functional Sinus Node Dysfunction. Circ. Arrhythmia Electrophysiol. 2021, 14, e009747. [Google Scholar] [CrossRef]
- Thurber, C.J.; Sneider, D.R.; Sauer, W.H.; Kapur, S. Recurrent vasovagal syncope following successful cardioneuroablation. Heart Rhythm Case Rep. 2022, 8, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Sarabanda, A.V.; Melo, S.L.; Rivarola, E.; Hachul, D.; Scanavacca, M. Anatomically guided atrial ganglionated plexus ablation evaluated by extracardiac vagal stimulation for vagally mediated atrioventricular block. Heart Rhythm Case Rep. 2021, 7, 301–305. [Google Scholar] [CrossRef]
- Suenaga, H.; Murakami, M.; Tani, T.; Saito, S. Frequent neurally mediated reflex syncope in a young patient with dextrocardia: Efficacy of catheter ablation of the superior vena cava-aorta ganglionated plexus. J. Arrhythmia 2014, 31, 172–176. [Google Scholar] [CrossRef]
- Rebecchi, M.; de Ruvo, E.; Strano, S.; Sciarra, L.; Golia, P.; Martino, A.; Calò, L. Ganglionated plexi ablation in right atrium to treat cardioinhibitory neurocardiogenic syncope. J. Interv. Card. Electrophysiol. 2012, 34, 231–235. [Google Scholar] [CrossRef]
- Pachon M, J.C.; Pachon M, E.I.; Lobo, T.J.; Pachon, M.J.C.; Pachon, M.Z.C.; Vargas, R.N.A.; Manrique, R.M.; Jatene, A.D. Syncopal High-Degree AV Block Treated with Catheter RF Ablation without Pacemaker Implantation. Pacing Clin. Electrophysiol. 2006, 29, 318–322. [Google Scholar] [CrossRef]
- Aksu, T.; Golcuk, S.E.; Guler, T.E.; Yalin, K.; Erden, I. Functional permanent 2:1 atrioventricular block treated with cardioneuroablation: Case report. Heart Rhythm Case Rep. 2015, 1, 58–61. [Google Scholar] [CrossRef]
- Debruyne, P. “Cardio-Neuromodulation” with a Multi-Electrode Irrigated Catheter A Potential New Approach for Patients with Cardio-Inhibitory Syncope. J. Cardiovasc. Electrophysiol. 2016, 27, 1110–1113. [Google Scholar] [CrossRef]
- Osorio, J.; Doud, D.M.; Aksu, T. Fractionation Mapping by Using a High-density Catheter to Map Ganglionated Plexus Sites during Sinus Rhythm. J. Innov. Card. Rhythm Manag. 2021, 12, 7–8. [Google Scholar] [CrossRef]
- John, L.; Mullis, A.; Payne, J.; Tung, R.; Aksu, T.; Winterfield, J. Fractionation Mapping of the Ganglionated Plexi for Cardioneuroablation. J. Innov. Card. Rhythm Manag. 2021, 12, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Yalin, K. Selective vagal innervation principles of ganglionated plexi: Step-by-step cardioneuroablation in a patient with vasovagal syncope. J. Interv. Card. Electrophysiol. 2021, 60, 453–458. [Google Scholar] [CrossRef]
- Singhal, R.; Lo, L.-W.; Lin, Y.-J.L.; Chang, S.-L.; Hu, Y.-F.; Chao, T.-F.; Chung, F.-P.; Chiou, C.-W.; Tsao, H.-M.; Chen, S.-A. Intrinsic Cardiac Autonomic Ganglionated Plexi within Epicardial Fats Modulate the Atrial Substrate Remodeling: Experiences with Atrial Fibrillation Patients Receiving Catheter Ablation. Acta Cardiol. Sin. 2016, 32, 174–184. [Google Scholar] [CrossRef]
- Mateos, J.C.P.; Mateos, E.I.P.; Lobo, T.J.; Pachón, M.Z.C.; Mateos, J.C.P.; Pachón, D.Q.V.; Vargas, R.N.A.; Piegas, L.S.; Jatene, A.D. Radiofrequency catheter ablation of atrial fibrillation guided by spectral mapping of atrial fibrillation nests in sinus rhythm. Arq. Bras. Cardiol. 2007, 89, 140–150. [Google Scholar]
- Chang, H.-Y.; Lo, L.-W.; Lin, Y.-J.; Lee, S.-H.; Chiou, C.-W.; Chen, S.-A. Relationship Between Intrinsic Cardiac Autonomic Ganglionated Plexi and the Atrial Fibrillation Nest. Circ. J. 2014, 78, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.C.P.; Mateos, E.I.P.; Higuti, C.; Peña, T.G.S.; Lobo, T.J.; Pachón, C.T.C.; Mateos, J.C.P.; Acosta, J.C.Z.; Ortencio, F.; Amarante, R. Cardioneuroablation: Catheter Vagal Denervation as a New Therapy for Cardioinhibitory Syncope. J. Card. Arrhythm. 2020, 32, 182–196. [Google Scholar] [CrossRef]
- Debruyne, P.; Wijns, W. Cardio-Neuromodulation: The Right-Sided Approach. JACC Clin. Electrophysiol. 2017, 3, 1056–1057. [Google Scholar] [CrossRef]
- Debruyne, P. Letter by Debruyne regarding article “Selective ablation of atrial ganglionated plexus attenuates vasovagal reflex in a canine model”. Pacing Clin. Electrophysiol. 2019, 42, 390. [Google Scholar] [CrossRef]
- Hou, Y.; Scherlag, B.J.; Lin, J.; Zhou, J.; Song, J.; Zhang, Y.; Patterson, E.; Lazzara, R.; Jackman, W.M.; Po, S.S. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm 2007, 4, 56–63. [Google Scholar] [CrossRef]
- Li, L.; Po, S.; Yao, Y. Cardioneuroablation for Treating Vasovagal Syncope: Current Status and Future Directions. Arrhythmia Electrophysiol. Rev. 2023, 12, e18. [Google Scholar] [CrossRef]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, A.; Vitulano, N.; Troisi, F.; Quadrini, F.; Romanazzi, I.; Calvi, V.; Grimaldi, M. Pulsed Field Ablation to Treat Atrial Fibrillation: A Review of the Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ueda, M.; Watanabe, M.; Ishimura, M.; Kajiyama, T.; Hashiguchi, N.; Kanaeda, T.; Nakano, M.; Hiranuma, Y.; Ishizaka, T.; et al. Identification of Left Atrial Ganglionated Plexi by Dense Epicardial Mapping as Ablation Targets for the Treatment of Concomitant Atrial Fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 1336–1341. [Google Scholar] [CrossRef]
- Goff, Z.D.; Laczay, B.; Yenokyan, G.; Sivasambu, B.; Sinha, S.K.; Marine, J.E.; Ashikaga, H.; Berger, R.D.; Akhtar, T.; Spragg, D.D.; et al. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J. Cardiovasc. Electrophysiol. 2019, 30, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Vesela, J.; Osmancik, P.; Herman, D.; Prochazkova, R. Changes in heart rate variability in patients with atrial fibrillation after pulmonary vein isolation and ganglionated plexus ablation. Physiol. Res. 2019, 68, 49–57. [Google Scholar] [CrossRef]
- Baysal, E.; Mutluer, F.O.; Dagsali, A.E.; Kumrulu, U.C.; Huang, H.D.; Aksu, T. Improved health-related quality of life after cardioneuroablation in patients with vasovagal syncope. J. Interv. Card. Electrophysiol. 2022, 1–8. [Google Scholar] [CrossRef]
- Calò, L.; Rebecchi, M.; Sciarra, L.; De Luca, L.; Fagagnini, A.; Zuccaro, L.M.; Pitrone, P.; Dottori, S.; Porfirio, M.; de Ruvo, E.; et al. Catheter Ablation of Right Atrial Ganglionated Plexi in Patients with Vagal Paroxysmal Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2012, 5, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Scanavacca, M.; Rivarola, E.W.; Torres, R.V.A.; Hardy, C.; Wu, T.C.; Darrieux, F.; Pisani, C.; Hachul, D. Sinus Node Artery Occlusion during Cardiac Denervation Procedures. JACC Case Rep. 2022, 4, 1169–1175. [Google Scholar] [CrossRef] [PubMed]

| Technique | N | Age (y) | Diagnosis | Approach | Procedure Time (min) | Follow-Up Duration (months) | Syncope Recurrence | Heart Rate Variability (p-Value) | Year, Reference |
|---|---|---|---|---|---|---|---|---|---|
| SA + AA * | 6 | 47.5 ± 16 | NMS | LA, RA, SVC | 38.9 ± 15.4 | 9.2 | 0 | 2005 [37] | |
| SA + AA * | 7 | 47.5 ± 16 | Functional high degree AVB | LA, RA, SVC | 38.9 ± 15.4 | 9.2 | 0 | 2005 [37] | |
| SA (FFT) + AA * | 13 | 47.5 ± 16 | Sinus node dysfunction | LA, RA, SVC | 38.9 ± 15.4 | 9.2 | 0 | 0.003 | 2005 [37] |
| SA + AA | 43 | 32.9 ± 15 | NMS | SVC, RA, inferior–posterior interatrial septum | NR | 21.7 ± 11 | 3 (6.9%) | 0.001 | 2011 [52] |
| SA (FFT) + HFS * | 7 | 42.7 ± 14.7 | AVB | RA in 6 patients, followed by LA in 1 patient | 121.2 ± 16.4 (for all patients in this study) | 6 | 1 (14.3%) | NR | 2016 [53] |
| SA (FFT) + HFS * | 8 | 42.7 ± 14.7 | NMS | LA then RA | 121.2 ± 16.4 (for all patients in this study) | 12.3 ± 3.4 | 0 | 0.001 (SDNN 6 months post procedure) | 2016 [53] |
| SA (FFT) + HFS * | 7 | 42.7 ± 14.7 | SND | LA then RA | 121.2 ± 16.4 (for all patients in this study) | 9.5 ± 3.1 | 0 | 0.001 (SDNN 6 months post procedure) | 2016 [53] |
| Fractionation * Mapping | 12 | NR | VVS | NR | NR | 12 | 0 | SD | 2019 [54] |
| SA + HFS * | 8 | NR | VVS | NR | NR | 12 | 2 | SD | 2019 [54] |
| SA + AA | 83 | 47.3 ± 17 | AF VVS | RA, LA | 237.2 ± 38 | 40 | 0 | 0.001 | 2020 [55] |
| PVI | 67 | 53.2 ± 11.3; 55.2 ± 11.6 | Paroximal AF | LA | 105.2 ± 22.3; 126.0 ± 26.7 | 12 | 18 (54.5%) | NR | 2011 [48] |
| HFS + PVI | 83 | NR | AF | NR | NR | 22 | NR | NR | 2009 [19] |
| HFS (selective vagal denervation) | 10 | 50.4 ± 6.4 | Recurrent NMS | LA | 50.2 ± 3.8 | 30 ± 16 | 0 | 0.002 | 2012 [23] |
| Synchronized HFS * | 20 | 50–69 | Paroximal AF, AVB | LA | NR | NR | NR | NR | 2013 [22] |
| Continuous HFS * | 10 | 54–68 | Persistent AF, AVB | LA | NR | NR | NR | NR | 2013 [22] |
| HFS | 11 | 45.9 ± 10.9 | Symptomatic SB | LA, RA, SVC | NR | 18 ± 6 | Significant symptom improvement | 0.001 | 2015 [24] |
| HFS * | 10 | 50.4 ± 6.4 | VVS | LA | 50.2 ± 3.8 | 36.4 ± 22.2 | 0 | 0.751 | 2016 [21] |
| AA * | 47 | 41.7 ± 14.1 | VVS | LA | 43.7 ± 6.1 | 36.4 ± 22.2 | 5 | 0.751 | 2016 [21] |
| HFS * | 43 | NR | VVS | NR | NR | NR | NR | SD | 2022 [56] |
| HFS * | 40 | NR | AF | NR | NR | NR | NR | SD | 2022 [56] |
| AA * | 42 | 51.2 ± 15.3 | VVS | LA | NR | 8 | 16% | SD | 2022 [57] |
| HFS * | 66 | 51.2 ± 15.3 | VVS | LA | NR | 8 | 16% | SD | 2022 [57] |
| HFS | 28 | NR | VVS | LA | NR | NR | NR | SD | 2021 [58] |
| HFS + AA | 115 | NR | VVS | LA | NR | 21.4 ± 13.1 | NR | SD | 2019 [59] |
| AA | 14 | 34.0 ± 13.8 | VVS, advanced AVB, sinus arrest | LA, RV, LA | 112 ± 15 | 22.5 ± 11.3 | 0 VVS; 0 sinus arrest; 4 AV block | 0.002 (SDNN 30) days post-procedure); 0.002 (pNN > 50, 30 days post-procedure) | 2017 [50] |
| AA | 18 | 36.9 ± 11.2 | VVS | RA | NR | 34.1 ± 6.1 | 3 (16.6%) | 0.001 | 2021 [60] |
| ‡ AA | 62 | 47.8 ± 12.6 | Symptomatic SB | LA, RA, SVC | NR | 12 | Significant symptom improvement in | SD | 2017 [61] |
| AA | 26 | 41.8 ± 15.4 | VVS + PVCs | LA | NR | 10.6 ± 6.8 | 1 (3.8%) | 2021 [51] | |
| † NR | 51 | NR | VVS | NR | NR | 22 | 2 (3.9%) | NR | 2022 [62] |
| NR | 123 | 42.2 ± 17.1 | VVS | LA | NR | 48 ± 13.2 | 33 (26.8%) | NR | 2022 [63] |
| ‡ CardNM (computed tomographic scan + electro-anatomical mapping) | 20 | 41.4 ± 18.8 | NMS | RA, SVC, coronary sinus | 7 ± 4 | 6 | 4 (20%) | 0.001 | 2018 [64] |
| ‡ CardNM (computed tomographic scan + electro-anatomical mapping) | 50 | 42.4 ± 17 | NMS | RA, SVC, coronary sinus | 8 ± 4 | 12 | 13 (26%) | 0.001 | 2021 [65] |
| Technique | Age (y), (Sex) | Diagnosis | Approach | Procedure Time (min) | Follow-Up Duration (Months) | Syncope Recurrence | Heart Rate Variability (p-Value) | Year, Reference |
|---|---|---|---|---|---|---|---|---|
| HFS | 20 (F) | VVS | RA, LA | NR | 7 | 1 | SD | 2022 [66] |
| HFS | 52 (F) | VVS | RA, LA | 18.8 | 18 | 0 | NR | 2020 [20] |
| HFS (selective vagal denervation) | 57 (F) | VVS | RA, LA | NR | 12 | 0 | SD | 2012 [40] |
| HFS | 15 (F) | NMS | RA, RV, LA | NR | 13 | 3 | SD | 2009 [41] |
| Electro-anatomical mapping CARTO3 | 38 (M) | AVB | RA, LV, coronary sinus, His bundle, LA | NR | 11 | NR | NR | 2021 [67] |
| Electro-anatomical mapping CARTO3 | 35 (F) | NMS and AVB | SA and AV nodes (RA, SVC, R, L, interatrial septum, LA) | NR | 10 | 2 | NR | 2016 [42] |
| Electro-anatomical mapping CARTO3 + spectral mapping | 17 (M) | NMS | SVC | NR | 12 | 0 | NR | 2015 [68] |
| Electro-anatomical mapping CARTO3 * | 31 (F) | VVS | RA, SVC, IVC, sinus AV nodes | NR | 8 | 0 | NS | 2012 [69] |
| Electro-anatomical mapping CARTO3 * | 45 (F) | VVS | RA, SVC, IVC, sinus AV nodes | NR | 8 | 0 | NS | 2012 [69] |
| Spectral mapping (fibrillar myocardium) | 23 (F) | AVB | RA, His bundle region, AV and sinus nodes | NR | 21 | NR | SD | 2006 [70] |
| Spectral mapping + HFS | 55 (F) | AVB | RA, SVC | 75 | 12 | 0 | NR | 2015 [71] |
| Spectral mapping (fibrillar myocardium) | 38 (M) | Vagal mediated AVB | Sinus nodes, AV nodes (R, L inter-atrial septum innervation) | 87 | 15 | NA | SD | 2016 [39] |
| ‡ CardNM (computed tomographic scan + electro-anatomical mapping) | 16 (F) | VVS | LA | 3 | 22 | 0 | SD | 2016 [72] |
| Fractionation mapping software (Ensite Precision, Abbott https://www.cardiovascular.abbott/us/en/hcp/products/electrophysiology/mapping-systems/ensite.html, accessed on 6 August 2023) | 18 (M) | VVS | LA, RA | NR | NR | NR | NR | 2021 [73] |
| Fractionation mapping software (Ensite Precision, Abbott) | 20 (F) | VVS | RA | 11.4 | 1 | 0 | SD | 2021 [74] |
| Fractionation mapping software (Ensite Precision, Abbott) | 46 (F) | VVS | RA | NR | NR | NR | NR | 2021 [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarkoni, M.; Rehman, W.u.; Bajwa, A.; Yarkoni, A.; Rehman, A.u. Ganglionated Plexus Ablation Procedures to Treat Vasovagal Syncope. Int. J. Mol. Sci. 2023, 24, 13264. https://doi.org/10.3390/ijms241713264
Yarkoni M, Rehman Wu, Bajwa A, Yarkoni A, Rehman Au. Ganglionated Plexus Ablation Procedures to Treat Vasovagal Syncope. International Journal of Molecular Sciences. 2023; 24(17):13264. https://doi.org/10.3390/ijms241713264
Chicago/Turabian StyleYarkoni, Merav, Wajeeh ur Rehman, Ata Bajwa, Alon Yarkoni, and Afzal ur Rehman. 2023. "Ganglionated Plexus Ablation Procedures to Treat Vasovagal Syncope" International Journal of Molecular Sciences 24, no. 17: 13264. https://doi.org/10.3390/ijms241713264






