Photocatalytic and Photothermal Antimicrobial Mussel-Inspired Nanocomposites for Biomedical Applications
Abstract
:1. Introduction
2. Results
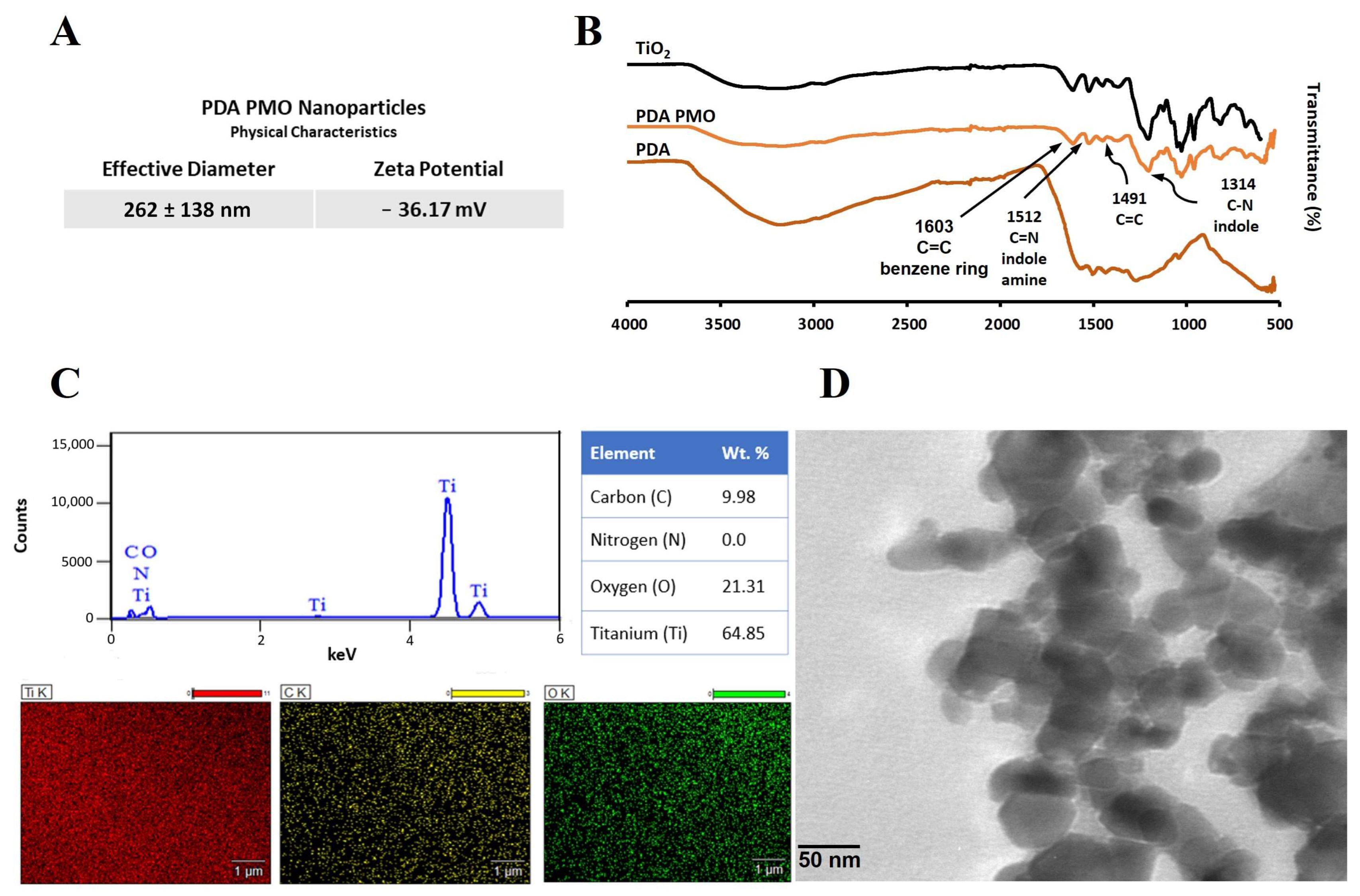
2.1. Characterization of PDA PMO
2.2. Characterization of HADA Hydrogel and HADA PDA PMO Nanocomposites
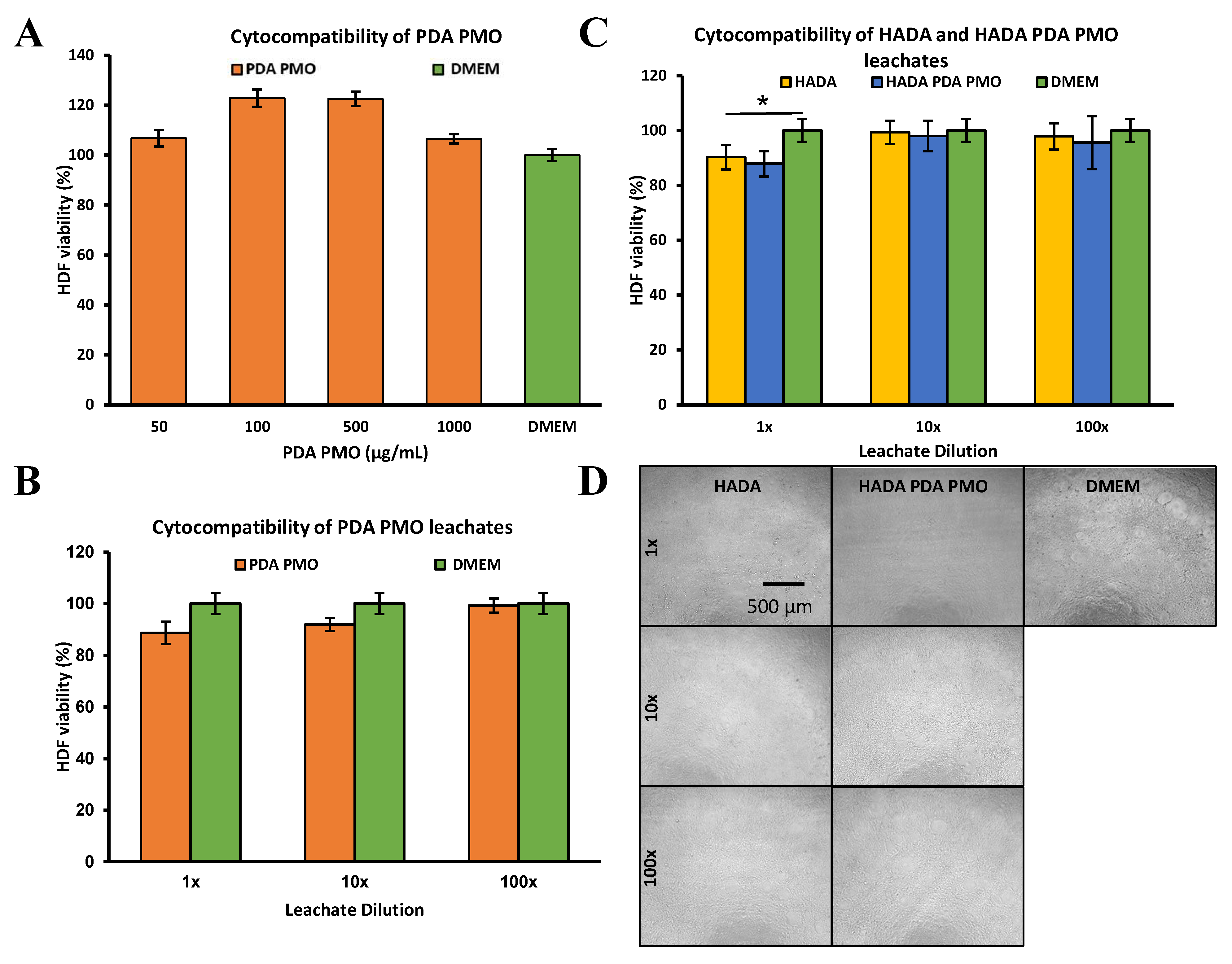
2.3. Cytocompatibility of Nanoparticles, Hydrogels, and Nanocomposites
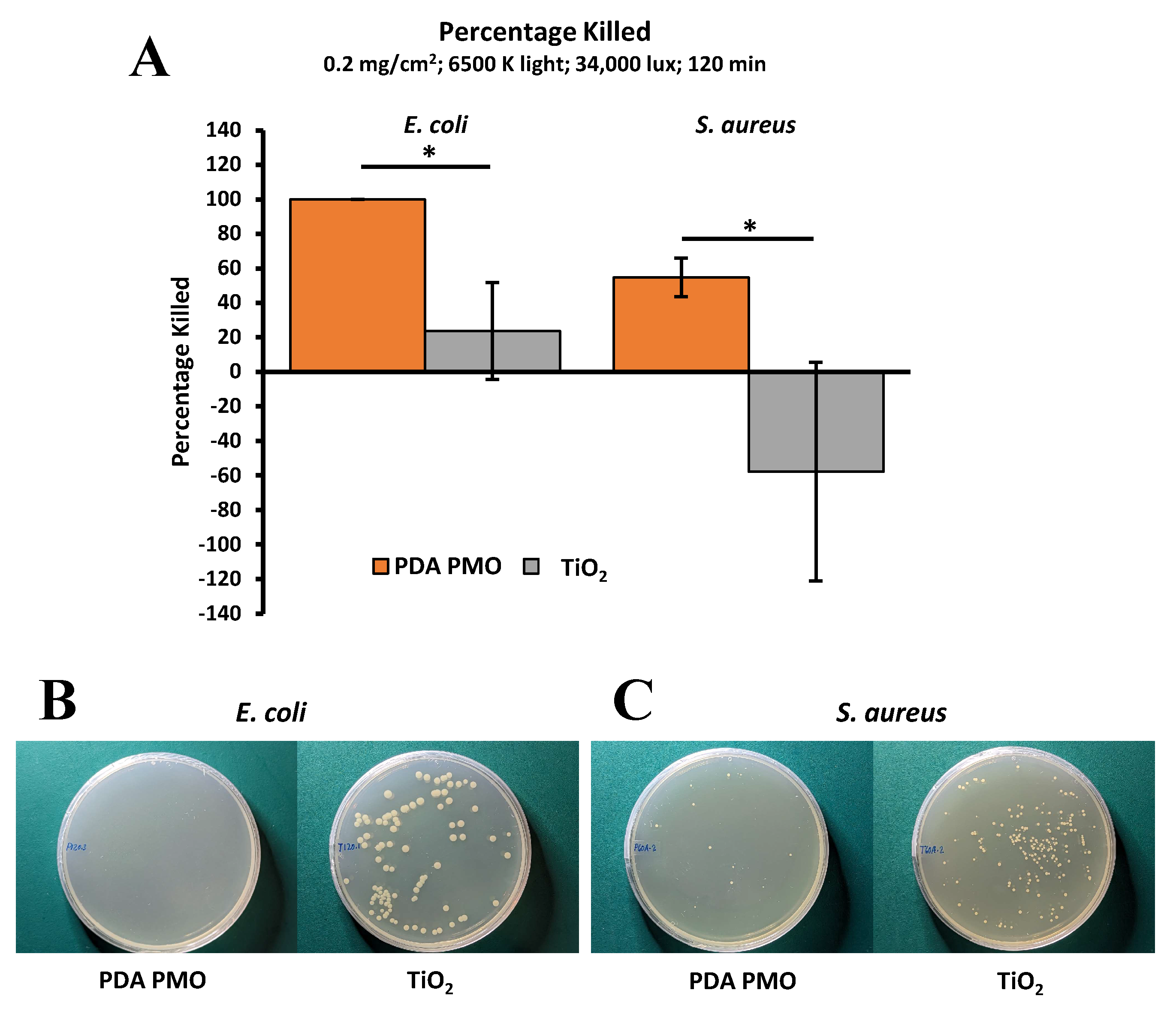
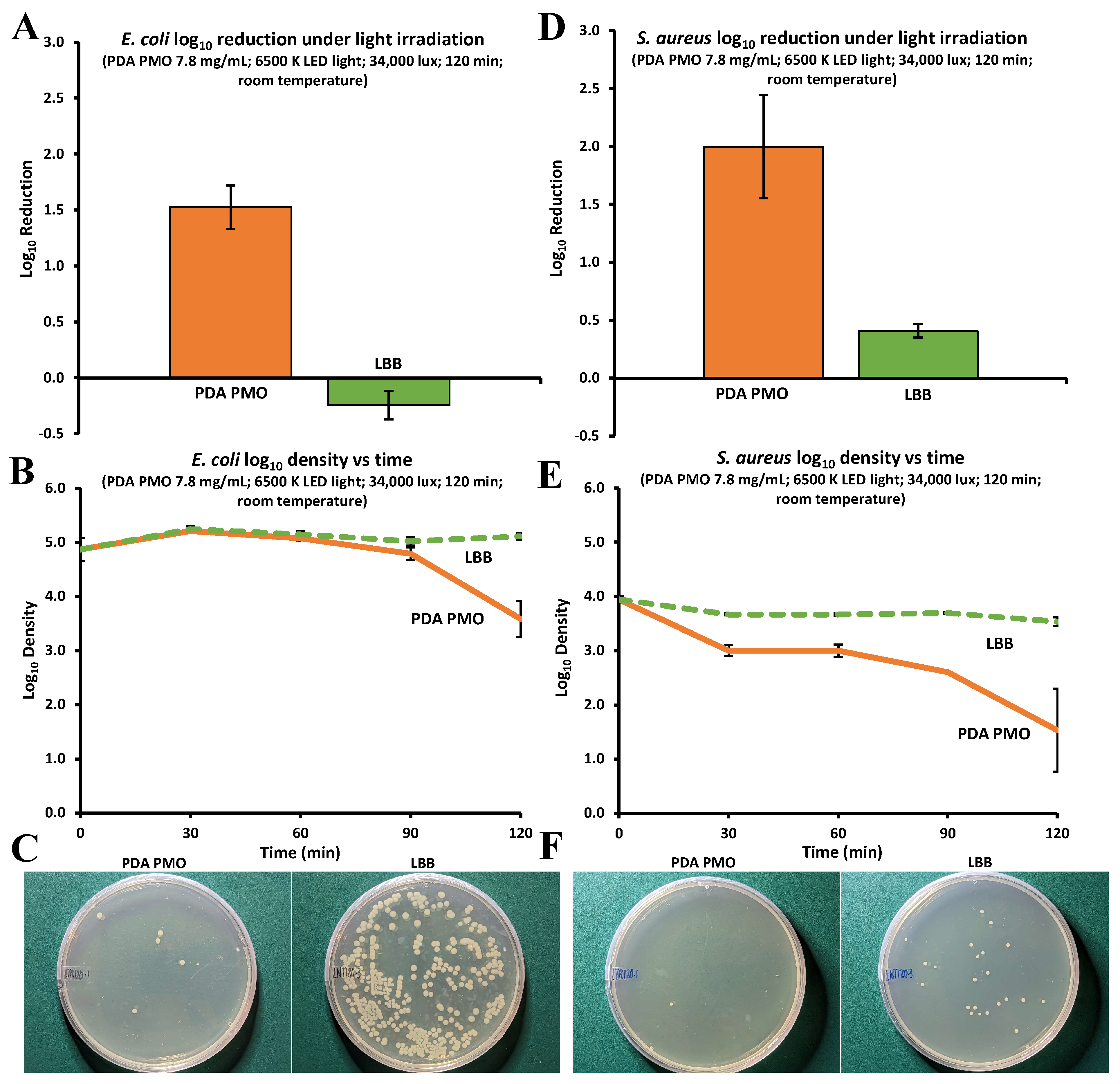
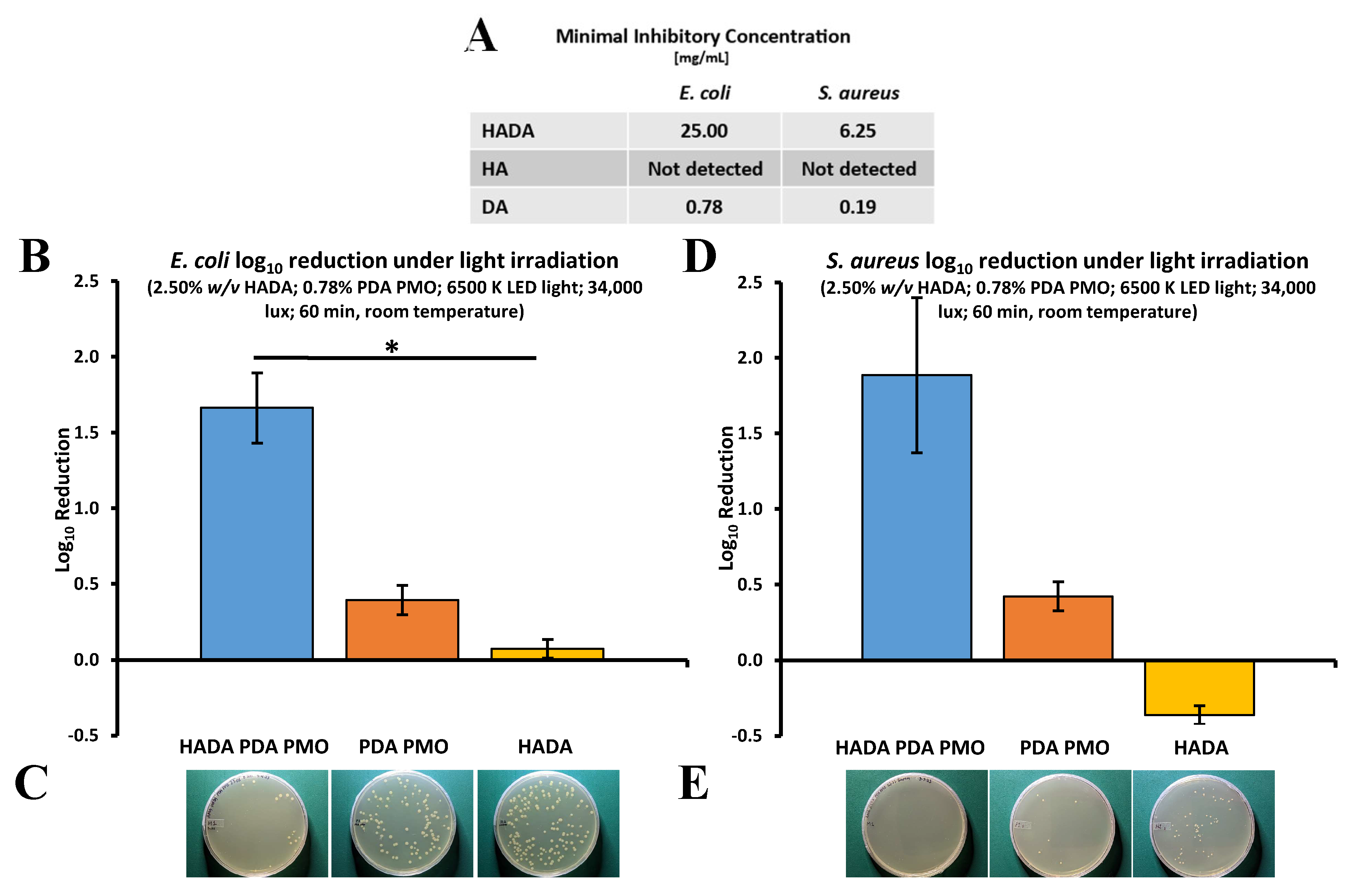
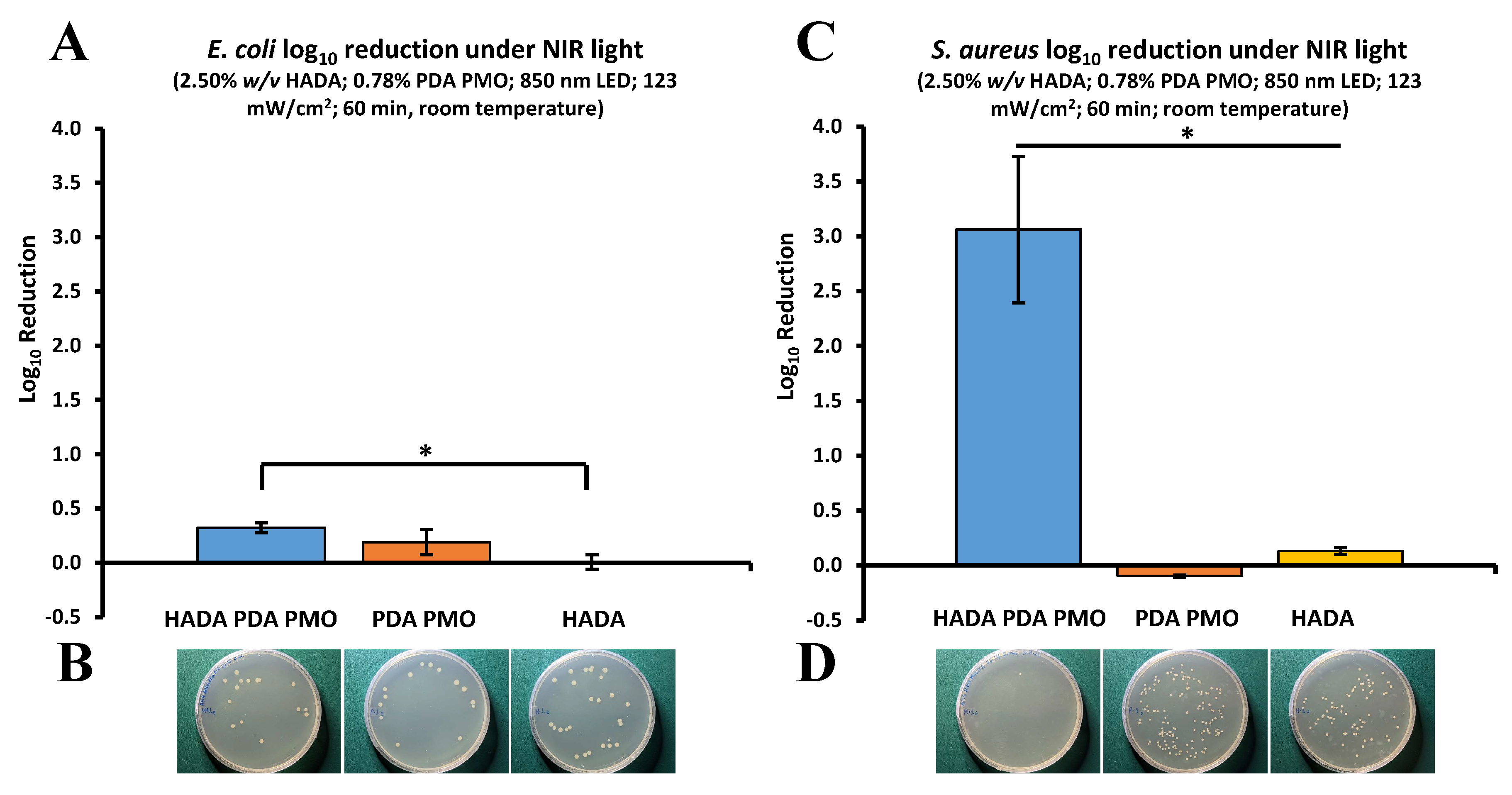
2.4. Antimicrobial Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Elaboration of Photo Responsive Nanoparticles
4.3. Characterization of Photo Responsive Nanoparticles
4.4. HADA Synthesis
4.5. Formulation and Characterization of Hydrogel and Nanocomposites
4.6. Cytocompatibility Studies
4.7. Antimicrobial Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, T.; Liu, J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. Int. J. Mol. Sci. 2022, 23, 7278. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, X.; Qu, J.; Xiong, F.; Xuan, H.; Jin, Y.; Yuan, H. Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite. Int. J. Mol. Sci. 2022, 23, 7712. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Douglas, J.J.M. Penicillin treatment of syphilis: Clearing away the shadow on the land. JAMA J. Am. Med. Assoc. 2009, 301, 769–771. [Google Scholar] [CrossRef]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Hurkacz, M.; Dobrek, L.; Wiela-Hojeńska, A. Antibiotics and the Nervous System-Which Face of Antibiotic Therapy Is Real, Dr. Jekyll (Neurotoxicity) or Mr. Hyde (Neuroprotection)? Molecules 2021, 26, 7456. [Google Scholar] [CrossRef]
- Kaur, K.; Reddy, S.; Barathe, P.; Shriram, V.; Anand, U.; Proćków, J.; Kumar, V. Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review. Front. Microbiol. 2021, 12, 747019. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Stegger, M.; Wirth, T.; Andersen, P.S.; Skov, R.L.; De Grassi, A.; Simões, P.M.; Tristan, A.; Petersen, A.; Aziz, M.; Kiil, K.; et al. Origin and evolution of European community-acquired methicillin-resistant Staphylococcus aureus. mBio 2014, 5, e01044-14. [Google Scholar] [CrossRef]
- Vandenbroucke-Grauls, C.M.J.E.; Kluytmans, J.A.J.W. Tracing the origins of antibiotic resistance. Adv. Matern. Nat. Med. 2022, 28, 638–640. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; Johnson, S.C.; Fell, F.; Hackett, S.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, E.; Sakowicz, T. An Alternative to Antibiotics: Selected Methods to Combat Zoonotic Foodborne Bacterial Infections. Curr. Microbiol. 2021, 78, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, Y.H.; Powers, Z.M.; Kao, C.Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Yang, S.C.; Hsu, C.Y.; Sung, J.T.; Fang, J.Y. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules 2021, 26, 6392. [Google Scholar] [CrossRef]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Personalized Therapeutic Cocktail of Wild Environmental Phages Rescues Mice from Acinetobacter baumannii Wound Infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z.; Tetyana, P.; Hlatshwayo, T.; Mlambo, M.; et al. Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arab. J. Chem. 2022, 15, 103798. [Google Scholar] [CrossRef]
- Zhao, Y.; Dunn, A.; Lin, J.; Shi, D. Chapter 13—Photothermal Effect of Nanomaterials for Efficient Energy Applications. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Wang, X., Chen, X., Eds.; Elsevier: Berlin/Heidelberg, Germany, 2019; pp. 415–434. [Google Scholar]
- Andoy, N.M.O.; Jeon, K.; Kreis, C.T.; Sullan, R.M.A. Multifunctional and Stimuli-Responsive Polydopamine Nanoparticle-Based Platform for Targeted Antimicrobial Applications. Adv. Funct. Mater. 2020, 30, 2004503. [Google Scholar] [CrossRef]
- Aguilar-Ferrer, D.; Szewczyk, J.; Coy, E. Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: Physico-chemical properties and perspectives. Catal. Today 2022, 397, 316–349. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.-H.; Yang, L.; Huang, C.-C.; Chen, L.; Wang, W.-C.; Chen, G.-W.; Yan, J.; Sawettanun, S.; Lin, C.-H. Improved Anticancer Photothermal Therapy Using the Bystander Effect Enhanced by Antiarrhythmic Peptide Conjugated Dopamine-Modified Reduced Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 2017, 6, 1600804. [Google Scholar] [CrossRef]
- Muller, C.; Berber, E.; Lutzweiler, G.; Ersen, O.; Bahri, M.; Lavalle, P.; Ball, V.; Vrana, N.E.; Barthes, J. Polyarginine Decorated Polydopamine Nanoparticles With Antimicrobial Properties for Functionalization of Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 982. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793–801. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Xu, Y.; Li, Y.; Zhai, X.; Su, P.; Ma, Q.; Zhang, H. PDA-Based Drug Delivery Nanosystems: A Potential Approach for Glioma Treatment. Int. J. Nanomed. 2022, 17, 3751–3775. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef]
- Hao, Y.N.; Zheng, A.Q.; Guo, T.T.; Shu, Y.; Wang, J.H.; Johnson, O.; Chen, W. Glutathione triggered degradation of polydopamine to facilitate controlled drug release for synergic combinational cancer treatment. J. Mater. Chem. B 2019, 7, 6742–6750. [Google Scholar] [CrossRef]
- Fan, S.; Lin, W.; Huang, Y.; Xia, J.; Xu, J.-F.; Zhang, J.; Pi, J. Advances and Potentials of Polydopamine Nanosystem in Photothermal-Based Antibacterial Infection Therapies. Front. Pharmacol. 2022, 13, 829712. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, X.; Zhou, L.; Su, Y.; Dong, C.-M. A Sweet Polydopamine Nanoplatform for Synergistic Combination of Targeted Chemo-Photothermal Therapy. Macromol. Rapid Commun. 2015, 36, 916–922. [Google Scholar] [CrossRef]
- Wu, R.; Wang, H.; Hai, L.; Wang, T.; Hou, M.; He, D.; He, X.; Wang, K. A photosensitizer-loaded zinc oxide-polydopamine core-shell nanotherapeutic agent for photodynamic and photothermal synergistic therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 189–192. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Zhao, W.; Zhao, C. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873. [Google Scholar] [CrossRef]
- Li, F.; Huang, K.; Chang, H.; Liang, Y.; Zhao, J.; Yang, S.; Liu, F. A polydopamine coated nanoscale FeS theranostic platform for the elimination of drug-resistant bacteria via photothermal-enhanced Fenton reaction. Acta Biomater. 2022, 150, 380–390. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef]
- Liu, C.; Ling, J.; Yang, L.-Y.; Ouyang, X.-K.; Wang, N. Chitosan-based carbon nitride-polydopamine-silver composite dressing with antibacterial properties for wound healing. Carbohydr. Polym. 2023, 303, 120436. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Yeung, K.L.; Leung, W.K.; Yao, N.; Cao, S. Reactivity and antimicrobial properties of nanostructured titanium dioxide. Catal. Today 2009, 143, 218–224. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Rozhkova, E.; Rajh, T. Dynamics of Localized Charges in Dopamine-Modified TiO2 and their Effect on the Formation of Reactive Oxygen Species. J. Am. Chem. Soc. 2009, 131, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Zaleska, A. Doped-TiO2: A Review. Recent. Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Roeselová, M.; Vieceli, J.; Dang, L.X.; Garrett, B.C.; Tobias, D.J. Hydroxyl Radical at the Air−Water Interface. J. Am. Chem. Soc. 2004, 126, 16308–16309. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Das, R.; Sarkar, S.; Chakraborty, S.; Choi, H.; Bhattacharjee, C. Remediation of Antiseptic Components in Wastewater by Photocatalysis Using TiO2 Nanoparticles. Ind. Eng. Chem. Res. 2014, 53, 3012–3020. [Google Scholar] [CrossRef]
- Sun, X.; Yan, L.; Xu, R.; Xu, M.; Zhu, Y. Surface modification of TiO2 with polydopamine and its effect on photocatalytic degradation mechanism. Colloids Surfaces. A Physicochem. Eng. Asp. 2019, 570, 199–209. [Google Scholar] [CrossRef]
- Wang, T.; Xia, M.; Kong, X. The Pros and Cons of Polydopamine-Sensitized Titanium Oxide for the Photoreduction of CO2. Catalysts 2018, 8, 215. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, H.; Liu, X.; Zheng, Y.; Li, Z.; Li, C.; Yeung, K.W.K.; Zhu, S.; Liang, Y.; Cui, Z.; et al. Photoresponsive Materials for Antibacterial Applications. Cell Rep. Phys. Sci. 2020, 1, 100245. [Google Scholar] [CrossRef]
- Mao, W.X.; Lin, X.J.; Zhang, W.; Chi, Z.X.; Lyu, R.W.; Cao, A.M.; Wan, L.J. Core-shell structured TiO2@polydopamine for highly active visible-light photocatalysis. Chem. Commun. 2016, 52, 7122–7125. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef]
- Ahuja, A.; Khar, R.K.; Ali, J. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 2008, 23, 489–515. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial Effects of Hyaluronic Acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Castano, O.; Perez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.A.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Lee, S.Y.; Kim, H.J.; Lee, K.; Lee, J.; Kim, D.D.; Cho, H.J. Polypseudorotaxane and polydopamine linkage-based hyaluronic acid hydrogel network with a single syringe injection for sustained drug delivery. Carbohydr. Polym. 2021, 266, 118104. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Soto-Garcia, L.; Yaman, S.; Kuriakose, A.; Rivera, A.U.; Jones, V.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Polydopamine nanoparticles and hyaluronic acid hydrogels for mussel-inspired tissue adhesive nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 134, 112589. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Polega, E.; Thomson, K.; Bhuiyan, M.S.A.; Pinnaratip, R.; Trought, M.; Kendrick, C.; Gao, Y.; Perrine, K.A.; Pan, L.; et al. Antibacterial Properties of Mussel-Inspired Polydopamine Coatings Prepared by a Simple Two-Step Shaking-Assisted Method. Front. Chem. 2019, 7, 631. [Google Scholar] [CrossRef]
- Gao, B.; Chen, L.; Zhao, Y.; Yan, X.; Wang, X.; Zhou, C.; Shi, Y.; Xue, W. Methods to prepare dopamine/polydopamine modified alginate hydrogels and their special improved properties for drug delivery. Eur. Polym. J. 2019, 110, 192–201. [Google Scholar] [CrossRef]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing fluorescence lifetime standards using two-photon excitation and time-domain instrumentation: Rhodamine B, coumarin 6 and lucifer yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef]
- Jakimińska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of Rhodamine B to Rhodamine-110—The mechanism revisited. J. Photochem. Photobiol. A Chem. 2022, 433, 114176. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Lih, E.; Choi, S.G.; Ahn, D.J.; Joung, Y.K.; Han, D.K. Optimal conjugation of catechol group onto hyaluronic acid in coronary stent substrate coating for the prevention of restenosis. J. Tissue Eng. 2016, 7, 2041731416683745. [Google Scholar] [CrossRef]
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Pernodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Brassolatti, P.; de Almeida Rodolpho, J.M.; Franco de Godoy, K.; de Castro, C.A.; Flores Luna, G.L.; Dias de Lima Fragelli, B.; Pedrino, M.; Assis, M.; Nani Leite, M.; Cancino-Bernardi, J.; et al. Functionalized Titanium Nanoparticles Induce Oxidative Stress and Cell Death in Human Skin Cells. Int. J. Nanomed. 2022, 17, 1495–1509. [Google Scholar] [CrossRef]
- Saquib, Q.; Al-Khedhairy, A.A.; Siddiqui, M.A.; Abou-Tarboush, F.M.; Azam, A.; Musarrat, J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2012, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2(anatase)/TiO2(rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. Int. Ed. Engl. 2002, 41, 2811–2813. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Q.; Qiu, J.; Wang, F.; Lin, W.; Zhu, F.; Wei, C.; Zhou, N.; Ouyang, G. Enhanced Photocatalytic Degradation of Environmental Pollutants under Visible Irradiation by a Composite Coating. Env. Sci. Technol. 2017, 51, 5137–5145. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.; Filip, C.; Hadade, N.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-F.; Hong, J.-D. Dopamine-Melanin Nanofilms for Biomimetic Structural Coloration. Biomacromolecules 2015, 16, 660–666. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.-H.; Yang, M.; Baek, M.-J.; Kim, M.-H.; Lee, J.; Khademhosseini, A.; Kim, D.-D.; Cho, H.-J. Ferrous sulfate-directed dual-cross-linked hyaluronic acid hydrogels with long-term delivery of donepezil. Int. J. Pharm. 2020, 582, 119309. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic Acid Catechol: A Biopolymer Exhibiting a pH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, S.Y.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Kim, M.-H.; Kim, H.-J.; Lee, J.; Lee, K.; et al. Monopotassium phosphate-reinforced in situ forming injectable hyaluronic acid hydrogels for subcutaneous injection. Int. J. Biol. Macromol. 2020, 163, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Cibrao, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured Polymeric Coatings Based on Chitosan and Dopamine-Modified Hyaluronic Acid for Biomedical Applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.W. Catechol-Functionalized Hyaluronic Acid Hydrogels Enhance Angiogenesis and Osteogenesis of Human Adipose-Derived Stem Cells in Critical Tissue Defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, J.; Lee, J.Y. Universal surface modification using dopamine-hyaluronic acid conjugates for anti-biofouling. Int. J. Biol. Macromol. 2020, 151, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wan, H.; Kaper, H.J.; Sharma, P.K. Dopamine-conjugated hyaluronic acid delivered via intra-articular injection provides articular cartilage lubrication and protection. J. Colloid Interface Sci. 2022, 619, 207–218. [Google Scholar] [CrossRef]
- Melnik, T.; Ben Ameur, S.; Kanfar, N.; Vinet, L.; Delie, F.; Jordan, O. Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. Int. J. Mol. Sci. 2022, 23, 5706. [Google Scholar] [CrossRef]
- Sato, T.; Aoyagi, T.; Ebara, M.; Auzély-Velty, R. Catechol-modified hyaluronic acid: In situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym. Bull. 2017, 74, 4069–4085. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Gupta, S.; Namdev, K.R.; Banerjee, A.; Srivastava, A. Polydopamine and dopamine interfere with tetrazolium-based cytotoxicity assays and produce exaggerated cytocompatibility inferences. Biomater. Sci. 2021, 9, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- Tennant, J.R. Evaluation of the Trypan Blue Technique for Determination of Cell Viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef]
- Tsaousis, K.T.; Kopsachilis, N.; Tsinopoulos, I.T.; Dimitrakos, S.A.; Kruse, F.E.; Welge-Luessen, U. Time-dependent morphological alterations and viability of cultured human trabecular cells after exposure to Trypan blue. Clin. Exp. Ophthalmol. 2013, 41, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.K.; Yeung, C.K.; Lai, T.Y.; Chan, K.P.; Pang, C.P. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Br. J. Ophthalmol. 2004, 88, 1590–1594. [Google Scholar] [CrossRef]
- Chan, L.L.-Y.; Rice, W.L.; Qiu, J. Observation and quantification of the morphological effect of trypan blue rupturing dead or dying cells. PLoS ONE 2020, 15, e0227950. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.-Y.; Kuksin, D.; Laverty, D.J.; Saldi, S.; Qiu, J. Morphological observation and analysis using automated image cytometry for the comparison of trypan blue and fluorescence-based viability detection method. Cytotechnology 2015, 67, 461–473. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Pandey, N.; Hakamivala, A.; Xu, C.; Hariharan, P.; Radionov, B.; Huang, Z.; Liao, J.; Tang, L.; Zimmern, P.; Nguyen, K.T.; et al. Biodegradable Nanoparticles Enhanced Adhesiveness of Mussel-Like Hydrogels at Tissue Interface. Adv. Healthc. Mater. 2018, 7, e1701069. [Google Scholar] [CrossRef]
- Liu, L.; Ye, P.; Jiang, T.; Dai, S.; Zhou, L.; Zhang, L.; Cui, J.; Wang, Z.; Liu, J.; Yang, P.; et al. A novel micropattern platform constructed by TiO2 oxidation of PDA. Colloids Surf. B Biointerfaces 2023, 223, 113141. [Google Scholar] [CrossRef]
- Tedja, R.; Lim, M.; Amal, R.; Marquis, C. Effects of serum adsorption on cellular uptake profile and consequent impact of titanium dioxide nanoparticles on human lung cell lines. ACS Nano 2012, 6, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Mzimela, N.; Tichapondwa, S.; Chirwa, E. Visible-light-activated photocatalytic degradation of rhodamine B using WO3 nanoparticles. RSC Adv. 2022, 12, 34652–34659. [Google Scholar] [CrossRef]
- Luo, M.; Xu, J.; Xu, W.; Zheng, Y.; Wu, G.; Jeong, T. Photocatalytic Activity of MoS2 Nanoflower-Modified CaTiO3 Composites for Degradation of RhB under Visible Light. Nanomaterials 2023, 13, 636. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Lei, Z.; Liu, L.; Zhang, J.; You, H.; Zhang, H.; Wang, Z. Facile Preparation of Doxorubicin-Loaded Upconversion@Polydopamine Nanoplatforms for Simultaneous In Vivo Multimodality Imaging and Chemophotothermal Synergistic Therapy. Adv. Healthc. Mater. 2015, 4, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.; Zhu, D.; Yu, X.; Ouyang, X.; Xie, Z.; Zhao, Y.; et al. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.; Hamilton, J.W.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Miao, Y.-E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Tomić, A.; Sinisa, M. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period. Technol. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Srinivasan, C.; Somasundaram, N. Bactericidal and detoxification effects of irradiated semiconductor catalyst, TiO2. Curr. Sci. 2003, 85, 1431–1438. [Google Scholar]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Envion. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, J.; Michta, E.; Rybicka, M.; Grinholc, M.; Gwizdek-Wiśniewska, A.; Bielawski, K.P. Superoxide dismutase is upregulated in Staphylococcus aureus following protoporphyrin-mediated photodynamic inactivation and does not directly influence the response to photodynamic treatment. BMC Microbiol. 2010, 10, 323. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial Actions of Reactive Oxygen Species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Gopal, J.; Muraleedharan, P.; Tyagi, A.K.; Raj, B. Physics and chemistry of photocatalytic titanium dioxide: Visualization of bactericidal activity using atomic force microscopy. Curr. Sci. 2006, 90, 1378–1383. [Google Scholar]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar] [PubMed]
- Tyo, A.; Welch, S.; Hennenfent, M.; Fooroshani, P.K.; Lee, B.P.; Rajachar, R. Development and Characterization of an Antimicrobial Polydopamine Coating for Conservation of Humpback Whales. Front. Chem. 2019, 7, 618. [Google Scholar] [CrossRef]
- Iqbal, Z.; Lai, E.P.C.; Avis, T.J. Antimicrobial effect of polydopamine coating on Escherichia coli. J. Mater. Chem. 2012, 22, 21608–21612. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus of Colloidal Polydopamine Prepared by Carbon Dot Stimulated Polymerization of Dopamine. Nanomaterials 2019, 9, 1731. [Google Scholar] [CrossRef]
- Chang, R.; Zhao, D.; Zhang, C.; Liu, K.; He, Y.; Guan, F.; Yao, M. Nanocomposite multifunctional hyaluronic acid hydrogel with photothermal antibacterial and antioxidant properties for infected wound healing. Int. J. Biol. Macromol. 2023, 226, 870–884. [Google Scholar] [CrossRef]
- Han, D.; Han, Y.; Li, J.; Liu, X.; Yeung, K.W.K.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; et al. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl. Catal. B Environ. 2020, 261, 118248. [Google Scholar] [CrossRef]
- Qi, L.; Guo, B.; Lu, Q.; Gong, H.; Wang, M.; He, J.; Jia, B.; Ren, J.; Zheng, S.; Lu, Y. Preparation and Photocatalytic and Antibacterial Activities of Micro/Nanostructured TiO2-Based Photocatalysts for Application in Orthopedic Implants. Front. Mater. 2022, 9, 914905. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Liu, Z.; Wang, Y.; Lin, D.; Chen, L.; Dai, J.; Lin, K.; Shen, S.G. Polydopamine nanoparticles as dual-task platform for osteoarthritis therapy: A scavenger for reactive oxygen species and regulator for cellular powerhouses. Chem. Eng. J. 2021, 417, 129284. [Google Scholar] [CrossRef]
- Shi, J.-L.; Lang, X. Assembling polydopamine on TiO2 for visible light photocatalytic selective oxidation of sulfides with aerial O2. Chem. Eng. J. 2020, 392, 123632. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Faria, M.; Primc, G.; Pottathara, Y.; Leskovšek, M.; Gorjanc, M.; Mozetič, M.; Thomas, S.; et al. Nanofibrils vs nanocrystals bio-nanocomposites based on sodium alginate matrix: An improved-performance study. Heliyon 2020, 6, e03266. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Liang, H.; Jia, Z.; Zhang, H.; Wang, X.; Wang, J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B. Appl. Surf. Sci. 2017, 422, 1–10. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Reaction Mechanism and Degradation Pathway of Rhodamine 6G by Photocatalytic Treatment. Water Air Soil. Pollut. 2017, 228, 291. [Google Scholar] [CrossRef]
- Ariyanti, D.; Maillot, M.; Gao, W. TiO2 used as photocatalyst for rhodamine B degradation under solar radiation. Int. J. Mod. Phys. B 2017, 31, 1744095. [Google Scholar] [CrossRef]
- Algubury, H.; Alsaady, H. Photcatalytic Degradation of Rhodamine b using Titanium Dioxide. Int. J. Multidiscip. Curr. Res. 2015, 33, 98–104. [Google Scholar]
- Lu, X.; Shi, S.; Li, H.; Gerhard, E.; Lu, Z.; Tan, X.; Li, W.; Rahn, K.M.; Xie, D.; Xu, G.; et al. Magnesium oxide-crosslinked low-swelling citrate-based mussel-inspired tissue adhesives. Biomaterials 2020, 232, 119719. [Google Scholar] [CrossRef]
- EPA. Interim Method for Evaluating the Efficacy of Antimicrobial Surface Coatings. Available online: https://www.epa.gov/pesticide-analytical-methods/antimicrobial-testing-methods-procedures-interim-method-evaluating (accessed on 1 March 2022).
- Hamilton, M. The Log Reduction (LR) Measure of Disinfectant Efficacy. Available online: https://biofilm.montana.edu/documents/KSA-SM-07.pdf (accessed on 1 March 2022).
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Garcia, L.F.; Guerrero-Rodriguez, I.D.; Hoang, L.; Laboy-Segarra, S.L.; Phan, N.T.K.; Villafuerte, E.; Lee, J.; Nguyen, K.T. Photocatalytic and Photothermal Antimicrobial Mussel-Inspired Nanocomposites for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 13272. https://doi.org/10.3390/ijms241713272
Soto-Garcia LF, Guerrero-Rodriguez ID, Hoang L, Laboy-Segarra SL, Phan NTK, Villafuerte E, Lee J, Nguyen KT. Photocatalytic and Photothermal Antimicrobial Mussel-Inspired Nanocomposites for Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(17):13272. https://doi.org/10.3390/ijms241713272
Chicago/Turabian StyleSoto-Garcia, Luis F., Ingrid D. Guerrero-Rodriguez, Luu Hoang, Samantha Lauren Laboy-Segarra, Ngan T. K. Phan, Enrique Villafuerte, Juhyun Lee, and Kytai T. Nguyen. 2023. "Photocatalytic and Photothermal Antimicrobial Mussel-Inspired Nanocomposites for Biomedical Applications" International Journal of Molecular Sciences 24, no. 17: 13272. https://doi.org/10.3390/ijms241713272







