Underlying Molecular Mechanism and Construction of a miRNA-Gene Network in Idiopathic Pulmonary Fibrosis by Bioinformatics
Abstract
:1. Introduction
2. Results
2.1. The Identification of DEGs
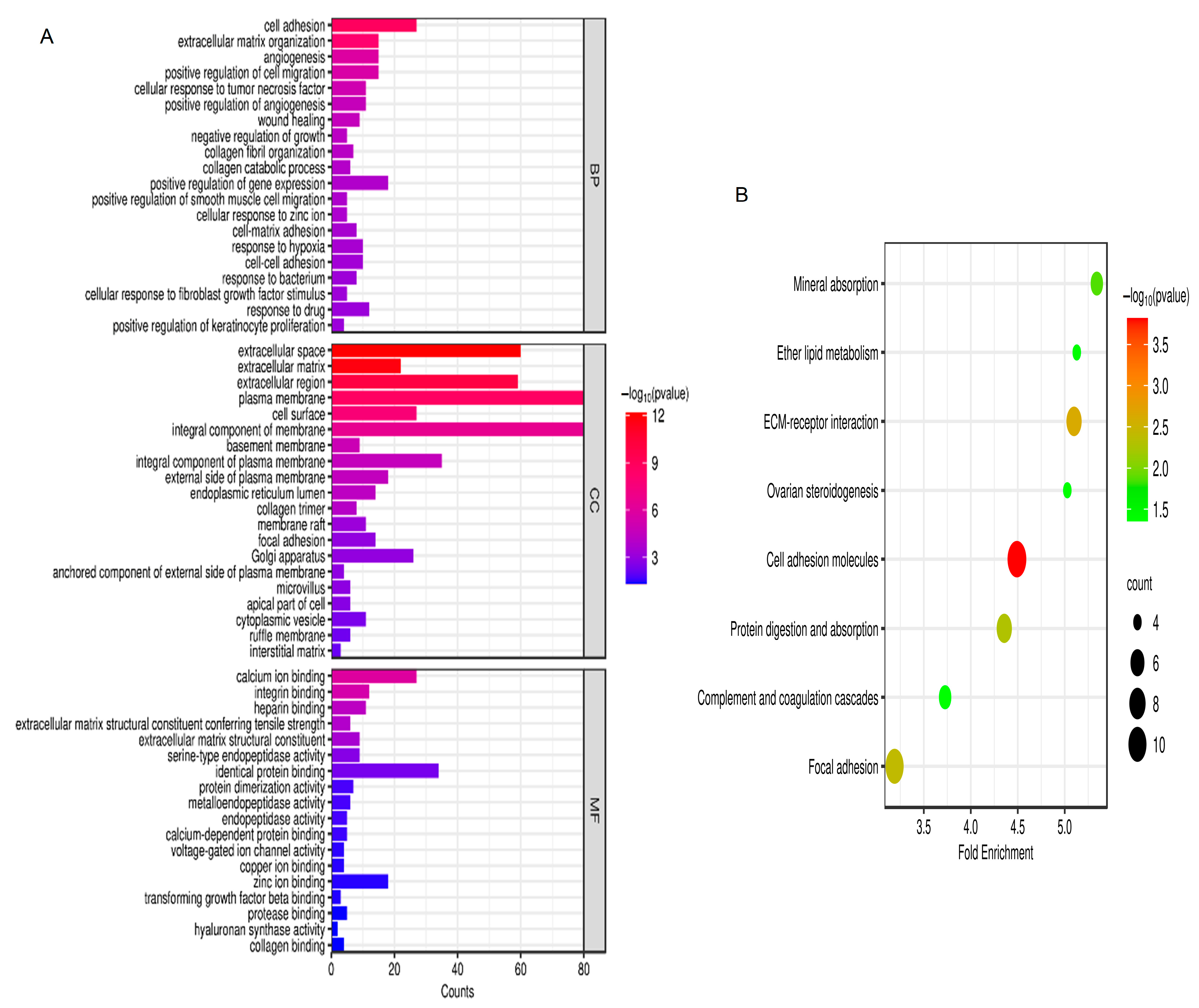
2.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
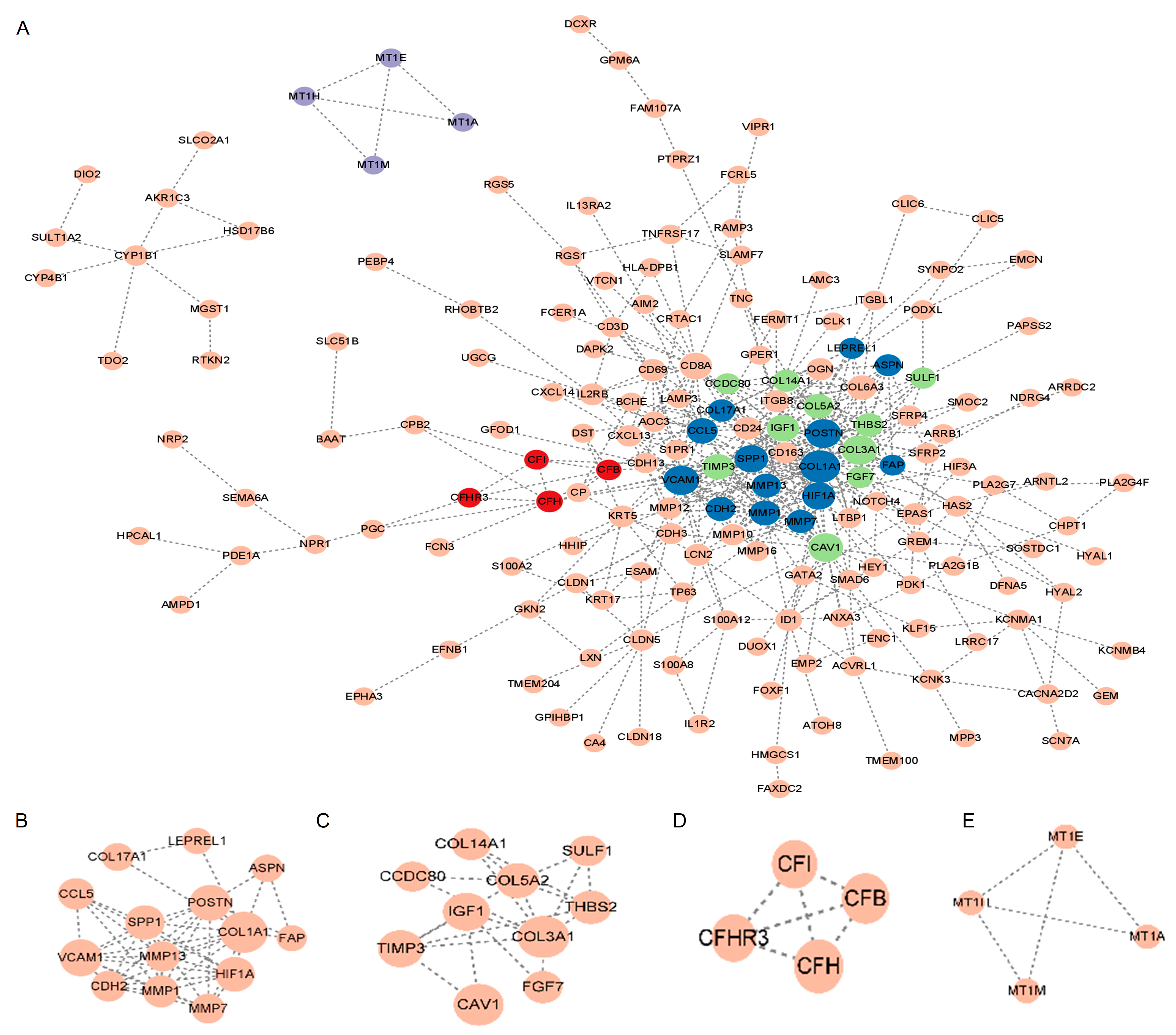
2.3. Protein–Protein Interaction (PPI) Network
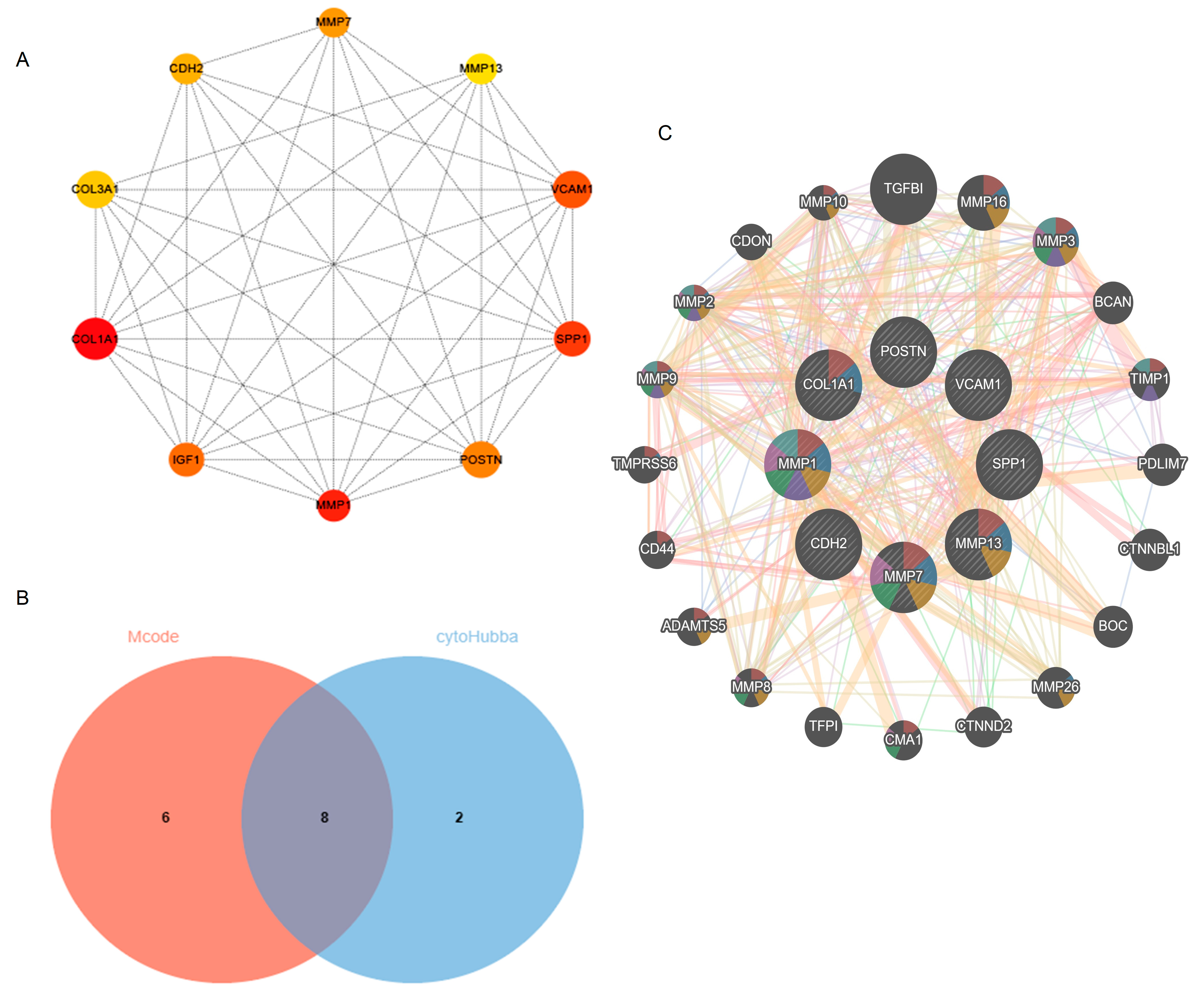
2.4. Hub Gene Selection
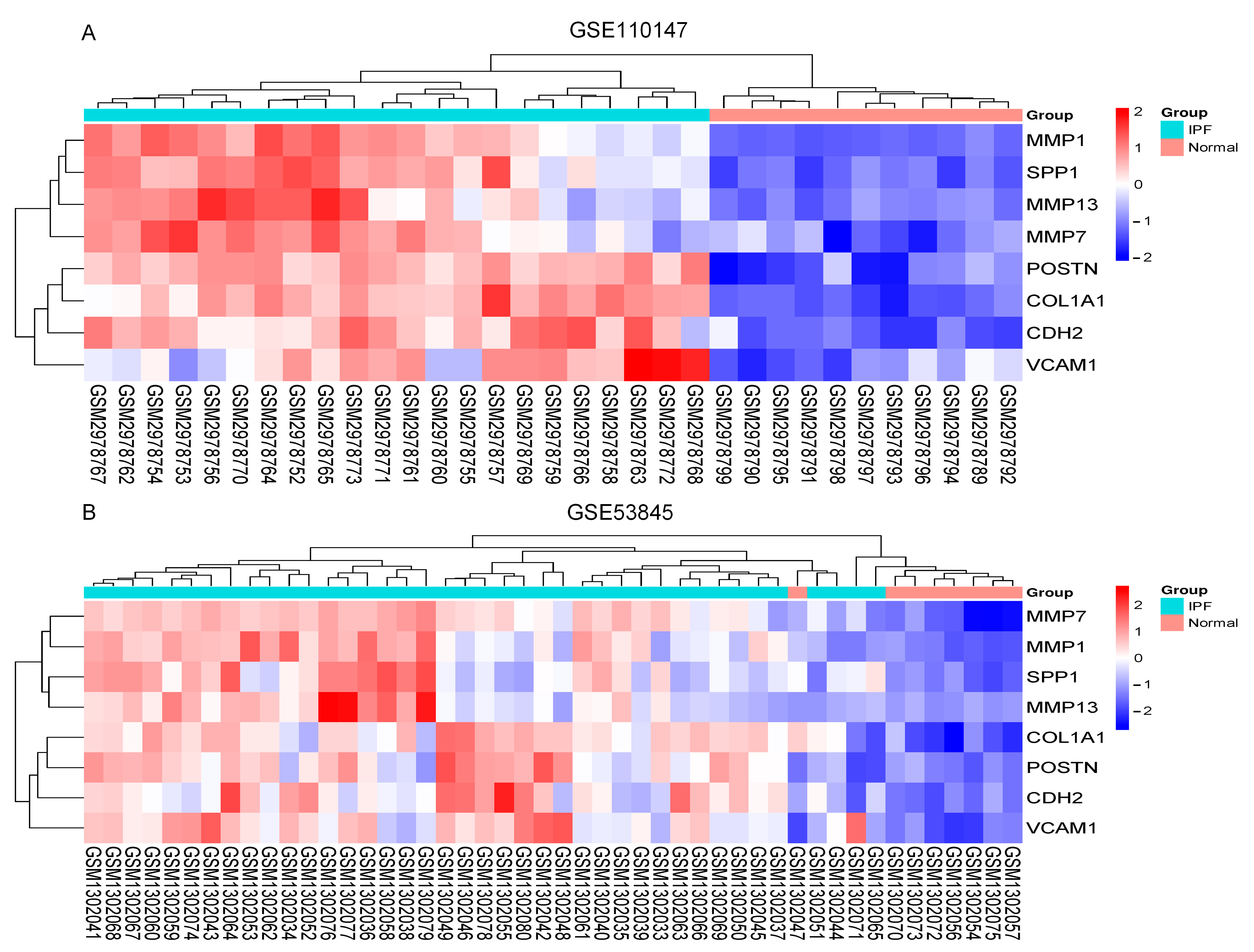
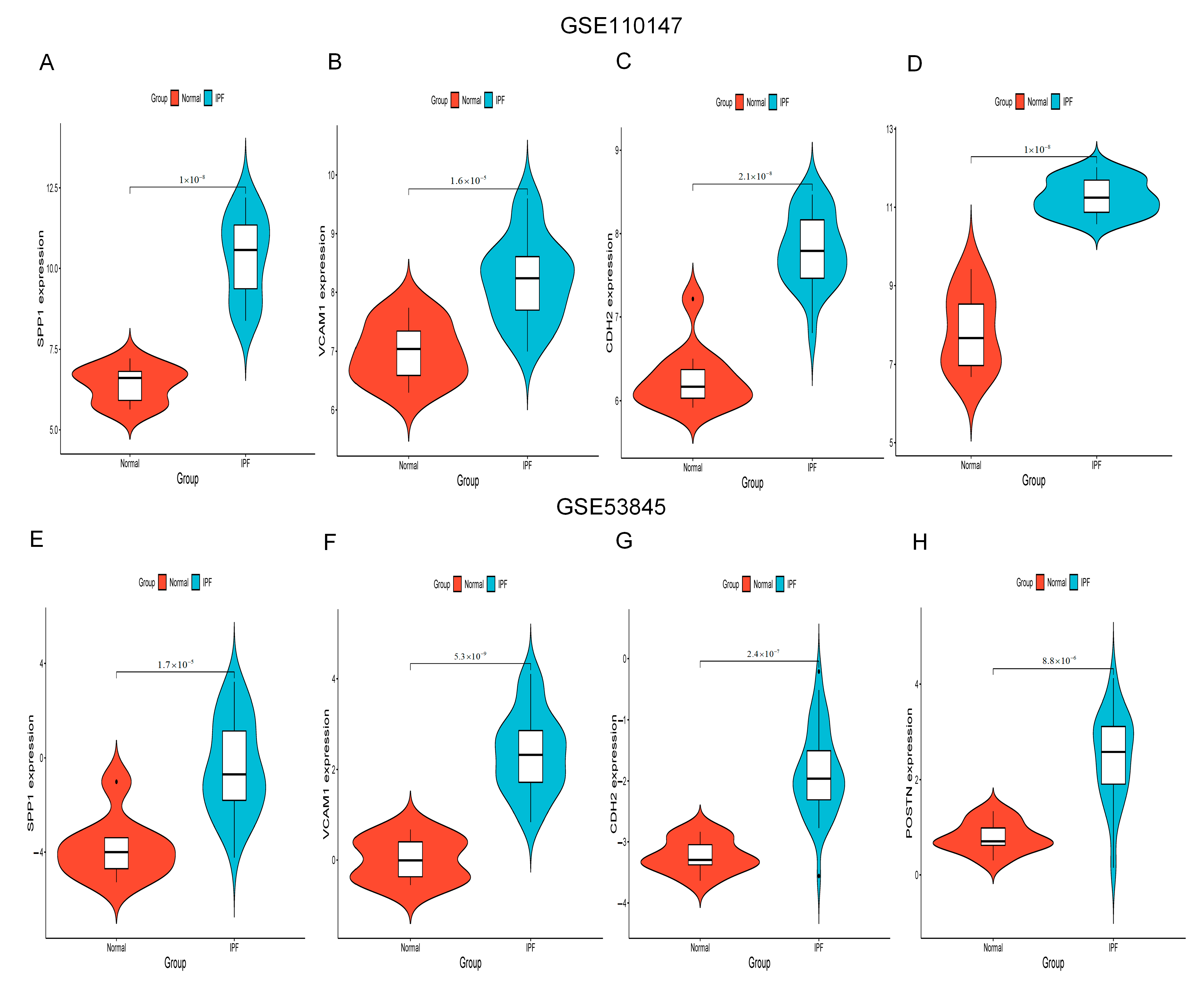
2.5. Increased Expression of Hub Gene in IPF Lung Tissues
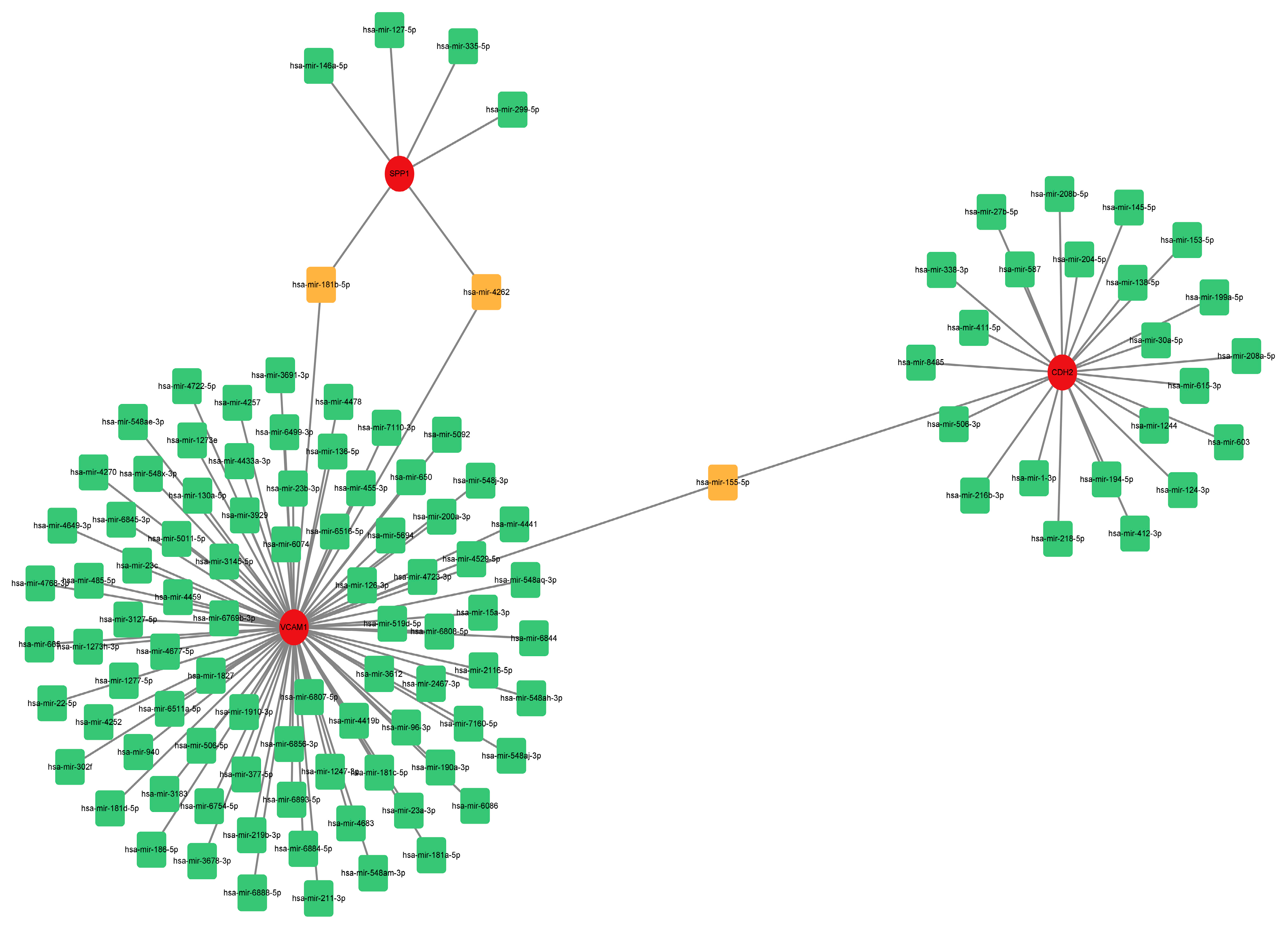
2.6. Construction of a microRNA (miRNA)-Gene Network
3. Discussion
4. Materials and Methods
4.1. Data Download
4.2. DEGs Screening and Data Processing
4.3. KEGG and GO Enrichment Analysis
4.4. PPI Network
4.5. Hub Genes
4.6. Animals and BLM-Induced Mouse Model
4.7. Collection of Lung Tissue
4.8. Pathological Staining
4.9. RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
4.10. MiRNAs Associated with Hub Genes
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Calabrese, F.; Lunardi, F.; Tauro, V.; Pezzuto, F.; Fortarezza, F.; Vedovelli, L.; Faccioli, E.; Balestro, E.; Schiavon, M.; Esposito, G.; et al. RNA Sequencing of Epithelial Cell/Fibroblastic Foci Sandwich in Idiopathic Pulmonary Fibrosis: New Insights on the Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Deskin, B.; Rehan, M.; Yadav, S.; Matsunaga, Y.; Lasky, J.A.; Thannickal, V.J. Novel mediators of idiopathic pulmonary fibrosis. Clin. Sci. 2022, 136, 1229–1240. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Giudici, F.; Wade, B.; Santagiuliana, M.; Salton, F.; Confalonieri, P.; Simbolo, M.; Scarpa, A.; Tollot, S.; et al. Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics 2021, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef]
- Confalonieri, P.; Volpe, M.C.; Jacob, J.; Maiocchi, S.; Salton, F.; Ruaro, B.; Confalonieri, M.; Braga, L. Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF). Cells 2022, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Akalin, P.K. Introduction to bioinformatics. Mol. Nutr. Food Res. 2006, 50, 610–619. [Google Scholar] [CrossRef]
- Gauthier, J.; Vincent, A.T.; Charette, S.J.; Derome, N. A brief history of bioinformatics. Brief. Bioinform. 2019, 20, 1981–1996. [Google Scholar]
- Cecchini, M.J.; Hosein, K.; Howlett, C.J.; Joseph, M.; Mura, M. Comprehensive gene expression profiling identifies distinct and overlapping transcriptional profiles in non-specific interstitial pneumonia and idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Xiao, K.; Zhang, X.; Wang, P.; Xiao, S.; Qi, H.; Meng, L.; Zhang, X.; Shen, F. Bioinformatics analysis on differentially expressed genes of alveolar macrophage in IPF. Exp. Lung Res. 2019, 45, 288–296. [Google Scholar] [CrossRef]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Cottin, V. Genetics of idiopathic pulmonary fibrosis: From mechanistic pathways to personalised medicine. J. Med. Genet. 2017, 54, 93–99. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Islam, S.; Caporarello, N.; Kostallari, E.; Dielis, K.; Tschumperlin, D.J.; Hirsova, P.; Ibrahim, S.H. Liver sinusoidal endothelial cell expressed vascular cell adhesion molecule 1 promotes liver fibrosis. Front. Immunol. 2022, 13, 983255. [Google Scholar] [PubMed]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar]
- Johnson, A.; DiPietro, L.A. Apoptosis and angiogenesis: An evolving mechanism for fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 3893–3901. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar]
- Hu, Q.; Saleem, K.; Pandey, J.; Charania, A.N.; Zhou, Y.; He, C. Cell Adhesion Molecules in Fibrotic Diseases. Biomedicines 2023, 11, 1995. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Shin, S.K.; Choi, M.S. Ursolic Acid Attenuates Hepatic Steatosis, Fibrosis, and Insulin Resistance by Modulating the Circadian Rhythm Pathway in Diet-Induced Obese Mice. Nutrients 2018, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.M.; Waters, C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, Y.; Zhang, X.; Wu, Y.; Song, Y.; Zhang, F.; Zhang, J.; Sun, H. Time-resolved proteome and transcriptome of paraquat-induced pulmonary fibrosis. Pulm. Pharmacol. Ther. 2022, 75, 102145. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]
- Rosas, I.O.; Richards, T.J.; Konishi, K.; Zhang, Y.; Gibson, K.; Lokshin, A.E.; Lindell, K.O.; Cisneros, J.; Macdonald, S.D.; Pardo, A.; et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008, 5, e93. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Kang, L.; Wang, Z.; Wang, Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm. Med. 2017, 17, 63. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, D.; Shen, J.; Yan, Y.; Gong, C.; Gu, J.; Xue, H.; Qian, Y.; Zhang, W.; He, X.; et al. Up-regulation of VCAM1 Relates to Neuronal Apoptosis After Intracerebral Hemorrhage in Adult Rats. Neurochem. Res. 2015, 40, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Agassandian, M.; Tedrow, J.R.; Sembrat, J.; Kass, D.J.; Zhang, Y.; Goncharova, E.A.; Kaminski, N.; Mallampalli, R.K.; Vuga, L.J. VCAM-1 is a TGF-β1 inducible gene upregulated in idiopathic pulmonary fibrosis. Cell Signal. 2015, 27, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- László, Z.I.; Lele, Z. Flying under the radar: CDH2 (N-cadherin), an important hub molecule in neurodevelopmental and neurodegenerative diseases. Front. Neurosci. 2022, 16, 972059. [Google Scholar]
- Beaven, E.; Kumar, R.; Bhatt, H.N.; Esquivel, S.V.; Nurunnabi, M. Myofibroblast specific targeting approaches to improve fibrosis treatment. Chem. Commun. (Camb. Engl.) 2022, 58, 13556–13571. [Google Scholar] [CrossRef]
- O’Regan, A. The role of osteopontin in lung disease. Cytokine Growth Factor. Rev. 2003, 14, 479–488. [Google Scholar] [CrossRef]
- Depalle, B.; McGilvery, C.M.; Nobakhti, S.; Aldegaither, N.; Shefelbine, S.J.; Porter, A.E. Osteopontin regulates type I collagen fibril formation in bone tissue. Acta Biomater. 2021, 120, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Matsuda, K.; Uehara, T. Osteopontin enhances the migration of lung fibroblasts via upregulation of interleukin-6 through the extracellular signal-regulated kinase (ERK) pathway. Biol. Chem. 2020, 401, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, D.N.; Moore, B.B. The role of periostin in lung fibrosis and airway remodeling. Cell. Mol. Life Sci. CMLS 2017, 74, 4305–4314. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, R.; Zhao, M.; Garcia-Gomez, A.; Ballestar, E. miRNAs as Therapeutic Targets in Inflammatory Disease. Trends Pharmacol. Sci. 2019, 40, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Kyriakidis, K.; Tsezou, A. MicroRNAs and the Diagnosis of Childhood Acute Lymphoblastic Leukemia: Systematic Review, Meta-Analysis and Re-Analysis with Novel Small RNA-Seq Tools. Cancers 2022, 14, 3976. [Google Scholar] [CrossRef]
- Wang, K.; Ren, Y.; Liu, Y.; Zhang, J.; He, J.J. miR-4262 Promotes Proliferation and Invasion of Human Breast Cancer Cells Through Directly Targeting KLF6 and KLF15. Oncol. Res. 2017, 25, 277–283. [Google Scholar] [CrossRef]
- Guo, L.; Tan, K.; Luo, Q.; Bai, X. Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn. J. Basic. Med. Sci. 2020, 20, 372–380. [Google Scholar] [CrossRef]
- Wu, L.; Pu, L.; Zhuang, Z. miR-155-5p/FOXO3a promotes pulmonary fibrosis in rats by mediating NLRP3 inflammasome activation. Immunopharmacol. Immunotoxicol. 2023, 45, 257–267. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- DePianto, D.J.; Chandriani, S.; Abbas, A.R.; Jia, G.; N’Diaye, E.N.; Caplazi, P.; Kauder, S.E.; Biswas, S.; Karnik, S.K.; Ha, C.; et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax 2015, 70, 48–56. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics (Oxf. Engl.) 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Bandettini, W.P.; Kellman, P.; Mancini, C.; Booker, O.J.; Vasu, S.; Leung, S.W.; Wilson, J.R.; Shanbhag, S.M.; Chen, M.Y.; Arai, A.E. MultiContrast Delayed Enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: A clinical validation study. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2012, 14, 83. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef]


| Data Set | Platforms | Sample | Normal | IPF | General Information |
|---|---|---|---|---|---|
| GSE110147 | GPL6244 | 33 | 11 | 22 | disease state, tissue |
| GSE53845 | GPL6480 | 48 | 8 | 40 | disease state, tissue, diagnosis, source, gender, sample type |
| NO. | Gene Symbol | Full Name | Function |
|---|---|---|---|
| 1 | MMP13 | Matrix Metallopeptidase 13 | Plays a role in the degradation of extracellular matrix proteins including fibrillar collagen, fibronectin, TNC and ACAN. |
| 2 | SPP1 | Secreted Phosphoprotein 1 | Major non-collagenous bone protein that binds tightly to hydroxyapatite. Appears to form an integral part of the mineralized matrix. Probably important to cell–matrix interaction. |
| 3 | MMP7 | Matrix Metallopeptidase 7 | Degrades casein, gelatins of types I, III, IV, and V, and fibronectin. Activates procollagenase. |
| 4 | VCAM1 | Vascular Cell Adhesion Molecule 1 | Cell adhesion glycoprotein predominantly expressed on the surface of endothelial cells that plays an important role in immune surveillance and inflammation. Acts as a major regulator of leukocyte adhesion to the endothelium through interaction with different types of integrins. |
| 5 | CDH2 | Cadherin 2 | Calcium-dependent cell adhesion protein; preferentially mediates homotypic cell–cell adhesion by dimerization with a CDH2 chain from another cell. Cadherins may thus contribute to the sorting of heterogeneous cell types. |
| 6 | COL1A1 | Collagen Type I Alpha 1 Chain | Type I collagen is a member of group I collagen (fibrillar forming collagen). |
| 7 | POSTN | Periostin | Induces cell attachment and spreading and plays a role in cell adhesion. Enhances incorporation of BMP1 in the fibronectin matrix of connective tissues, and subsequent proteolytic activation of lysyl oxidase LOX (By similarity). |
| 8 | MMP1 | Matrix Metallopeptidase 1 | Cleaves collagens of types I, II, and III at one site in the helical domain. Also cleaves collagens of types VII and X. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhang, Y.; Hou, Y.; Li, H.; He, J.; Zhao, H.; Sun, X.; Liu, Y. Underlying Molecular Mechanism and Construction of a miRNA-Gene Network in Idiopathic Pulmonary Fibrosis by Bioinformatics. Int. J. Mol. Sci. 2023, 24, 13305. https://doi.org/10.3390/ijms241713305
Zheng S, Zhang Y, Hou Y, Li H, He J, Zhao H, Sun X, Liu Y. Underlying Molecular Mechanism and Construction of a miRNA-Gene Network in Idiopathic Pulmonary Fibrosis by Bioinformatics. International Journal of Molecular Sciences. 2023; 24(17):13305. https://doi.org/10.3390/ijms241713305
Chicago/Turabian StyleZheng, Shuping, Yan Zhang, Yangfan Hou, Hongxin Li, Jin He, Hongyan Zhao, Xiuzhen Sun, and Yun Liu. 2023. "Underlying Molecular Mechanism and Construction of a miRNA-Gene Network in Idiopathic Pulmonary Fibrosis by Bioinformatics" International Journal of Molecular Sciences 24, no. 17: 13305. https://doi.org/10.3390/ijms241713305
APA StyleZheng, S., Zhang, Y., Hou, Y., Li, H., He, J., Zhao, H., Sun, X., & Liu, Y. (2023). Underlying Molecular Mechanism and Construction of a miRNA-Gene Network in Idiopathic Pulmonary Fibrosis by Bioinformatics. International Journal of Molecular Sciences, 24(17), 13305. https://doi.org/10.3390/ijms241713305






