Exploring the Skin Brain Link: Biomarkers in the Skin with Implications for Aging Research and Alzheimer’s Disease Diagnostics
Abstract
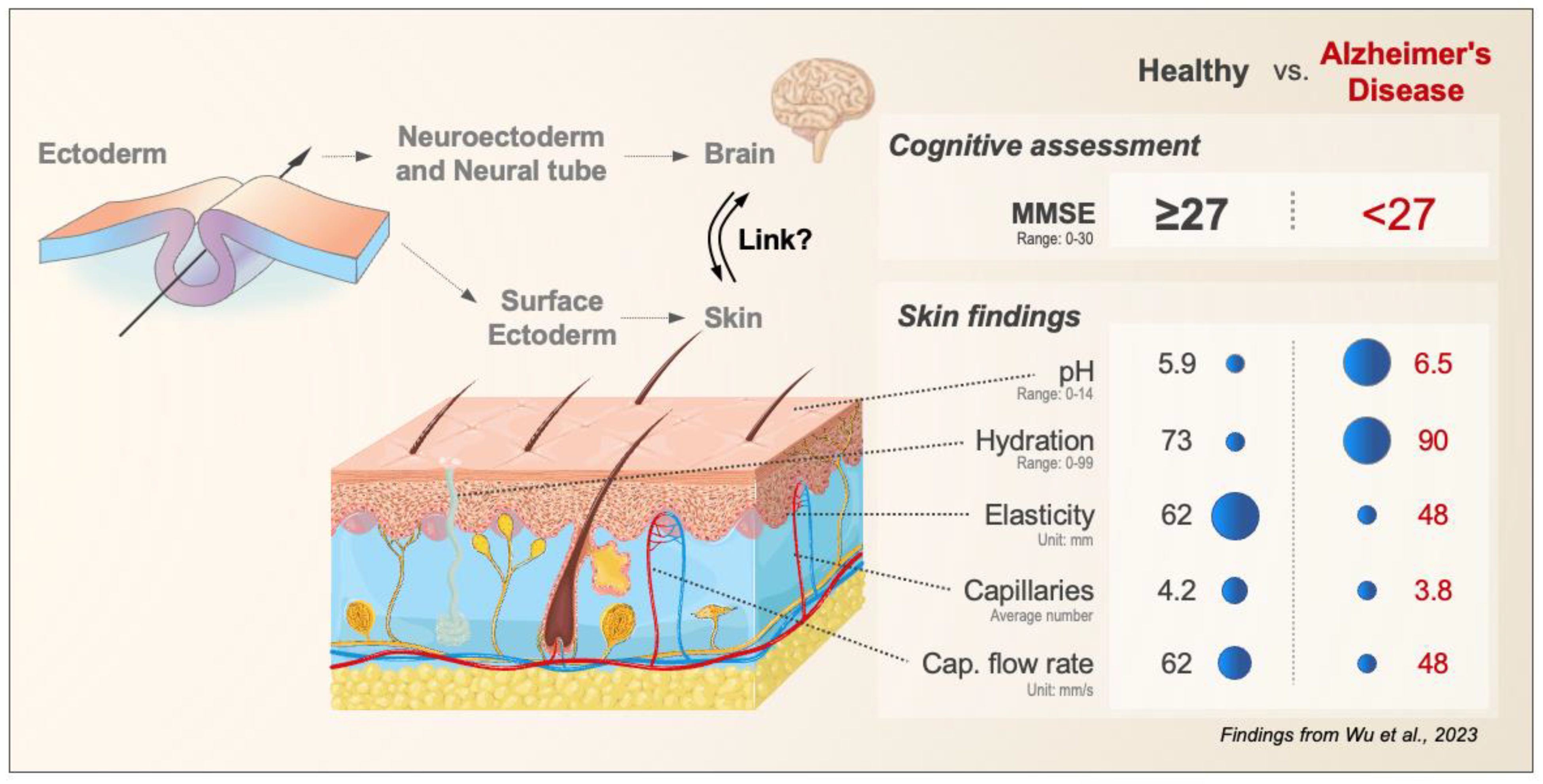
:1. Introduction
2. Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Wu, C.Y.; Ho, C.Y.; Yang, Y.H. Developing Biomarkers for the Skin: Biomarkers for the Diagnosis and Prediction of Treatment Outcomes of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8478. [Google Scholar] [CrossRef]
- Bacigalupo, I.; Mayer, F.; Lacorte, E.; Di Pucchio, A.; Marzolini, F.; Canevelli, M.; Di Fiandra, T.; Vanacore, N. A Systematic Review and Meta-Analysis on the Prevalence of Dementia in Europe: Estimates from the Highest-Quality Studies Adopting the DSM IV Diagnostic Criteria. J. Alzheimers Dis. 2018, 66, 1471–1481. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- O’bryant, S.E.; Lacritz, L.H.; Hall, J.; Waring, S.C.; Chan, W.; Khodr, Z.G.; Massman, P.J.; Hobson, V.; Cullum, C.M. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Arch. Neurol. 2010, 67, 746–749. [Google Scholar] [CrossRef]
- Spemann, H. Über Korrelationen in der Entwicklung des Auges/on correlations in the developemt of the eye. Verh. Anat. Ges. Jena 1901, 15, 61–79. [Google Scholar]
- Spemann, H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren/on induction of embryo anlagen by implantation of organizers of other species. Arch. Mikrosk. Anat. Entwicklungsmech. 1924, 100, 599–638. [Google Scholar] [CrossRef]
- Pijuan-Sala, B.; Griffiths, J.A.; Guibentif, C.; Hiscock, T.W.; Jawaid, W.; Calero-Nieto, F.J.; Mulas, C.; Ibarra-Soria, X.; Tyser, R.C.V.; Ho, D.L.L.; et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019, 566, 490–495. [Google Scholar] [CrossRef]
- Gaspard, N.; Vanderhaeghen, P. Mechanisms of neural specification from embryonic stem cells. Curr. Opin. Neurobiol. 2010, 20, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, P.K.; Das, J.M. Neuroanatomy, Neural Tube Development and Stages. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Hopper, A.D.; Jalal, M.; Munir, A. Recent advances in the diagnosis and management of pancreatic neuroendocrine tumours. Front. Gastroenterol. 2019, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.P.; McMillin, J.M.; Aceto, T., Jr.; Bruins, G. A newly recognized neuroectodermal syndrome of familial alopecia, anosmia, deafness, and hypogonadism. Am. J. Med. Genet. 1983, 15, 497–506. [Google Scholar] [CrossRef]
- Swarup, M.S.; Gupta, S.; Singh, S.; Prakash, A.; Mehndiratta, A.; Garg, A. Phakomatoses: A pictorial review. Indian J. Radiol. Imaging 2020, 30, 195–205. [Google Scholar] [CrossRef]
- Agache, P.G.; Monneur, C.; Leveque, J.L.; De Rigal, J. Mechanical properties and Young’s modulus of human skin in vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Escoffier, C.; de Rigal, J.; Rochefort, A.; Vasselet, R.; Leveque, J.L.; Agache, P.G. Age-related mechanical properties of human skin: An in vivo study. J. Investig. Dermatol. 1989, 93, 353–357. [Google Scholar] [CrossRef]
- Hall, C.M.; Moeendarbary, E.; Sheridan, G.K. Mechanobiology of the brain in ageing and Alzheimer’s disease. Eur. J. Neurosci. 2021, 53, 3851–3878. [Google Scholar] [CrossRef]
- Ma, J.; Ma, C.; Li, J.; Sun, Y.; Ye, F.; Liu, K.; Zhang, H. Extracellular Matrix Proteins Involved in Alzheimer’s Disease. Chemistry 2020, 26, 12101–12110. [Google Scholar] [CrossRef]
- Morgan, C.; Inestrosa, N.C. Interactions of laminin with the amyloid beta peptide. Implications for Alzheimer’s disease. Braz. J. Med. Biol. Res. 2001, 34, 597–601. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E.; Hossini, A.M. Skin Mirrors Brain: A Chance for Alzheimer’s Disease Research. Adv. Exp. Med. Biol. 2021, 1339, 371–380. [Google Scholar] [CrossRef]
- Fenske, N.A.; Lober, C.W. Structural and functional changes of normal aging skin. J. Am. Acad. Dermatol. 1986, 15 Pt 1, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, D.; Tang, K.; Sun, Q. The Relationship between Alzheimer’s Disease and Skin Diseases: A Review. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Bertalan, G.; Becker, J.; Tzschätzsch, H.; Morr, A.; Herthum, H.; Shahryari, M.; Greenhalgh, R.D.; Guo, J.; Schröder, L.; Alzheimer, C.; et al. Mechanical behavior of the hippocampus and corpus callosum: An attempt to reconcile ex vivo with in vivo and micro with macro properties. J. Mech. Behav. Biomed. Mater. 2023, 138, 105613. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Cogswell, P.M.; Trzasko, J.D.; Manduca, A.; Senjem, M.L.; Meyer, F.B.; Ehman, R.L.; Huston, J.I. Identification of Normal Pressure Hydrocephalus by Disease-Specific Patterns of Brain Stiffness and Damping Ratio. Investig. Radiol. 2020, 55, 200–208. [Google Scholar] [CrossRef]
- Hiscox, L.V.; Johnson, C.L.; McGarry, M.D.; Perrins, M.; Littlejohn, A.; van Beek, E.J.; Roberts, N.; Starr, J.M. High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults. Neurobiol. Aging 2018, 65, 158–167. [Google Scholar] [CrossRef]
- Urban, M.W.; Chen, S.; Greenleaf, J.F. Error in estimates of tissue material properties from shear wave dispersion ultrasound vibrometry. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 748–758. [Google Scholar] [CrossRef]
- Bridwell, D.A.; Wu, L.; Eichele, T.; Calhoun, V.D. The spatiospectral characterization of brain networks: Fusing concurrent EEG spectra and fMRI maps. Neuroimage 2013, 69, 101–111. [Google Scholar] [CrossRef]
- Murphy, M.C.; Jones, D.T.; Jack, C.R., Jr.; Glaser, K.J.; Senjem, M.L.; Manduca, A.; Felmlee, J.P.; Carter, R.E.; Ehman, R.L.; Huston, J. Regional brain stiffness changes across the Alzheimer’s disease spectrum. Neuroimage Clin. 2016, 10, 283–290. [Google Scholar] [CrossRef]
- Cecchi, C.; Fiorillo, C.; Sorbi, S.; Latorraca, S.; Nacmias, B.; Bagnoli, S.; Nassi, P.; Liguri, G. Oxidative stress and reduced antioxidant defenses in peripheral cells from familial Alzheimer’s patients. Free Radic. Biol. Med. 2002, 33, 1372–1379. [Google Scholar] [CrossRef]
- Jameson, C.; Boulton, K.A.; Silove, N.; Nanan, R.; Guastella, A.J. Ectodermal origins of the skin-brain axis: A novel model for the developing brain, inflammation, and neurodevelopmental conditions. Mol. Psychiatry 2023, 28, 108–117. [Google Scholar] [CrossRef]
- Koster, M.I.; Roop, D.R. Asymmetric cell division in skin development: A new look at an old observation. Dev. Cell 2005, 9, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Tournoy, J.; Bossuyt, X.; Snellinx, A.; Regent, M.; Garmyn, M.; Serneels, L.; Saftig, P.; Craessaerts, K.; De Strooper, B.; Hartmann, D. Partial loss of presenilins causes seborrheic keratosis and autoimmune disease in mice. Hum. Mol. Genet. 2004, 13, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, N.; Fucci, N. The current status of sweat testing for drugs of abuse: A review. Curr. Med. Chem. 2013, 20, 545–561. [Google Scholar] [CrossRef]
- Ray, T.R.; Ivanovic, M.; Curtis, P.M.; Franklin, D.; Guventurk, K.; Jeang, W.J.; Chafetz, J.; Gaertner, H.; Young, G.; Rebollo, S.; et al. Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med. 2021, 13, 587. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; Vecillas, L.d.L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34 (Suppl. S28), e13854. [Google Scholar] [CrossRef]
- Mishra, A.; Greaves, R.; Massie, J. The relevance of sweat testing for the diagnosis of cystic fibrosis in the genomic era. Clin. Biochem. Rev. 2005, 26, 135–153. [Google Scholar]
- Prakashan, D.; Ramya, P.R.; Gandhi, S. A Systematic Review on the Advanced Techniques of Wearable Point-of-Care Devices and Their Futuristic Applications. Diagnostics 2023, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Wang, N.; Rajan, S.; Kern, D.; Palma, J.A.; Kaufmann, H.; Freeman, R. Cutaneous alpha-Synuclein Signatures in Patients with Multiple System Atrophy and Parkinson Disease. Neurology 2023, 100, e1529–e1539. [Google Scholar] [CrossRef]
- Rodríguez-Leyva, I.; Chi-Ahumada, E.G.; Carrizales, J.; Rodríguez-Violante, M.; Velázquez-Osuna, S.; Medina-Mier, V.; Martel-Gallegos, M.G.; Zarazúa, S.; Enríquez-Macías, L.; Castro, A.; et al. Parkinson disease and progressive supranuclear palsy: Protein expression in skin. Ann. Clin. Transl. Neurol. 2016, 3, 191–199. [Google Scholar] [CrossRef]
- Qiang, L.; Fujita, R.; Yamashita, T.; Angulo, S.; Rhinn, H.; Rhee, D.; Doege, C.; Chau, L.; Aubry, L.; Vanti, W.B.; et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell 2011, 146, 359–371. [Google Scholar] [CrossRef]
- Bruzelius, A.; Kidnapillai, S.; Drouin-Ouellet, J.; Stoker, T.; Barker, R.A.; Rylander Ottosson, D. Reprogramming Human Adult Fibroblasts into GABAergic Interneurons. Cells 2021, 10, 3450. [Google Scholar] [CrossRef] [PubMed]
- Mollinari, C.; De Dominicis, C.; Lupacchini, L.; Sansone, L.; Caprini, D.; Casciola, C.M.; Wang, Y.; Zhao, J.; Fini, M.; Russo, M.; et al. Detection of Pathological Markers of Neurodegenerative Diseases following Microfluidic Direct Conversion of Patient Fibroblasts into Neurons. Int. J. Mol. Sci. 2022, 23, 2147. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Umegaki-Arao, N.; Guo, Z.; Liu, L.; Higgins, C.A.; Christiano, A.M. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS ONE 2013, 8, e77673. [Google Scholar] [CrossRef] [PubMed]
- Gunhanlar, N.; Shpak, G.; Van Der Kroeg, M.; Gouty-Colomer, L.A.; Munshi, S.T.; Lendemeijer, B.; Ghazvini, M.; Dupont, C.; Hoogendijk, W.J.G.; Gribnau, J.; et al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry 2018, 23, 1336–1344. [Google Scholar] [CrossRef]
- McKinney, C.E. Using induced pluripotent stem cells derived neurons to model brain diseases. Neural. Regen. Res. 2017, 12, 1062–1067. [Google Scholar] [CrossRef]
- Chirila, F.V.; Xu, G.; Fontaine, D.; Kern, G.; Khan, T.K.; Brandt, J.; Konishi, Y.; Nebe-Von-Caron, G.; White, C.L.; Alkon, D.L. Morphometric imaging biomarker identifies Alzheimer’s disease even among mixed dementia patients. Sci. Rep. 2022, 12, 17675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klostermeier, S.; Li, A.; Hou, H.X.; Green, U.; Lennerz, J.K. Exploring the Skin Brain Link: Biomarkers in the Skin with Implications for Aging Research and Alzheimer’s Disease Diagnostics. Int. J. Mol. Sci. 2023, 24, 13309. https://doi.org/10.3390/ijms241713309
Klostermeier S, Li A, Hou HX, Green U, Lennerz JK. Exploring the Skin Brain Link: Biomarkers in the Skin with Implications for Aging Research and Alzheimer’s Disease Diagnostics. International Journal of Molecular Sciences. 2023; 24(17):13309. https://doi.org/10.3390/ijms241713309
Chicago/Turabian StyleKlostermeier, Stefanie, Annie Li, Helen X. Hou, Ula Green, and Jochen K. Lennerz. 2023. "Exploring the Skin Brain Link: Biomarkers in the Skin with Implications for Aging Research and Alzheimer’s Disease Diagnostics" International Journal of Molecular Sciences 24, no. 17: 13309. https://doi.org/10.3390/ijms241713309
APA StyleKlostermeier, S., Li, A., Hou, H. X., Green, U., & Lennerz, J. K. (2023). Exploring the Skin Brain Link: Biomarkers in the Skin with Implications for Aging Research and Alzheimer’s Disease Diagnostics. International Journal of Molecular Sciences, 24(17), 13309. https://doi.org/10.3390/ijms241713309





